Abstract
The rice husk has the potential to be used for converting agricultural wastes into renewable energy. Therefore, this study aims to improve the hydrolysis of rice husk through Hydrogen Peroxide (HP) and Combined Hydrogen Peroxide–Aqueous Ammonia (CHPA) pretreatments. The removal of lignin from rice husks was determined using SEM–EDS examination of the samples. At a specific concentration of H2O2, (CHPA) pretreatment eliminated a significantly larger amount of lignin from biomass. The percentage of lignin removal of HP varied from 48.25 to 66.50, while CHPA ranged from 72.22 to 85.73. Hence, the use of batch kinetics of lignin removal of both pretreatments is recommended, where the kinetic parameters are determined by fitting the experimental data. Based on the results, the activation energies for HP and CHPA pretreatments were 9.96 and 7.44 kJ/mol, which showed that the24 model is appropriate for the experimental data. The increase in temperatures also led to a higher pretreatment value, indicating their positive correlation. Meanwhile, CHPA pretreatment was subjected to enzymatic hydrolysis of 6% enzyme loading for the production of 6.58 g glucose/L at 25 h.
1. Introduction
Recently, the need for alternative sources of energy has become important to effectively reduce human reliance on fossil fuels. Previous studies showed that 23.7% of total energy demand is obtained from renewable energies [1,2]. The desire to achieve a long-term sustainable economy that emphasizes the “reuse” of biobased raw resources rather than the traditional industrial method of “get, create, and waste” has also stimulated the use of renewable energy sources [3]. Meanwhile, lignocellulose is one of the most abundant renewable energy sources due to its wide variety of applications. Out of these materials, agricultural commodities have great potential to be used for biofuel production, which has been an essential new strategy in many countries. The conversion of lignocellulosic biomass to bioethanol has stimulated the interest of several studies. The progress of bioethanol production is beneficial because it is economical and protects the environment. It is also profitable for human society’s long-term growth and compatible with the current low green concept [4]. Compared to bioethanol generated from sugar and starch, cellulose-based bioethanol is not produced at an economic cost. Therefore, the implementation of a cost reduction approach is needed [5]. Pretreatment and hydrolysis are the two fundamental phases in fuel generation, based on the bioconversion into various sugars. Several studies improved fermentable carbohydrate recapture from sugar breakdown during the pretreatment phase.
The complicated and resistant nature of agricultural wastes is a critical issue in the production of the second generation of bioethanol. This is because the complex composition of lignocellulosic biomass prevents its effective application. Several studies involved multiple pretreatment procedures to handle the immune nature of the lignocellulosic biomass regularly. Therefore, there is a need for an environmentally friendly, effective, and economic pretreatment strategy [6]. The synthesis of bioethanol has been carried out using several lignocellulosic biomasses such as wheat straw [7,8], paddy straw [9], sugarcane bagasse [10], corn stover [11,12], and palm empty fruit bunch [13]. However, the by-product of the rice milling industry has received little attention such as rice husk.
The dissolution of lignocellulosic components and their biotechnological pathway to bioethanol requires pretreatment. Energy usage and the cost of a pretreatment procedure are also required to determine the commercial feasibility of the method [14]. Furthermore, the pretreatment process must not alter the structural similarities of the lignocellulosic elements. During this process, lignin’s natural design frequently changes, allowing access to valorization. Compared to procedures, alkaline pretreatment has numerous benefits such as low operating conditions and time, which significantly boost enzyme hydrolysis [15]. A previous study on lignin showed that alkaline pretreatment or its combination with other reagents was able to remove lignin [16].
Alkaline pretreatment positively affected wood products and eliminated the acetyl group from hemicellulose [17] as well as gave fewer inhibitors at low operating conditions than acid pretreatment [18]. It also promotes significant lignin reduction and more highly porous creation. Furthermore, alkaline pretreatment enhances cellulose accessibility by disturbing a fraction of the lignocellulosic. Yang et al. [19] investigated the influence of alkali on bamboo pretreatment in a combination of hot water and NaOH at 0.5–2% (w/v). A single-stage HP pretreatment of biomass was also carried out using a large load of H2O2, approximately 10% w/v [20]. After the AHP treatment, significant structural changes occur in the physiology of the substrates, which includes the removal of lignin and hemicellulose, increasing the porosity area for easy attack by recombinant cellulases [21]. The combined action of H2O2 and ammonia increased ammonolysis while decreasing oxidation [21]. The micrographs of hydrogen peroxide–ammonia fiber expansion (H-AFEX)-treated giant reed showed that the changed porosity structure enhanced the enzyme accessible surface area and subsequent enzymatic hydrolysis [4].
Lignin removal is the most common chemical process that occurs during pretreatment. Several studies investigated the kinetics of lignin removal from biomass such as corn stover [22], wheat straw [23,24,25], rice husk [26], pine or deodar sawdust [27], oil palm empty fruit bunch [28,29,30], oak wood [31], cedarwood [32], and sugarcane bagasse [33,34,35,36] to improve pretreatment selectivity. However, the kinetics of lignin removal of hydrogen peroxide (HP) and the combination of hydrogen peroxide–aqueous ammonia (CHPA) pretreatments of rice husk have not been carried out. Therefore, this study examined the composition of HP and CHPA pretreated rice husks using an approximation approach. The SEM–EDS analysis established the morphological configuration and the content of samples, namely C, O, and Si.
The kinetic of pretreatment stage is essential to determine the economics of a process of reactor investment costs. In a pseudohomogeneous system with biomass, the H2O2 solution produces a pseudohomogeneous system, and the pretreatment is a first-order reaction [27], which use two separate kinetic models. One of the models considers biomass as several kinds of polymers, with distinct phases of pretreatment assigned to various rate-controlling mechanisms of the whole process [35]. Meanwhile, the consecutive model assumes that biomass comprises several polymer components that can degrade sequentially. In this model, there are three types of lignin, namely initial, bulk, and residual lignins that react in sequential order. It also proposes that distinct kinds of lignin degrade consecutively at the start and respond as pretreatment progress [25,27,35]. The use of HP and CHPA pretreatments of rice husk and the kinetics of two different techniques were examined and verified in this study. The results help determine pretreatment parameters, efficiently manage the process, and optimize the operating parameters using HP and aqueous ammonia.
2. Materials and Methods
2.1. Materials
The rice husks used were obtained from the Pemulutan Village of Ogan Ilir in South Sumatra, Indonesia. The samples collected were dried in direct sunshine for one day, screened through a sieve, and fed into a crusher (screen size: 35 mesh).
2.2. Experimental Methods
This study used two different kinds of procedures, namely standard HP and CHPA pretreatment to evaluate kinetics and the influence of variables. The standard HP pretreatment used 3% of H2O2 and its adjustment to a pH of 11.5 with NaOH [37]. Subsequently, the rice husk was immersed in an HP solution with a solid-to-H2O2 solution ratio of 1:10 (w/v). A total of 5 experimental setups were created in 5 different Erlenmeyers with glass stoppers and cooked for 48 h at various temperatures, namely 30 °C, 40 °C, 50 °C, 60 °C, and 80 °C.
Meanwhile, another pretreatment process was carried out consecutively with 5 separate procedures using CHPA. The rice husks were first immersed in a 3% H2O2 solution (pH = 11.5) with a ratio of 1:10 (w/v) and autoclaved for 48 h at 30 °C, 40 °C, 50 °C, 60 °C, and 80 °C, respectively. After cooling the pretreated sample, the solid phase was collected, neutralized with distilled water, and dehydrated at 40 °C. Subsequently, a 20% (v/v) aqueous ammonia solution at 100 °C for 5 h pretreated the solid phase [38]. The pretreated sample was cooled, while the solid fraction was filtered, rinsed with distilled water for neutralization, and dried at 40 °C.
2.3. Sampling and Lignin Quantification
During the pretreatment procedure, a portion of the solid phase and the H2O2 solution was taken after each of the predefined time intervals to determine the changes in the properties over time. A ratio of 1:10 (w/v) was kept in the reactor during sampling of the test length to maintain testing procedures consistently. After every withdrawal, the samples were rinsed in deionized water and dehydrated at 40 °C.
2.4. Pretreatment Parameters and Kinetics
In this study, both HP and CHPA pretreatments of rice husks investigated kinetic data at different temperatures, namely 30 °C, 40 °C, 50 °C, 60 °C, and 80 °C. Chesson’s method [39] determined the lignin composition of pretreated rice husk at reaction times of 6, 24, and 48 h for each isothermal condition.
The quantity of lignin released from pretreated materials at various temperatures was used to analyze pretreatment kinetics. Meanwhile, the percentage of lignin removed each given time (t) was estimated using the Equation below [40]:
where CL0 is the initial lignin in untreated rice husk, CL(t) is the lignin in pretreated rice husk at any time t, L(t) is the percentage of lignin removal at any time.
Since the pretreatment is considered as a first-order reaction, the reaction rate can be expressed as:
where K is the constant of pretreatment rate. By solving Equation (2) with the initial condition (L = 0 at t = 0), the following time-dependent formulation of lignin solubilization ratio (L) is obtained:
Therefore, plotting–ln (1 − L) versus time t, where intercept (0) shows the K value of the slope.
It has been discovered that the removal of the total lignin component from biomass is very difficult. Therefore, a new measure, which is known as the degree of pretreatment (Dd) is being investigated. This parameter is used to predict a given biomass’s maximal lignin solubilization ratio when a specific pretreatment method is used [25,27]. The kinetic model described in Equation (2) is expressed below:
With the Dd limit set to 0 ≤ Dd ≤ 1. The integration of Equation (4) can be represented as follows:
Therefore, the value of Dd is calculated for each of the operational pretreatment parameters by plotting L versus [1 − exp (−K·t)].
The activation energy value can describe the relationship between the rate constant and temperature through the Arrhenius law [27]:
where A is the Arrhenius constant, Ea is the activation energy, R is the gas constant (8.314 kJ/mole∙K), and T is the absolute temperature (K).
The activation energy (Ea) of pretreatment is calculated from the logarithmic form of the Arrhenius equation (ln K = ln A − Ea/RT) by plotting ln K against 1/T with the slope equal to Ea/R. Meanwhile, ln A is the intercept where the Arrhenius constant is calculated. The pretreatment kinetics are established by assuming the material contained only one kind of lignin.
2.5. Characterization of Rice Husk
The micrographs of the cross-section of untreated and pretreated samples were observed using an SEM–EDS microscope Phenom Pro X. The SEM images are taken using an accelerating voltage of 15 kV. Subsequently, the quantities of cellulose, hemicellulose, and lignin were evaluated by Chesson standard methods [39]. In this process, 1 g (a) of rice husks were combined in 150 mL deionized water for 1 h at 100 °C. Vacuum filtration was used to recover the solid, rinsed with 300 mL of deionized water, dehydrated at 80 °C, and weighed (b). Rice husks were combined with 150 mL 1 N H2SO4 for one h at 100 °C, which were carried out in the same order as the preceding stage (c). The dried rice husks were combined with 10 mL of 72% H2SO4 for 4 h and 150 mL of 1 N H2SO4 was inserted. The mixture was maintained for one h in an oil bath at 100 °C under reflux. The solid was then separated, rinsed, and dehydrated similarly as described in the preceding phase (d). In the last stage, the rice husks were heated at 600 °C for 6 h and weighed (e). The equations below were used to calculate the quantities of hemicellulose, cellulose, and lignin in the structure:
2.6. Preparation of Crude Enzyme from Aspergillus niger
There was 12.5% sucrose, 0.25% (NH4)2SO4, and 0.2% KH2PO4 in 100 mL of inoculum culture, and 1 M HCl was used to alter the pH to 3. The wire loop’s end was also soaked in 96% ethanol and torched in a Bunsen burner flame until it became crimson. Aspergillus niger culture and PDA media were collected with a wire loop submerged in a liquid medium using an aseptic room for all aspects of the process. The liquid medium was wrapped with cotton and incubated at 30 °C for 24 h. Subsequently, 20 g of rice husk (dry weight) was placed in a 250 mL beaker glass and prepared with medium supplemented urea (0.03 g), MgSO4·7H2O (0.005 g), and KH2PO4 (0.0023 g). A total of 80 mL of aquadet was prepared in a medium with a pH of 5, autoclaved at 121 °C for 15 min, and chilled gradually. A total of 10 mL suspended Aspergillus niger spores were added to the medium and incubated for 96 h at 37 °C. After incubation, the contents of these flasks were aseptically centrifuged and used for enzymatic hydrolysis [38], while cellulase activity was determined [41].
2.7. Enzymatic Hydrolysis
A total of 20 g CHPA pretreated sample was placed in a 1000 mL Erlenmeyer flask. Subsequently, a medium containing 5 g/L yeast, 7.5 g/L (NH4)2SO4, 3.5 g/L K2HPO4, 0.75 g/L MgSO4·7H2O, and 1 g/L CaCl2·2H2O was supplied with a mass to volume ratio of 1:10 and a pH of 5. The mixture was sterilized by autoclaving for 60 min at 121 °C [42], the liquid was chilled, and an enzyme at varying concentrations of 2, 4, and 6% (v/w) was added. The hydrolysis reaction was carried out at 50 °C for 25 h, while agitated at 200 RPM, and the temperature was also reduced to 30 °C. The DNS technique was used to determine glucose concentration in hydrolysis assay [43]. A UV-VIS spectrophotometer SPEKOL 1500 at 540 nm was used to determine the reducing sugars.
3. Results
3.1. Effect of Pretreatment Temperature and Time
Lignin reduction is a thermo-sensitive procedure that varies widely with different temperature pretreatment settings [27]. The reduction mechanism of lignin and other related parts of biomass varies significantly based on the sample type. A specific pretreatment setting is much less efficient for a model with higher lignocellulosic resistance. However, the condition can be used for the pretreatment of samples with a more porous lignocellulosic hetero-matrix. Since the degree of pretreatment is related to heating temperature, rice husk is only treated with HP at 30 °C, 40 °C, 50 °C, 60 °C, and 80 °C). This showed that pretreatment is more efficient at higher temperatures. It was discovered that enhanced heat energy raises the kinetic energy of compounds, causing a more significant number of combinations to move quicker, which leads to the breaking of a substantial number of bonds [26].
Table 1 shows the rice husk composition after each HP pretreatment stage evaluated by the Chesson method [39]. Each analysis was repeated twice to ensure that variances were less than 3%. Raw rice husk data considerably correlated with softwood composition, where the percentages of the three main polymers ranged from 25 to 40% and less cellulose than hardwood [44]. The relative concentrations of three components, namely cellulose, hemicellulose, and lignin were significantly raised during pretreatment, thereby reducing the contaminants in rice husk [45]. Furthermore, the lignin percentage of biomass changes with each phase of HP pretreatment. The cellulose content of rice husk after HP pretreatment steadily rose from 22.34 to 33.69% as the temperature increased from 30 to 80 °C. The relative contents of hemicellulose also increased by 4.5 to 8.04%. This indicated that the pretreatment used in this study was light and did not affect the lignocellulose structural composition [45].

Table 1.
Effect of various HP Pretreatment temperatures and times on the rice husks composition.
HP pretreated the rice husk, break lignin and hemicellulose without changing the cellulose crystallinity. It also dissociates into superoxide radicals and hydroxyl ions. Subsequently, the ether and ester linkages are exposed in the polymer structure, which dissolves the lignin and hemicelluloses [46].
Table 2 shows the rice husk composition after each CHPA pretreatment stage evaluated by the Chesson method [39]. The results showed that the lignin percentage of biomass reduces significantly with each phase of CHPA pretreatment. The cellulose content of CHPA pretreated rice husk steadily increased from 54.23 to 63.84% as the pretreatment temperature increased from 30 to 80 °C for 48 h. The relative contents of hemicellulose also increased 2.25% to 14.82% and the highest lignin reduction was 81.97% at 80 °C for 48 h. As expected, the percentages of cellulose and hemicellulose in rice husks processed with CHPA were impressively higher than those in untreated husks. This occurred because some of the lignin in the husks was removed during the processing. These results showed that lignin reduction is directly related to the quantity of time spent during pretreatment.

Table 2.
Effect of CHPA pretreatment temperature and time on the composition of the rice husk samples.
Meanwhile, the equilibrium time for maximal lignin removal varied with high temperatures. From a macroscopic perspective, CHPA pretreatment is highly effective, as the brown color of the rice husk hue fades away, leaving a white substance. Although the change indicated the elimination of lignin, it is not completely visible in the case of HP pretreatment.
Figure 1 shows that the pretreatment of rice husk with HP at 30 °C takes 48 h to reduce 48.25% of lignin. Meanwhile, with CHPA pretreatment, rice husk reduces 72.22% of its lignin at 30 °C for 48 h (Figure 2). This is because CHPA pretreated rice husk has a more porous and distorted structure, as well as hydroxyl radicals and superoxide anions, which break a huge amount of inter and intra-chain links in a shorter time. Moreover at 40, 50, 60, and 80 °C, HP removes lignin 55.48, 59.39, 59.61, and 66.50% in 48 h, respectively, while CHPA removes lignin 76.60, 78.25, 79.51, and 85.73% in 48 h, respectively, at 40, 50, 60, and 80 °C. The substances and oxygen radicals’ ions (HP pretreatment) permeate gradually through the cell wall during the early phases of the process, which leads to chemical reactions. The lignin absorbs the solvent in the pretreatment and diffuses from the cell membrane to the bulk solution. Moreover, soluble molecules mainly comprise acidic by-products and monomeric sugars that were produced during the pretreatment. This often causes diffusion-controlled rigidity in the outlet paths of lignin from the cellulose and the entry point of the solvent, which is used for HP pretreatment into metabolic pathways of punctured cell walls. Therefore, the concentration of reaction products in the system inhibits subsequent diffusion over time. The increase in the percentage of lignin reduction is comparable, which is in the following order: HP [48.25 (30 °C) 55.48 (40 °C): 59.39 (50 °C) 59.61 (60 °C): 66.50 (80 °C)], CHPA [72.22 (30 °C): 76.60 (40 °C): 78.25 (50 °C): 79.51 (60 °C): 85.73 (80 °C)].
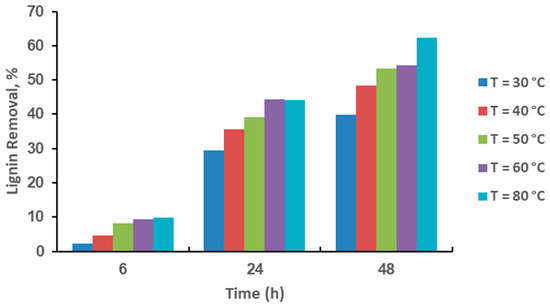
Figure 1.
Effects of HP pretreatment time and temperature on lignin reduction.
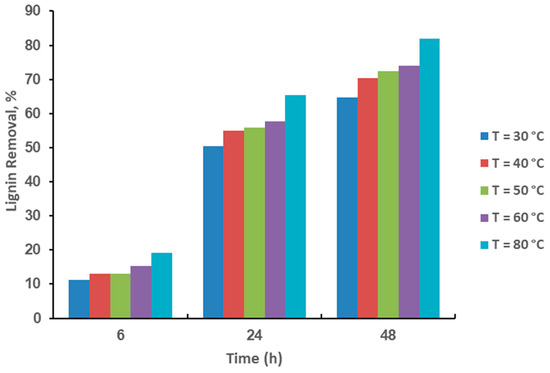
Figure 2.
Effects of CHPA pretreatment time and temperature on lignin reduction.
The two critical CHPA stages are HP and aqueous ammonia. After HP pretreatment, the time-dependent change of lignin was observed in CHPA. The subsequent materials are pretreated at 100 °C for 5 h with a 20% aqueous ammonia, while lignin removal was monitored in real-time and used to estimate pretreatment kinetics parameters. Figure 2 shows the time-dependent change of pretreatment patterns of rice husk at various temperatures. This was carried out to determine the best influence of pretreatment time and temperature on the sample. After CHPA pretreatment, lignin reduction varied from 72.22 to 85.73% of the baseline lignin in untreated rice husks.
3.2. Calculation of Kinetic Parameters
Table 3 shows the predicted kinetic parameters for each pretreatment condition based on linear regression of experimental data. Based on the results, all these HP and CHPA rate constants rise as the temperature increases. Meanwhile, higher temperature causes more reactant interactions, increasing the reaction rate with higher constant values. The table also showed that less activation energy is needed to start the reaction for CHPA pretreated samples. However, higher activation energy is required for samples pretreated with HP pretreatment to start the reaction. The reaction rate (K) rises with increasing temperature in HP and CHPA pretreatments. The reaction rate is rapid because the mixtures give fast impregnation of the biomass as differences in surface tension control its transport, which enables the heating agents to reach the surface area within the solid quickly. This action removes the requirement for the separation of the impregnation stage, leading to a significant reduction in overall heating time.

Table 3.
Effect of varying pretreatment temperatures on kinetic parameters of lignin removal by linear regression of experimental data.
Furthermore, under alkali pretreatments, phenolic hydroxyl groups and a small fraction of aliphatic hydroxyl groups together with certain C H links are charged [26]. Since the basicity of molecules changes with temperature, reaction rates rise as the temperature increases, which leads to extra vibrational energy transferred to the links. The kinetic constant (K) increases with the temperature, and the results are identical to those discovered in other studies [26,27], specifically for CHPA pretreatment. Meanwhile, the activation energies of pretreatment modeled by several authors are compared in Table 4.

Table 4.
Several authors modeled activation energies of pretreatment at different raw materials.
3.3. Scanning Electron Microscopy (SEM)–Energy Dispersive Spectroscopy (EDS)
In this study, SEM–EDS was used to examine the pretreated rice husk. Table 5 shows that the carbon content of rice husk increased from 47.60% to 57.27% and 73.27% for HP and CHPA pretreatments, respectively. The increase of carbon content showed that the pretreatment also improved cellulose, while processing lowered the silica concentration. The lignocellulosic surface includes phytoliths and silica frames unaffected by decomposition that protect the structures from cellulose degradation before pretreatment [47].

Table 5.
Effect of untreated and pretreated (HP and CHPA) rice husk on element composition.
The concentrations and distribution of carbohydrates, as well as lignin in raw and processed biomass solid particles, need to be characterized [48]. These results are beneficial for optimizing pretreatment conditions, which influence enzymatic hydrolysis that is not only linked to the degree of pretreatment [49]. Figure 3 shows the SEM pictures of untreated and pretreated rice husks. For the SEM examination, the materials used include the control biomass, HP and CPHA which both pretreated with H2O2 3% at temperature 80 °C for 48 h, and 20% (v/v) aqueous ammonia solution at 100 °C for 5 h. Figure 3A shows a Scanning Electron Microscope of untreated rice husk. Due to the degradation of lignin bonds during the pretreatments, the surfaces of pretreated rice husk had several holes, and cellulose crystalline structure was reduced, which increased the total surface area for the enzyme-substrate complex arrangement [50,51]. The SEM images show that the treated biomass is distorted (Figure 3B,C) due to the high temperature of HP preparation. The primary goal of the pretreatment is to remove lignin and silica, which provide mechanical and chemical robustness to the biomass structures. However, the rheological behavior of the rice husks alters after pretreatment and the lignocellulosic shape of rice husks prepared with H2O2 was changed. The lignin layer was partially removed from the rice husk fiber in numerous locations after CHPA treatments to show the cellulose structures. According to SEM–EDS analysis, the amount of silica in the samples prepared with HP was 6.02%.
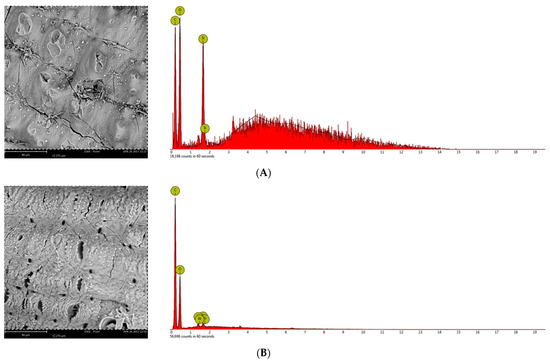
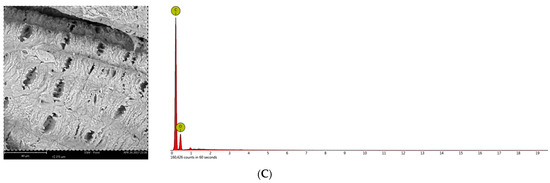
Figure 3.
SEM pictures and EDS analysis of rice husk face: (A) Unpretreated rice husk; (B) after H2O2 pretreatment with 3% (v/v) H2O2 at 80 °C for 48 h; and (C) after combined H2O2 -aqueous ammonia treatment with 3% (v/v) H2O2 at 80 °C for 48 h and 20% (v/v) Aqueous ammonia at 100 °C for 5 h. The scale bars are 80 μm.
Meanwhile, Si was not discovered in the samples prepared with CHPA. This indicated that lignin and silica were removed in large quantities from the rice husk during processing. Many superoxide radicals and hydroxide ions can degrade the cell walls by adding hydrophilic carboxyl groups and cleaving specific inter-unit linkages as the material is exposed for a prolonged time [27] to dissolve the lignin and hemicellulose. After the pretreatment stages, lignin and hemicelluloses are steadily reduced from the matrix, which makes the structure more porous, deformed, brittle, and ideal for enzymatic hydrolysis. Furthermore, in each phase of CHPA, the continuous decrease of cellulose chains plays a critical role in pore spaces.
3.4. Validation of Pretreatment Kinetics Model
The two equations were applied to match the experimental results and the effectiveness of pretreatment kinetics models was assessed in terms of determination coefficient (R2). Figure 4 compares experimental data with theoretical data, which was estimated using Equations (2) and (4). In this model, the maximal potential of lignin solubility at a certain pretreatment degree was assumed. This is because all lignin from biomass under a given pretreatment condition cannot be easily extracted, therefore, the degree of pretreatment was proposed. It can also be affected by various variables such as the solvent, the treatment conditions, and the biomass structure [52]. The element was not considered in the traditional pretreatment kinetics model of Equation (2). Compared to the model described by Equation (4), the quality of fit, as measured by the numeric value of R2 (Table 6), is substantially better for the model as shown by Equation (4).
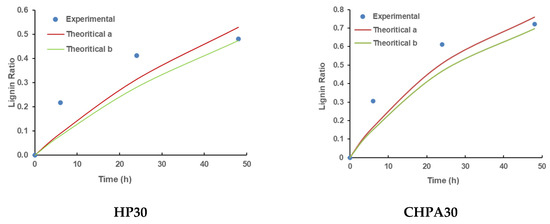
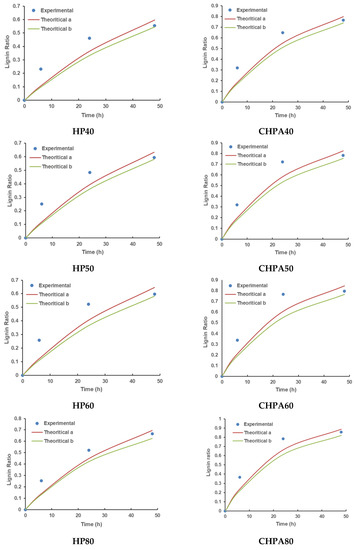
Figure 4.
Validation of kinetics models of rice husk pretreatment using experimental data; theoretical (a) depicts experimental data suited to Equation (2), while theoretical (b) shows the same adapted to Equation (4) including the degree of pretreatment (Dd). (HP30, HP40, HP50, HP60, and HP80 Hydrogen Peroxide Pretreatments at 30 °C, 40 °C, 50 °C, 60 °C, and 80 °C, respectively; CHPA30, CHPA40, CHPA50, CHPA60, and CHPA80 Combination Hydrogen Peroxide–Aqueous Ammonia Pretreatments at 30 °C, 40 °C, 50 °C, 60 °C, and 80 °C, respectively).

Table 6.
The values of prediction coefficients (R2) were acquired after estimating the parameters of two alternative pretreatment kinetics models, Equations (2) and (4).
3.5. Effects of Hydrolysis Time on Glucose Concentration of CHPA Pretreated Sample
The glucose produced from CHPA pretreated rice husk was evaluated using the DNS technique and the transient behavior is shown in Figure 5. The increased glucose production is due to a rise in enzyme concentration from 2% to 6% for a given substrate concentration. After 25 h, hydrolysis experiments with 2%, 4%, and 6% enzyme concentration yielded glucose concentrations of 4.20, 5.53, and 6.58 g/L, respectively. Meanwhile, further increases in enzyme concentration frequently led to higher glucose recovery. The enhanced glucose production is related to a higher enzyme concentration per substrate unit. This is the usual tendency of chemical reactions, where a rise in the reacting molecules pushes the process ahead to generate the products. The product was formed quicker than the dilution effect caused by the added water flow at the start of the reaction, which enabled the product to accumulate and rise in concentration [53]. Greater attention required more vigorous agitation to prevent severe pressures on the enzyme that can produce denaturation. However, a higher substrate loading with improved yield can be required for industrial applications. A previous study showed that the enzymatic hydrolysis of lignocellulose that binds polysaccharide chains and catalyzes the process is facilitated by enzymes [54]. However, most studies did not provide recommendations for enzyme loading and hydrolytic duration, which are the two crucial parameters for optimal enzyme use in actual applications [55].
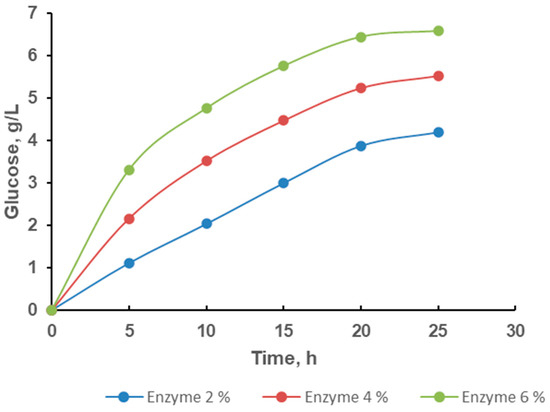
Figure 5.
Effects of hydrolysis time on glucose concentration of CHPA pretreated sample.
The impact of cellulose hydrolysis highly correlated with the effect of pretreatment, where the less lignin in the biomass, the simpler it is to enzymatically hydrolyze. A previous study [40] discovered that the combined impact of HP and ammonia breaks lignin and also reduces its polydispersity index (PI), leading to homogeneous lignin fragments. This showed that ammonia damaged the ester link preferentially, while HP destroyed the resinol structure selectively. Both ammonia and HP have the potential to act on the lignin side chain’s alkyl ether and aryl ether linkages, however, they had a negligible effect on the p-coumaran structure. Furthermore, their synergy on lignin indicated the fundamental mechanism of the pretreatment and provided insight into lignin structure and biomass resistance to increase enzymatic hydrolysis. The existence of lignin has a detrimental impact on the yield of enzymatic hydrolysis. This is because unproductive lignin adsorption on the enzyme leads to the creation of a lignin–enzyme complex, which is ineffective for the process [56]. Since the lignin concentration was reduced in this study using CHPA pretreatment, the hydrolysis impact was enhanced, producing a higher glucose concentration.
4. Conclusions
The rice husks were processed with HP and CHPA pretreatment to remove lignin and silica, which contribute mechanical characteristics to the biomass structure and impair enzymatic hydrolysis. This study investigated the influence of pretreatment temperature and duration on lignin. The results showed that temperature and length of pretreatment were the significant factors affecting lignin from rice husk. At elevated temperatures, hydrogen bonds between molecules break, which allows HP and aqueous ammonia to permeate readily into the rice husk structure, facilitating lignin removal. The maximum lignin reduction was 81.97% after 48 h pretreatment at 80 °C and the silica in the paddy husks was also eliminated. Based on the images acquired by scanning electron microscopy, it was discovered that the rice husk structure becomes pretreated and distorted as the degree of pretreatment increases. At relatively moderate temperatures, the exact mechanism is responsible for depolymerization and thermal breakdown of cellulose.
Furthermore, it was discovered that the first-order pseudo-kinetic model can be used globally to explain the kinetics of pretreatment for a range of chemical pretreatment procedures and feedstocks. The kinetic parameters are determined by fitting experimental data and the results indicated that the model is appropriate. The activation energies for HP and CHPA pretreatments were 13.68 and 8.18 kJ/mol, respectively. The higher temperatures lead to a higher degree of pretreatment value, indicating their positive correlation. The optimum glucose production (6.58 g/L) was attained with CHPA pretreated at 25 h hydrolysis time and 6% enzyme loading.
Author Contributions
Conceptualization, N.N., H.H. (Hasanudin Hasanudin) and H.H. (Hermansyah Hermansyah); methodology, N.N. and H.H. (Hermansyah Hermansyah); software, N.N.; validation, N.N., H.H. (Hasanudin Hasanudin), and H.H. (Hermansyah Hermansyah); formal analysis, N.N.; investigation, N.N.; resources, N.N., H.H. (Hasanudin Hasanudin), and H.H. (Hermansyah Hermansyah); data curation, N.N.; writing—original draft preparation, N.N.; writing—review and editing, N.N. and A.F.; visualization, N.N.; supervision, H.H. (Hasanudin Hasanudin) and H.H. (Hermansyah Hermansyah); project administration, H.H. (Hasanudin Hasanudin) and H.H. (Hermansyah Hermansyah). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Direktorat Sumber Daya-DIKTI, Ministry of Education and Culture, Indonesia grant number 299/E4.1/AK.04.PT/2021 (as Research Contract: Skema Penelitian Terapan, No. 0164.01/UN9/SB3.LP2M.PT/2021) And The APC was funded by Direktorat Sumber Daya-DIKTI, Ministry of Education and Culture, Indonesia grant number 299/E4.1/AK.04.PT/2021 (as Research Contract: Skema Penelitian Terapan, No. 0164.01/UN9/SB3.LP2M.PT/2021).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
All of authors are grateful for the financial funding presented by Direktorat Sumber Daya-DIKTI, Ministry of Education and Culture, Indonesia, for the research project No. 299/E4.1/AK.04.PT/2021, 12 July 2021 (as Research Contract: Skema Penelitian Terapan, No. 0164.01/UN9/SB3.LP2M.PT/2021, 13 July 2021).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent trends in the pretreatment of lignocellulosic biomass for value-added products. Front. Energy Res. 2018, 6, 141. [Google Scholar] [CrossRef]
- Rezania, S.; Oryani, B.; Cho, J.; Talaiekhozani, A.; Sabbagh, F.; Hashemi, B.; Rupani, P.F.; Mohammadi, A.A. Different pretreatment technologies of lignocellulosic biomass for bioethanol production: An overview. Energy 2020, 199, 117457. [Google Scholar] [CrossRef]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Emerging technologies for the pretreatment of lignocellulosic biomass. Bioresour. Technol. 2018, 262, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Qiao, X.; Cao, Y.; Shao, Q. Application of hydrogen peroxide presoaking prior to ammonia fiber expansion pretreatment of energy crops. Fuel 2017, 205, 184–191. [Google Scholar] [CrossRef]
- Liu, C.G.; Xiao, Y.; Xia, X.X.; Zhao, X.Q.; Peng, L.; Srinophakun, P.; Bai, F.W. Cellulosic ethanol production: Progress, challenges and strategies for solutions. Biotechnol. Adv. 2019, 37, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Kassaye, S.; Pant, K.K.; Jain, S. Synergistic effect of ionic liquid and dilute sulphuric acid in the hydrolysis of microcrystalline cellulose. Fuel Process. Technol. 2016, 148, 289–294. [Google Scholar] [CrossRef]
- Qiu, J.; Tian, D.; Shen, F.; Hu, J.; Zeng, Y.; Yang, G.; Zhang, Y.; Deng, S.; Zhang, J. Bioethanol production from wheat straw by phosphoric acid plus hydrogen peroxide (PHP) pretreatment via simultaneous saccharification and fermentation (SSF) at high solid loadings. Bioresour. Technol. 2018, 268, 355–362. [Google Scholar] [CrossRef]
- Lauberte, L.; Telysheva, G.; Cravotto, G.; Andersone, A.; Janceva, S.; Dizhbite, T.; Arshanitsa, A.; Jurkjane, V.; Vevere, L.; Grillo, G.; et al. Lignin-Derived antioxidants as value-added products obtained under cavitation treatments of the wheat straw processing for sugar production. J. Clean. Prod. 2021, 303, 126369. [Google Scholar] [CrossRef]
- Gan, Y.-Y.; Zhou, S.-L.; Dai, X.; Wu, H.; Xiong, Z.-Y.; Qin, Y.-H.; Ma, J.; Yang, L.; Wu, Z.-K.; Wang, T.-L.; et al. Effect of iron salt type and dosing mode on Fenton-based pretreatment of rice straw for enzymatic hydrolysis. Bioresour. Technol. 2018, 265, 394–398. [Google Scholar] [CrossRef]
- Yu, N.; Tan, L.; Sun, Z.Y.; Nishimura, H.; Takei, S.; Tang, Y.Q.; Kida, K. Bioethanol from sugarcane bagasse: Focused on optimum of lignin content and reduction of enzyme addition. Waste Manag. 2018, 76, 404–413. [Google Scholar] [CrossRef]
- Wojtusik, M.; Villar, J.C.; Ladero, M.; Garcia-Ochoa, F. Physico-chemical kinetic modelling of hydrolysis of a steam-explosion pretreated corn stover: A two-step approach. Bioresour. Technol. 2018, 268, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Truong, N.P.V.; Kim, T.H. Effective saccharification of corn stover using low-liquid aqueous ammonia pretreatment and enzymatic hydrolysis. Molecules 2018, 23, 1050. [Google Scholar] [CrossRef] [PubMed]
- Polprasert, S.; Choopakar, O.; Elefsiniotis, P. Bioethanol production from pretreated palm empty fruit bunch (PEFB) using sequential enzymatic hydrolysis and yeast fermentation. Biomass Bioenergy 2021, 149, 106088. [Google Scholar] [CrossRef]
- Mankar, A.R.; Pandey, A.; Modak, A.; Pant, K. Pretreatment of lignocellulosic biomass: A review on recent advances. Bioresour. Technol. 2021, 334, 125235. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef]
- Robak, K.; Balcerek, M. Current state-of-the-art in ethanol production from lignocellulosic feedstocks. Microbiol. Res. 2020, 240, 126534. [Google Scholar] [CrossRef]
- Wirawan, F.; Cheng, C.-L.; Lo, Y.-C.; Chen, C.-Y.; Chang, J.-S.; Leu, S.-Y.; Lee, D.-J. Continuous cellulosic bioethanol co-fermentation by immobilized Zymomonas mobilis and suspended Pichia stipitis in a two-stage process. Appl. Energy 2020, 266, 114871. [Google Scholar] [CrossRef]
- Fan, Z.; Lin, J.; Wu, J.; Zhang, L.; Lyu, X.; Xiao, W.; Gong, Y.; Xu, Y.; Liu, Z. Vacuum-assisted black liquor-recycling enhances the sugar yield of sugarcane bagasse and decreases water and alkali consumption. Bioresour. Technol. 2020, 309, 123349. [Google Scholar] [CrossRef]
- Yang, H.; Shi, Z.; Xu, G.; Qin, Y.; Deng, J.; Yang, J. Bioethanol production from bamboo with alkali-catalyzed liquid hot water pretreatment. Bioresour. Technol. 2019, 274, 261–266. [Google Scholar] [CrossRef]
- Alvarez-Vasco, C.; Zhang, X. Alkaline hydrogen peroxide pretreatment of softwood: Hemicellulose degradation pathways. Bioresour. Technol. 2013, 150, 321–327. [Google Scholar] [CrossRef]
- Zhao, C.; Qiao, X.; Shao, Q.; Hassan, M.; Ma, Z.; Yao, L. Synergistic effect of hydrogen peroxide and ammonia on lignin. Ind. Crop. Prod. 2020, 146, 112177. [Google Scholar] [CrossRef]
- Gao, J.; Yang, X.; Wan, J.; He, Y.; Chang, C.; Ma, X.; Bai, J. Delignification Kinetics of Corn Stover with Aqueous Ammonia Soaking Pretreatment. BioResources 2016, 11, 2403–2416. [Google Scholar] [CrossRef]
- Siddiqi, M.H.; Liu, X.-M.; Hussain, M.A.; Qureshi, T.; Tabish, A.N.; Lateef, H.U.; Zeb, H.; Farooq, M.; Nawaz, S.; Nawaz, S. Evaluation of physiochemical, thermal and kinetic properties of wheat straw by demineralising with leaching reagents for energy applications. Energy 2022, 238, 122013. [Google Scholar] [CrossRef]
- Bhattarai, S.; Bottenus, D.; Ivory, C.F.; Gao, A.H.; Bule, M.; Garcia-Perez, M.; Chen, S. Simulation of the ozone pretreatment of wheat straw. Bioresour. Technol. 2015, 196, 78–87. [Google Scholar] [CrossRef]
- Dong, L.; Zhao, X.; Liu, D. Kinetic modeling of atmospheric formic acid pretreatment of wheat straw with ‘potential degree of reaction’ models. RSC Adv. 2015, 5, 20992–21000. [Google Scholar] [CrossRef]
- Dagnino, E.P.; Felissia, F.E.; Chamorro, E.; Area, M.C. Studies on lignin extraction from rice husk by a soda-ethanol treatment: Kinetics, separation, and characterization of products. Chem. Eng. Res. Des. 2018, 129, 209–216. [Google Scholar] [CrossRef]
- Baksi, S.; Sarkar, U.; Saha, S.; Ball, A.K.; Kuniyal, J.C.; Wentzel, A.; Birgen, C.; Preisig, H.A.; Wittgens, B.; Markussen, S. Studies on delignification and inhibitory enzyme kinetics of alkaline peroxide pretreated pine and deodar saw dust. Chem. Eng. Process. Process Intensif. 2019, 143, 107607. [Google Scholar] [CrossRef]
- Yiin, C.L.; Yusup, S.; Quitain, A.T.; Uemura, Y.; Sasaki, M.; Kida, T. Thermogravimetric analysis and kinetic modeling of low-transition-temperature mixtures pretreated oil palm empty fruit bunch for possible maximum yield of pyrolysis oil. Bioresour. Technol. 2018, 255, 189–197. [Google Scholar] [CrossRef]
- Yiin, C.L.; Yusup, S.; Quitain, A.T.; Uemura, Y.; Sasaki, M.; Kida, T. Delignification kinetics of empty fruit bunch (EFB): A sustainable and green pretreatment approach using malic acid-based solvents. Clean Technol. Environ. Policy 2018, 20, 1987–2000. [Google Scholar] [CrossRef]
- Shamjuddin, A.; Ab Rasid, N.S.; Raissa, M.M.M.; Abu Zarin, M.A.; Omar, W.N.N.W.; Syahrom, A.; Januddi, M.A.F.M.S.; Amin, N.A.S. Kinetic and dynamic analysis of ozonolysis pretreatment of empty fruit bunch in a well-mixed reactor for sugar production. Energy Convers. Manag. 2021, 244, 114526. [Google Scholar] [CrossRef]
- Halder, P.; Patel, S.; Kundu, S.; Hakeem, I.G.; Marzbali, M.H.; Pramanik, B.; Shah, K. Dissolution reaction kinetics and mass transfer during aqueous choline chloride pre-treatment of oak wood. Bioresour. Technol. 2021, 322, 124519. [Google Scholar] [CrossRef]
- Kawamata, Y.; Yoshikawa, T.; Aoki, H.; Koyama, Y.; Nakasaka, Y.; Yoshida, M.; Masuda, T. Kinetic analysis of delignification of cedar wood during organosolv treatment with a two-phase solvent using the unreacted-core model. Chem. Eng. J. 2019, 368, 71–78. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, R.; Liu, D. Evaluation of the mass transfer effects on delignification kinetics of atmospheric acetic acid fractionation of sugarcane bagasse with a shrinking-layer model. Bioresour. Technol. 2018, 261, 52–61. [Google Scholar] [CrossRef]
- Chang, X.; Bai, Y.; Wu, R.; Liu, D.; Zhao, X. Heterogeneity of lignocellulose must be considered for kinetic study: A case on formic acid fractionation of sugarcane bagasse with different pseudo-homogeneous kinetic models. Renew. Energy 2020, 162, 2246–2258. [Google Scholar] [CrossRef]
- Shatalov, A.A.; Pereira, H. Kinetics of organosolv delignification of fibre crop Arundo donax L. Ind. Crop. Prod. 2005, 21, 203–210. [Google Scholar] [CrossRef]
- Huang, G.; Peng, W.; Yang, S.; Yang, C. Delignification kinetic modeling of NH4OH-KOH-AQ pulping for bagasse. Ind. Crop. Prod. 2018, 123, 740–745. [Google Scholar] [CrossRef]
- Banerjee, G.; Car, S.; Scott-Craig, J.S.; Hodge, D.B.; Walton, J.D. Alkaline peroxide pretreatment of corn stover: Effects of biomass, peroxide, and enzyme loading and composition on yields of glucose and xylose. Biotechnol. Biofuels 2011, 4, 16. [Google Scholar] [CrossRef]
- Novia, N.; Said, M.; Jannah, A.M.; Pebriantoni, P.; Bayu, M. Aqueous Ammonia Soaking-Dilute Acid Pretreatment to Produce Bioethanol from Rice Husk. Technol. Rep. Kansai Univ. 2020, 62, 891–900. [Google Scholar]
- Datta, R. Acidogenic fermentation of lignocellulose-acid yield and conversion of components. Biotechnol. Bioeng. 1981, 23, 2167–2170. [Google Scholar] [CrossRef]
- Xing, W.; Xu, G.; Dong, J.; Han, R.; Ni, Y. Novel dihydrogen-bonding deep eutectic solvents: Pretreatment of rice straw for butanol fermentation featuring enzyme recycling and high solvent yield. Chem. Eng. J. 2018, 333, 712–720. [Google Scholar] [CrossRef]
- Nathan, V.K.; Rani, M.E.; Rathinasamy, G.; Dhiraviam, K.N.; Jayavel, S. Process optimization and production kinetics for cellulase production by Trichoderma viride VKF3. Springerplus 2014, 3, 92. [Google Scholar] [CrossRef]
- Li, H.; Kim, N.J.; Jiang, M.; Kang, J.W.; Chang, H.N. Simultaneous saccharification and fermentation of lignocellulosic residues pretreated with phosphoric acid-acetone for bioethanol production. Bioresour. Technol. 2009, 100, 3245–3251. [Google Scholar] [CrossRef]
- Miller, G.L. Use of DinitrosaIicyIic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Rapado, P.; Faba, L.; Ordóñez, S. Influence of delignification and reaction conditions in the aqueous phase transformation of lignocellulosic biomass to platform molecules. Bioresour. Technol. 2021, 321, 124500. [Google Scholar] [CrossRef]
- Liu, K.-X.; Li, H.-Q.; Zhang, J.; Zhang, Z.-G.; Xu, J. The effect of non-structural components and lignin on hemicellulose extraction. Bioresour. Technol. 2016, 214, 755–760. [Google Scholar] [CrossRef]
- Betts, W.B.; Dart, R.K.; Ball, A.S.; Pedlar, S.L. Biosynthesis and Structure of Lignocellulose. In Biodegradation; Springer: London, UK, 1991; pp. 139–155. [Google Scholar]
- Kim, T.H.; Taylor, F.; Hicks, K.B. Bioethanol production from barley hull using SAA (soaking in aqueous ammonia) pretreatment. Bioresour. Technol. 2008, 99, 5694–5702. [Google Scholar] [CrossRef]
- Russo, M.; Procentese, A.; Montagnaro, F.; Marzocchella, A. Effect of enzymes adsorption on enzymatic hydrolysis of coffee silverskin: Kinetic characterization and validation. Biochem. Eng. J. 2022, 180, 108364. [Google Scholar] [CrossRef]
- Niglio, S.; Procentese, A.; Russo, M.E.; Piscitelli, A.; Marzocchella, A. Integrated enzymatic pretreatment and hydrolysis of apple pomace in a bubble column bioreactor. Biochem. Eng. J. 2019, 150, 107306. [Google Scholar] [CrossRef]
- Ang, T.N.; Ngoh, G.C.; Chua, A.S.M. Comparative study of various pretreatment reagents on rice husk and structural changes assessment of the optimized pretreated rice husk. Bioresour. Technol. 2013, 135, 116–119. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Caparanga, A.R.; Ordono, E.E.; Villaflores, O.B.; Pouriman, M. Effect of ammonium carbonate pretreatment on the enzymatic digestibility, structural characteristics of rice husk and bioethanol production via simultaneous saccharification and fermentation process with Saccharomyces cerevisiae Hansen 2055. Ind. Crop. Prod. 2017, 101, 84–91. [Google Scholar] [CrossRef]
- Ji, Q.; Yu, X.; Yagoub, A.E.-G.A.; Chen, L.; Zhou, C. Efficient removal of lignin from vegetable wastes by ultrasonic and microwave-assisted treatment with ternary deep eutectic solvent. Ind. Crop. Prod. 2020, 149, 112357. [Google Scholar] [CrossRef]
- Al-Mardeai, S.; Elnajjar, E.; Hashaikeh, R.; Kruczek, B.; Al-Zuhair, S. Dynamic model of simultaneous enzymatic cellulose hydrolysis and product separation in a membrane bioreactor. Biochem. Eng. J. 2021, 174, 108107. [Google Scholar] [CrossRef]
- Olivieri, G.; Wijffels, R.; Marzocchella, A.; Russo, M. Bioreactor and bioprocess design issues in enzymatic hydrolysis of lignocellulosic biomass. Catalysts 2021, 11, 680. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Song, M.; Topakas, E.; Yu, Q.; Yuan, Z.; Wang, Z.; Guo, Y. Combining Michaelis-Menten theory and enzyme deactivation reactions for the kinetic study of enzymatic hydrolysis by different pretreated sugarcane bagasse. Process Biochem. 2021, 105, 72–78. [Google Scholar] [CrossRef]
- Shen, X.-J.; Wen, J.-L.; Mei, Q.-Q.; Chen, X.; Sun, D.; Yuan, T.-Q.; Sun, R.-C. Facile fractionation of lignocelluloses by biomass-derived deep eutectic solvent (DES) pretreatment for cellulose enzymatic hydrolysis and lignin valorization. Green Chem. 2019, 21, 275–283. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).