Enterococcus faecium s6 Enabled Efficient Homofermentative Lactic Acid Production from Xylan-Derived Sugars
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Fermentation Media
2.2. Inoculum Preparation and Lactic Acid Production from Xylose
2.3. Lactic Acid Production from Xylan and Xylooligosaccharides
2.4. β-d-Xylosidase Activity
2.5. Analytical Methods
3. Results and Discussion
3.1. Lactic Acid Fermentation Pattern at Different Xylose Concentrations
3.2. Improved LA Fermentation in Repeated Batch Process
3.3. Utilization of Xylan and Xylooligosaccharides (XOS)
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Z.; Li, Y.; Zhang, J.; Peng, N.; Liang, Y.; Zhao, S. High-titer lactic acid production by Pediococcus acidilactici PA204 from corn stover through fed-batch simultaneous saccharification and fermentation. Microorganisms 2020, 8, 1491. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, M.A.; Sonomoto, K. Opportunities to overcome the current limitations and challenges for efficient microbial production of optically pure lactic acid. J. Biotechnol. 2016, 236, 176–192. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, M.A.; Hassan, S.E.-D.; Alrefaey, H.M.A.; Elsakhawy, T. Efficient co-utilization of biomass-derived mixed sugars for lactic acid production by Bacillus coagulans Azu-10. Fermentation 2021, 7, 28. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Hassan, S.E.-D.; Fouda, A.; Radwan, A.A.; Barghoth, M.G.; Desouky, S.G. Evaluating the effect of lignocellulose-derived microbial inhibitors on the growth and lactic acid production by Bacillus coagulans Azu-10. Fermentation 2021, 7, 17. [Google Scholar] [CrossRef]
- Hu, C.Y.; Chi, D.J.; Chen, S.S.; Chen, Y.C. The direct conversion of xylan to lactic acid by Lactobacillus brevis transformed with a xylanase gene. Green Chem. 2011, 13, 1729–1734. [Google Scholar] [CrossRef]
- Walton, S.L.; Bischoff, K.M.; van Heiningen, A.R.; van Walsum, G.P. Production of lactic acid from hemicellulose extracts by Bacillus coagulans MXL-9. J. Ind. Microbiol. Biotechnol. 2010, 37, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Dysvik, A.; La Rosa, S.L.; Buffetto, F.; Liland, K.H.; Myhrer, K.S.; Rukke, E.O.; Westereng, B. Secondary lactic acid bacteria fermentation with wood-derived xylooligosaccharides as a tool to expedite sour beer production. J. Agric. Food Chem. 2019, 68, 301–314. [Google Scholar] [CrossRef]
- Chaillou, S.; Bor, Y.C.; Batt, C.A.; Postma, P.W.; Pouwels, P.H. Molecular cloning and functional expression in Lactobacillus plantarum 80 of xylT, encoding the d-xylose-H+ symporter of Lactobacillus brevis. Appl. Environ. Microbiol. 1998, 64, 4720–4728. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, K.; Komiyama, A.; Sonomoto, K.; Ishizaki, A.; Hall, S.; Stanbury, P. Two different pathways for d-xylose metabolism and the effect of xylose concentration on the yield coefficient of l-lactate in mixed-acid fermentation by the lactic acid bacterium Lactococcus lactis IO-1. Appl. Microbiol. Biotechnol. 2002, 60, 160–167. [Google Scholar]
- Bustos, G.; Moldes, A.B.; Cruz, J.M.; Domínguez, J.M. Influence of the metabolism pathway on lactic acid production from hemicellulosic trimming vine shoots hydrolyzates using Lactobacillus pentosus. Biotechnol. Prog. 2005, 21, 793–798. [Google Scholar] [CrossRef]
- Ohara, H.; Owaki, M.; Sonomoto, K. Xylooligosaccharide fermentation with Leuconostoc lactis. J. Biosci. Bioeng. 2006, 101, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, M.A.; Tashiro, Y.; Zendo, T.; Hanada, K.; Shibata, K.; Sonomoto, K. Efficient homofermentative l-(+)-lactic acid production from xylose by a novel lactic acid bacterium, Enterococcus mundtii QU 25. Appl. Environ. Microbiol. 2011, 77, 1892–1895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Rahman, M.A.; Tashiro, Y.; Zendo, T.; Sakai, K.; Sonomoto, K. Enterococcus faecium QU 50: A novel thermophilic lactic acid bacterium for high-yield l-lactic acid production from xylose. FEMS Microbiol. Lett. 2015, 362, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alrefaey, H.; Abdel-Rahman, M.A.; Hassan, S.E.D.; El-Din, M.N.; Azab, M.S. Sequential optimization of the fermentation factors with integrating seed culture adaptation for increased biorefinery of beet molasses to lactic acid. Biomass Convers. Biorefin. 2021, 11, 1013–1028. [Google Scholar] [CrossRef]
- Nielsen, S.S. Total carbohydrate by phenol-sulfuric acid method. In Food Analysis Laboratory Manual; Food Science Text Series; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Klongklaew, A.; Unban, K.; Kanpiengjai, A.; Wongputtisin, P.; Pamueangmun, P.; Shetty, K.; Khanongnuch, C. Improvement of enantiomeric l-lactic acid Production from mixed hexose-pentose sugars by coculture of Enterococcus mundtii WX1 and Lactobacillus rhamnosus SCJ9. Fermentation 2021, 7, 95. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Recent advances in lactic acid production by microbial fermentation processes. Biotechnol. Adv. 2013, 31, 877–902. [Google Scholar] [CrossRef]
- Selim, M.T.; Salem, S.S.; Fouda, A.; El-Gamal, M.S.; Abdel-Rahman, M.A. Use of corn-steep water effluent as a promising substrate for lactic acid production by Enterococcus faecium strain WH51-1. Fermentation 2021, 7, 111. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Tashiro, Y.; Zendo, T.; Sonomoto, K. Improved lactic acid productivity by an open repeated batch fermentation system using Enterococcus mundtii QU 25. RSC Adv. 2013, 3, 8437–8445. [Google Scholar] [CrossRef]
- Oh, H.; Wee, Y.J.; Yun, J.S.; Ryu, H.W. Lactic acid production through cell-recycle repeated-batch bioreactor. Appl. Biochem. Biotechnol. 2003, 107, 603–613. [Google Scholar] [CrossRef]
- Wee, Y.J.; Yun, J.S.; Kim, D.; Ryu, H.W. Batch and repeated batch production of l(+)-lactic acid by Enterococcus faecalis RKY1 using wood hydrolyzate and corn steep liquor. J. Ind. Microbiol. Biotechnol. 2006, 33, 431. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Hassan, S.E.D.; Azab, M.S.; Mahin, A.A.; Gaber, M.A. High improvement in lactic acid productivity by new alkaliphilic bacterium using repeated batch fermentation integrated with increased substrate concentration. BioMed Res. Int. 2019, 2019, 7212870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mladenović, D.; Pejin, J.; Kocić-Tanackov, S.; Djukić-Vuković, A.; Mojović, L. Enhanced lactic acid production by adaptive evolution of Lactobacillus paracasei on agro-industrial substrate. Appl. Biochem. Biotechnol. 2019, 187, 753–769. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Lactic acid production from lignocellulose- derived sugars using lactic acid bacteria: Overview and limits. J. Biotechnol. 2011, 156, 286–301. [Google Scholar] [CrossRef] [PubMed]
- Shinkawa, S.; Okano, K.; Yoshida, S.; Tanaka, T.; Ogino, C.; Fukuda, H.; Kondo, A. Improved homo l-lactic acid fermentation from xylose by abolishment of the phosphoketolase pathway and enhancement of the pentose phosphate pathway in genetically modified xylose-assimilating Lactococcus lactis. Appl. Microbiol. Biotechnol. 2011, 91, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Iliev, I.; Vasileva, T.; Bivolarski, V.; Momchilova, A.; Ivanova, I. Metabolic profiling of xylooligosaccharides by Lactobacilli. Polymers 2020, 12, 2387. [Google Scholar] [CrossRef]


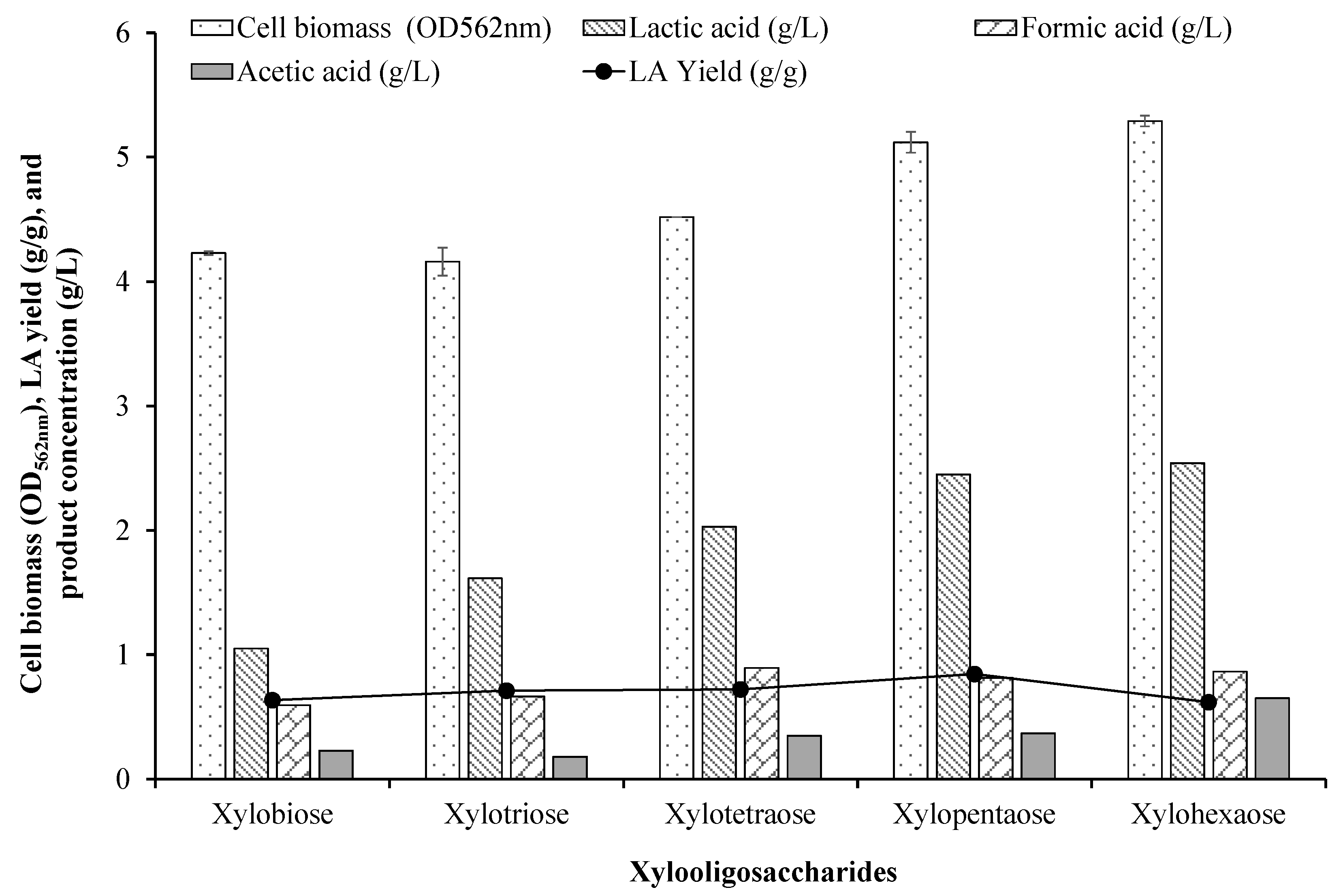
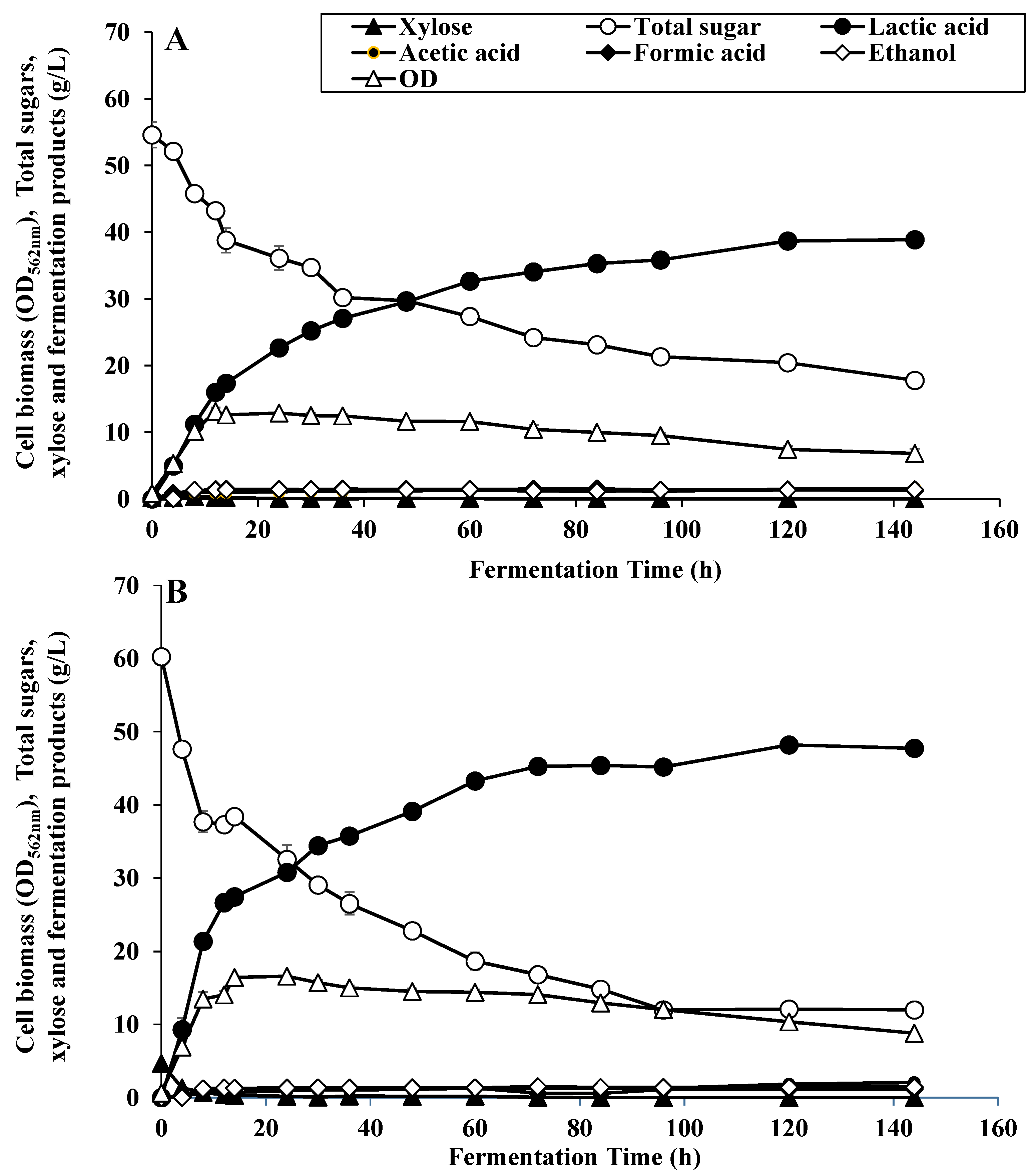
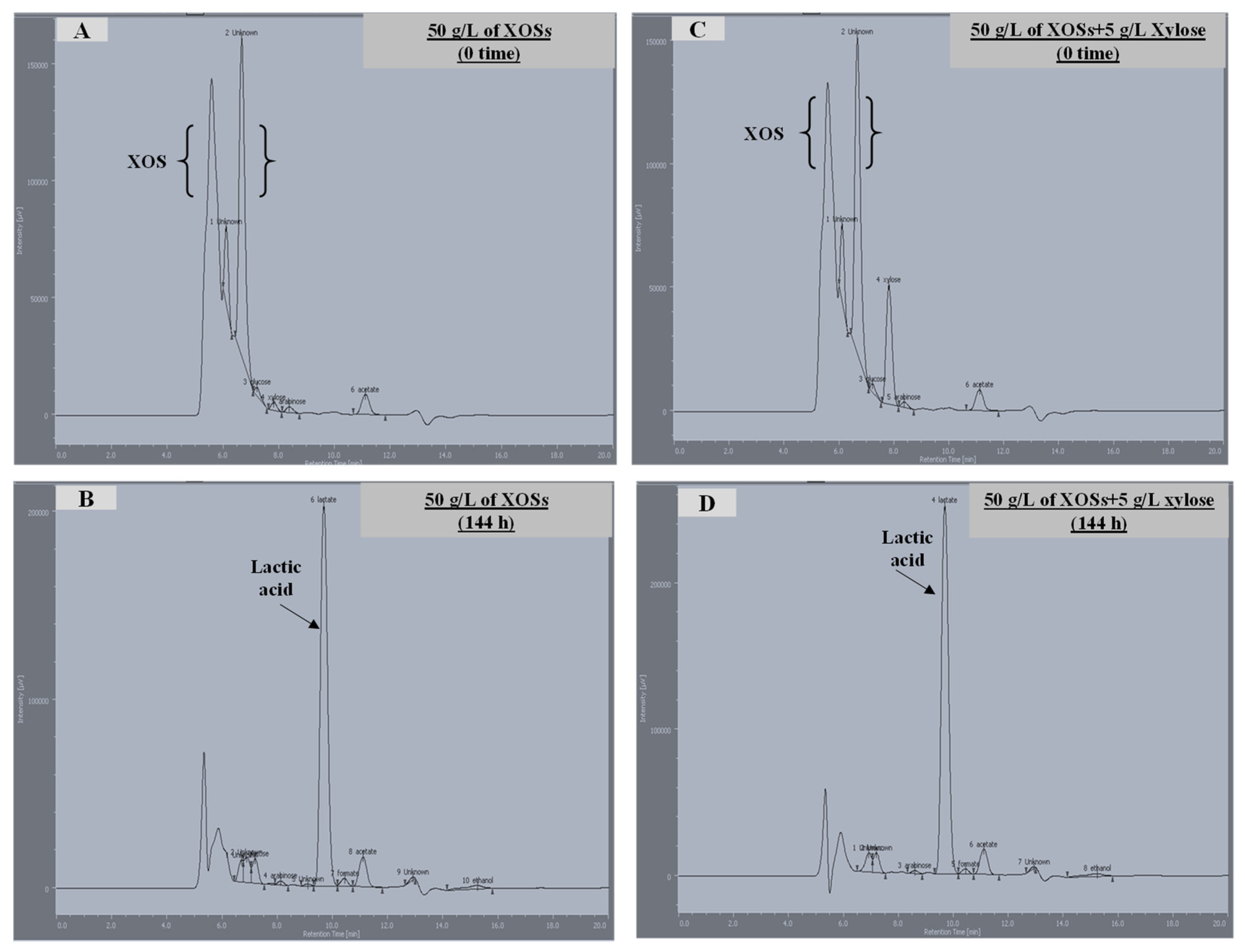
| Run | Initial Xylose (g/L) | Time | OD562nm | Residual Xylose (g/L) | Lactic Acid (g/L) | Acetic Acid (g/L) | Formic Acid (g/L) | Ethanol (g/L) | Lactic Acid Yield (g/g) | Lactic Acid Productivity (g/L.h) | Max. Lactic Acid Productivity (g/L.h) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 50 | 12 | 12.2 | 3.30 | 50.8 | 0.0 | 0.0 | 0.0 | 0.984 | 4.24 | 5.74 |
| 2 | 7 | 17.0 | 2.90 | 47.1 | 0.0 | 0.0 | 0.0 | 1.00 | 6.73 | 7.80 | |
| 3 | 6 | 28.0 | 1.50 | 49.5 | 0.0 | 0.0 | 0.0 | 1.02 | 8.26 | 9.37 | |
| 4 | 5 | 27.5 | 2.50 | 47.1 | 0.0 | 0.0 | 1.04 | 0.993 | 9.43 | 12.0 | |
| 5 | 4.5 | 39.0 | 2.30 | 49.8 | 0.0 | 0.466 | 1.18 | 1.04 | 11.0 | 13.1 | |
| 6 | 4.5 | 41.1 | 0.880 | 49.9 | 0.0 | 0.564 | 2.89 | 1.01 | 11.0 | 15.5 | |
| 7 | 4.5 | 45.5 | 1.20 | 50.8 | 0.0 | 0.584 | 0.0 | 1.04 | 11.2 | 16.1 | |
| 8 | 4 | 46.3 | 1.40 | 49.4 | 0.0 | 0.54 | 0.0 | 1.01 | 12.3 | 18.1 | |
| 9 | 4 | 51.3 | 1.60 | 48.2 | 0.0 | 0.496 | 0.0 | 0.996 | 12.0 | 16.3 | |
| 10 | 4 | 50.0 | 1.21 | 51.3 | 0.0 | 0.480 | 1.35 | 1.05 | 12.8 | 16.9 | |
| 11 | 3.5 | 48.9 | 1.80 | 47.5 | 0.0 | 0.0 | 0.0 | 0.987 | 13.5 | 17.0 | |
| 12 | 3.5 | 52.1 | 2.01 | 48.6 | 0.0 | 0.0 | 0.0 | 1.01 | 13.8 | 17.4 | |
| 13 | 3.5 | 57.4 | 1.80 | 50.2 | 0.0 | 0.540 | 1.94 | 1.04 | 14.3 | 18.3 | |
| 14 | 72 | 6 | 62.9 | 3.00 | 66.8 | 0.0 | 0.0 | 5.77 | 0.956 | 11.1 | 11.9 |
| 15 | 5 | 60.4 | 5.40 | 66.8 | 0.0 | 0.0 | 5.88 | 0.990 | 13.3 | 17.7 | |
| 16 | 4 | 59.9 | 3.40 | 63.3 | 0.0 | 0.514 | 1.29 | 0.911 | 15.8 | 20.7 | |
| 17 | 4 | 67.4 | 4.02 | 63.6 | 0.0 | 0.570 | 0.0 | 0.924 | 15.9 | 21.1 | |
| 18 | 80 | 5 | 68.6 | 5.24 | 77.9 | 0.0 | 0.620 | 3.24 | 1.024 | 15.5 | 25.6 |
| 19 | 5 | 74.9 | 5.48 | 80.7 | 0.0 | 0.472 | 0.0 | 1.025 | 16.5 | 24.8 | |
| 20 | 5 | 63.4 | 4.00 | 79.1 | 0.0 | 0.0 | 4.2 | 1.02 | 15.8 | 33.5 | |
| 21 | 5 | 66.1 | 2.00 | 79.0 | 0.0 | 0.0 | 0.0 | 0.996 | 15.8 | 28.9 | |
| 22 | 6 | 69.0 | 2.21 | 81.1 | 0.4 | 0.0 | 0.0 | 1.02 | 13.5 | 31.9 | |
| 23 | 8 | 57.4 | 6.05 | 75.5 | 0.1 | 0.0 | 0.0 | 1.00 | 9.4 | 22.2 | |
| 24 | 17 | 46.1 | 10.0 | 62.0 | 0.0 | 0.0 | 0.0 | 0.869 | 3.64 | 16.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Rahman, M.A. Enterococcus faecium s6 Enabled Efficient Homofermentative Lactic Acid Production from Xylan-Derived Sugars. Fermentation 2022, 8, 134. https://doi.org/10.3390/fermentation8030134
Abdel-Rahman MA. Enterococcus faecium s6 Enabled Efficient Homofermentative Lactic Acid Production from Xylan-Derived Sugars. Fermentation. 2022; 8(3):134. https://doi.org/10.3390/fermentation8030134
Chicago/Turabian StyleAbdel-Rahman, Mohamed Ali. 2022. "Enterococcus faecium s6 Enabled Efficient Homofermentative Lactic Acid Production from Xylan-Derived Sugars" Fermentation 8, no. 3: 134. https://doi.org/10.3390/fermentation8030134
APA StyleAbdel-Rahman, M. A. (2022). Enterococcus faecium s6 Enabled Efficient Homofermentative Lactic Acid Production from Xylan-Derived Sugars. Fermentation, 8(3), 134. https://doi.org/10.3390/fermentation8030134






