Abstract
The aim of this study was to evaluate the microbial changes and biogenic amine (BA) formation in rapeseed meal (RP) during fermentation with a bacterial starter combination of Lactiplantibacillus plantarum-LUHS122 and -LUHS135, Lacticaseibacillus casei-LUHS210, Lentilactobacillus farraginis-LUHS206, Pediococcus acidilactici-LUHS29, and Liquorilactobacillus uvarum-LUHS245. Sampling was carried out after 12 h and 7, 14, 21, and 28 days of cultivation under conditions of constant changes to the substrate, with a change frequency of 12 h. The highest lactic acid bacteria (LAB) and yeast/mould counts were established in RP fermented for 14 days (8.29 and 4.34 log10 CFU/g, respectively); however, the lowest total enterobacteria count was found in RP fermented for 12 h (3.52 log10 CFU/g). Further metagenomic analysis showed that Lactobacillus spp. were the most prevalent species in fermented RP. The changes in microbial community in RP led to differences in BA formation. Putrescine and phenylethylamine were found in all fermented RP samples, while the contents of some other amines increased with prolonged fermentation. Finally, the use of combined fermentation could ensure Lactobacillus spp. domination; however, other parameters should be controlled due to the formation of undesirable compounds.
1. Introduction
Fermented feed has been a part of animal diet for thousands of years and evolved through the need for new higher-value products, as well as to extend the shelf life and improve product safety via the inhibition of spoilage caused by pathogenic and opportunistic bacteria and fungi [1,2,3,4]. Lactic acid bacteria (LAB) are natural constituents of many fermented products; their main metabolites are organic acids, diacetyl, and ethanol [5]. In addition, many LAB are desirable intestinal microorganisms because they metabolise carbohydrates and reduce environmental pH, which leads to the suppression of the growth of pathogenic bacteria [6]. Our previous studies showed that Lactiplantibacillus plantarum-LUHS122 and -LUHS135, Lacticaseibacillus casei-LUHS210, Lentilactobacillus farraginis-LUHS206, Pediococcus acidilactici-LUHS29, and Liquorilactobacillus uvarum-LUHS245 strains inhibit a variety of pathogenic and opportunistic microorganisms in vitro, including methicillin-resistant Staphylococcus aureus, as well as fungi [7]. For this reason, the application of combinations of starter LAB strains has become very attractive. Until now, many probiotics producers have suggested compositions of probiotic strains for feed fermentation; however, combined culture fermentation requires an optimum balance of the LAB [8]. According to Hesseltine [9], on application of a single starter, it might be challenging to control the microbial environment due to the rates at which substrates are utilised, contamination from the outside environment, and other unstable factors; therefore, combinations of starter cultures are used to ensure the stability of the fermentation process. However, different strains possess different properties, and when a combination is used, it is difficult to predict which strains will survive at the end of the process. Additionally, the metabolic pathways of the LAB that are used could be related to the fermentation conditions (concentrations of nutrients, acidity parameters, other microorganisms, etc.).
Biogenic amines (BA) can be used as important chemical markers, which are associated with changes of microbial environment. In fermented substrates, BA are formed as a result of the decarboxylation of free amino acids by both Gram-negative and Gram-positive microorganisms showing decarboxylase activity [10]. The most common amines in fermented stock are histamine, tryptamine, putrescine, cadaverine, 2-phenylethylamine, tyramine, spermidine, and spermine [11,12]. Despite BA being involved in physiological processes in mammals, a high concentration of these compounds can be toxic. Additionally, separate BA can increase the toxicity of each other. For this reason, it is necessary to control both the fermentation process and its end products.
Although combined culture fermentation is used to ensure the fermentation process is safe and stable, changes in the microbial profile and metabolite formation might be quite difficult to predict. Studies on BA production in fermented feed, especially rapeseed meal, are very scarce. In the present study, a combination of newly isolated LAB strains with antimicrobial activities from a spontaneous rye sourdough was used for rapeseed fermentation. Knowledge on the microbial profile changes and BA formation in fermented rapeseed is valuable for the production of feed fermented with combined starter cultures. Furthermore, taking into consideration that the main parameter that is controlled in fermented substrate is Lactobacillus count rather than the profile, the BA could be important and sensitive chemical markers to show the changes (both desirable and undesirable) in Lactobacillus communities in fermentable substrates. Moreover, we hypothesised that despite it being possible to obtain high numbers of viable LAB in the end product (fermented feed stock), the biogenic amine concentration in the end-product could be related to changes in the microorganism community.
2. Materials and Methods
2.1. Lactic Acid Bacteria Strains and Rapeseed Meal Used in Experiments
The L. plantarum LUHS122, L. casei LUHS210, L. faraginis LUHS206, P. acidilactici LUHS29, L. plantarum LUHS135, and L. uvarum LUHS245 strains were obtained from the Lithuanian University of Health Sciences collection (Kaunas, Lithuania). Our previous studies showed that the above-mentioned strains inhibit various pathogenic and opportunistic microorganisms and are suitable for fermentation of various substrates [6,7,13,14]. The characteristics of the LAB strains used, including the inhibition of strains of pathogenic and opportunistic bacteria, as well as fungi, are described by Bartkiene et al. [7]. In addition, our previous studies showed that fermentation of feed with these strains had a positive influence on health and faecal microbial profile of piglets [4,15,16]. The above-mentioned LAB strains were stored at −80 °C in a Microbank system (Pro-Lab Diagnostics, UK) and separately propagated in de Man–Rogosa–Sharpe (MRS) broth (CM 0359, Oxoid Ltd., Hampshire, UK) at 30 ± 3 °C for 48 h before their use for feed fermentation.
Rapeseed meal (RP) was obtained from the SME ‘Ustukiu malunas’ (Pasvalys, Lithuania).
The LAB strains LUHS122, LUHS210, LUHS206, LUHS29, LUHS135, and LUHS245 were used for fermentation of RP. The RP, water, and a suspension of combined LAB strains (LAB in equal parts by volume, total 3% of dry matter of the RP mass (v/m)) containing 8.9 log10 CFU/mL) were fermented at 30 ± 2 °C for 12 h. Anaerobic conditions were attained by incubation of the fermentable substrate in anaerobic jars (Oxoid, Basingstoke, Hampshire, UK) with GasPak Plus™ (BBL, Cockeysville, MD, USA). For 100 g of RP, 60 mL of water was used. Further, the pure starter culture combination was not used for fermentation. After every 12 h of fermentation, one-third of the fermented RP mass was used as a starter for the second stage of fermentation, and two-thirds of the fermented mass was removed and used for analysis. Samples for analysis were taken after 12 h and 7, 14, 21, and 28 days of fermentation. Further RP fermentation cycles were performed at 30 ± 2 °C until the 28th day. Non-fermented RP was used as a control (RPnf). The principal scheme of the sample preparation process is given in Figure 1.
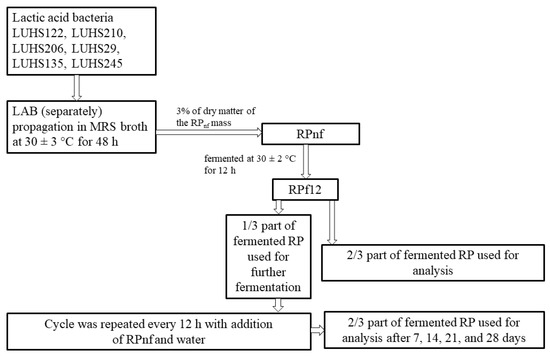
Figure 1.
The principal scheme of the fermented rapeseed meal sample preparation process (LUHS122—L. plantarum; LUHS210—L. casei; LUHS206—L. farraginis; LUHS29—P. acidilactici; LUHS135—L. plantarum; LUHS245—L. uvarum; RPnf—non-fermented rapeseed meal; RPf12—12-h-fermented rapeseed meal).
2.2. Evaluation of Acidity and Microbiological Characteristics of Samples
The pH was measured using a pH electrode (PP-15; Sartorius, Goettingen, Germany). For evaluation of the concentrations of L(+) and D(−) lactic acid isomers, a specific Megazyme assay kit (Megazyme Int., Bray, Ireland) was used. Counts of LAB, total bacteria (TBC), enterobacteria (TEC), and mould/yeast (M/Y) in samples were determined according to Bartkiene et al. [17].
2.3. Determination of Biogenic Amine Content in Samples
The extraction and determination of BA in RP samples followed the procedures developed by Ben-Gigirey et al. [18] with some modifications, as described by Bartkiene et al. [19]. The following BA were analysed: tryptamine, phenylethylamine, cadaverine, putrescine, histamine, tyramine, spermine and spermidine. The standard BA solutions were prepared by dissolving known amounts of each BA (including internal standard—1.7-diamino-heptane) in 20 mL of deionised water. Briefly, 5 g of sample was extracted with 10 mL of perchloric acid (0.4 mol/L) twice. The derivatization of sample extracts and standards was performed using a dansyl chloride solution in acetonitrile (10 mg/mL) as a reagent. A Varian ProStar HPLC system (Varian Corp., Palo Alto, CA, USA) equipped with a ProStar 325 UV/VIS Detector and Galaxy software (Agilent, Santa Clara, CA, USA) was used for analysis. A Discovery® HS C18 column (150 × 4.6 mm, 5 µm; SupelcoTM Analytical, Bellefonte, PA, USA) was used to separate BA. Ammonium acetate (0.1 mol/L) and acetonitrile were used as the mobile phases at a flow rate of 0.8 mL/min. The sample volume injected was 20 µL and the amines were monitored at 254 nm. The BA were identified based on their retention times in comparison to their corresponding standards.
2.4. Metagenomic Analysis of Rapeseed Meal Samples
Non-fermented and fermented (after 12 h and 7, 14, 21, and 28 days) RP samples were taken for microbial profiling analysis. A Quick-DNA Fecal/Soil Microbe Kit (Zymo Research, Irvine, CA, USA) was used for total DNA extraction according to the manufacturer’s instructions. The initial quantity and quality of the DNA were controlled using a Nano Drop 2000 spectrophotometer (Thermo Fisher, Waltham, MA, USA). Metagenomic analysis was performed in an independent service laboratory (Baseclear, Leiden, The Netherlands). During data analysis, reads of plant origin were dropped from the total amount of reads and data were analysed only for those bacteria for which the prevalence was at least 10 reads.
2.5. Statistical Analysis
Microbiological results are expressed as the means (n = 5) ± standard deviations (SD); physicochemical results are expressed as the means (n = 3) ± SD. To evaluate the effect of fermentation duration, the data were analysed by one-way analysis of variance (ANOVA) and Tukey’s honestly significant difference (HSD) procedure as post hoc tests. A linear Pearson’s correlation was used to quantify the strength of the relationship between the variables. The correlation coefficients were calculated using the statistical package SPSS for Windows (v15.0, SPSS, Chicago, IL, USA). Correlation strength interpretation was performed in accordance with [20]. Results were recognised as statistically significant at p ≤ 0.05.
3. Results and Discussion
3.1. Acidity Parameters of Rapeseed Meal Samples
The acidity parameters of RP samples are shown in Figure 2 (a—total lactic acid content, D(−) and L(+) lactic acid isomer concentration (g/100 g); b—pH). The highest concentration of lactic acid was found in RP samples fermented for 12 h (2.01 g/100 g). In other samples, the average lactic acid concentration was 1.39 g/100 g; in control samples it was 0.1 g/100 g. The L(+) and D(−) lactic acid isomer ratio was 0.92 in RPf12 and RPf7, 0.84 in RPf14, and 0.95 in RPf21 and RPf28. Very strong negative correlations were found between pH and D(−) lactic acid isomer and L(+) lactic acid isomer (r = −0.915, p = 0.0001; r = −0.903, p = 0.0001, respectively). One-way ANOVA indicated that the duration of fermentation has a significant effect on all the RP acidity parameters analysed (p = 0.0001).
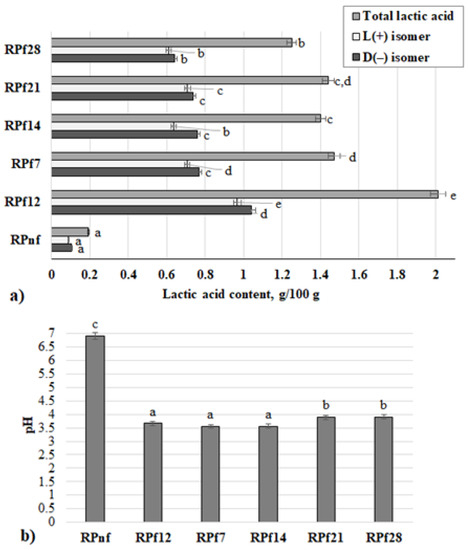
Figure 2.
Acidity parameters of rapeseed meal samples: (a) total lactic acid content, D(−) and L(+) lactic acid isomer concentration (g/100 g); (b) pH) (RPnf—non-fermented rapeseed meal; RPf12—12-h-fermented rapeseed meal; RP 7, 14, 21, 28—rapeseed meal samples fermented for 7, 14, 21, and 28 days respectively. Superscripts: a–e Mean values with different letters within a column are significantly different (p ≤ 0.05).
The observed correlation between pH and lactic acid concentration in RP samples can be explained by the fact that the acidification of the fermentable substrate mainly depends on the production of lactic acid, which is a result of sugar metabolism by LAB strains [21]. Other end products of fermentation, such as acetic acid and carbon dioxide, could also contribute to the reduction in pH. Similar pH values were obtained by Ashayerizadeh et al. [22] for RP fermented with L. acidophilus, Bacillus subtilis, and Aspergillus niger for 25 days. Wang et al. [23] showed that 9.5 g/100 g of lactic acid could be obtained in RP after 72 h of fermentation with B. licheniformis, Candida utilis, and Lactobacillus. The growth and productivity of LAB are limited by the medium pH and nutrient availability [24], and this could explain why the prolonged fermentation decreased the amount of lactic acid produced in RP. Homo- or heterofermentative utilisation of the substrate by Lactobacillus may lead to the production of D(−) and L(+) lactic acid isomers or a racemic mixture of both, while homofermentative Pediococcus species produce only a mixture of these isomers [25]. Moreover, the content of each isomer in a racemic mixture can also be affected by the substrate composition, changes in pH and temperature, or fermentation mode [26]. Narayanan et al. [27] found that L(+) isomers produced by Lactobacillus can be converted to the D(−) isomer by racemase when the former accumulates. In this study, a mixture of selected LAB strains was used; therefore, a racemic mixture of both lactic acid isomers was determined in all RP samples.
3.2. Microbiological Parameters of the Fermented Samples
The microbiological parameters of RP samples are given in Figure 3. The highest LAB count was found after 14 days of fermentation (average of 8.29 log10 CFU/g). After 21 and 28 days of fermentation, the average LAB counts were 11.6% and 24.4% lower than that in samples fermented for 14 days, respectively. Significant correlations were found between the LAB count and acidity parameters D(−) (r = 0.802, p = 0.0001), L(+) (r = 0.767, p = 0.0001), lactic acid concentration (r = 0.786, p = 0.0001), and pH (r = −0.937, p = 0.0001). One-way ANOVA showed that the duration of fermentation has a significant effect on the LAB count in RP samples (p = 0.0001).
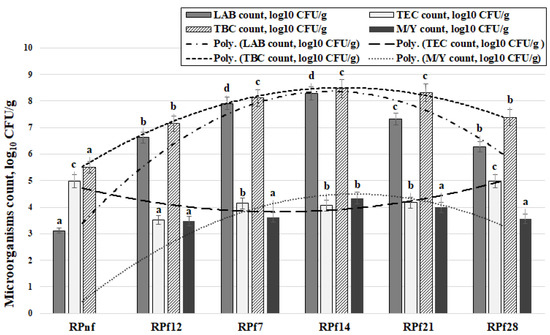
Figure 3.
Microbiological parameters of rapeseed meal samples (RPnf—non-fermented rapeseed meal; RPf12—12-h-fermented rapeseed meal; RPf 7, 14, 21, 28—rapeseed meal samples fermented for 7, 14, 21, and 28 days, respectively; LAB—lactic acid bacteria; TEC—total enterobacteria count; TBC—total bacteria count; M/Y—mould and yeast count; CFU—colony forming units. Superscripts: a–d Mean values with different letters within a column are significantly different (p ≤ 0.05)).
Currently, LAB are used in sustainable agriculture systems due to their high biological potential. Recently, many scientists have paid a great deal of attention to feed fermented with different LAB [28,29]. LAB form a significant group in the bacterial community, as they successfully reduce pH to a point where undesirable microorganisms are no longer able to grow, and may also control nutrient loss in feed [21]. LAB have the ability to convert citric acid into lactic acid, and acetic acid causes a decrease in substrate pH. The low pH contributes to the maintenance of a microbiologically stable environment, which can be dominated by acid-tolerant LAB species [30]. LAB utilise water-soluble carbohydrates and convert them into mixtures of organic acids. The use of LAB in starter cultures can enhance food or feed quality while minimising the loss of nutrients, such as carbohydrates, crude protein, volatile compounds, free fatty acids, and minerals. Therefore, the use of selected LAB additives enhances the fermentation process, leading to the control of dry matter loss and pathogenic activity [31].
The highest TEC counts were found in non-fermented RP samples and after 28 days of fermentation (average of 4.97 log10 CFU/g). The lowest TEC count was found in RP samples after 12 h of fermentation (3.52 log10 CFU/g); after 7, 14, and 21 days of fermentation, the average TEC count was 4.13 log10 CFU/g. In contrast to the findings for LAB counts, negative correlations were found between TEC and D(−) (r = −0.802, p = 0.0001), L(+) (r = −0.774, p = 0.0001), and lactic acid concentration (r = −0.756, p = 0.0001); a positive correlation was established between TEC and pH (r = −0.611, p = 0.007). Additionally, a moderate negative correlation was established between LAB count and TEC (r = −0.583, p = 0.011). One-way ANOVA showed that the duration of fermentation has a significant effect on TEC in RP samples (p = 0.0001).
Enterobacteria, yeasts, and moulds are undesirable microorganisms that may cause spoilage of fermented food or feed. Moreover, undesirable microorganisms can decrease the nutritional quality and also have an impact on human and animal health and animal production quality [21,32]. Lactobacilli play a crucial role in the bio-preservation of feed and nutrient quality through the production of organic acids and the inhibition of deleterious spoilage by the remaining microbial community. Additionally, LAB effectively control secondary fermentation within inoculated forage. Due to their lower pH, antimicrobial compounds (organic acids and bacteriocins) may eliminate undesirable pathogens. For example, secondary fermentation in silage is an undesirable acidification process that is carried out mainly by enterobacteria, clostridia (butyric acid producers), and yeasts (ethanol producers) [21,28,33]. It should be highlighted that during fermentation it is necessary to control the amount of enterobacteria due to their ability to initiate the formation of BA. The amount of Enterobacteriaceae may be correlated with the concentrations of various BA formed in fermentable substrates. The formation of BA in fermentable substrates is related to pH, storage, and processing conditions; counts of pathogenic bacteria (e.g., enterobacteria); and other factors [34,35,36].
No yeast and mould (Y/M) populations were found in non-fermented RP samples. The highest Y/M count was found in samples fermented for 14 days (4.34 log10 CFU/g); in other samples, the average Y/M count was 3.33 log10 CFU/g. The changes in Y/M count could be due to the ability of LAB to produce different acids [37]. However, yeasts can improve the growth of LAB, as well as being stable in media, as they can use bacterial end products as energy sources [14,37]. For instance, yeasts use LAB metabolites such as lactic acid, glucose, and galactose as energy sources [38]. Yeasts also synthesise metabolites that promote the development of LAB strains in media, establishing a mutualistic relationship between yeasts and LAB [14,37].
These findings are in agreement with the correlations found in this study. A very strong positive correlation was found between LAB and M/Y counts (r = 0.956, p = 0.0001), and a moderate negative correlation was established between Y/M and TEC (r = −0.530, p = 0.024). Similar tendencies of the correlations were established for M/Y as were found for LAB counts. A negative correlation was found between the M/Y counts in samples and lactic acid concentrations (r = −0.756, p = 0.0001), and a very strong negative correlation was found between M/Y counts and pH (r = −0.970, p = 0.007). One-way ANOVA showed that the duration of fermentation has a significant effect on M/Y counts in RP samples (p = 0.0001).
The TBC in non-fermented RP samples was 5.51 log10 CFU/g, while in fermented samples it was 30.2% higher on average.
Fermentation is associated with high counts of viable LAB and TBC because fermentation lowers the pH of the substrate due to the organic acids produced by LAB [39,40]. An acidic substrate is unsuitable for the growth of pathogenic bacteria, and this process reduces the counts of pathogenic Enterobacteriaceae strains; however, they are replaced by a higher LAB count, and in addition to this the TBC also increases [3,4,41].
However, comparing samples taken after 12 h and 28 days of fermentation with those taken after 7, 14, and 21 days of fermentation, a higher TBC was found in samples in the middle of the experiment (on average 12.5% higher). A very strong positive correlation was found between TBC and LAB counts (r = 0.972, p = 0.0001). Therefore, it can be stated that the main microorganisms in fermented RP were LAB; to verify these results, further metagenomic analysis was performed. Additionally, similar correlations were observed for TBC as were found for LAB counts between TBC and D(−) (r = 0.701, p = 0.001), L(+) (r = 0.675, p = 0.002), lactic acid concentration (r = 0.695, p = 0.001), and pH (r = −0.879, p = 0.0001).
3.3. Biogenic Amine Formation in Fermented Rapeseed Meal
The BA content in RP samples is shown in Figure 4. Tryptamine was found only in one sample (after 28 days of fermentation: 18.8 mg/kg). Phenylethylamine was found in all the tested RP samples; however, the average content of this BA was 8 times higher in fermented samples than in non-fermented samples. Additionally, comparing samples taken after 12 h and 7 and 14 days of fermentation with those taken after 21 and 28 days of fermentation, the earlier samples showed 2.5 times lower phenylethylamine concentration (average of 35.2 mg/kg). Putrescine was found only in fermented samples, at 383 mg/kg after 12 h, then a similar value after 7 days (384 mg/kg) and the lowest value after 14 days (287 mg/kg). However, after 21 and 28 days of fermentation, the average putrescine contents in RP samples increased (in comparison with samples fermented for 14 days) by 19.2% and 43.5%, respectively. Cadaverine, histamine, and tyramine were found at two sampling points, after 21 and 28 days of fermentation, with higher concentrations of these BA being found in samples fermented for 28 days (averages of 45.4%, 40.6%, and 44.2% higher, respectively). In contrast to cadaverine, tyramine, and histamine, spermidine was established only in non-fermented samples (132 mg/kg), and no spermine was found in RP samples.
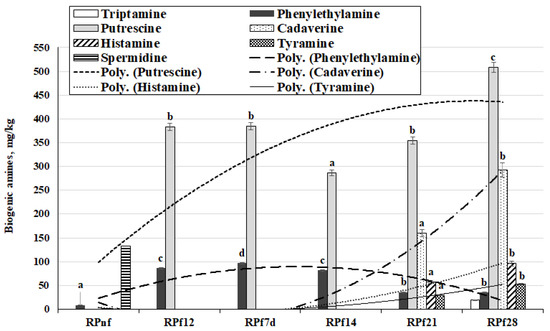
Figure 4.
Biogenic amine concentrations in rapeseed meal samples (RPnf—non-fermented rapeseed meal; RPf12—12-h-fermented rapeseed meal; RPf 7, 14, 21, 28—rapeseed meal samples fermented for 7, 14, 21, and 28 days, respectively. Superscripts: a–d Mean values with different letters within a column are significantly different (p ≤ 0.05)).
Significant correlations were established between phenylethylamine and spermidine and all acidity and microbiological parameters analysed (Table 1). Additionally, significant correlations were found between the putrescine concentration in samples and most of the parameters analysed (except TEC), as well as moderate positive correlations of cadaverine, histamine, and tyramine with TEC.

Table 1.
Pearson correlations (r) and significance (p) between biogenic amine (BA) concentrations in rapeseed meal (RP) samples and acidity, as well as microbiological parameters.
BA formation occurs through the decarboxylation of amino acids catalysed by exogenous decarboxylases of fermentative or contaminating microorganisms [35]. The intensity of BA formation is strongly affected by the presence of decarboxylase-positive bacteria, the contents of free amino acids and endogenous or microbial proteases, and environmental conditions such as temperature and pH [42]. Histamine and tyramine are the most toxic ones, but BA toxicity usually occurs at high levels [43]. However, no specific legislation covers BA levels in food, except for histamine in fishery products [35]. Spoilage microorganisms, especially enterobacteria and Pseudomonas, can produce histamine and cadaverine [43]. This could explain the increases in putrescine, histamine, and cadaverine contents in RP samples after 21 and 28 days of fermentation and the significant positive correlations observed between several BA (histamine and cadaverine) and TEC. The mentioned BA could also be accumulated by LAB, which are the major producers of tyramine, while moulds and yeasts might also contribute to BA production [43].
Significant correlations of putrescine and phenylethylamine with the mould/yeast count were found in this study. The observed correlations between putrescine content and LAB count or acidity parameters suggest that high levels of putrescine in all fermented RP could also be related to LAB activity. The same possible explanation may be applied to the formation of phenylethylamine in fermented RP. Moreover, the RP contains a high level of arginine, which is a precursor of putrescine, and a significant amount of phenylalanine, which can be converted to phenylethylamine [44]. Additionally, decarboxylase activity can be induced in acidic conditions as the cell response to acid stress; therefore, the production of BA may increase [42]. Spermidine, which was determined only in non-fermented RP, can be found naturally in all raw plant materials [45]. Studies involving BA analyses in fermented feed, especially rapeseed meal, are very scarce. A comparison of the obtained results with other authors is complicated due to the different experimental conditions, such as the substrate type, duration and mode of fermentation, and LAB strains used. In the study by Lau et al. [46], BA were not detected in liquid feed (50% canola meal, 25% wheat, and 25% barley) after 24 h fermentation with a mixture of Lactiplantibacillus plantarum, Pediococcus pentosaceus, and Lactobacillus lactis. Canibe et al. [47] found a higher total content of biogenic amines (tyramine, putrescine, cadaverine, and histamine) in 5-day-fermented liquid feed of barley and wheat (1186 mg/kg of DM) compared to dry feed (71 mg/kg of DM).
One-way ANOVA showed that the duration of fermentation has a significant effect on the concentrations of all BA analysed in RP samples (p = 0.0001).
3.4. Microbial Profiles of the Non-Fermented and Fermented Rapeseed Meal
The bacterial profile of rapeseed meal before the fermentation is given in Figure 5. Before fermentation the most predominant genus in rapeseed meal content was Phytoplasma (94.5%). It was detected in 11,646 reads of Phytoplasma from the total of 12,329 bacterial reads. The second most prevalent genus was Pantoea (1.9%). The amount of any other bacterial genera in rapeseed meal was below 0.6%, as presented in Figure 5.
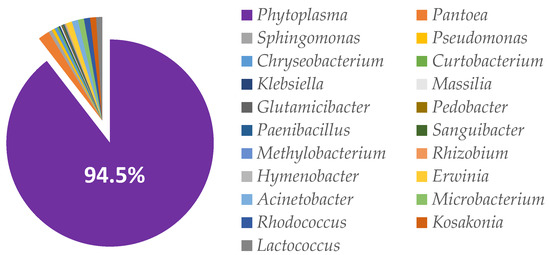
Figure 5.
Bacterial profile at the genus level within rapeseed meal before the fermentation.
The amounts of Lactobacillus spp. as DNA reads obtained after metagenomic sequencing of 16S rRNA in RP samples are presented in Figure 6 and Figure 7.
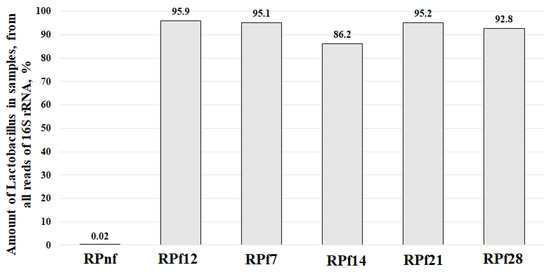
Figure 6.
Percental numbers of Lactobacillus in rapeseed meal samples (RPnf—non-fermented rapeseed meal; RPf12—12-h-fermented rapeseed meal; RPf 7, 14, 21, 28—rapeseed meal samples fermented for 7, 14, 21, and 28 days, respectively).
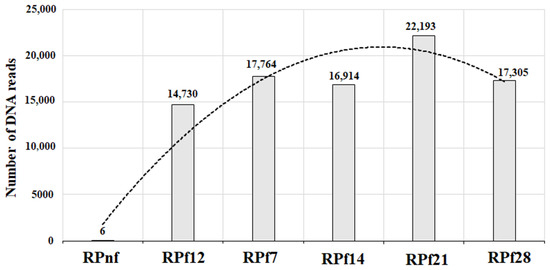
Figure 7.
Numbers of Lactobacillus in fermented samples of animal feed during the course of experiment (RPnf—non-fermented rapeseed meal; RPf12—12-h-fermented rapeseed meal; RPf 7, 14, 21, 28—rapeseed meal fermented for 7, 14, 21, and 28 days respectively).
Figure 6 presents the percentages of Lactobacillus spp. from all DNA reads detected in the analysed samples. The amounts of lactobacilli in samples at different stages varied from 86.2% to 95.9%. The remaining percentages (4.1–13.8%) of DNA reads of 16S rRNA sequences included not only those for bacteria but also sequences of plant origin, meaning that the amount of other bacterial genera in fermented samples was low (Supplementary Files S1–S6). Therefore, during the whole process of fermentation, no other genera except Lactobacillus were actively multiplied. The small amounts of different bacterial species presented in samples can be treated as contaminants, which are always found in non-sterile food or feed products.
As can be seen in Figure 6, the amounts of lactobacilli during different time periods were very similar, except for day 14, when there was an obvious decrease in their percentage. This can be explained by the analysis of the DNA reads (Supplementary File S4), which shows an increased percentage (6.8%) of plant (Brassicaceae) DNA detected together with bacterial DNA and included in the presented data. The amounts of lactobacilli detected 2 weeks before and 2 weeks after day 14 demonstrate the stable and high amounts of Lactobacillus spp. in samples. The prevalence of other bacteria in all fermented samples during all experiments was very low, with the highest being for bifidobacteria (2.19%), which were detected on day 28 of RP fermentation (Supplementary File S6).
P. acidilactici, which was used for fermentation, was detected only after 12 h of fermentation and accounted for 0.27% of all bacteria. From day 7, no Pediococcus species were detected in fermented samples. According to previous data, Pediococcus species are quite resistant to different environmental conditions, including low pH [48], but many other factors may influence their inability to survive in mixed bacterial communities, such as Lactobacillus bacteriocins used in a cocktail of bacteria for fermentation, the slow assimilation of nutrients and slow reproduction rate, and the non-optimal composition of nutrients presented in RP.
As can be seen from Figure 7, the number of lactobacilli in fermented RP samples had a tendency to increase from 12 h of fermentation up to 21 days and then decreased slightly but remained high enough on day 28. This means that the Lactobacillus strains used for RP fermentation are able to survive, multiply, and maintain homeostasis in fermented meal for a prolonged period.
4. Conclusions
The duration of fermentation had a significant effect on all RP parameters analysed (p = 0.0001). A very strong positive correlation was obtained between TBC and LAB counts in fermented RP samples (r = 0.972, p = 0.0001); therefore, it can be stated that the main microorganisms in fermented RP are LAB. Additionally, metagenomic analysis showed that Lactobacillus spp. are the most prevalent species in fermented RP. However, changes in the microbial community in RP led to differences in the formation of BA and possibly other metabolites. Tryptamine was found only in RP fermented for 28 days, phenylethylamine was found in all tested RP sample, and putrescine was found only in fermented samples. Cadaverine, histamine, and tyramine were found in samples fermented for 21 and 28 days. Spermidine was established only in non-fermented samples (132 mg/kg). Finally, it can be stated that the use of combined fermentation can ensure the domination of Lactobacillus spp.; however, other parameters (i.e., the concentration of D(−) lactic acid) should be controlled because of the formation of undesirable compounds. Further studies are needed to indicate the chemical markers for sensitive control of combined fermentation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation8040136/s1. Supplementary File S1. Rape expeller non-fermented seed meal. Supplementary File S2. Rape expeller fermented after 12 h. Supplementary File S3. Rape expeller fermented after 7 days. Supplementary File S4. Rape expeller fermented after 14 days. Supplementary File S5. Rape expeller fermented after 21 days. Supplementary File S6. Rape expeller fermented after 28 days.
Author Contributions
Conceptualization and methodology, E.B. and R.G.; investigation, M.R., D.K., E.Z, V.S., S.B. and L.V.; writing—original draft preparation, E.B., F.Ö. and M.R; writing—review and editing, E.B., R.G., M.R., D.K. and F.Ö.; visualization, E.Z. and V.S.; supervision, E.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of the current study are available from the corresponding author, upon reasonable request.
Acknowledgments
The authors gratefully acknowledge the EUREKA Network Project E!13309 ‘SUSFEETECH’ (Nr. 01.2.2-MITA-K-702-05-0001) and COST Action CA18101 ‘Sourdough Biotechnology Network towards Novel, Healthier, and Sustainable Food and Bioprocesses’.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ross, R.P.; Morgan, S.; Hill, C. Preservation and fermentation: Past, present and future. Int. J. Food Microbiol. 2002, 79, 3–16. [Google Scholar] [CrossRef] [Green Version]
- Macori, G.; Cotter, P.D. Novel insights into the microbiology of fermented dairy foods. Curr. Opin. Biotechnol. 2018, 49, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Vadopalas, L.; Ruzauskas, M.; Lele, V.; Starkute, V.; Zavistanaviciute, P.; Zokaityte, E.; Bartkevics, V.; Pugajeva, I.; Reinolds, I.; Badaras, S.; et al. Combination of Antimicrobial Starters for Feed Fermentation: Influence on Piglet Feces Microbiota and Health and Growth Performance, Including Mycotoxin Biotransformation in vivo. Front. Vet. Sci. 2020, 7, 528990. [Google Scholar] [CrossRef]
- Vadopalas, L.; Ruzauskas, M.; Lele, V.; Starkute, V.; Zavistanaviciute, P.; Zokaityte, E.; Bartkevics, V.; Badaras, S.; Klupsaite, D.; Mozuriene, E.; et al. Pigs’ Feed Fermentation Model with Antimicrobial Lactic Acid Bacteria Strains Combination by Changing Extruded Soya to Biomodified Local Feed Stock. Animals 2020, 10, 783. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, P.M.; Kuniyoshi, T.M.; Oliveira, R.P.; Hill, C.; Ross, R.P.; Cotter, P.D. Antimicrobials for food and feed; a bacteriocin perspective. Curr. Opin. Biotechnol. 2020, 61, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Bartkiene, E.; Lele, V.; Sakiene, V.; Zavistanaviciute, P.; Ruzauskas, M.; Bernatoniene, J.; Jakstas, V.; Viskelis, P.; Zadeike, D.; Juodeikiene, G. Improvement of the antimicrobial activity of lactic acid bacteria in combination with berries/fruits and dairy industry by-products. J. Sci. Food Agric. 2019, 99, 3992–4002. [Google Scholar] [CrossRef]
- Bartkiene, E.; Lele, V.; Ruzauskas, M.; Domig, K.J.; Starkute, V.; Zavistanaviciute, P.; Bartkevics, V.; Pugajeva, I.; Klupsaite, D.; Juodeikiene, G.; et al. Lactic Acid Bacteria Isolation from Spontaneous Sourdough and Their Characterization Including Antimicrobial and Antifungal Properties Evaluation. Microorganisms 2020, 8, 64. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-J.; Choi, Y.-J.; Lee, M.J.; Park, S.J.; Oh, S.J.; Yun, Y.-R.; Min, S.G.; Seo, H.-Y.; Park, S.-H.; Lee, M.-A. Effects of combining two lactic acid bacteria as a starter culture on model kimchi fermentation. Food Res. Int. 2020, 136, 109591. [Google Scholar] [CrossRef]
- Hesseltine, C.W. Applications of Biotechnology in Traditional Fermented Foods; National Academies Press: Washington, DC, USA, 1992; ISBN 978-0-309-04685-5. [Google Scholar]
- Wójcik, W.; Łukasiewicz, M.; Puppel, K. Biogenic amines: Formation, action and toxicity—A review. J. Sci. Food Agric. 2021, 101, 2634–2640. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; Kim, M.-J.; Moon, B. Various biogenic amines in Doenjang and changes in concentration depending on boiling and roasting. Appl. Biol. Chem. 2017, 60, 273–279. [Google Scholar] [CrossRef]
- Ly, D.; Mayrhofer, S.; Schmidt, J.-M.; Zitz, U.; Domig, K.J. Biogenic Amine Contents and Microbial Characteristics of Cambodian Fermented Foods. Foods 2020, 9, 198. [Google Scholar] [CrossRef] [Green Version]
- Bartkiene, E.; Bartkevics, V.; Krungleviciute, V.; Pugajeva, I.; Zadeike, D.; Juodeikiene, G. Lactic Acid Bacteria Combinations for Wheat Sourdough Preparation and Their Influence on Wheat Bread Quality and Acrylamide Formation. J. Food Sci. 2017, 82, 2371–2378. [Google Scholar] [CrossRef]
- Bartkiene, E.; Bartkevics, V.; Lele, V.; Pugajeva, I.; Zavistanaviciute, P.; Mickiene, R.; Zadeike, D.; Juodeikiene, G. A concept of mould spoilage prevention and acrylamide reduction in wheat bread: Application of lactobacilli in combination with a cranberry coating. Food Control 2018, 91, 284–293. [Google Scholar] [CrossRef]
- Bartkiene, E.; Zokaityte, E.; Lele, V.; Starkute, V.; Zavistanaviciute, P.; Klupsaite, D.; Cernauskas, D.; Ruzauskas, M.; Bartkevics, V.; Pugajeva, I.; et al. Combination of Extrusion and Fermentation with Lactobacillus plantarum and L. uvarum Strains for Improving the Safety Characteristics of Wheat Bran. Toxins 2021, 13, 163. [Google Scholar] [CrossRef]
- Vadopalas, L.; Badaras, S.; Ruzauskas, M.; Lele, V.; Starkute, V.; Zavistanaviciute, P.; Zokaityte, E.; Bartkevics, V.; Klupsaite, D.; Mozuriene, E.; et al. Influence of the Fermented Feed and Vaccination and Their Interaction on Parameters of Large White/Norwegian Landrace Piglets. Animals 2020, 10, 1201. [Google Scholar] [CrossRef] [PubMed]
- Bartkiene, E.; Ruzauskas, M.; Lele, V.; Zavistanaviciute, P.; Bernatoniene, J.; Jakstas, V.; Ivanauskas, L.; Zadeike, D.; Klupsaite, D.; Viskelis, P.; et al. Development of antimicrobial gummy candies with addition of bovine colostrum, essential oils and probiotics. Int. J. Food Sci. Technol. 2018, 53, 1227–1235. [Google Scholar] [CrossRef]
- Ben-Gigirey, B.; De Sousa, J.M.V.B.; Villa, T.G.; Velázquez, J.B. Histamine and Cadaverine Production by Bacteria Isolated from Fresh and Frozen Albacore (Thunnus alalunga). J. Food Prot. 1999, 62, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Bartkiene, E.; Bartkevics, V.; Rusko, J.; Starkute, V.; Bendoraitiene, E.; Zadeike, D.; Juodeikiene, G. The effect of Pediococcus acidilactici and Lactobacillus sakei on biogenic amines formation and free amino acid profile in different lupin during fermentation. LWT 2016, 74, 40–47. [Google Scholar] [CrossRef]
- Evans, T.G.; Reed, S.S.; Hibbs, J.B. Nitric Oxide Production in Murine Leishmaniasis: Correlation of Progressive Infection with Increasing Systemic Synthesis of Nitric Oxide. Am. J. Trop. Med. Hyg. 1996, 54, 486–489. [Google Scholar] [CrossRef]
- Kim, D.; Lee, K.D.; Choi, K.C. Role of LAB in silage fermentation: Effect on nutritional quality and organic acid production—An overview. AIMS Agric. Food 2021, 6, 216–234. [Google Scholar] [CrossRef]
- Ashayerizadeh, A.; Dastar, B.; Shargh, M.S.; Mahoonak, A.S.; Zerehdaran, S. Fermented rapeseed meal is effective in controlling Salmonella enterica serovar Typhimurium infection and improving growth performance in broiler chicks. Vet. Microbiol. 2017, 201, 93–102. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Ng, C.-C.; Su, H.; Tzeng, W.-S.; Shyu, Y.-T. Probiotic potential of noni juice fermented with lactic acid bacteria and bifidobacteria. Int. J. Food Sci. Nutr. 2009, 60, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Rawoof, S.A.A.; Kumar, P.S.; Vo, D.-V.N.; Devaraj, K.; Mani, Y.; Devaraj, T.; Subramanian, S. Production of optically pure lactic acid by microbial fermentation: A review. Environ. Chem. Lett. 2021, 19, 539–556. [Google Scholar] [CrossRef]
- Liptáková, D.; Matejčeková, Z.; Valík, Ľ. Lactic Acid Bacteria and Fermentation of Cereals and Pseudocereals. In Fermentation Processes; Jozala, A.F., Ed.; Intech Publisher: London, UK; Rijeka, Croatia, 2017; pp. 223–254. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Ding, Y.; Dong, H.; Hou, H.; Zhang, X. Distribution of Phenolic Acids and Antioxidant Activities of Different Bran Fractions from Three Pigmented Wheat Varieties. J. Chem. 2018, 2018, 6459243. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, A.; Narayanan, N.; Roychoudhury, P.K. L (+) lactic acid fermentation and its product polymerization. Electron. J. Biotechnol. 2004, 7, 167–178. [Google Scholar] [CrossRef]
- Kung, L., Jr.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef]
- Soundharrajan, I.; Park, H.S.; Rengasamy, S.; Sivanesan, R.; Choi, K.C. Application and Future Prospective of Lactic Acid Bacteria as Natural Additives for Silage Production—A Review. Appl. Sci. 2021, 11, 8127. [Google Scholar] [CrossRef]
- Agyirifo, D.S.; Wamalwa, M.; Otwe, E.P.; Galyuon, I.; Runo, S.; Takrama, J.; Ngeranwa, J. Metagenomics analysis of cocoa bean fermentation microbiome identifying species diversity and putative functional capabilities. Heliyon 2019, 5, e02170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pot, B.; Salvetti, E.; Mattarelli, P.; Felis, G.E. The potential impact of the Lactobacillus name change: The results of an expert meeting organised by the Lactic Acid Bacteria Industrial Platform (LABIP). Trends Food Sci. Technol. 2019, 94, 105–113. [Google Scholar] [CrossRef]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Govea, F.E.; Muck, R.E.; Broderick, G.A.; Weimer, P.J. Lactobacillus plantarum effects on silage fermentation and in vitro microbial yield. Anim. Feed Sci. Technol. 2013, 179, 61–68. [Google Scholar] [CrossRef]
- Ekici, K.; Omer, A. Biogenic amines formation and their importance in fermented foods. BIO Web Conf. 2020, 17, 00232. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Capillas, C.; Herrero, A.M. Impact of Biogenic Amines on Food Quality and Safety. Foods 2019, 8, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Özogul, F.; Hamed, I. The importance of lactic acid bacteria for the prevention of bacterial growth and their biogenic amines formation: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1660–1670. [Google Scholar] [CrossRef] [PubMed]
- García, C.; Rendueles, M.; Díaz, M. Liquid-phase food fermentations with microbial consortia involving lactic acid bacteria: A review. Food Res. Int. 2019, 119, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Bintsis, T. Lactic acid bacteria as starter cultures: An update in their metabolism and genetics. AIMS Microbiol. 2018, 4, 665–684. [Google Scholar] [CrossRef]
- Agarussi, M.C.N.; Pereira, O.G.; De Paula, R.A.; Da Silva, V.P.; Roseira, J.P.S.; Silva, F.F.E. Novel lactic acid bacteria strains as inoculants on alfalfa silage fermentation. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Vadopalas, L.; Zokaityte, E.; Zavistanaviciute, P.; Gruzauskas, R.; Starkute, V.; Mockus, E.; Klementaviciute, J.; Ruzauskas, M.; Lele, V.; Cernauskas, D.; et al. Supplement Based on Fermented Milk Permeate for Feeding Newborn Calves: Influence on Blood, Growth Performance, and Faecal Parameters, including Microbiota, Volatile Compounds, and Fatty and Organic Acid Profiles. Animals 2021, 11, 2544. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, T.; Tang, H.; Li, X.; Chen, Y.; Zhang, L.; Zhang, J. Probiotic potential and amylolytic properties of lactic acid bacteria isolated from Chinese fermented cereal foods. Food Control 2020, 111, 107057. [Google Scholar] [CrossRef]
- Gardini, F.; Özogul, Y.; Suzzi, G.; Tabanelli, G.; Özogul, F. Technological Factors Affecting Biogenic Amine Content in Foods: A Review. Front. Microbiol. 2016, 7, 1218. [Google Scholar] [CrossRef] [Green Version]
- Barbieri, F.; Montanari, C.; Gardini, F.; Tabanelli, G. Biogenic Amine Production by Lactic Acid Bacteria: A Review. Foods 2019, 8, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, J.; Skrede, A.; Mydland, L.T.; Øverland, M. Fractionation of rapeseed meal by milling, sieving and air classification—Effect on crude protein, amino acids and fiber content and digestibility. Anim. Feed Sci. Technol. 2017, 230, 143–153. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Esparza, N.C.; Latorre-Moratalla, M.L.; Comas-Basté, O.; Toro-Funes, N.; Veciana-Nogués, M.T.; Vidal-Carou, M.C. Polyamines in Food. Front. Nutr. 2019, 6, 108. [Google Scholar] [CrossRef] [PubMed]
- Lau, N.; Hummel, J.; Kramer, E.; Hünerberg, M. Fermentation of liquid feed with lactic acid bacteria reduces dry matter losses, lysine breakdown, formation of biogenic amines, and phytate-phosphorus. Transl. Anim. Sci. 2022, 6, txac007. [Google Scholar] [CrossRef] [PubMed]
- Canibe, N.; Højberg, O.; Badsberg, J.H.; Jensen, B.B. Effect of feeding fermented liquid feed and fermented grain on gastrointestinal ecology and growth performance in piglets. J. Anim. Sci. 2007, 85, 2959–2971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olajugbagbe, T.E.; Odukoya, S.O.A.; Omafuvbe, B.O. Evaluation of the Effects of Pediococcus acidilactici Isolated from Wara, a Nigerian Milk Product, in the Prevention of Diarrhea and the Modulation of Intestinal Microflora in Wistar Rats. Asian J. Med. Health 2020, 18, 94–106. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).