Abstract
Paper mulberry (Broussonetia papyrifera L.) is an unconventional forage with high crude protein content and is widely used in China. In order to evaluate the pattern of fermentation quality and the aerobic stability when inoculated with lactic acid bacteria, laboratory-scale silage was prepared. The experimental groups included a control group (CK), a Lactobacillus plantarum ‘LC365283’ (selected from paper mulberry silage) treatment (L1), a commercial inoculant Lactobacillus plantarum treatment (GF), a commercial inoculant Lactobacillus buchneri treatment (FR), a 3% molasses treatment (MO), a 150 U/g cellulase treatment (CE), and their combinations (MO + L1, MO + GF, MO + FR, CE + L1, CE + GF, and CE + FR). The changes in bacterial community and composition of the fermentation products were evaluated after being ensiled for 30 days and unsealed for 1, 3, 5, and 7 days. Compared with the CK, MO and CE, the silages treated with L1, MO + L1, and CE + L1 showed higher lactic acid concentrations, lower pH values, and lower ammonia nitrogen concentrations (p < 0.05). During the first 3 days of aerobic exposure, the pH values and organic acid content changed slightly in all treatments. The present study suggests that addition of L1 was better than commercial inoculum, and the paper mulberry silages could be well preserved after being unsealed for 3 days.
1. Introduction
Paper mulberry (Broussonetia papyrifera L.) is widely used as forage in the Asia-Pacific region [1] due to its high crude protein (CP) content (27.17%), yield (52.5 t per hm2 per year), strong adaptability, and in vitro organic matter digestibility (50.88%) [2]). Sun et al. reported that paper mulberry was used to replace Medicago sativa L. to alleviate the shortage of high-protein forage [3]. Due to the adaptability of paper mulberry to continuous growth and periodic harvesting, ensiling is the main approach in paper mulberry production when other processing technologies, such as hay-making, are costly or risky [4]. Until now, paper mulberry silage has been added in the daily ration of dairy cows [5] and Hu lambs [6], and this revealed that paper mulberry silage could improve the feeding characteristics, growth performance, antioxidant capacity, and immunity of the livestock. Therefore, well-preserved paper mulberry silage with high aerobic stability is conducive to the development of animal husbandry.
However, the principal ensiling method for paper mulberry is challenging due its low water-soluble carbohydrate (WSC) concentration and poor lactic acid bacteria (LAB) community composition [7]. In addition, as a ligneous plant, paper mulberry has high fiber content. Thus, a better processing method for paper mulberry silage should be explored. A previous researcher reported that commercial LAB inoculants could improve the fermentation quality of paper mulberry silage [8], while the LAB strain screened from the plant itself might be more adaptable. At the same time, few publications have focused on the aerobic stability of paper mulberry silage.
Therefore, the Lactobacillus plantarum ‘LC365283’ (L1), selected from paper mulberry silage was used in this study for its high rate of acid production and strong growth ability. Two commercial inoculants were selected for comparison with L1: GF (mainly contains Lactobacillus plantarum) and FR (mainly contains Lactobacillus buchneri) were used to improve the aerobic stability of paper mulberry silages [9]. In addition, molasses and cellulase were used to supplement sufficient substrate. The effects of the selected LAB strain were evaluated by examining the fermentation products and microbial communities in the paper mulberry silages and during aerobic exposure.
2. Materials and Methods
2.1. Silage Materials and Ensiling
The paper mulberry leaves were harvested without wilting in a rainy and hot period (June) at the experimental base in Luoyang Academy of Agriculture and Forestry Science, Henan Province China (34.39 N, 112.12 E, elevation 250 m, annual mean temperature 15 °C, average annual precipitation 603 mm). The harvested plants were 1.5 to 2.0 m high and were 3 years old. The samples were immediately chopped into 2–3 cm pieces. The baseline chemical and microbiological characteristics of the pre-ensiled paper mulberry samples are shown in Table 1.

Table 1.
Chemical composition of paper mulberry.
Lactobacillus plantarum strain ‘LC365283’ (L1) was isolated from paper mulberry and selected for its high acid production rate. After isolation, Lactobacillus plantarum strain ‘LC365283’ was preserved by freeze-drying. The physiological and biochemical properties of L1 showed that it could grow at both low (5 °C) and high temperature (50 °C) under low pH (3.0) and in 6.5% NaCl solution. Commercial additive GF was obtained from Sichuan Gaofuji Biotechnology Co., Ltd., which mainly contains Lactobacillus plantarum. Another commercial inoculant, FR, was obtained from Lallemand Inc., Montreal, Quebec, Canada, which contains Lactobacillus buchneri. The molasses (MO) used in this experiment was obtained from Weifang Lvlong Biotechnology Co., Ltd. (Weifang, China). The cellulase (CE) was produced by Trichoderma viride and obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China)., and the unit of enzyme activity was 25 units/mg of DM.
Among the additives, inoculated additives were diluted with sterile water and mixed homogeneously into the chopped forage; then, they were inoculated at a concentration of 6 log CFU/g of FM. At the same time, the same volume water (1 mL) was added to the CK silage. The experimental groups were subjected to the following treatments: (1) CK, control without additive; (2) L1, Lactobacillus plantarum ‘LC365283’; (3) GF, commercial inoculant Lactobacillus plantarum; (4) FR, commercial inoculant Lactobacillus buchneri; (5) MO, applied at a concentration of 3%; (6) MO + L1, 3% MO combined with L1; (7) MO + GF, 3% MO combined with commercial inoculant GF; (8) MO + FR, 3% MO combined with commercial inoculant FR; (9) CE, cellulase applied at a concentration of 150 U/g; (10) CE + L1, 150 U/g CE combined with L1; (11) CE + GF, 150 U/g CE combined with GF; and (12) CE + FR, 150 U/g CE combined with FR. Between each 2 silage treatments, gloves were changed to prevent bacterial contamination, and 300 g of material was packed into a 0.5 L plastic jar (Hewanglan Paper and Plastic Products Factory, Beijing, China).
For each treatment, 3 replications were performed to measure the silage quality (3 × 12 = 36 jars). Another 3 jars for each treatment were prepared for measuring pH values, organic acid content, ammonia nitrogen (NH3-N) content, and microbial community (3 × 12 = 36 jars in total) after exposure to the air. The jars were stored at room temperature for 30 days.
2.2. Aerobic Stability
After opening the 36 jars for aerobic exposure, 167 g silage was removed from each jar to make the silo loose. The buckets were covered with a layer of cheesecloth to allow air infiltration of the silage mass while preventing drying and protecting it from contamination [10]. A sample of 10 g of each silage sample was collected to measure pH values, yeast counts, and organic acid and NH3-N contents after exposure for 1, 3, 5, and 7 days.
2.3. Chemical and Fermentation Characteristics
After opening the jars (36 jars for silage quality), 10 g samples of each treatment were taken and blended with 90 g distilled water, followed by extraction in a 4 °C refrigerator for 24 h and filtering through 4 layers of gauze and qualitative filter papers to obtain the extracts of the silage samples [11]. The filtrate was used to measure organic acid contents, NH3-N concentration, and pH values. The organic acid content, including lactic acid, acetic acid, propionic acid, and butyric acid, was measured via HPLC (column: Shodex RS Pak KC-811; Showa Denko K.K., Kawasaki, Japan; detector: DAD, 210 nm, SPD-20A; Shimadzu Co., Ltd., Kyoto, Japan; eluent: 3 mmol/L HClO4, 10 mL/min; temperature: 50 °C) [12]. NH3-N concentration was determined with the phenol–sodium-hypochlorite method [13]. The pH values were determined using a pH meter (FiveEasy 20K; Mettler-Toledo International Inc., Greifensee, Switzerland).
Another 10 g sample of each treatment was taken for measuring the bacteria community by next-generation sequencing and stored in a −80 °C freezer. At the same time, 10 g of silage was sampled and blended with 90 mL of sterilized water to enumerate microorganisms on agar plates.
The residual material was oven-dried at 65 °C for 48 h to measure the DM [14] and ground to pass through a 1 mm screen for nutritional quality analysis. Neutral detergent fiber (NDF) and acid detergent fiber (ADF) were measured according to Van Soest et al. with an ANKOM 2000 Fiber Analyzer (ANKOM Technology, New York, NY, USA) [15]. In the NDF measurements, a heat-tolerant enzyme and sodium sulfite were also used. The water-soluble carbohydrate (WSC) content was determined with the anthrone method [16]. Crude protein (CP) content was calculated by multiplying total nitrogen (TN) by 6.25, and the TN content was determined according to the Kjeldahl procedure [17]. The buffering capacity (BC) was measured by suspending 1 g of sample in 100 mL distilled water for 30 min, followed by titration to pH 4.0 with lactic acid (0.1 mol/L) [18].
2.4. Enumeration of Microorganisms on Agar Plates
A sample of 10 g of pre-ensiled forage and silage was sampled and blended with 90 mL of sterilized water and serially diluted in sterilized distilled water from 10−1 to 10−5 [19]. The number of lactic acid bacteria (LAB) was measured by MRS agar at 37 °C for 48 h. Yeast was counted on rose–bengal agar after incubation at 28 °C for 48 h, and coliform bacteria were counted on eosin-methylene blue agar at 37 °C for 48 h. All microbial data were transformed to log10 on an FM basis. The 3 media were obtained from Beijing Aoboxing Biotechnology Co., Ltd., Beijing, China.
2.5. Analysis of Complex Bacterial Community by Next-Generation Sequencing
The samples stored at −80 °C were sent to Biomarker Technologies Co., Ltd. (Beijing, China). The pre-ensiled materials and silage samples were added to 10 mmol/L of sterilized phosphate-buffered saline (pH 7.4), and DNA extraction was performed. The V3 + V4 regions of the bacteria’s 16S ribosomal RNA gene were amplified by PCR (95 °C for 5 min, followed by 30 cycles at 95 °C for 30 s, 57 °C for 30 s, 72 °C for 40 s, and a final extension at 72 °C for 7 min) using primers 335F (CADACTCCTACGGGAGGC) and 769R (ATCCTGTTTGMTMCCCVCRC) [20]. To minimize PCR bias, PCR reactions for each sample were conducted in triplicate, and mixtures of the 3 PCR products were used for DNA concentration determination and sequencing. The DNA samples were paired-end sequenced (2 × 250) on an Illumina MiSeq platform. For quality control purposes, any sequences that contained mismatches and ambiguous reads in primers were removed.
2.6. Calculations and Statistical Analysis
The data on chemical composition were analyzed by two-way analysis of variance to evaluate the effects of treatment (T), days after exposure to the air (D), and their interaction (D × T). The means were then tested for significant differences by Duncan’s multiple range method. All statistical analyses were performed using the general linear model procedure of SAS 9.0 (SAS Institute, Cary, NC, USA, 2002). Significance was declared at p < 0.05 unless otherwise noted.
3. Results
3.1. Fermentation Quality of Paper Mulberry Silages
When the L1-treated silage was unsealed at 30 days after storage, a lower pH value (4.53) was found (p < 0.05) among the treatment groups with single microbial additives (CK, L1, GF, FR) (Table 2). The addition of CE + L1 decreased the pH value and NH3-N content significantly (p < 0.05) in the treatment groups combined with CE (CE, CE + L1, CE + GF, and CE + FR). The pH value was lower in the treatments combined with MO (MO, MO + L1, MO + GF, and MO + FR) compared with those with single microbial additive or those combined with CE (p < 0.05). The ratio of lactic acid/acetic acid in the silage treated with MO was the highest, while the MO + L1-, MO + GF-, and MO + FR-treated silages were second highest compared with other treatments (p < 0.05). The addition of MO and MO + FR enhanced propionic acid production significantly (p < 0.05).

Table 2.
Effects of additive on the fermentation quality of paper mulberry silages.
3.2. Nutrient Substance of Paper Mulberry Silages
The water-soluble carbohydrate (WSC) content in silage groups treated with MO (MO, MO + L1, MO + GF, and MO + FR) was higher (p < 0.05) than that in other treatments (Table 3). The concentration of dry matter, neutral detergent fiber, acid detergent fiber, and cellulose had no significant differences among the treatments.

Table 3.
Effects of additive on the nutrient substances of paper mulberry silages.
3.3. Bacterial Community Structure in Paper Mulberry Silages
The change in bacterial community structure of paper mulberry was displayed at the genus (Figure 1a) and species level (Figure 1b). Pseudomonas (19.63%), Methylobacterium (12.95%), Sphingomonas (11.84%), and Lachnospiraceae (10.78%) were the predominant bacteria present in paper mulberry. After 30 days of ensiling, the proportion of Pseudomonas, Methylobacterium, Sphingomonas, and Lachnospiraceae decreased to <0.2%, while Lactobacillus dominated in the paper mulberry silage.
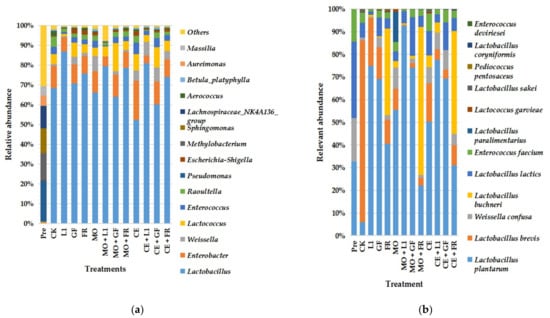
Figure 1.
Relative abundance of paper mulberry silage bacterial communities. (a) At genus level; (b) at species level; L1, Lactobacillus plantarum ‘LC365283’; GF, commercial inoculant mainly containing Lactobacillus plantarum; FR, commercial inoculant containing Lactobacillus buchneri; MO, molasses; CE, cellulase.
The main microbiota in the CK group were Lactobacillus (63.49%, Figure 1a), while the main species of Lactobacillus was Lactobacillus brevis (80.09%, Figure 1b). In the FR groups (FR, MO + FR, and CE + FR), the proportion of Lactobacillus buchneri accounted for 38.33%, 65.32%, 45.45%, respectively. According to the results of bacterial community structure at species level, the proportion of Lactobacillus plantarum was more than 50% in the silages treated with L1, GF, MO + L1 MO + GF, CE + L1, and CE + GF, and that in CK was lower than 10%.
3.4. Aerobic Stability of Paper Mulberry Silages
3.4.1. pH Value of Paper Mulberry Silages after Exposure to Air
Except for the MO + GF-treated silage, the pH values changed slightly in other treatments during the first 3 days after aerobic exposure (Figure 2). The pH value increased markedly from 4.64 to 6.23 after exposure for 7 days in the CK-treated silage; additionally, the pH value increased from 4.15 to 7.18 in the MO-treated silage. Less of an increase in pH value was observed in the CE-treated silage (increased from 4.56 to 5.57) compared with that in the CE- and MO-treated silages. The uptrend in pH values in the L1- and MO + L1-treated silages was similar with that in the MO-treated silage. The uptrend in pH values in the FR- and CE + FR-treated silages was the least, which increased from 4.88 to 5.21 and 4.81 to 5.11, respectively.
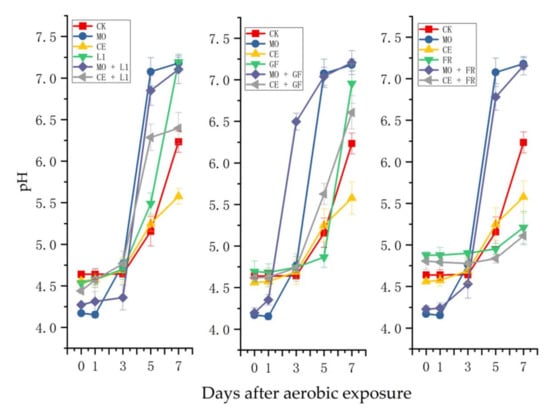
Figure 2.
Changes in pH value of the paper mulberry silages during 7-day spoilage test. L1, Lactobacillus plantarum ‘LC365283’; GF, commercial inoculant mainly containing Lactobacillus plantarum; FR, commercial inoculant containing Lactobacillus buchneri; MO, molasses; CE, cellulase.
3.4.2. Fermentation Products of Paper Mulberry Silages after Exposure to Air
The fermentation products of paper mulberry during aerobic exposure are presented in Figure 3. The lactic acid concentration in the silages treated with CK increased slightly at the first day of exposure and decreased sharply from the third day (Figure 3a). The lactic acid content of the silages treated with MO decreased from the first day of exposure, and the final content was similar with that of the CK. Lactic acid concentration decreased during days 1–3 and increased during days 3–7 in the CE-treated silage. In addition, an uptrend of lactic acid concentration was observed in the silages treated with CE + L1 and CE + FR.
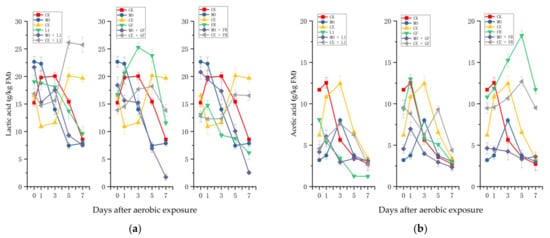
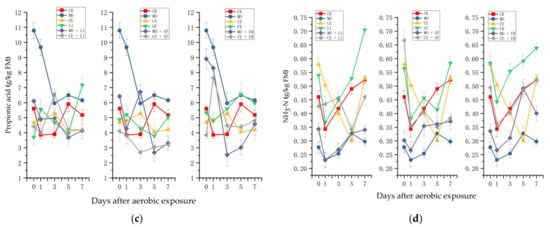
Figure 3.
Changes in (a) lactic acid; (b) acetic acid; (c) propionic acid; and (d) NH3-N content of the paper mulberry silages in the 7-day spoilage test. L1, Lactobacillus plantarum ‘LC365283’; GF, commercial inoculant mainly containing Lactobacillus plantarum; FR, commercial inoculant containing Lactobacillus buchneri; MO, molasses; CE, cellulase; FM, fresh matter.
During the aerobic exposure, acetic acid showed an increasing trend at first and then decreased in most of the treatments (Figure 3b). The acetic acid concentration in CK increased slightly on the first day and decreased to 2.73 g/kg of FM after 7 days of exposure. The acetic acid content of the silage treated with MO increased during the first 3 days of exposure, while it decreased to 3.02 g/kg of FM at the end; the acetic acid in the CE-treated silage showed a similar trend with that of the CK-treated silage. It is noteworthy that the FR- (11.72 g/kg of FM) and CE + FR-treated (9.51 g/kg of FM) silages had higher acetic acid contents compared with other treatments.
As shown in Figure 3c, the propionic acid concentration in the CK-treated silage decreased in the first 3 days of exposure and then increased. In the first 3 days of exposure, the propionic acid content decreased sharply and then remained stable at 6 g/kg of FM in the MO-treated silage. The change in the propionic acid content in the silage treated with CE was slight (from 4.69 to 4.21 g/kg of FM) during the exposure. The silages only treated with lactic acid bacteria inoculants (L1. GF, FR) showed a decreasing trend at first and then increased.
During the aerobic exposure, the NH3-N content was decreasing at first and then increased in most of the treatments (Figure 3d). After 7 days of exposure, the NH3-N content in the silage only treated with lactic acid bacteria was much higher than that in the CK-, MO-, CE-treated silages.
3.4.3. Yeast and Mold Counts of Paper Mulberry Silages after Exposure to Air
As shown in Figure 4a, yeast in the CK- and CE-treated silages was not detected in the 7 days of exposure. In the first 3 days of exposure, no yeast was found in the MO-treated silage, while it increased to 5.09 log CFU/g of FM at the end. Additionally, in the MO + L1-, MO + GF-, MO + FR-, CE + GF- and CE + FR-treated silages yeast was detected after exposure to the air.
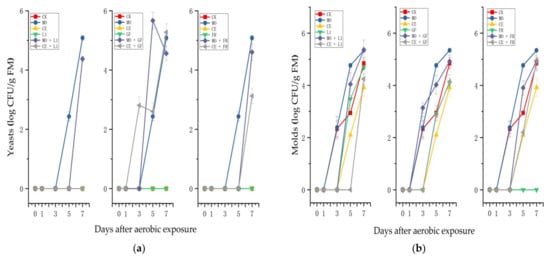
Figure 4.
Changes in (a) yeast counts; (b) mold counts of paper mulberry silages in the 7-day spoilage test. L1, Lactobacillus plantarum ‘LC365283’; GF, commercial inoculant mainly containing Lactobacillus plantarum; FR, commercial inoculant containing Lactobacillus buchneri; MO, molasses; CE, cellulase; FM, fresh matter.
After 1 day of aerobic exposure, the mold count increased sharply in the CK treatment, and by the 7th day, the mold counts reached 4.85 log CFU/g of FM (Figure 4b). The mold count in the MO treatment showed (5.34 log CFU/g of FM in the end) similar changes to those in the CK treatment. After the third day of aerobic exposure, the mold count began to rise and finally reached 3.92 log CFU/g of FM in the CE-treated silage. At the aerobic stage, no molds were detected in the silage treated with FR.
4. Discussion
4.1. Effects of Lactic Acid Bacteria on Fermentation Quality in Paper Mulberry Silages
The rapid establishment and growth of LAB during the fermentation process are important. When the fermentation substrate content reached 65 g/kg of DM [21] and the LAB number reached 5 log CFU/g of FM [22], well-preserved silages could be obtained. However, the WSC content and LAB numbers in paper mulberry were 39.61 g/kg of DM and 4.57 log CFU/g of FM, respectively, which were lower than the recommended levels. Therefore, the fermentation quality of paper mulberry silages should be improved in this study according to the characteristics of the raw materials.
After 30 days of ensiling, the pH value of the L1-treated silage was lower only in the treatment group of the LAB strain, which revealed that the LAB strain screened from paper mulberry was more suitable for fermentation. However, the silages treated with molasses (MO, MO + L1, and MO + FR) had lower pH values and higher ratios of lactic acid and acetic acid compared with the other treatments, which reached the standard of well-preserved silage (pH < 4.20). Compared with the silages treated with molasses, the fermentation quality in the L1- and GF-treated silages was worse. Therefore, the main reason for poor fermentation quality in paper mulberry is the lack of substrate. This result is the same with Muck [23], who reported that insufficient WSC content might lead to poor fermentation quality following the addition of LAB inoculant. Additionally, the NH3-N content of the MO-treated silage was lower than that of CK. This result might be explained by Dong et al., who reported that protease deactivation occurred at relatively low pH, and low pH could suppress the activity of Enterobacteria, Clostridia, or other microorganisms to reduce the consumption of crude protein [24].
Cellulase had no effects on improving the paper mulberry silage quality in this study, which was similar to the research reported by Liu et al. [25] and Meeske et al. [26], i.e., that cellulase neither improved fermentation quality nor affected fiber degradability in TMR and oat silage. However, the previous studies on the effects of cellulase on silage quality also showed inconsistent results. For example, Chen et al. reported that cellulase was found to decrease pH value and increased the lactic acid content in kudzu [27], and Sun et al. obtained the same results for sugarcane top silage [28]. Thus, the instability of cellulase in silage might be caused by many factors, such as the source of enzyme, amount of enzyme added, or the characteristics of plant fiber.
The FR-treated silage had higher acetic acid content compared with that of the CK-treated silage. This can be explained by the words of Muck et al., who concluded that facultative hetero-fermentative LAB could control the early fermentation period and convert lactic acid to acetic acid [29]. The acetic acid possessed the function of improving aerobic stability [30], and this accelerated better performance during aerobic exposure in the paper mulberry silage treated with FR.
4.2. Effects of Lactic Acid Bacteria on Aerobic Stability in Paper Mulberry Silages
When the fermentation was completed and silage was exposed to the air during feeding out, heating is usually initiated by yeast or molds, and changes occur in the chemical substrates and microbial communities of silages [31]. In this study, the WSC and lactic acid content were higher in the treatments group of MO (MO, MO + L1, MO + GF, and MO + FR), and the fermentation quality deteriorated sharply at the aerobic exposure stage; this may be caused by yeast or molds, which can utilize the abundant WSC and lactic acid in the MO group.
In this study, the pH values increased insignificantly in the first 3 days of aerobic exposure, except for the treatments with added MO. This may be explained by low WSC content in paper mulberry and a large amount of hetero-fermentation LAB attached, which can produce acetic acid to inhibit the activity of yeast and molds [32]. The experiment’s data also supported this view in that the concentration of acetic acid was high in the paper mulberry silages, except for the MO group.
After 7 days of exposure, slighter increases in pH values were observed in the FR- and CE + FR-treated silages. This may be explained by McDonald et al., who reported that hetero-fermentation LAB could promote acetate production and aerobic stability [33]. In this study, the acetic acid content in the FR or CE + FR-treated silages was slightly higher than that of the other treatments after exposure to the air for 7 days. Additionally, the pH value in CE-treated silage showed less increase compared with other treatments, which was similar with the results of Nkosi et al., who reported that cellulase (4.05) could decrease pH values compared with control (4.92), Lactobacillus plantrum addition (5.4), and Lactobacillus buchneri (4.23) [34].
The concentration of lactic acid increased in the CE-, CE + L1- and CE + FR-treated silages. This result is consistent with Kibe et al., who reported that lactic acid bacteria could produce lactic acid under aerobic conditions and part of the fiber was degraded to soluble sugars by cellulase; [35] Han et al. also supported this view [36]. At the same time, Britt et al. found the lactic acid content of corn silage was the highest after 14 days of aerobic exposure, which was caused by the LAB producing lactic acid with residual WSC [37].
5. Conclusions
The Lactobacillus plantarum ‘LC365283’ selected from paper mulberry silage was more suitable for silage compared with commercial LAB strain. Silages treated with CE or CE + FR had lower pH values and yeast counts after 7 days of aerobic exposure, and they improved the aerobic stability of paper mulberry silages. Furthermore, the pH values or mold counts of paper mulberry silages remained stable during the first 3 days of aerobic exposure in all treatments. Therefore, the fermentation quality of paper mulberry silages could remain for 3 days after unsealing.
Author Contributions
Conceptualization, Y.-C.Z. and F.-Y.Y.; methodology, X.-K.W. and Y.-L.Z.; data curation, Y.-C.Z.; writing—original draft preparation, Y.-C.Z.; writing—review and editing, K.-K.N.; project administration, F.-Y.Y. and Y.-L.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research & Development Program of China (Grant No. 2021YFD1300300).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the first author. The data are not publicly available due to restrictions by the research group.
Acknowledgments
In this section, you can acknowledge any support given which is not covered by the author contribution or funding sections. This may include administrative and technical support, or donations in kind (e.g., materials used for experiments).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Peng, X.; Wu, Q.; Teng, L.; Tang, F.; Pi, Z.; Shen, S. Transcriptional regulation of the paper mulberry under cold stress as revealed by a comprehensive analysis of transcription factors. BMC Plant Biol. 2015, 15, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obour, R.; Oppong, K.; Abebrese, I.K. Chemical composition and nutritive value of an invasive exotic species Broussonetia Papyrifera in Ghana. J. Nat. Sci. Res. 2017, 20, 45–53. [Google Scholar]
- Sun, W.T.; Huang, Y.; Wu, C.R.; Peng, C.; Zheng, Y.L.; Chen, C.; Hao, J. Addition of lactic acid bacteria can promote the quality and feeding value of Broussonetia papyrifera (Paper Mulberry) Silage. Fermentation 2022, 8, 25. [Google Scholar] [CrossRef]
- Wang, X.K.; Liu, H.; Xie, Y.X.; Zhang, Y.C.; Lin, Y.L.; Zheng, Y.L.; Yang, X.P.; Wang, N.W.; Ni, K.K.; Yang, F.Y. Effect of Sucrose and Lactic Acid Bacteria Additives on Fermentation Quality, Chemical Composition and Protein Fractions of Two Typical Woody Forage Silages. Agriculture 2021, 11, 256. [Google Scholar] [CrossRef]
- Si, B.; Tao, H.; Zhang, X.; Guo, J.; Cui, K.; Tu, Y.; Diao, Q. Effect of Broussonetia papyrifera L. (paper mulberry) silage on dry matter intake, milk composition, antioxidant capacity and milk fatty acid profile in dairy cows. Asian-Australas. J. Anim. Sci. 2018, 31, 1259–1266. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Y.; Guo, C.Z.; Wang, L.; Chen, F.; Dong, X.W.; Li, X.M.; Ni, K.K.; Yang, F.Y. Effect of paper mulberry silage on the growth performance, rumen microbiota and muscle fatty acid composition in Hu lambs. Fermentation 2021, 7, 286. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Li, D.X.; Wang, X.K.; Lin, Y.L.; Zhang, Q.; Chen, X.Y.; Yang, F.Y. Fermentation dynamics and diversity of bacterial community in four typical woody forages. Ann. Microbiol. 2018, 69, 233–240. [Google Scholar] [CrossRef]
- Cheng, Q.; Chen, Y.; Bai, S.; Chen, L.; You, M.; Zhang, K.; Li, P.; Chen, C. Study on the bacterial community structure and fermentation characteristics of fresh and ensiled paper mulberry. Anim. Sci. J. 2021, 92, e13656. [Google Scholar] [CrossRef]
- Nishino, N.; Hattori, H.; Kishida, Y. Alcoholic fermentation and its prevention by Lactobacillus buchneri in whole crop rice silage. Lett. Appl. Microbiol. 2007, 44, 538–543. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, X.; Zhao, M.; Yu, Z. Lactic bacteria for enhancing the fermentation quality and aerobic stability of Leymus chinensis silage. Grass Forage Sci. 2015, 71, 472–481. [Google Scholar] [CrossRef]
- Ren, F.Y.; He, R.C.; Zhou, X.K.; Gu, Q.C.; Xia, Z.S.; Liang, M.Z.; Zhou, J.H.; Lin, B.; Zou, C.X. Dynamic changes in fermentation profiles and bacterial community composition during sugarcane top silage fermentation: A preliminary study. Bioresour. Technol. 2019, 285, 121315. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.F. Studies of the Factors of Affecting Alfalfa Silage Quality and the Utilization of Alfalfa Silage in Dairy Cows Diet. Ph.D. Thesis, China Agricultural University, Beijing, China, 2005. [Google Scholar]
- Broderick, G.A.; Kang, J.H. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef]
- Zhang, L.Y. Feed Analysis and Quality Test Technology, 4th ed.; China Agricultural University: Beijing, China, 2002; pp. 46–47. [Google Scholar]
- Van Soest, P.J.; Robersom, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Murphy, R.P. A method for the extraction of plant samples and determination of total soluble carbohydrates. J. Sci. Food Agric. 1958, 9, 714–717. [Google Scholar] [CrossRef]
- Yang, S. Feed Analysis and Quality Test Technology, 1st ed.; China Agricultural University: Beijing, China, 1999; pp. 16–27. [Google Scholar]
- Herrmann, C.; Idler, C.; Heiermann, M. Improving aerobic stability and biogas production of maize silage using silage additives. Bioresour. Technol. 2015, 197, 393–403. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Zi, X.J.; Zhou, H.L.; Hou, G.Y.; Cai, Y.M. Effects of sucrose, glucose, molasses and cellulase on fermentation quality and in vitro gas production of king grass silage. Anim. Feed. Sci. Tech. 2014, 197, 206–212. [Google Scholar] [CrossRef]
- Zhang, Q. Studies on Screening, Mechanism of Action of Lactic Acid Bacteria for Forage Ensiling. Ph.D. Thesis, China Agricultural University, Beijing, China, 2016. [Google Scholar]
- Zhang, Q.; Wu, B.Y.L.; Nishino, N.; Wang, X.G.; Yu, Z. Fermentation and microbial population dynamics during the ensiling of native grass and subsequent exposure to air. Anim. Sci. J. 2016, 87, 389–397. [Google Scholar] [CrossRef]
- Waroon, K.; Suradej, P.; David, H.; Cai, Y.M. Natural lactic acid bacteria population of tropical grasses and their fermentation factor analysis of silage prepared with cellulase and inoculants. J. Dairy Sci. 2016, 99, 9768–9781. [Google Scholar] [CrossRef] [Green Version]
- Muck, R.E. Silage microbiology and its control through additives. Rev. Bras. Zootec. 2010, 39, 183–191. [Google Scholar] [CrossRef] [Green Version]
- Dong, Z.H.; Yuan, X.J.; Wen, A.Y.; Desta, S.T.; Shao, T. Effects of calcium propionate on the fermentation quality and aerobic stability of alfalfa silage. Asian-Australas. J. Anim. Sci. 2017, 30, 1278–1284. [Google Scholar] [CrossRef]
- Liu, Q.H.; Li, X.Y.; Seare, T.D.; Zhang, J.G.; Shao, T. Effects of Lactobacillus plantarum and fibrolytic enzyme on the fermentation quality and in vitro digestibility of total mixed rations silage including rape straw. J. Integrat. Agr. 2016, 15, 2087–2096. [Google Scholar] [CrossRef] [Green Version]
- Meeske, R.; Merwe, G.D.; Greyling, J.F.; Cruywagen, C.W. The effect of adding an enzyme containing lactic acid bacterial inoculant to big round bale oat silage on intake, milk production and milk composition of Jersey cows. Anim. Feed. Sci. Technol. 2002, 97, 159–167. [Google Scholar] [CrossRef]
- Chen, X.Z.; Li, W.Y.; Gao, C.F.; Zhang, X.P.; Weng, B.Q.; Cai, Y.M. Silage preparation and fermentation quality of kudzu, sugarcane top and their mixture treated with lactic acid bacteria, molasses and cellulase. Anim. Sci. J. 2017, 88, 1715–1721. [Google Scholar] [CrossRef]
- Sun, Q.Z.; Gao, F.Q.; Yu, Z.; Tao, Y.; Zhao, S.F.; Cai, Y.M. Fermentation quality and chemical composition of shrub silage treated with lactic acid bacteria inoculants and cellulase additives. Anim. Sci. J. 2012, 83, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Muck, R.E.; Nadeau, E.M.G.; Mcallister, T.A.; Contreras-Govea, F.E.; Santos, M.C.; Kung, L. Silage review: Recent advances and future uses of silage additives. J. Dairy Sci. 2018, 101, 3980–4000. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.C.; Li, D.X.; Wang, X.K.; Lin, Y.L.; Zhang, Q.; Chen, X.Y.; Yang, F.Y. Fermentation quality and aerobic stability of mulberry silage prepared with lactic acid bacteria and propionic acid. Anim. Sci. J. 2019, 90, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Ranjit, N.K.; Taylor, C.C.; Kung, L. Effect of Lactobacillus buchneri 40788 on the fermentation, aerobic stability and nutritive value of maize silage. Grass Forage Sci. 2002, 57, 73–81. [Google Scholar] [CrossRef]
- Zhao, J.; Tao, X.X.; Wang, S.R.; Li, J.F.; Shao, T. Effect of sorbic acid and dual-purpose inoculants on the fermentation quality and aerobic stability of high dry matter rice straw silage. J. Appl. Microbiol. 2021, 130, 1456–1465. [Google Scholar] [CrossRef]
- McDonald, P.A.R.; Henderson, H.S.J.E. The Biochemistry of Silage, 2nd ed.; Chalcombe Publications: Bucks, UK, 1991. [Google Scholar]
- Nkosi, B.D.; Vadlani, P.V.; Brijwani, K.; Nanjunda, A.; Meeske, R. Effects of bacterial inoculants and an enzyme on the fermentation quality and aerobic stability of ensiled whole-crop sweet sorghum. S. Afr. J. Anim. Sci. 2012, 42, 232–240. [Google Scholar] [CrossRef] [Green Version]
- Kibe, K.; Ewart, J.M.; Mcdonald, P. Chemical studies with silage microorganisms in artificial media and sterile herbages. J. Sci. Food Agr. 1977, 28, 335–364. [Google Scholar] [CrossRef]
- Han, L.Y.; Li, J.; Na, R.S.; Yu, Z.; Zhou, H. Effect of two additives on the fermentation, in vitro digestibility and aerobic security of Sorghum-sudangrass hybrid silages. Grass Forage Sci. 2013, 70, 185–194. [Google Scholar] [CrossRef]
- Britt, D.G.; Huber, J.T.; Rogers, A.L. Fungal growth and acid production during fermentation and refermentation of organic acid treated corn silages. J. Dairy Sci. 1975, 58, 532–539. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).