Assessment of Feed Value of Chicory and Lucerne for Poultry, Determination of Bioaccessibility of Their Polyphenols and Their Effects on Caecal Microbiota
Abstract
:1. Introduction
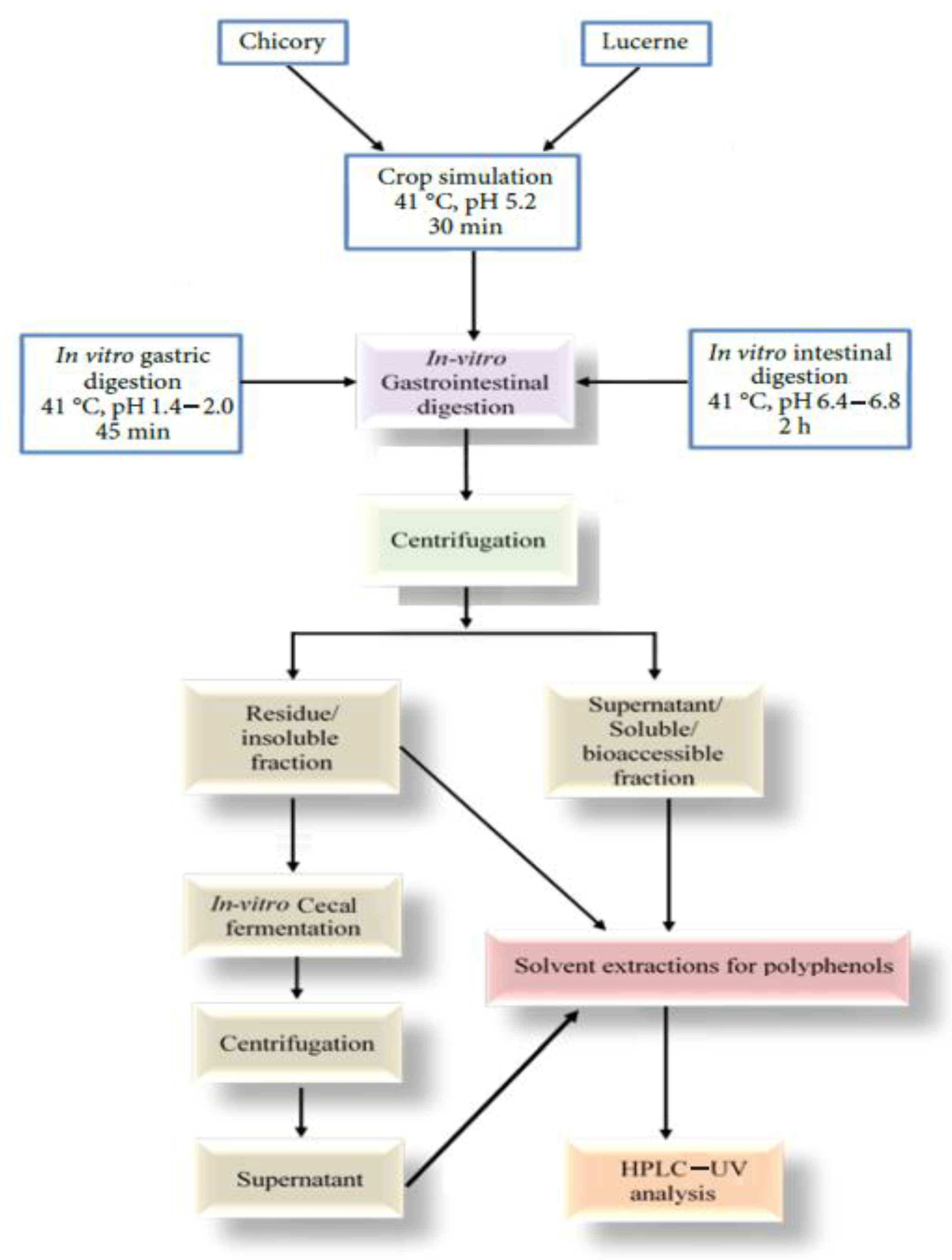
2. Materials and Methods
2.1. In Vitro Digestion
2.1.1. Sample Preparation
2.1.2. Gastrointestinal Digestion
2.2. In Vitro Cecal Fermentation
2.2.1. Cecal Slurry Preparation
2.2.2. Basal Media Preparation
2.2.3. Cecal Fermentation
2.3. Antioxidant Potential of Digestive Fractions
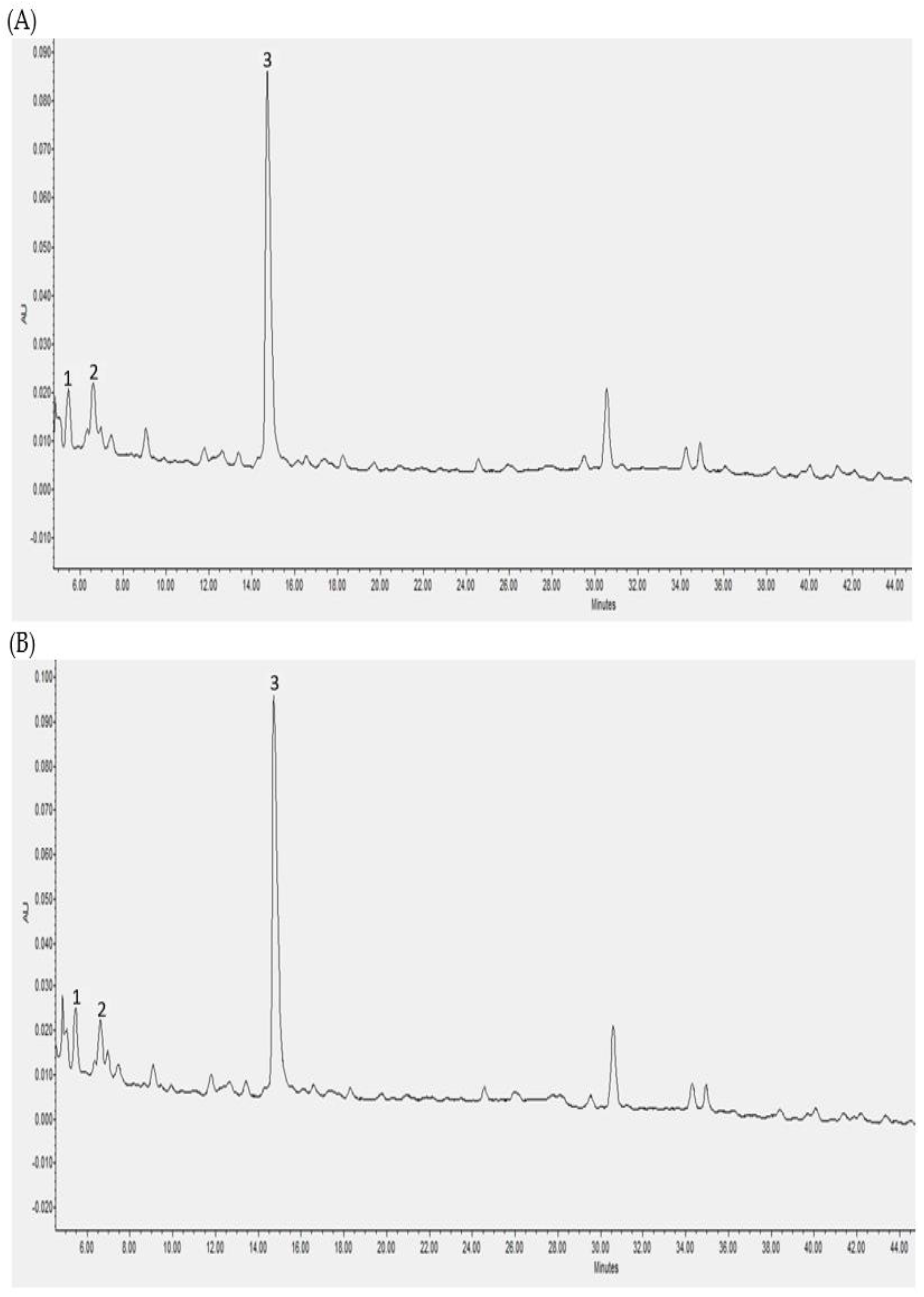
2.4. Separation and Analysis of Polyphenols
2.4.1. Quantification of Individual Polyphenols
2.4.2. Determination of Bioaccessibility of Polyphenols
2.5. Short-chain Fatty Acids Analysis
2.6. In Vitro Gas Production
2.7. 16S rRNA Sequencing and Analysis
2.8. Statistical Analysis
3. Results
3.1. Total Phenolic Content of Chicory and Lucerne during In Vitro Digestion and Cecal Fermentation
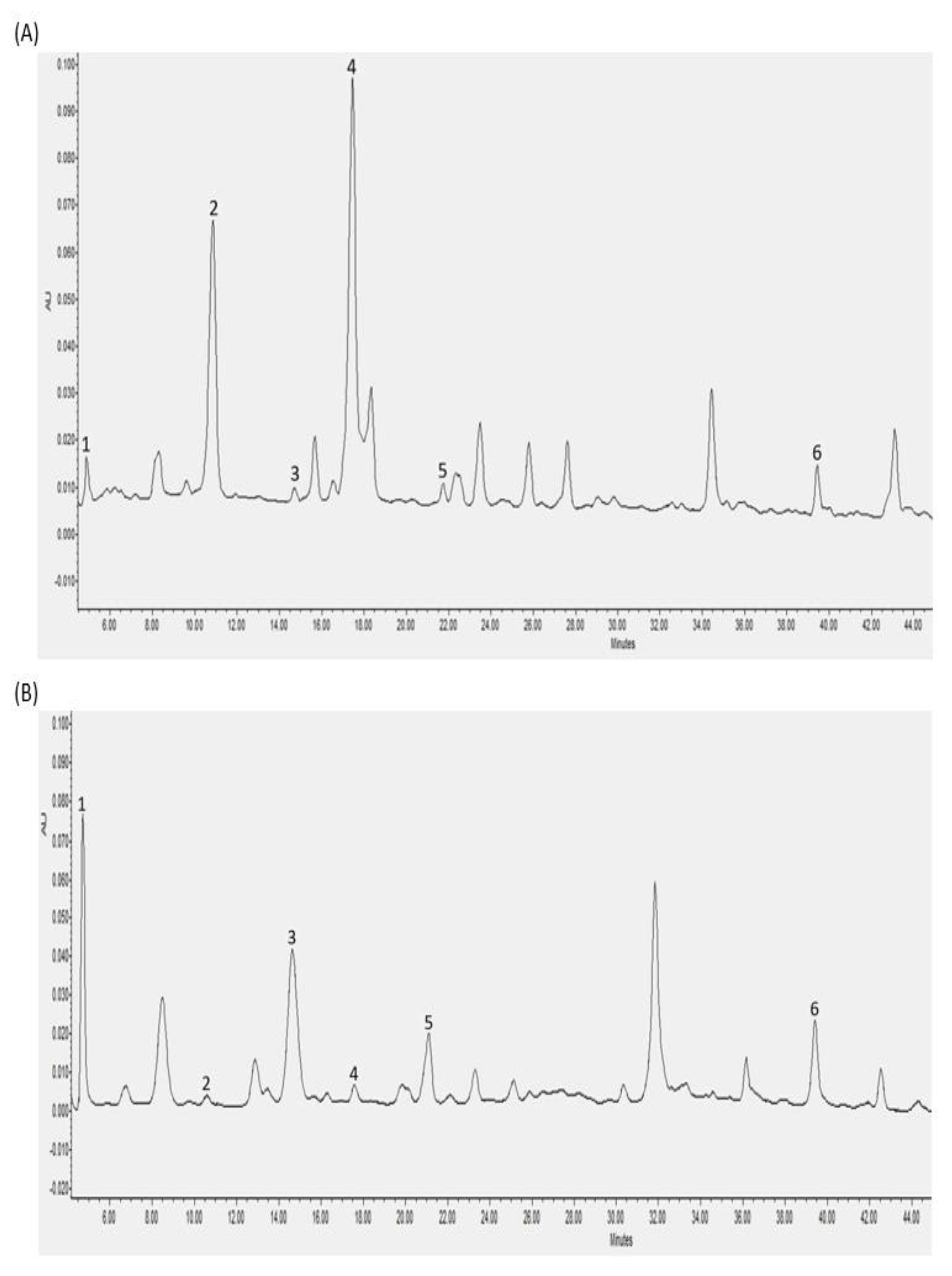
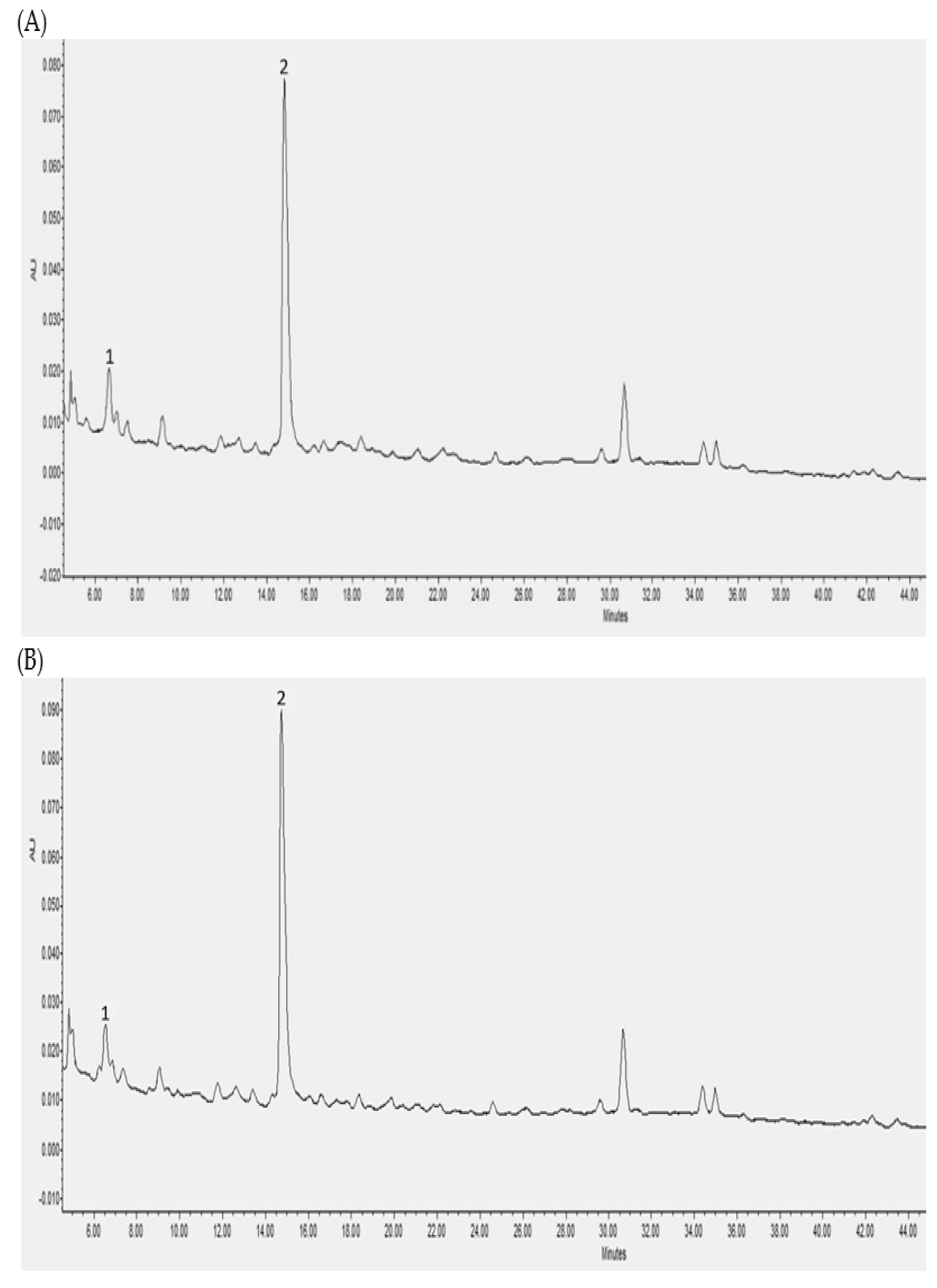
3.2. Individual Polyphenols Identified and Quantified after In Vitro Digestion and Their Bioaccessibility
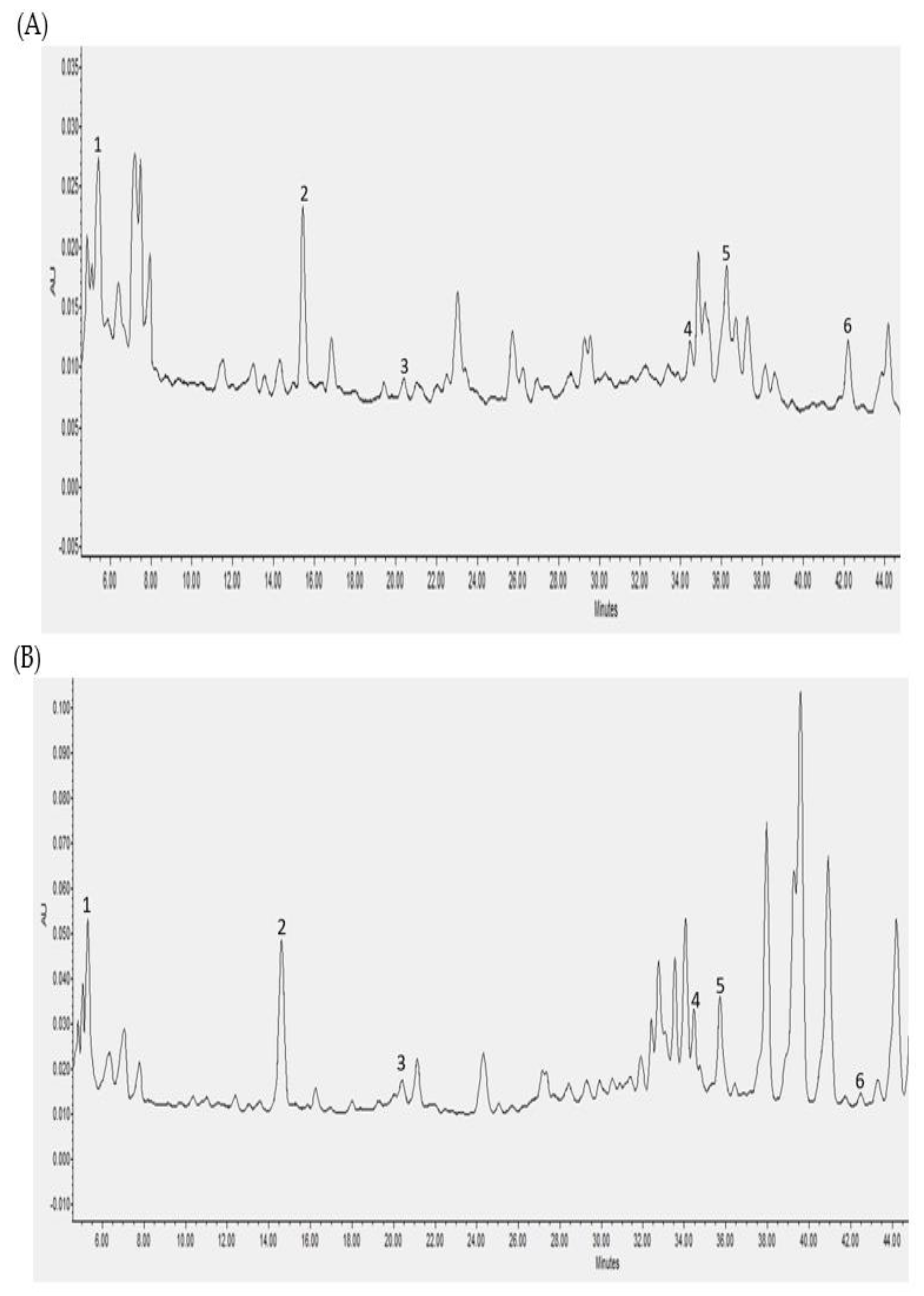
3.3. Individual Polyphenols Identified and Quantified after Cecal Fermentation
3.4. Antioxidant Activities of Chicory and Lucerne during In Vitro Digestion and Cecal Fermentation
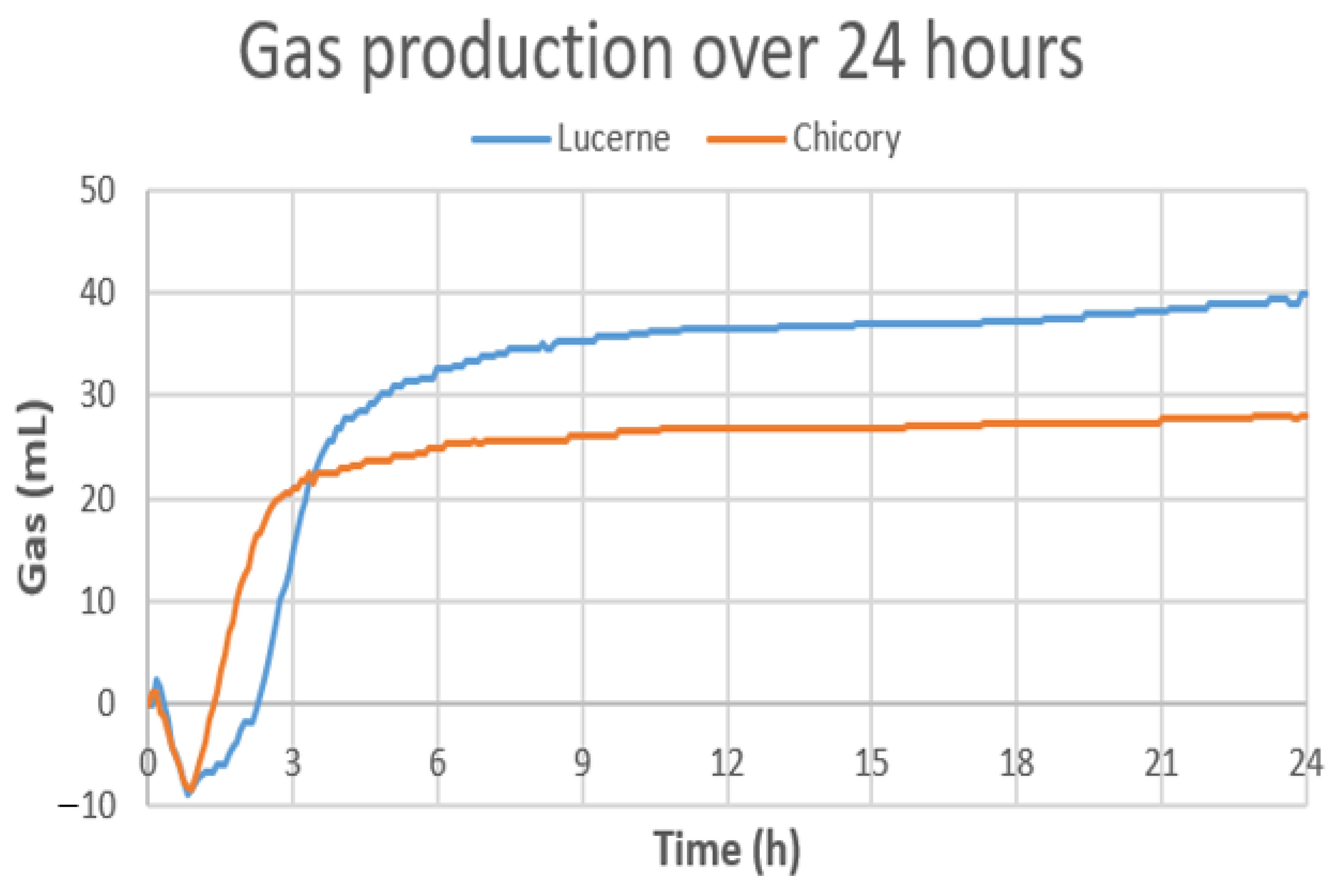
3.5. Gas Production with Chicory and Lucerne during In Vitro Cecal Fermentation
3.6. Short-chain Fatty Acids Production during In Vitro Cecal Fermentation of Chicory and Lucerne
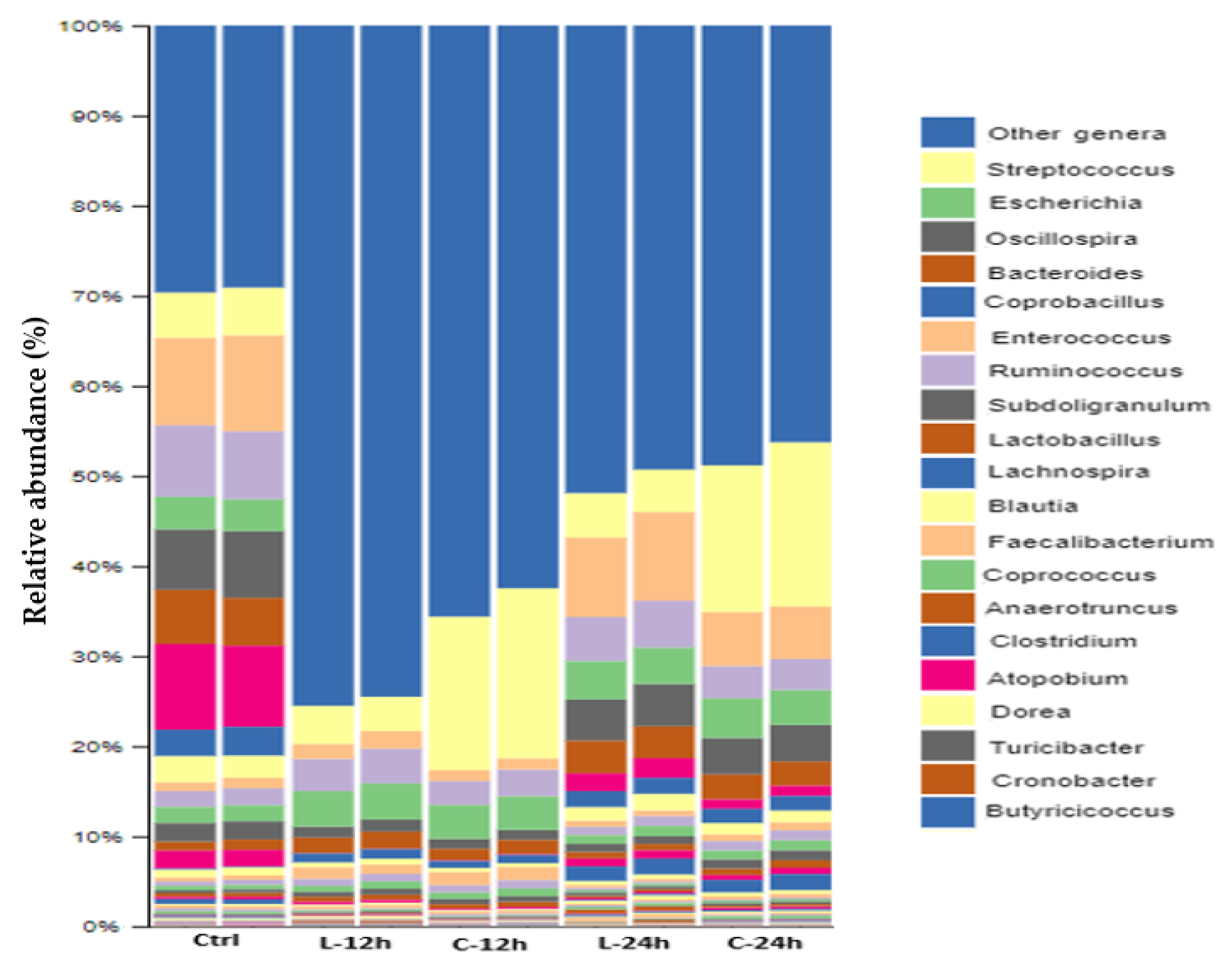
3.7. Modulatory Effects of Chicory and Lucerne on Gut Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mielmann, A. The utilisation of lucerne (Medicago sativa): A review. Br. Food J. 2013, 115, 590–600. [Google Scholar] [CrossRef]
- Iqbal, Y.; Cottrell, J.J.; Suleria, H.A.R.; Dunshea, F.R. Gut microbiota-polyphenol interactions in chicken: A review. Animals 2020, 10, 1391. [Google Scholar] [CrossRef] [PubMed]
- Nwafor, I.; Shale, K.; Achilonu, M. Chemical composition and nutritive benefits of chicory (Cichorium intybus) as an ideal complementary and/or alternative livestock feed supplement. Sci. World J. 2017, 2017, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faramarzzadeh, M.; Behroozlak, M.; Samadian, F.; Vahedi, V. Effects of chicory powder and butyric acid combination on performance, carcass traits and some blood parameters in broiler chickens. Iran. J. Appl. Anim. Sci. 2017, 7, 139–145. [Google Scholar]
- Khoobani, M.; Hasheminezhad, S.H.; Javandel, F.; Nosrati, M.; Seidavi, A.; Kadim, I.T.; Laudadio, V.; Tufarelli, V. Effects of dietary chicory (Chicorium intybus L.) and probiotic blend as natural feed additives on performance traits, blood biochemistry, and gut microbiota of broiler chickens. Antibiotics 2019, 9, 5. [Google Scholar] [CrossRef] [Green Version]
- Gaskins, H.R.; Collier, C.T.; Anderson, D.B. Antibiotics as growth promotants: Mode of action. Anim. Biotechnol. 2002, 13, 29–42. [Google Scholar] [CrossRef]
- Jeurissen, S.H.; Lewis, F.; van der Klis, J.D.; Mroz, Z.; Rebel, J.M.; ter Huurne, A.A. Parameters and techniques to determine intestinal health of poultry as constituted by immunity, integrity, and functionality. Curr. Issues Intest. Microbiol. 2002, 3, 1–14. [Google Scholar]
- Kohl, K.D. Diversity and function of the avian gut microbiota. J. Comp. Physiol. B 2012, 182, 591–602. [Google Scholar] [CrossRef]
- Iqbal, Y.; Ponnampalam, E.N.; Suleria, H.A.; Cottrell, J.J.; Dunshea, F.R. LC-ESI/QTOF-MS profiling of chicory and lucerne polyphenols and their antioxidant activities. Antioxidants 2021, 10, 932. [Google Scholar] [CrossRef]
- Brenes, A.; Viveros, A.; Goni, I.; Centeno, C.; Sayago-Ayerdy, S.G.; Arija, I.; Saura-Calixto, F. Effect of grape pomace concentrate and vitamin E on digestibility of polyphenols and antioxidant activity in chickens. Poult. Sci. 2008, 87, 307–316. [Google Scholar] [CrossRef]
- Tuohy, K.M.; Conterno, L.; Gasperotti, M.; Viola, R. Up-regulating the human intestinal microbiome using whole plant foods, polyphenols, and/or fiber. J. Agric. Food Chem. 2012, 60, 8776–8782. [Google Scholar] [CrossRef] [PubMed]
- Espín, J.C.; González-Sarrías, A.; Tomás-Barberán, F.A. The gut microbiota: A key factor in the therapeutic effects of (poly) phenols. Biochem. Pharmacol. 2017, 139, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; Mahgoub, S.A.; Hussein, M.M.; Saadeldin, I.M. Improving growth performance and health status of meat-type quail by supplementing the diet with black cumin cold-pressed oil as a natural alternative for antibiotics. Environ. Sci. Pollut. Res. 2018, 25, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela-Grijalva, N.V.; Pinelli-Saavedra, A.; Muhlia-Almazan, A.; Domínguez-Díaz, D.; González-Ríos, H. Dietary inclusion effects of phytochemicals as growth promoters in animal production. J. Anim. Sci. Technol. 2017, 59, 8. [Google Scholar] [CrossRef] [Green Version]
- Surai, P. Polyphenol compounds in the chicken/animal diet: From the past to the future. J. Anim. Physiol. Anim. Nutr. 2014, 98, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef] [Green Version]
- Sang, V.T.; Dai Hung, N.; Se-Kwon, K. Pharmaceutical properties of marine polyphenols: An overview. Acta Pharm. Sci. 2019, 57, 217–242. [Google Scholar] [CrossRef] [Green Version]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef]
- Hernandez-Patlan, D.; Solis-Cruz, B.; Méndez-Albores, A.; Latorre, J.D.; Hernandez-Velasco, X.; Tellez, G.; López-Arellano, R. Comparison of PrestoBlue® and plating method to evaluate antimicrobial activity of ascorbic acid, boric acid and curcumin in an in vitro gastrointestinal model. J. Appl. Microbiol. 2018, 124, 423–430. [Google Scholar] [CrossRef]
- Latorre, J.D.; Hernandez-Velasco, X.; Kuttappan, V.A.; Wolfenden, R.E.; Vicente, J.L.; Wolfenden, A.D.; Bielke, L.R.; Prado-Rebolledo, O.F.; Morales, E.; Hargis, B.M.; et al. Selection of Bacillus spp. for cellulase and xylanase production as direct-fed microbials to reduce digesta viscosity and Clostridium perfringens proliferation using an in vitro digestive model in different poultry diets. Front. Vet. Sci. 2015, 2, 25. [Google Scholar] [CrossRef]
- Karunaratne, N.D.; Abbott, D.A.; Chibbar, R.N.; Hucl, P.J.; Pozniak, C.J.; Classen, H.L. In vitro assessment of the starch digestibility of western Canadian wheat market classes and cultivars. Can. J. Anim. Sci. 2018, 98, 463–476. [Google Scholar] [CrossRef] [Green Version]
- Lei, F.; Yin, Y.; Wang, Y.; Deng, B.; Yu, H.D.; Li, L.; Xiang, C.; Wang, S.; Zhu, B.; Wang, X. Higher-level production of volatile fatty acids in vitro by chicken gut microbiotas than by human gut microbiotas as determined by functional analyses. Appl. Environ. Microbiol. 2012, 78, 5763–5772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, S.; Augustin, M.A.; Shen, Z.; Ng, K.; Sanguansri, L.; Ajlouni, S. Bioaccessibility of curcuminoids in buttermilk in simulated gastrointestinal digestion models. Food Chem. 2015, 179, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Menke, K.H. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Develop. 1988, 28, 7–55. [Google Scholar]
- Iqbal, Y.; Ponnampalam, E.N.; Cottrell, J.J.; Suleria, H.A.; Dunshea, F.R. Extraction and characterization of polyphenols from non-conventional edible plants and their antioxidant activities. Food Res. Int. 2022, 157, 111205. [Google Scholar] [CrossRef]
- Gu, C.; Suleria, H.A.R.; Dunshea, F.R.; Howell, K. Dietary lipids influence bioaccessibility of polyphenols from black carrots and affect microbial diversity under simulated gastrointestinal digestion. Antioxidants 2020, 9, 762. [Google Scholar] [CrossRef]
- Huang, C.-B.; Xiao, L.; Xing, S.-C.; Chen, J.-Y.; Yang, Y.-W.; Zhou, Y.; Chen, W.; Liang, J.-B.; Mi, J.-D.; Wang, Y. The microbiota structure in the cecum of laying hens contributes to dissimilar H2S production. BMC Genom. 2019, 20, 770. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [Green Version]
- Bokulich, N.A.; Dillon, M.R.; Zhang, Y.; Rideout, J.R.; Bolyen, E.; Li, H.; Albert, P.S.; Caporaso, J.G. q2-longitudinal: Longitudinal and paired-sample analyses of microbiome data. MSystems 2018, 3, e00219-00218. [Google Scholar] [CrossRef] [Green Version]
- Ed Nignpense, B.; Francis, N.; Blanchard, C.; Santhakumar, A.B. Bioaccessibility and bioactivity of cereal polyphenols: A review. Foods 2021, 10, 1595. [Google Scholar] [CrossRef] [PubMed]
- Mrduljaš, N.; Krešić, G.; Bilušić, T. Polyphenols: Food sources and health benefits. In Functional Food-Improve Health Through Adequate Food; Hueda, M.C., Ed.; IntechOpen: London, UK, 2017; pp. 23–41. [Google Scholar]
- Chaudhary, J.; Jain, A.; Manuja, R.; Sachdeva, S. A comprehensive review on biological activities of p-hydroxy benzoic acid and its derivatives. Int. J. Pharm. Sci. Rev. Res. 2013, 22, 109–115. [Google Scholar]
- Kara, K.; Guclu, B.K.; Senturk, M.; Eren, M.; Baytok, E. Effects of catechin and copper or their combination in diet on productive performance, egg quality, egg shelf-life, plasma 8-OHdG concentrations and oxidative status in laying quail (Coturnix coturnix japonica). J. Appl. Anim. Res. 2021, 49, 97–103. [Google Scholar] [CrossRef]
- Si, H.; Wang, X.; Zhang, L.; Parnell, L.D.; Admed, B.; LeRoith, T.; Ansah, T.-A.; Zhang, L.; Li, J.; Ordovás, J.M.; et al. Dietary epicatechin improves survival and delays skeletal muscle degeneration in aged mice. FASEB J. 2019, 33, 965–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, J.; Song, J.; Liu, L.; Xue, B.; Tian, G.; Yang, Y. Effect of epigallocatechin gallate on growth performance and serum biochemical metabolites in heat-stressed broilers. Poult. Sci. 2018, 97, 599–606. [Google Scholar] [CrossRef]
- Santos, D.I.; Saraiva, J.M.A.; Vicente, A.A.; Moldão-Martins, M. Methods for determining bioavailability and bioaccessibility of bioactive compounds and nutrients. In Innovative Thermal and Non-Thermal Processing, Bioaccessibility and Bioavailability of Nutrients and Bioactive Compounds; Barba, F.J., Saraiva, J.M.A., Cravotto, G., Lorenzo, J.M., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 23–54. [Google Scholar]
- Zheng, M.; Mao, P.; Tian, X.; Guo, Q.; Meng, L. Effects of dietary supplementation of alfalfa meal on growth performance, carcass characteristics, meat and egg quality, and intestinal microbiota in Beijing-you chicken. Poult Sci 2019, 98, 2250–2259. [Google Scholar] [CrossRef]
- Han, G.G.; Kim, E.B.; Lee, J.; Lee, J.-Y.; Jin, G.; Park, J.; Huh, C.-S.; Kwon, I.-K.; Kil, D.Y.; Choi, Y.-J. Relationship between the microbiota in different sections of the gastrointestinal tract, and the body weight of broiler chickens. Springerplus 2016, 5, 911. [Google Scholar] [CrossRef] [Green Version]
- Johnson, T.J.; Youmans, B.P.; Noll, S.; Cardona, C.; Evans, N.P.; Karnezos, T.P.; Ngunjiri, J.M.; Abundo, M.C.; Lee, C.-W. A consistent and predictable commercial broiler chicken bacterial microbiota in antibiotic-free production displays strong correlations with performance. Appl. Environ. Microbiol. 2018, 84, e00362-00318. [Google Scholar] [CrossRef] [Green Version]
- Pan, D.; Yu, Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes 2014, 5, 108–119. [Google Scholar] [CrossRef]
- Caly, D.L.; D’Inca, R.; Auclair, E.; Drider, D. Alternatives to antibiotics to prevent necrotic enteritis in broiler chickens: A microbiologist’s perspective. Front. Microbiol. 2015, 6, 1336. [Google Scholar] [CrossRef] [Green Version]
- Eeckhaut, V.; Wang, J.; Parys, A.; Haesebrouck, F.; Joossens, M.; Falony, G.; Raes, J.; Ducatelle, R.; Immerseel, F. The probiotic Butyricicoccus pullicaecorum reduces feed conversion and protects from potentially harmful intestinal microorganisms in broilers. Front. Microbiol. 2016, 7, 1416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bovera, F.; Morra, F.; Di Meo, C.; Nizza, A. Use of the in vitro gas production technique to study feed digestibility in domesticated ostriches (Struthio camelus var. domesticus). Ann. Anim. Sci. 2006, 16, 769–777. [Google Scholar]
- Polansky, O.; Sekelova, Z.; Faldynova, M.; Sebkova, A.; Sisak, F.; Rychlik, I. Important metabolic pathways and biological processes expressed by chicken cecal microbiota. Appl. Environ. Microbiol. 2016, 82, 1569–1576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jha, R.; Mishra, P. Dietary fiber in poultry nutrition and their effects on nutrient utilization, performance, gut health, and on the environment: A review. J. Anim. Sci. Biotechnol. 2021, 12, 51. [Google Scholar] [CrossRef]
- Sun, B.; Hou, L.; Yang, Y. The development of the gut microbiota and short-chain fatty acids of layer chickens in different growth periods. Front. Vet. Sci. 2021, 8, 699. [Google Scholar] [CrossRef]
- Dunislawska, A.; Slawinska, A.; Stadnicka, K.; Bednarczyk, M.; Gulewicz, P.; Jozefiak, D.; Siwek, M. Synbiotics for broiler chickens—In vitro design and evaluation of the influence on host and selected microbiota populations following in ovo delivery. PLoS ONE 2017, 12, e0168587. [Google Scholar] [CrossRef] [Green Version]
- Meimandipour, A.; Shuhaimi, M.; Soleimani, A.; Azhar, K.; Hair-Bejo, M.; Kabeir, B.; Javanmard, A.; Anas, O.M.; Yazid, A. Selected microbial groups and short-chain fatty acids profile in a simulated chicken cecum supplemented with two strains of Lactobacillus. Poult. Sci. 2010, 89, 470–476. [Google Scholar] [CrossRef]
- Khatibjoo, A.; Mahmoodi, M.; Fattahnia, F.; Akbari-Gharaei, M.; Shokri, A.-N.; Soltani, S. Effects of dietary short-and medium-chain fatty acids on performance, carcass traits, jejunum morphology, and serum parameters of broiler chickens. J. Appl. Anim. Res. 2018, 46, 492–498. [Google Scholar] [CrossRef]
- Panda, A.; Rao, S.; Raju, M.; Sunder, G.S. Effect of butyric acid on performance, gastrointestinal tract health and carcass characteristics in broiler chickens. Asian-Australas. J. Anim. Sci. 2009, 22, 1026–1031. [Google Scholar] [CrossRef]
- Maki, J.J.; Klima, C.L.; Sylte, M.J.; Looft, T. The microbial pecking order: Utilization of intestinal microbiota for poultry health. Microorganisms 2019, 7, 376. [Google Scholar] [CrossRef] [Green Version]
- Du, K.; Bereswill, S.; Heimesaat, M.M. A literature survey on antimicrobial and immune-modulatory effects of butyrate revealing non-antibiotic approaches to tackle bacterial infections. Eur. J. Microbiol. Immunol. 2021, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sunkara, L.T.; Achanta, M.; Schreiber, N.B.; Bommineni, Y.R.; Dai, G.; Jiang, W.; Lamont, S.; Lillehoj, H.S.; Beker, A.; Teeter, R.G. Butyrate enhances disease resistance of chickens by inducing antimicrobial host defense peptide gene expression. PLoS ONE 2011, 6, e27225. [Google Scholar] [CrossRef] [PubMed]
- Sunkara, L.T.; Jiang, W.; Zhang, G. Modulation of antimicrobial host defense peptide gene expression by free fatty acids. PLoS ONE 2012, 7, e49558. [Google Scholar]
| TPC | Chicory (mg GAE/g of Digesta) | Lucerne (mg GAE/g of Digesta) |
|---|---|---|
| Undigested | 0.441 ± 0.04 b | 0.714 ± 0.01 a |
| Soluble gastric fraction | 0.400 ± 0.01 b | 0.491 ± 0.02 b |
| Soluble intestinal fraction | 0.540 ± 0.02 a | 0.690 ± 0.01 a |
| Soluble fermented fraction (12 h) | 0.510 ± 0.02 a | 0.542 ± 0.01 b |
| Soluble fermented fraction (24 h) | 0.500 ± 0.03 a | 0.544 ± 0.02 b |
| Compounds | Gastric | Intestinal | |||
|---|---|---|---|---|---|
| Chicory (µg/g) | Lucerne (µg/g) | Chicory (µg/g) | Lucerne (µg/g) | ||
| Gallic acid | FPP | 20.4 ± 0.8 d | 41.4 ± 1.2 a | 25.9 ± 0.3 c | 26.1 ± 1.3 b |
| BPP | 17.2 ± 0.1 d | 21.7 ± 0.1 b | 19.3 ± 1.2 c | 29.3 ± 3 a | |
| TPP | 37.6 | 63.1 | 45.2 | 55.4 | |
| % Bioaccessibility | 54.2% | 65.6% | 57.3% | 47.1% | |
| Protocatechuic acid | FPP | 38.1 ± 0.5 a | ND | 13.5 ± 0.2 b | ND |
| BPP | 30.4 ± 0.7 a | ND | 3.2 ± 0.1 b | ND | |
| TPP | 68.5 | ̶ | 16.7 | ̶ | |
| % Bioaccessibility | 55.6% | ̶ | 80.8% | ̶ | |
| Catechin | FPP | 18.6 ± 0.6 c | 18.7 ± 0.1 c | 27.6 ± 0.4 b | 44.3 ± 0.14 a |
| BPP | 13.4 ± 0.1 c | 11.8 ± 0.2 d | 14.1 ± 1.1 b | 14.9 ± 0.61 a | |
| TPP | 32 | 30.5 | 41.7 | 59.2 | |
| % Bioaccessibility | 58.1% | 61.3% | 66.2% | 74.8% | |
| p-hydroxybenzoic acid | FPP | 25.4 ± 0.2 a | ND | 14.8 ± 0.1 b | ND |
| BPP | 21.6 ± 0.1 a | ND | 1.5 ± 0.1 b | ND | |
| TPP | 47 | ̶ | 16.3 | ̶ | |
| % Bioaccessibility | 54.0% | ̶ | 90.8% | ̶ | |
| Epicatechin | FPP | 11.6 ± 0.2 a | 5.2 ± 0.1 d | 7.3 ± 0.1 b | 6.5 ± 0.2 c |
| BPP | 8.3 ± 0.1 a | 6.9 ± 0.2 b | 5.2 ± 0.1 c | 4.9 ± 0.1 d | |
| TPP | 19.9 | 12.1 | 12.5 | 11.4 | |
| % Bioaccessibility | 58.3% | 42.9% | 58.4% | 57.0% | |
| Epicatechin gallate | FPP | ND | 13.4 ± 0.1 b | ND | 14.2 ± 0.1 a |
| BPP | ND | 13.9 ± 0.1 a | ND | 12.6 ± 0.1 b | |
| TPP | ̶ | 27.3 | ̶ | 26.8 | |
| % Bioaccessibility | ̶ | 49.1% | ̶ | 52.9% | |
| Quercetin-3-galactoside | FPP | ND | 13.3 ± 0.3 b | ND | 15.1 ± 0.1 a |
| BPP | ND | 22.2 ± 0.1 a | ND | 10.3 ± 0.1 b | |
| TPP | ̶ | 35.5 | ̶ | 25.4 | |
| % Bioaccessibility | ̶ | 37.5% | ̶ | 59.4% | |
| Quercetin-3-rhamnoside | FPP | 5.5 ± 0.1 a | ND | 4.9 ± 0.1 b | ND |
| BPP | 38.2 ± 0.3 a | ND | 3.8 ± 0.1 b | ND | |
| TPP | 43.7 | ̶ | 8.7 | ̶ | |
| % Bioaccessibility | 12.6% | ̶ | 56.3% | ̶ | |
| Kaempferol-3-glucoside | FPP | ND | 12.2 ± 0.1 b | ND | 48.9 ± 0.1 a |
| BPP | ND | 21.1 ± 0.2 a | ND | 14.2 ± 0.1 b | |
| TPP | ̶ | 33.3 | ̶ | 63.1 | |
| % Bioaccessibility | ̶ | 36.6% | ̶ | 77.5% | |
| Compounds | Fermentation 12 h | Fermentation 24 h | ||
|---|---|---|---|---|
| Chicory (µg/g) | Lucerne (µg/g) | Chicory (µg/g) | Lucerne (µg/g) | |
| Gallic acid | - | 21.6 ± 0.2 b | - | 29.9 ± 2.8 a |
| Pyrogallol | 191.6 ± 16.5 c | 186.2 ± 2.8 d | 300.6 ± 22.7 b | 317.8 ± 8.1 a |
| Procyanidin B2 | 158.6 ± 1.8 d | 176.0 ± 0.2 b | 173.1 ± 0.7 c | 200.4 ± 5.1 a |
| Antioxidant Activity | ABTS (mg AAE/g) | DPPH (mg AAE/g) | RPA (mg AAE/g) | |||
|---|---|---|---|---|---|---|
| Chicory | Lucerne | Chicory | Lucerne | Chicory | Lucerne | |
| Undigested | 0.300 ± 0.010 a | 1.260 ± 0.020 a | 0.123 ± 0.010 b | 0.132 ± 0.010 ab | 0.347 ± 0.010 b | 0.573 ± 0.020 a |
| Soluble gastric fraction | 0.224 ± 0.003 b | 0.163 ± 0.002 d | 0.118 ± 0.008 b | 0.079 ± 0.003 bc | 0.300 ± 0.012 bc | 0.160 ± 0.005 cd |
| Soluble intestinal fraction | 0.303 ± 0.017 a | 0.362 ± 0.022 b | 0.038 ± 0.001 c | 0.026 ± 0.001 cd | 0.639 ± 0.078 a | 0.300 ± 0.007 b |
| Soluble fermented fraction (12 h) | 0.192 ± 0.008 bc | 0.258 ± 0.009 c | 0.195 ± 0.014 a | 0.164 ± 0.010 a | 0.236 ± 0.008 de | 0.135 ± 0.007 cd |
| Soluble fermented fraction (24 h) | 0.237 ± 0.043 b | 0.371 ± 0.027 b | 0.194 ± 0.036 a | 0.167 ± 0.002 a | 0.221 ± 0.008 de | 0.202 ± 0.002 c |
| SCFAs | Ctrl | Chicory | Lucerne | ||
|---|---|---|---|---|---|
| 12 h | 24 h | 12 h | 24 h | ||
| Acetic acid (mmol L−1) | 7.35 ± 0.09 a | 18.12 ± 0.05 bc | 22.56 ± 0.01 d | 18.25 ± 0.02 bc | 24.85 ± 0.01 e |
| Propionic acid (mmol L−1) | 1.40 ± 0.01 a | 1.95 ± 0.01 bc | 2.75 ± 0.01 d | 1.96 ± 0.01 bc | 2.97 ± 0.01 e |
| Butyric acid (mmol L−1) | 4.79 ± 0.02 a | 8.74 ± 0.01 b | 12.83 ± 0.01 d | 9.19 ± 0.01 c | 14.42 ± 0.01 e |
| Total SCFA (mmol L−1) | 13.54 a | 28.81 bc | 38.14 d | 29.40 bc | 42.24 e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, Y.; Ponnampalam, E.N.; Le, H.H.; Artaiz, O.; Muir, S.K.; Jacobs, J.L.; Cottrell, J.J.; Dunshea, F.R. Assessment of Feed Value of Chicory and Lucerne for Poultry, Determination of Bioaccessibility of Their Polyphenols and Their Effects on Caecal Microbiota. Fermentation 2022, 8, 237. https://doi.org/10.3390/fermentation8050237
Iqbal Y, Ponnampalam EN, Le HH, Artaiz O, Muir SK, Jacobs JL, Cottrell JJ, Dunshea FR. Assessment of Feed Value of Chicory and Lucerne for Poultry, Determination of Bioaccessibility of Their Polyphenols and Their Effects on Caecal Microbiota. Fermentation. 2022; 8(5):237. https://doi.org/10.3390/fermentation8050237
Chicago/Turabian StyleIqbal, Yasir, Eric N. Ponnampalam, Hieu Huu Le, Olivia Artaiz, Stephanie K. Muir, Joe L. Jacobs, Jeremy J. Cottrell, and Frank R. Dunshea. 2022. "Assessment of Feed Value of Chicory and Lucerne for Poultry, Determination of Bioaccessibility of Their Polyphenols and Their Effects on Caecal Microbiota" Fermentation 8, no. 5: 237. https://doi.org/10.3390/fermentation8050237
APA StyleIqbal, Y., Ponnampalam, E. N., Le, H. H., Artaiz, O., Muir, S. K., Jacobs, J. L., Cottrell, J. J., & Dunshea, F. R. (2022). Assessment of Feed Value of Chicory and Lucerne for Poultry, Determination of Bioaccessibility of Their Polyphenols and Their Effects on Caecal Microbiota. Fermentation, 8(5), 237. https://doi.org/10.3390/fermentation8050237








