The Volatile Compounds and Aroma Description in Various Rhizopus oligosporus Solid-State Fermented and Nonfermented Rice Bran
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Rice Bran Preparation
2.3. Rice Bran Fermentation
2.4. Headspace Solid Phase Microextraction (HS-SPME)/GC-MS Analysis
2.5. Aroma Description
2.6. Statistical Analysis
3. Results and Discussion
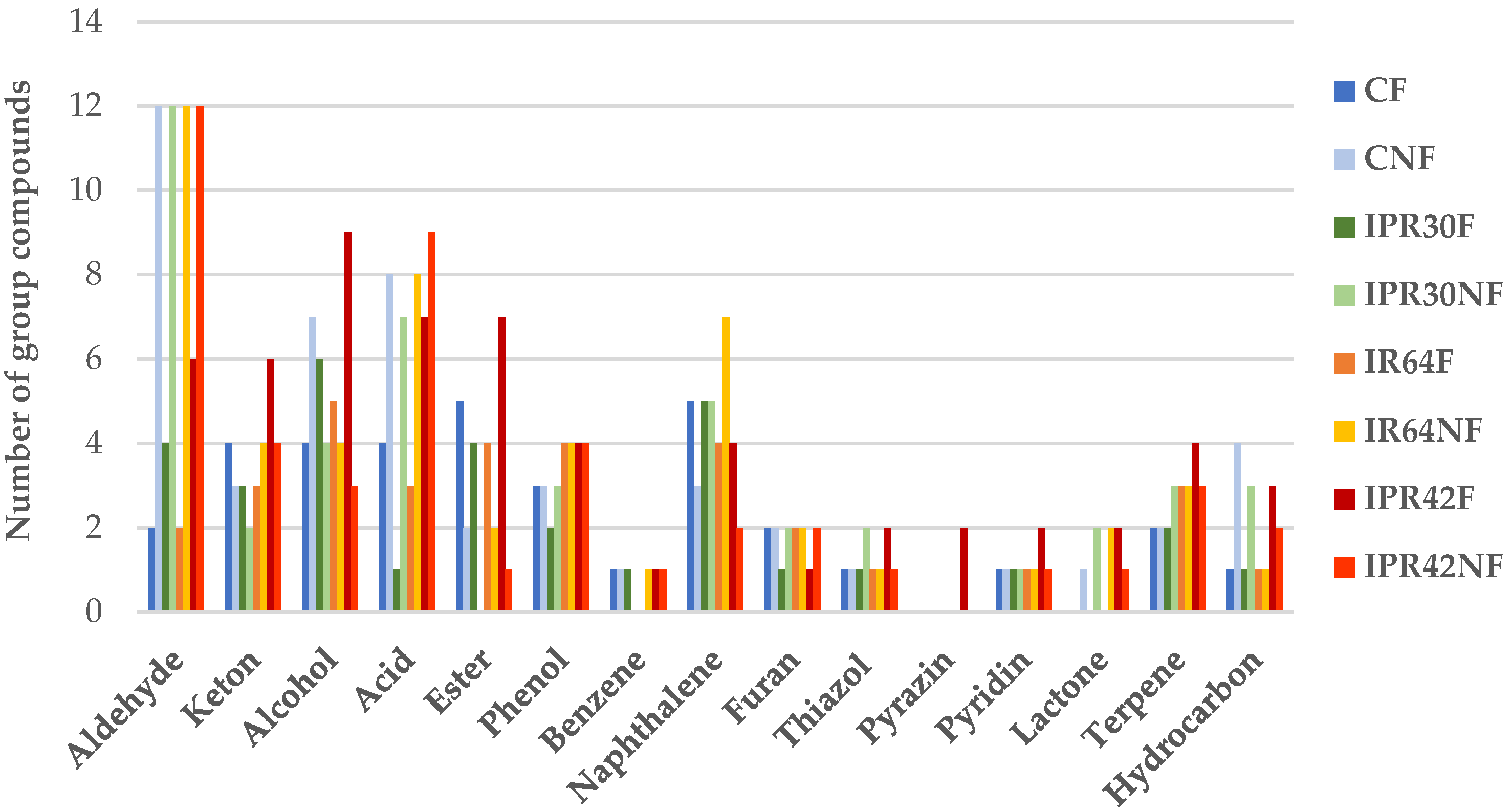
3.1. Volatiles of Fermented and Nonfermented Rice Bran
| No | Volatile Compounds | Code | LRI a | LRI Ref d | Identifi Cation e | Peak Area Percentage | Odor Description | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fermented | Nonfermented | |||||||||||||
| CF | IPR30F | IR64F | IPR42F | CNF | IPR30NF | IR64NF | IPR42NF | |||||||
| Aldehyde | ||||||||||||||
| 1 | Hexanal | Ad1 | 0 | 1065 [16] | MS + LRI | nd | 2403.09 | nd | nd | 0.636 | 3255 | 0.646 | 0.176 | grass, tallow, fat b |
| 2 | Heptanal | Ad2 | 1184 | 1186 [17] | MS + LRI | nd | nd | nd | nd | 0.443 | 0.388 | 0.329 | 0.431 | fat, citrus, rancid b |
| 3 | Octanal | Ad3 | 1287 | 1286 [18] | MS + LRI | nd | 0.001 | nd | nd | 0.277 | 0.146 | 0.417 | 0.473 | fat, soap, lemon, green b |
| 4 | Nonanal | Ad4 | 1391 | 1392 [17] | MS + LRI | nd | nd | nd | nd | 3.748 | 1860 | 2375 | 1214 | fat, citrus, green b |
| 5 | 3-Furaldehyde | Ad5 | 1425 | na | MS | nd | 0.002 | nd | nd | 0.351 | 0.730 | 0.342 | 0.254 | - |
| 6 | Furfural | Ad6 | 1448 | 1448 [18] | MS + LRI | 0.056 | 0.004 | 0.006 | 0.383 | 0.468 | 0.746 | 0.521 | 0.605 | bread, nutty, roasted, almond, sweet b |
| 7 | Decanal | Ad7 | 1495 | 1484 [17] | MS + LRI | nd | nd | nd | nd | 0.749 | 0.761 | 0.615 | 0.800 | soap, orange peel, tallow b |
| 8 | Benzaldehyde | Ad8 | 1517 | 1513 [17] | MS + LRI | nd | nd | nd | 0.251 | 1111 | 0.764 | 0.639 | 0.745 | almond, nutty, burnt sugar b |
| 9 | Benzene acetaldehyde | Ad9 | 1636 | 1643 [17] | MS + LRI | nd | nd | nd | nd | 0.309 | 0.377 | 0.370 | 0.425 | green, honey, alcoholic, chemical, sweet, caramel, bread, coffee, mosty c |
| 10 | 3-methylbenzaldehyde | Ad10 | 1644 | na | MS | nd | nd | nd | 0.302 | 0.540 | 0.473 | 0.370 | 0.763 | sweet fruity c |
| 11 | Cinnamaldehyde | Ad11 | 2046 | 2045 [19] | MS + LRI | nd | nd | nd | 0.663 | 0.062 | 0.209 | 0.151 | 0.394 | cinnamon, paint c |
| 12 | Vanillin | Ad12 | 2576 | 2578 [20] | MS + LRI | nd | nd | nd | nd | 0.153 | 0.261 | 0.135 | 0.288 | vanilla b |
| Ketone | ||||||||||||||
| 13 | 3-Penten-2-one | Kt1 | 1120 | na | MS | 0.546 | nd | nd | nd | nd | nd | nd | nd | fruity c |
| 14 | 3-Octen-2-one | Kt2 | 1388 | 1435 [21] | MS + LRI | 0.056 | 0.001 | nd | nd | 0.220 | nd | nd | nd | herbal c |
| 15 | 5-ethyldihydro-5-methyl-2(3H)-furanone | Kt3 | 1667 | 1684 [22] | MS + LRI | nd | nd | nd | 0.392 | nd | nd | 0.245 | nd | creamy c |
| 16 | 6,10-dimethyl (E) -5,9-undecadien-2-one | Kt4 | 1857 | 1865 [17] | MS + LRI | nd | nd | nd | 1612 | nd | nd | nd | 0.275 | - |
| 17 | 1-(1H-pyrrol-2-yl)-ethanone | Kt5 | 1977 | 1967 [23] | MS + LRI | nd | nd | 0.004 | 0.404 | nd | nd | 0.189 | 0.363 | musty c |
| 18 | dihydro-3-hydroxy-4,4-dimethyl-2(3H)-furanone | Kt6 | 2037 | na | MS | nd | nd | nd | 0.288 | nd | nd | nd | nd | cotton candy c |
| 19 | 6,10,14-trimethyl-2-Pentadecanone, | Kt7 | 2130 | 2110 [24] | MS + LRI | 0.030 | 0.005 | 0.005 | 0.324 | 0.258 | 0.379 | 0.393 | 0.330 | green, fat, floral |
| 20 | 5,6,7,7a-tetrahydro-4,4,7a-trimethyl-2(4H)-benzofuranone | Kt8 | 2368 | na | MS | 0.108 | 0.001 | 0.001 | 0.049 | 0.128 | 0.186 | 0.118 | 0.106 | fruity c |
| Alcohol | ||||||||||||||
| 21 | Ethanol | Al1 | 0 | 913 [25] | MS + LRI | nd | nd | nd | 2058 | nd | nd | nd | nd | sweet b |
| 22 | 2-methyl-1 propanol | Al2 | 110 | 1093 [26] | MS + LRI | nd | 0.010 | 0.017 | 1527 | 0.499 | nd | nd | nd | ethereal c |
| 23 | 3-methyl-1-butanol | Al3 | 1212 | 1220 [25] | MS + LRI | nd | 0.014 | nd | nd | nd | nd | nd | nd | fermented c |
| 24 | 1-Octen-3-ol | Al4 | 1453 | 1456 [27] | MS + LRI | nd | nd | nd | nd | 0.338 | nd | nd | nd | mushroom-like odor c |
| 25 | 2,3-Butanediol | Al5 | 1546 | 1494 [28] | MS + LRI | 1122 | 0.010 | 0.024 | 1933 | 0.420 | nd | nd | nd | creamy c |
| 27 | 4-ethyl-1,3-benzenediol | Al6 | 1572 | na | MS + LRI | nd | nd | nd | nd | 0.221 | 0.247 | 0.130 | nd | - |
| 28 | 2-Furanmethanol | Al7 | 1662 | 1686 [25] | MS + LRI | nd | nd | nd | 0.307 | 0.206 | nd | 0.789 | 0.330 | bready c |
| 29 | 2-hexadecanol | Al8 | 1745 | na | MS | nd | nd | nd | 0.262 | nd | 0.152 | nd | nd | - |
| 30 | benzyl alcohol | Al9 | 1882 | 1879 [17] | MS + LRI | nd | nd | nd | 0.397 | nd | nd | nd | nd | sweet, floral b |
| 31 | phenylethyl alcohol | Al10 | 1920 | 1920 [29] | MS + LRI | 0.430 | 0.001 | 0.006 | 0.653 | 0.142 | 0.138 | 0.113 | 0.252 | floral, slightly rose, sweet, clove-like c |
| 32 | Nicotinyl alcohol | Al11 | 2236 | na | MS | 0.906 | 0.009 | 0.010 | 1090 | 0.261 | 0.371 | 0.274 | 0.424 | green c |
| Acid | ||||||||||||||
| 33 | Acetic acid | As1 | 1449 | 1450 [16] | MS + LRI | nd | nd | nd | nd | 0.648 | 0.121 | 0.845 | 1630 | sour b |
| 34 | Butanoic acid | As2 | 1628 | 1628 [17] | MS + LRI | nd | nd | nd | 0.949 | 0.171 | nd | nd | 0.129 | cheesy, buttery c |
| 35 | Hexanoic acid | As3 | 1847 | 1846 [26] | MS + LRI | 0.273 | nd | 0.004 | 0.559 | 0.568 | 1823 | 0.373 | 1260 | fatty, sour fatty cheesy c |
| 36 | Heptanoic acid | As4 | 1955 | 1954 [30] | MS + LRI | nd | nd | nd | 0.148 | 0.105 | 0.323 | 0.116 | 1641 | cheesy, rancid, sour c |
| 37 | Octanoic acid | As5 | 2064 | 2065 [17] | MS + LRI | 0.244 | 0.002 | 0.003 | 0.276 | 0.102 | 0.450 | 0.139 | 0.340 | sweet, cheese b |
| 38 | Nonanoic acid | As6 | 2163 | 2177 [17] | MS + LRI | 0.053 | nd | nd | 0.055 | 0.653 | 0.508 | 0.439 | 0.259 | green, fat b |
| 39 | Dodecanoic acid | As7 | 2492 | 2502 [26] | MS + LRI | nd | nd | nd | nd | nd | 0.066 | 0.115 | 0.062 | - |
| 40 | Tetradecanoic acid | As8 | 2704 | 2706 [26] | MS + LRI | 0.083 | nd | nd | 0.109 | 0.261 | 0.351 | 0.502 | 0.137 | waxy, fatty, soapy, coconut c |
| Ester | ||||||||||||||
| 41 | Ethyl hexanoate | Es1 | 1233 | 1230 [31] | MS + LRI | 0.367 | nd | nd | 0.286 | nd | nd | nd | nd | apple peel, fruity b |
| 42 | Methyl tetradecanoat | Es2 | 2015 | 1994 [18] | MS + LRI | 0.448 | 0.004 | 0.005 | 0.247 | nd | nd | 0.122 | nd | orris |
| 43 | Isobutyl palmitat | Es3 | 2374 | na | MS | nd | 0.035 | 0.037 | 3587 | 0.081 | nd | nd | nd | grape b |
| 44 | 9-Octadecenoic acid methyl ester | Es4 | 2452 | na | MS | 0.042 | nd | nd | 0.148 | nd | nd | nd | nd | - |
| 45 | ethyl oleat | Es5 | 2052 | 2044 [32] | MS + LRI | 1233 | 0.007 | 0.006 | 3081 | nd | nd | nd | nd | fatty, fruity, oily c |
| 46 | 9,12-Octadecadienoic acid (Z,Z)-, methyl ester | Es6 | 2502 | na | MS | 0.047 | nd | nd | 2571 | 0.089 | nd | 0.113 | 0.075 | fatty c |
| 47 | Ethyl linoleate | Es7 | 2535 | 2491 [18] | MS + LRI | nd | 0.005 | 0.004 | 1954 | nd | nd | nd | nd | fatty, fruity, oily c |
| Phenol | ||||||||||||||
| 48 | 2-methoxyphenol | Ph1 | 1862 | 1872 [17] | MS + LRI | 1743 | 0.008 | 0.017 | 0.983 | nd | 0.606 | 0.207 | 0.114 | phenolic c |
| 49 | Butylated Hydroxytoluene | Ph2 | 1912 | 1912 [23] | MS + LRI | nd | nd | 0.003 | 0.256 | 0.070 | 0.147 | 0.101 | 0.121 | phenolic c |
| 50 | phenol | Ph3 | 2008 | 2002 [33] | MS + LRI | 1805 | 0.005 | 0.008 | 1325 | 0.050 | nd | 0.217 | 0.434 | phenol b |
| 51 | 2-Methoxy-4-vinylphenol | Ph4 | 2202 | 2200 [34] | MS + LRI | 0.070 | nd | 0.007 | 0.710 | 1030 | 1502 | 1754 | 0.771 | woody c |
| Benzene | ||||||||||||||
| 52 | Styrene | Bz1 | 1254 | 1250 [35] | MS + LRI | 0.306 | 0.003 | nd | 0.592 | 1094 | nd | 0.413 | 0.524 | balsamic, gasoline b |
| Naphthalene | ||||||||||||||
| 53 | Naphthalene | Na1 | 1738 | 1734 [17] | MS + LRI | 1078 | 0.009 | 0.012 | 1220 | 0.686 | 1390 | 0.978 | 1862 | tar b |
| 54 | 2-methylnaphthalene | Na2 | 1853 | 1877 [36] | MS + LRI | 0.383 | 0.002 | 0.002 | 0.326 | 0.112 | 0.256 | 0.110 | 0.153 | floral c |
| 55 | 1-methylnaphthalene | Na3 | 1889 | na | MS | 0.381 | 0.002 | 0.004 | 0.484 | 0.178 | 0.371 | 0.274 | nd | naphthyl c |
| 56 | 2,6-dimethylnaphthalene | Na4 | 1998 | na | MS | 0.067 | 0.001 | 0.001 | nd | nd | nd | 0.123 | nd | grass b |
| 57 | 2,7-dimethylnaphthalene | Na5 | 2006 | 2012 [36] | MS + LRI | 0.106 | 0.001 | nd | nd | nd | nd | 0.129 | nd | - |
| 58 | 2,3-dimethylnaphthalene | Na6 | 2073 | 2122 [37] | MS + LRI | nd | nd | nd | 0.102 | nd | 0.063 | 0.000 | nd | earthy c |
| 59 | 1,6,7-trimethylnaphthalene | Na7 | 2112 | 2122 [37] | MS + LRI | nd | nd | nd | nd | nd | 0.075 | 0.040 | nd | fruity strawberry |
| Furan | ||||||||||||||
| 60 | 2-Pentylfuran | Fr1 | 1229 | 1230 [38] | MS + LRI | 0.062 | 0.001 | 0.001 | nd | 0.208 | 0.157 | 0.242 | 0.114 | fruity, green, nutty, earthy, beany, vegetable c |
| 61 | 2,3-dihydro-Benzofuran | Fr2 | 2398 | 2392 [39] | MS + LRI | 0.092 | nd | 0.001 | 0.089 | 0.100 | 0.125 | 0.105 | 0.134 | musky odor |
| Thiazole | ||||||||||||||
| 62 | Thiazole | Th1 | 1804 | na | MS | nd | nd | nd | 0.178 | nd | 0.151 | nd | nd | fishy c |
| 63 | Benzothiazole | Th2 | 1964 | 1950 [39] | MS + LRI | 0.154 | nd | 0.002 | 0.371 | 0.238 | 0.315 | 0.335 | 0.366 | gasoline, rubber-like b |
| Pyrazine | ||||||||||||||
| 64 | 2,6-dimethylpyrazine | Pz1 | 1318 | 1308 [40] | MS + LRI | nd | nd | nd | 0.179 | nd | nd | nd | nd | - |
| 65 | trimethyl pyrazine | Pz2 | 1387 | 1433 [25] | MS + LRI | nd | nd | nd | 0.064 | nd | nd | nd | nd | roast, potato, must b |
| Pyridine | ||||||||||||||
| 66 | trimethyl-pyridine | Pr1 | 1373 | na | MS | 0.207 | 0.002 | 0.003 | 0.164 | 0.450 | 0.182 | 0.392 | 0.359 | nutty |
| 67 | 2-piperidinone | Pr2 | 1586 | na | MS | nd | nd | nd | 1308 | nd | nd | nd | nd | - |
| Lactone | ||||||||||||||
| 68 | Butyrolactone | Lc1 | 1623 | na | MS | nd | nd | nd | 0.351 | 0.100 | 0.159 | 0.122 | 0.230 | creamy-milk, oily, fatty c |
| 69 | Pantolactone | Lc2 | 2037 | 2033 [41] | MS + LRI | nd | nd | nd | 0.162 | nd | 0.746 | 0.416 | nd | cotton candy b |
| Terpene | ||||||||||||||
| 70 | 3-Carene | Tp1 | 1140 | 1138 [18] | MS + LRI | 0.390 | 0.004 | 0.005 | 0.355 | 0.794 | 0.577 | 0.701 | 1079 | lemon, resin b |
| 71 | D-Limonene | Tp2 | 1197 | 1190 [23] | MS + LRI | 0.979 | 0.009 | 0.040 | 1415 | 4755 | 1869 | 2404 | 3384 | lemon, orange b |
| 72 | Caryophyllene | Tp3 | 1593 | 1593 [42] | MS + LRI | nd | nd | 0.025 | 1344 | nd | 2371 | 0.478 | 0.614 | spicy c |
| 73 | Epizonarene | Tp4 | 1758 | na | MS | nd | nd | nd | 0.434 | nd | nd | nd | nd | - |
| Hydrocarbon | ||||||||||||||
| 74 | o-Xylene | Hd1 | 1134 | 1174 [18] | MS + LRI | nd | nd | nd | 0.280 | nd | nd | nd | 6674,91 | geranium b |
| 75 | o-Cymene | Hd2 | 1266 | 1260 [42] | MS + LRI | nd | nd | nd | 0.254 | nd | nd | nd | 6048,25 | - |
| 76 | Tetradecane | Hd3 | 1396 | 1400 [43] | MS + LRI | 0.185 | 0.002 | 0.005 | 0.216 | 0.671 | 0.876 | nd | 0.393 | alkane b |
| 77 | cyclodecanone | Hd4 | 1815 | na | MS | nd | nd | nd | nd | 0.156 | 0.180 | nd | nd | - |
| 78 | 2-Pentadecanone | Hd5 | 2025 | 1998 [44] | MS + LRI | nd | nd | nd | nd | 0.021 | 0.000 | 0.066 | 0.095 | floral c |
| 79 | Indole | Hd6 | 2454 | 2450 [42] | MS + LRI | nd | nd | nd | 0.280 | 2371.57 | nd | nd | nd | burnt, mothball b |
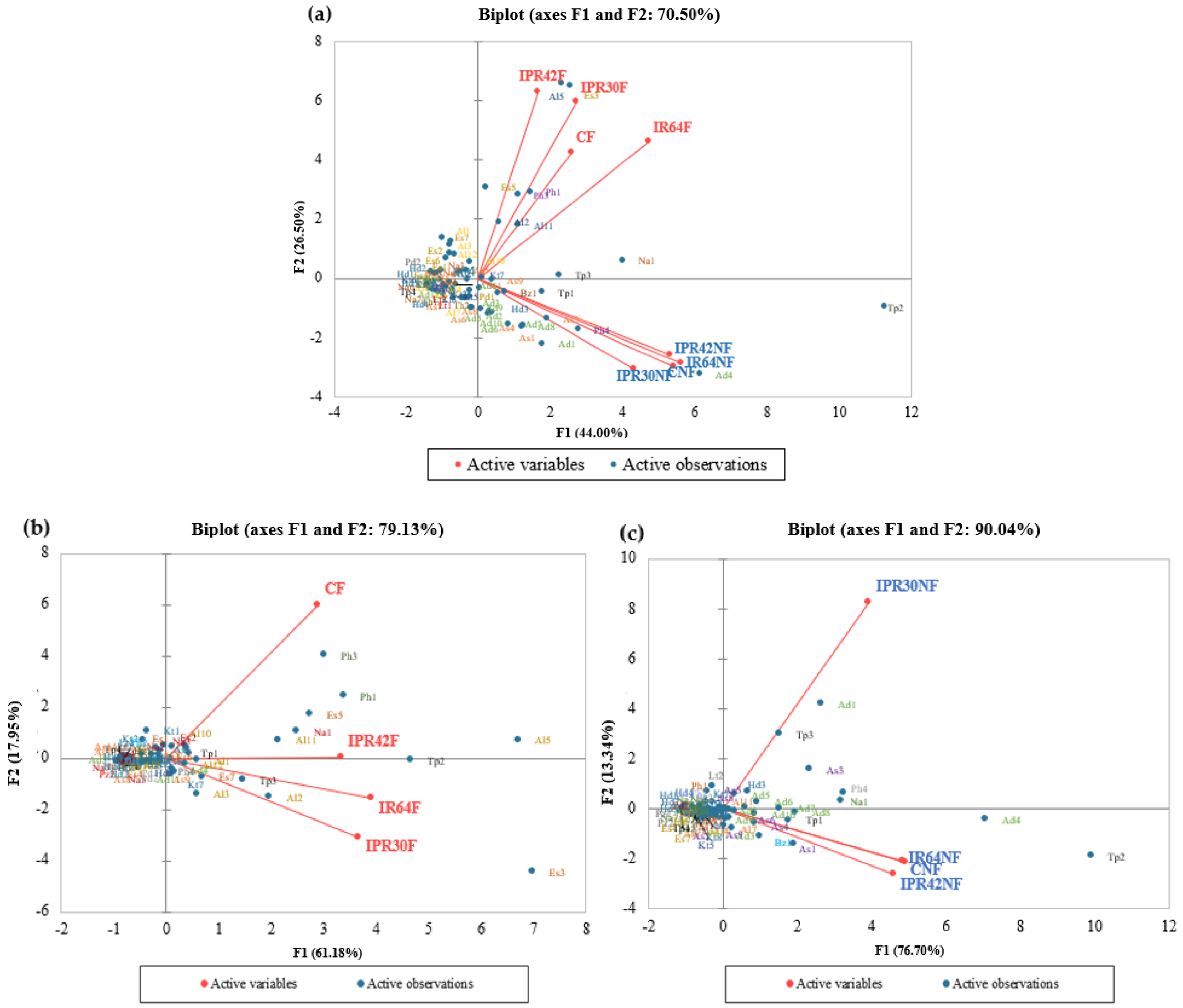
3.2. Principal Component Analysis of Fermented and Nonfermented Rice Bran
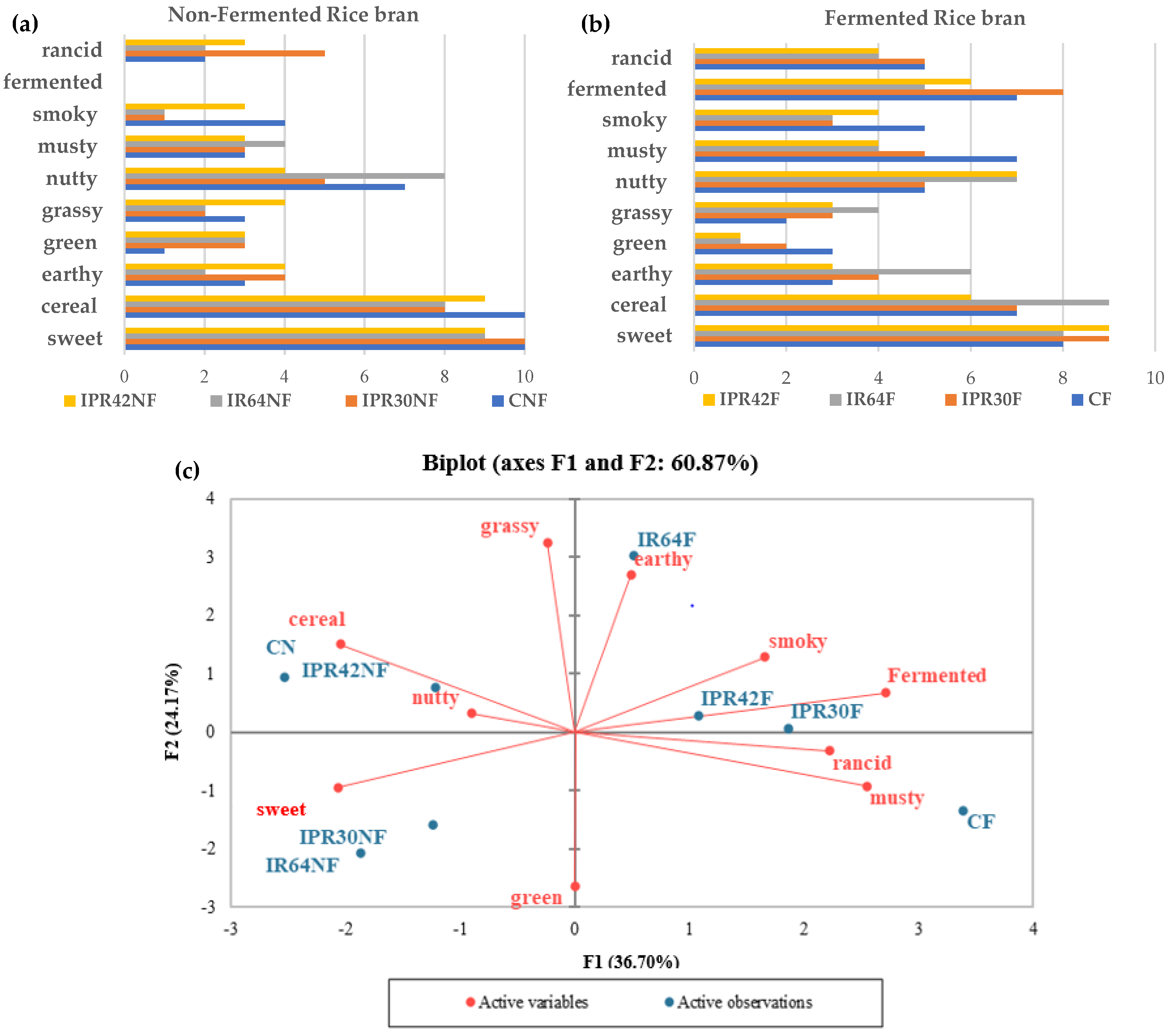
3.3. Aroma Description of Fermented and Nonfermented Rice Bran
3.4. Correlation Analysis between Volatile Compounds with Aroma Description (QDA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spaggiari, M.; Dall’Asta, C.; Galaverna, G.; Bilbao, M.D.D.C. Rice Bran By-Product: From Valorization Strategies to Nutritional Perspectives. Foods 2021, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Li, Y.; Pan, Q.; Fan, M.; Wang, L.; Qian, H. Analysis of the key aroma volatile compounds in rice bran during storage and processing via HS-SPME GC/MS. J. Cereal Sci. 2021, 99, 103178. [Google Scholar] [CrossRef]
- Lee, S.M.; Hwang, Y.R.; Kim, M.S.; Chung, M.S.; Kim, Y.-S. Comparison of Volatile and Nonvolatile Compounds in Rice Fermented by Different Lactic Acid Bacteria. Molecules 2019, 24, 1183. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Lim, H.J.; Chang, J.W.; Hurh, B.-S.; Kim, Y.-S. Investigation on the formations of volatile compounds, fatty acids, and γ-lactones in white and brown rice during fermentation. Food Chem. 2018, 269, 347–354. [Google Scholar] [CrossRef]
- Li, C.; Li, W.; Chen, X.; Feng, M.; Rui, X.; Jiang, M.; Dong, M. Microbiological, physicochemical and rheological properties of fermented soymilk produced with exopolysaccharide (EPS) producing lactic acid bacteria strains. LWT 2014, 57, 477–485. [Google Scholar] [CrossRef]
- Schmidt, C.G.; Gonçalves, L.M.; Prietto, L.; Hackbart, H.S.; Furlong, E.B. Antioxidant activity and enzyme inhibition of phenolic acids from fermented rice bran with fungus Rizhopus oryzae. Food Chem. 2014, 146, 371–377. [Google Scholar] [CrossRef]
- Ardiansyah; David, W.; Handoko, D.D.; Kusbiantoro, B.; Budijanto, S.; Shirakawa, H. Fermented rice bran extract improves blood pressure and glucose in stroke-prone spontaneously hypertensive rats. Nutr. Food Sci. 2019, 49, 844–853. [Google Scholar] [CrossRef]
- Ardiansyah; Nada, A.; Rahmawati, N.; Oktriani, A.; David, W.; Astuti, R.; Handoko, D.; Kusbiantoro, B.; Budijanto, S.; Shirakawa, H. Volatile Compounds, Sensory Profile and Phenolic Compounds in Fermented Rice Bran. Plants 2021, 10, 1073. [Google Scholar] [CrossRef]
- Direktorat Jenderal BP Holtikultura. Profil komoditas Padi. Ews.Kemendag.Go.Id. Indonesia. 2003. Available online: https://ews.kemendag.go.id/sp2kp-landing/assets/pdf/130827_ANL_UPK_Beras.pdf (accessed on 21 December 2021).
- Puri, R.; Khamrui, K.; Khetra, Y.; Malhotra, R.; Devraja, H.C. Quantitative descriptive analysis and principal component analysis for sensory characterization of Indian milk product cham-cham. J. Food Sci. Technol. 2015, 53, 1238–1246. [Google Scholar] [CrossRef]
- Ghosh, D.; Chattopadhyay, P. Application of principal component analysis (PCA) as a sensory assessment tool for fermented food products. J. Food Sci. Technol. 2011, 49, 328–334. [Google Scholar] [CrossRef]
- Dias, L.; Duarte, G.; Mariutti, L.; Bragagnolo, N. Aroma profile of rice varieties by a novel SPME method able to maximize 2-acetyl-1-pyrroline and minimize hexanal extraction. Food Res. Int. 2019, 123, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Kwak, H.S.; Jeong, Y.; Kim, M. Influence of Rice Varieties on Sensory Profile and Consumer Acceptance for Frozen-cooked Rice. Emir. J. Food Agric. 2015, 27, 793–800. [Google Scholar] [CrossRef]
- Zhao, Q.; Xue, Y.; Shen, Q. Changes in the major aroma-active compounds and taste components of Jasmine rice during storage. Food Res. Int. 2020, 133, 109160. [Google Scholar] [CrossRef]
- Hu, X.; Lu, L.; Guo, Z.; Zhu, Z. Volatile compounds, affecting factors and evaluation methods for rice aroma: A review. Trends Food Sci. Technol. 2020, 97, 136–146. [Google Scholar] [CrossRef]
- Tamura, H.; Boonbumrung, S.; Yoshizawa, T.; Varanyanond, W. Volatile Components of the Essential Oils in the Pulp of Four Yellow Mangoes (Mangifera indica L.) in Thailand. Food Sci. Technol. Res. 2000, 6, 68–73. [Google Scholar] [CrossRef][Green Version]
- Goodner, K. Practical retention index models of OV-101, DB-1, DB-5, and DB-Wax for flavor and fragrance compounds. LWT 2008, 41, 951–958. [Google Scholar] [CrossRef]
- Pino, J.A.; Marbot, R.; Fuentes, V. Characterization of Volatiles in Bullock’s Heart (Annona reticulata L.) Fruit Cultivars from Cuba. J. Agric. Food Chem. 2003, 51, 3836–3839. [Google Scholar] [CrossRef]
- Njoroge, S.M.; Ukeda, H.; Sawamura, M. Changes of the Volatile Profile and Artifact Formation in Daidai (Citrus aurantium) Cold-Pressed Peel Oil on Storage. J. Agric. Food Chem. 2003, 51, 4029–4035. [Google Scholar] [CrossRef]
- Culleré, L.; Escudero, A.; Cacho, J.; Ferreira, V. Gas Chromatography−Olfactometry and Chemical Quantitative Study of the Aroma of Six Premium Quality Spanish Aged Red Wines. J. Agric. Food Chem. 2004, 52, 1653–1660. [Google Scholar] [CrossRef]
- Ouzouni, P.K.; Koller, W.D.; Badeka, A.V.; Riganakos, K.A. Volatile compounds from the fruiting bodies of three Hygrophorus mushroom species from Northern Greece. Int. J. Food Sci. Technol. 2009, 44, 854–859. [Google Scholar] [CrossRef]
- In, H.C.; Se, Y.K.; Choi, H.K.; Kim, Y.S. Characterization of aroma-active compounds in raw and cooked pine-mushrooms (Tricholoma matsutake Sing.). J. Agric. Food Chem. 2006, 54, 6332–6335. [Google Scholar]
- Umano, K.; Hagi, Y.; Nakahara, K.; Shyoji, A.; Shibamoto, T. Volatile Chemicals Formed in the Headspace of a Heated D-Glucose/L-Cysteine Maillard Model System. J. Agric. Food Chem. 1995, 43, 2212–2218. [Google Scholar] [CrossRef]
- Wedge, D.E.; Klun, J.A.; Tabanca, N.; Demirci, B.; Ozek, T.; Baser, K.H.C.; Liu, Z.; Zhang, S.; Cantrell, C.L.; Zhang, J. Bioactivity-Guided Fractionation and GC/MS Fingerprinting of Angelica sinensis and Angelica archangelica Root Components for Antifungal and Mosquito Deterrent Activity. J. Agric. Food Chem. 2008, 57, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.L.; Sanz, J.; Martínez-Castro, I. Presence of some cyclitols in honey. Food Chem. 2004, 84, 133–135. [Google Scholar] [CrossRef]
- Dregus, M.; Engel, K.-H. Volatile Constituents of Uncooked Rhubarb (Rheum rhabarbarum L.) Stalks. J. Agric. Food Chem. 2003, 51, 6530–6536. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zorn, H.; Krings, U.; Berger, R.G. Characteristic Volatiles from Young and Aged Fruiting Bodies of Wild Polyporus sulfureus (Bull.:Fr.) Fr. J. Agric. Food Chem. 2005, 53, 4524–4528. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-S.; Liu, J.-B.; Yang, Z.-M.; Song, H.-L.; Liu, Y.; Zou, T.-T. Aroma-Active Compounds in Jinhua Ham Produced with Different Fermentation Periods. Molecules 2014, 19, 19097–19113. [Google Scholar] [CrossRef]
- Tang, H.; Ma, J.-K.; Chen, L.; Jiang, L.-W.; Xie, J.; Li, P.; He, J. GC-MS Characterization of Volatile Flavor Compounds in Stinky Tofu Brine by Optimization of Headspace Solid-Phase Microextraction Conditions. Molecules 2018, 23, 3155. [Google Scholar] [CrossRef]
- Mahajan, S.; Goddik, L.; Qian, M. Aroma Compounds in Sweet Whey Powder. J. Dairy Sci. 2004, 87, 4057–4063. [Google Scholar] [CrossRef]
- Selli, S.; Cabaroglu, T.; Canbas, A.; Erten, H.; Nurgel, C.; Lepoutre, J.; Gunata, Z. Volatile composition of red wine from cv. Kalecik Karasι grown in central Anatolia. Food Chem. 2004, 85, 207–213. [Google Scholar] [CrossRef]
- Lee, S.-J.; Ahn, B. Comparison of volatile components in fermented soybean pastes using simultaneous distillation and extraction (SDE) with sensory characterisation. Food Chem. 2009, 114, 600–609. [Google Scholar] [CrossRef]
- Lee, K.-G.; Lee, S.-E.; Takeoka, G.R.; Kim, J.-H.; Park, B.-S. Antioxidant activity and characterization of volatile constituents of beechwood creosote. J. Sci. Food Agric. 2005, 85, 1580–1586. [Google Scholar] [CrossRef]
- Xu, Y.; Fan, W.; Qian, M.C. Characterization of Aroma Compounds in Apple Cider Using Solvent-Assisted Flavor Evaporation and Headspace Solid-Phase Microextraction. J. Agric. Food Chem. 2007, 55, 3051–3057. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, J.; Zhu, Y.; Wang, X.; Shi, W. Volatile components present in different parts of grass carp. J. Food Biochem. 2018, 42, e12668. [Google Scholar] [CrossRef]
- Selli, S.; Rannou, C.; Prost, C.; Robin, J.; Serot, T. Characterization of Aroma-Active Compounds in Rainbow Trout (Oncorhynchus mykiss) Eliciting an Off-Odor. J. Agric. Food Chem. 2006, 54, 9496–9502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Mi, S.; Liu, R.-B.; Sang, Y.-X.; Wang, X.-H. Evaluation of Volatile Compounds during the Fermentation Process of Yogurts by Streptococcus thermophilus Based on Odor Activity Value and Heat Map Analysis. Int. J. Anal. Chem. 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- Chung, H.Y.; Ma, W.C.J.; Kim, J.-S.; Chen, F. Odor-Active Headspace Components in Fermented Red Rice in the Presence of a Monascus Species. J. Agric. Food Chem. 2004, 52, 6557–6563. [Google Scholar] [CrossRef]
- Wu, Y.; Pan, Q.; Qu, W.; Duan, C. Comparison of Volatile Profiles of Nine Litchi (Litchi chinensis Sonn.) Cultivars from Southern China. J. Agric. Food Chem. 2009, 57, 9676–9681. [Google Scholar] [CrossRef]
- Liu, M.; Liu, J.; He, C.; Song, H.; Liu, Y.; Zhang, Y.; Wang, Y.; Guo, J.; Yang, H.; Su, X. Characterization and comparison of key aroma-active compounds of cocoa liquors from five different areas. Int. J. Food Prop. 2017, 20, 2396–2408. [Google Scholar] [CrossRef]
- Chinnici, F.; Guerrero, E.D.; Sonni, F.; Natali, N.; Marín, R.N.; Riponi, C. Gas Chromatography−Mass Spectrometry (GC−MS) Characterization of Volatile Compounds in Quality Vinegars with Protected European Geographical Indication. J. Agric. Food Chem. 2009, 57, 4784–4792. [Google Scholar] [CrossRef]
- Lukić, I.; Lukić, M.; Žanetić, M.; Krapac, M.; Godena, S.; Bubola, K.B. Inter-varietal diversity of typical volatile and phenolic profiles of Croatian extra virgin olive oils as revealed by GC-IT-MS and UPLC-DAD analysis. Foods 2019, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Ao, Z.; Tang, T.; Tong, F.; Wei, Z.; Yang, F.; Shu, Y.; Liu, S.; Mai, K. Characterization of difference in muscle volatile compounds between triploid and diploid crucian carp. Aquac. Rep. 2021, 20, 100641. [Google Scholar] [CrossRef]
- Cayhan, G.G.; Selli, S. Characterization of the Key Aroma Compounds in Cooked Grey Mullet (Mugil cephalus) by Application of Aroma Extract Dilution Analysis. J. Agric. Food Chem. 2010, 59, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Mumpuni, P.D.; Ayustaningwarno, F. Analisis Kadar Tokoferol, γ-Oryzanol Dan Β-Karoten Serta Aktivitas Antioksidan Minyak Bekatul Kasar. J. Nutr. Coll. 2013, 2, 350–357. [Google Scholar] [CrossRef]
- Tylewicz, U.; Inchingolo, R.; Rodriguez-Estrada, M.T. Food Aroma Compounds. In Nutraceutical and Functional Food Components: Effects of Innovative Processing Techniques; Elsevier: Bologna, Italy, 2017; pp. 297–334. [Google Scholar] [CrossRef]
- Verma, D.K.; Srivastav, P.P. A paradigm of volatile aroma compounds in rice and their product with extraction and identification methods: A comprehensive review. Food Res. Int. 2020, 130, 108924. [Google Scholar] [CrossRef]
- Wei, C.-K.; Ni, Z.-J.; Thakur, K.; Liao, A.-M.; Huang, J.-H.; Wei, Z.-J. Aromatic effects of immobilized enzymatic oxidation of chicken fat on flaxseed (Linum usitatissimum L.) derived Maillard reaction products. Food Chem. 2020, 306, 125560. [Google Scholar] [CrossRef]
- Arsa, S.; Theerakulkait, C.; Cadwallader, K.R. Quantitation of Three Strecker Aldehydes from Enzymatic Hydrolyzed Rice Bran Protein Concentrates as Prepared by Various Conditions. J. Agric. Food Chem. 2019, 67, 8205–8211. [Google Scholar] [CrossRef]
- Yi, C.; Li, Y.; Zhu, H.; Liu, Y.; Quan, K. Effect of Lactobacillus plantarum fermentation on the volatile flavors of mung beans. LWT 2021, 146, 111434. [Google Scholar] [CrossRef]
- Al-Dalali, S.; Zheng, F.; Li, H.; Huang, M.; Chen, F. Characterization of volatile compounds in three commercial Chinese vinegars by SPME-GC-MS and GC-O. LWT 2019, 112, 108264. [Google Scholar] [CrossRef]
- Tomasik, P.; Horton, D. Enzymatic Conversions of Starch. In Advances in Carbohydrate Chemistry and Biochemistry; Elsevier: Amsterdam, The Netherlands, 2012; Volume 68. [Google Scholar]
| Variables | Furfural | Benzaldehyde | Benzene Acetaldehyde | 3-methylbenzaldehyde | 3-methyl-1-butanol | Octanoic Acid | Pantolactone |
|---|---|---|---|---|---|---|---|
| Sweet | 0.801 | 0.760 | 0.650 | 0.747 | 0.000 | −0.446 | 0.792 |
| Cereal | 0.493 | 0.575 | −0.086 | 0.691 | −0.309 | −0.471 | −0.143 |
| Earthy | −0.293 | −0.265 | −0.358 | −0.192 | 0.128 | −0.125 | 0.044 |
| Green | −0.350 | −0.429 | −0.574 | −0.229 | −0.051 | 0.138 | 0.415 |
| Grassy | −0.070 | 0.011 | 0.026 | −0.027 | 0.061 | −0.395 | −0.493 |
| Nutty | 0.241 | 0.281 | 0.381 | 0.120 | −0.286 | −0.306 | −0.267 |
| Musty | −0.583 | −0.462 | −0.401 | −0.539 | 0.261 | 0.842 | −0.216 |
| Smoky | 0.049 | 0.193 | 0.267 | 0.024 | 0.000 | 0.674 | −0.703 |
| Fermented | −0.569 | −0.471 | −0.153 | −0.620 | 0.657 | 0.703 | −0.203 |
| Rancid | −0.495 | −0.555 | −0.318 | −0.510 | 0.394 | 0.693 | 0.428 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Astuti, R.D.; Fibri, D.L.N.; Handoko, D.D.; David, W.; Budijanto, S.; Shirakawa, H.; Ardiansyah. The Volatile Compounds and Aroma Description in Various Rhizopus oligosporus Solid-State Fermented and Nonfermented Rice Bran. Fermentation 2022, 8, 120. https://doi.org/10.3390/fermentation8030120
Astuti RD, Fibri DLN, Handoko DD, David W, Budijanto S, Shirakawa H, Ardiansyah. The Volatile Compounds and Aroma Description in Various Rhizopus oligosporus Solid-State Fermented and Nonfermented Rice Bran. Fermentation. 2022; 8(3):120. https://doi.org/10.3390/fermentation8030120
Chicago/Turabian StyleAstuti, Retno Dwi, Dwi Larasatie Nur Fibri, Dody Dwi Handoko, Wahyudi David, Slamet Budijanto, Hitoshi Shirakawa, and Ardiansyah. 2022. "The Volatile Compounds and Aroma Description in Various Rhizopus oligosporus Solid-State Fermented and Nonfermented Rice Bran" Fermentation 8, no. 3: 120. https://doi.org/10.3390/fermentation8030120
APA StyleAstuti, R. D., Fibri, D. L. N., Handoko, D. D., David, W., Budijanto, S., Shirakawa, H., & Ardiansyah. (2022). The Volatile Compounds and Aroma Description in Various Rhizopus oligosporus Solid-State Fermented and Nonfermented Rice Bran. Fermentation, 8(3), 120. https://doi.org/10.3390/fermentation8030120










