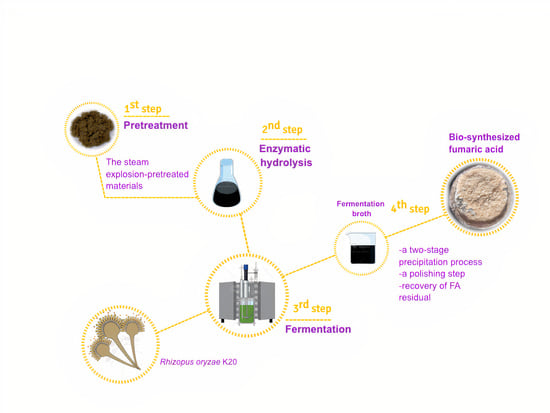
Recovery and Purification of Fumaric Acid from Fermented Oil Palm Empty Fruit Bunches Using a Simple Two-Stage Precipitation Procedure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material and Pretreatment
2.2. Microbial Culture
2.3. Separate Hydrolysis and Fermentation
2.4. Source of Glucose
2.5. Upscale Production of Fumaric Acid in an Air-Lift Fermenter
2.6. Fumaric Acid Precipitation
2.7. Removal of Contaminants from the Fumaric Acid Solution
2.8. Recovery of Residual Fumaric Acid from Dilute Solutions
2.9. Characterization of Bio-Synthesized Fumaric Acid
2.10. Analytical Methods
3. Results and Discussion
3.1. Source of Glucose
3.2. Upscale Fermentation of Fumaric Acid in an Air-Lift Fermenter
3.3. Recovery of Fumaric Acid from Fermented Oil Palm Empty Fruit Bunches
3.4. Removal of Contaminants from Fumaric Acid with Activated Charcoal
3.5. Recovery of Residual Fumaric Acid from Dilute Solutions
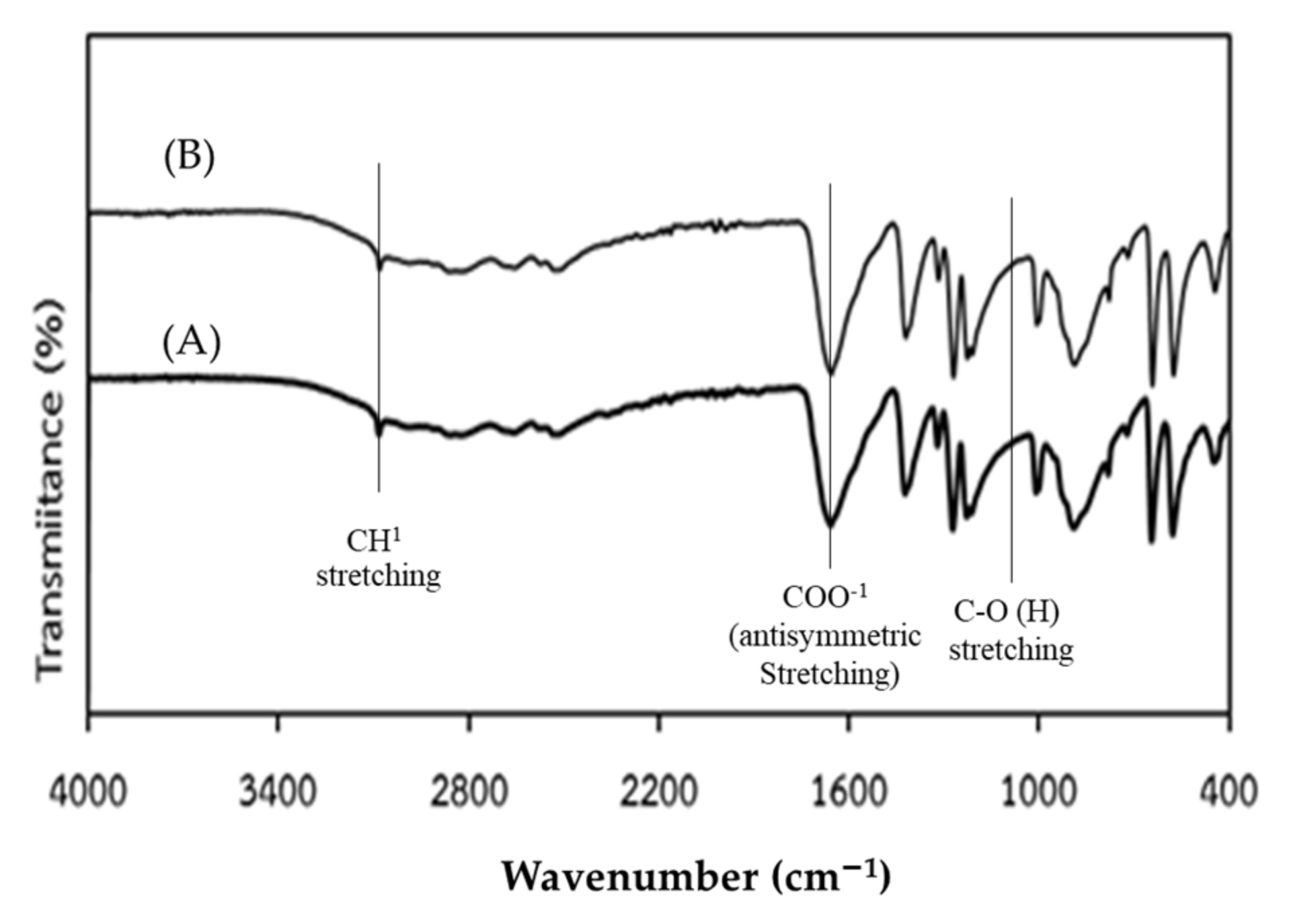
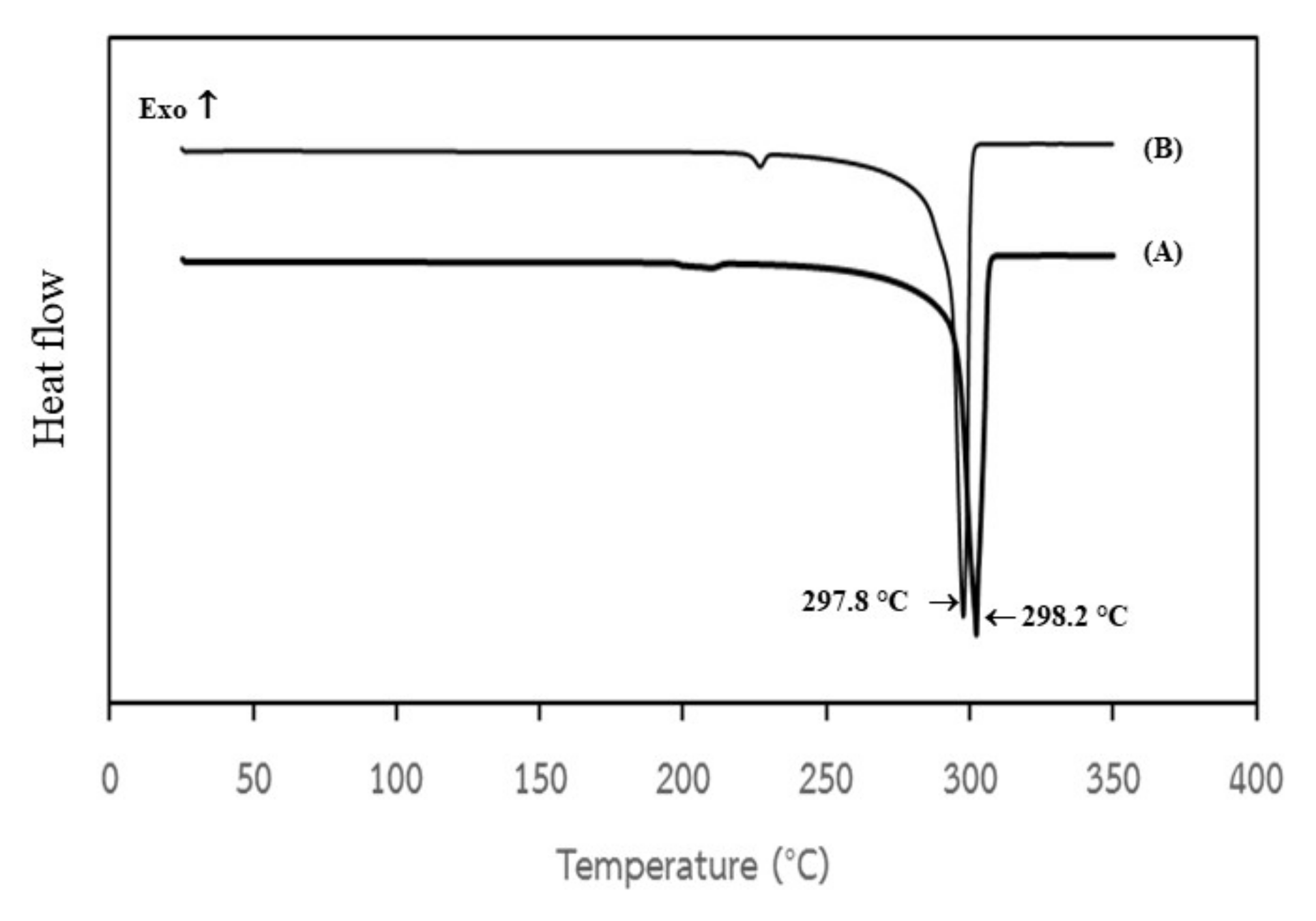
3.6. Characterization of Bio-Synthesized Fumaric Acid
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ji, X.J.; Huang, H.; Ouyang, P.K. Microbial 2,3-butanediol production: A state-of-the art review. Biotechnol. Adv. 2011, 29, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass; Volume I-Results of Screening for Potential Candidates from Sugars and Synthetic Gas (NREL/TP-510-35523); National Renewable Energy Laboratory: Golden, CO, USA, 2004.
- Fumaric acid Market-Growth, Trends, COVID-19 Impact, and Forecasts (2021–2026). Available online: https://www.mordorintelligence.com/industry-reports/fumaric-acid-market (accessed on 12 August 2021).
- Zhou, Y.; Du, J.; Tsao, G.T. Comparison of fumaric acid production by R. oryzae using different neutralizing agents. Bioproc. Biosyst. Eng. 2002, 25, 179–181. [Google Scholar]
- Liao, W.; Liu, Y.; Chen, S.L. Studying pellet formation of a filamentous fungus R. oryzae to enhance organic acid production. Appl. Biochem. Biotechnol. 2007, 137, 689–701. [Google Scholar] [PubMed]
- Deng, Y.; Li, S.; Xu, Q.; Gao, M.; Huang, H. Production of fumaric acid by simultaneous saccharification and fermentation of starchy materials with 2-deoxyglucose-resistant mutant strains of R. oryzae. Bioresour. Technol. 2012, 107, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, B.; Wang, Z.; Tang, Y.J.; Chen, T.; Zhao, X. Engineering Escherichia coli for fumaric acid production from glycerol. Bioresour. Technol. 2014, 174, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.K.; Kang, E.; Jeong, J.S.; Gong, G.; Choi, J.W.; Abimanyu, H.; Ahn, B.S.; Suh, D.J.; Choi, G.W. Production of anhydrous ethanol using oil palm empty fruit bunch in a pilot plant. Biomass Bioenergy 2014, 67, 99–107. [Google Scholar] [CrossRef]
- Lee, H.S.; Lee, H.; Ha, J.M.; Kim, J.; Suh, D.J. Production of aromatic compounds from oil palm empty fruit bunches by hydro- and solvothermolysis. Ind. Crop. Prod. 2015, 76, 104–111. [Google Scholar] [CrossRef]
- Medina, J.D.C.; Woiciechowski, A.; Filho, A.Z.; Nigam, P.S.; Ramos, L.P.; Soccol, C.R. Steam explosion pretreatment of oil palm empty fruit bunches (EFB) using autocatalytic hydrolysis: A biorefinery approach. Bioresour. Technol. 2016, 199, 173–180. [Google Scholar] [CrossRef]
- Engel, C.A.R.; Straathof, A.J.J.; Zijlmans, T.W.; Van Gulik, W.M.; Van der Wielen, L.A.M. Fumaric acid production by fermentation. Appl. Microbiol. Biotechnol. 2008, 78, 379–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, C.W.; Kim, D.I.; Choi, S.; Jang, J.W.; Lee, S.Y. Metabolic engineering of Escherichia coli for the production of fumaric acid. Biotechnol. Bioeng. 2013, 110, 2025–2034. [Google Scholar] [CrossRef]
- Siramon, P.; Punsuvon, V.; Vaithanomsat, P. Production of bioethanol from oil palm empty fruit bunch via acid impregnation-steam explosion pretreatment. Waste Biomass Valori. 2017, 9, 1407–1414. [Google Scholar] [CrossRef]
- Somogyi, M.J. Notes on sugar determination. J. Biol. Chem. 1952, 195, 19–23. [Google Scholar] [CrossRef]
- U-thai, P.; Vaithanomsat, P.; Boondaeng, A.; Talek, A.; Chuntranuluck, S. Production of Fumaric acid from Oil Palm Empty Fruit Bunch. 2015. KKU Res. J. 2016, 21, 221–228. [Google Scholar]
- Figueira, D.; Cavalheiro, J.; Ferreira, B.S. Purification of polymer-grade fumaric acid from fermented spent sulfite liquor. Fermentation 2017, 3, 13. [Google Scholar] [CrossRef] [Green Version]
- Liao, W.; Liu, Y.; Frear, C.; Chen, S.L. Co-production of fumaric acid and chitin from a nitrogen-rich lignocellulosic material-dairy manure-using a pelletized filamentous fungus R. oryzae ATCC20344. Bioresour. Technol. 2008, 99, 5859–5866. [Google Scholar] [CrossRef]
- Das, R.K.; Brar, S.K.; Verma, M. Fumaric acid: Production and application aspects. In Platform Chemical Biorefinery; Elsevier: Amsterdam, The Netherlands, 2016; pp. 133–157. [Google Scholar]
- Magnuson, J.K.; Lasure, L.L. Organic acid production by filamentous fungi. In Advances in Fungal Biotechnology for Industry, Agriculture and Medicine; Tkacz, J., Lange, L., Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 307–340. [Google Scholar]
- Carta, F.S.; Soccol, C.R.; Ramos, L.P.; Fontana, J.D. Production of fumaric acid by fermentation of enzymatic hydrolysates derived from Cassava bagasse. Bioresour. Technol. 1999, 68, 23–28. [Google Scholar] [CrossRef]
- Xu, Q.; Li, S.; Huang, H.; Wen, J. Key technologies for the industrial production of fumaric acid by fermentation. Biotechnol. Adv. 2012, 30, 1685–1696. [Google Scholar] [CrossRef]
- Riscaldati, E.; Moresi, M.; Federici, F.; Petruccioli, M. Direct ammonium fumarate production by Rhizopus arrhizus under phosphorous limitation. Biotechnol. Lett. 2000, 22, 1043–1047. [Google Scholar] [CrossRef]
- Fu, Y.Q.; Xu, Q.; Li, S.; Chen, Y.; Huang, H. Strain improvement of R. oryzae for over-production of fumaric acid by reducing ethanol synthesis pathway. Korean J. Chem. Eng. 2010, 27, 183–186. [Google Scholar] [CrossRef]
- Huang, L.P.; Wei, L.; Zang, R.; Xu, Z.N.; Cen, P.L. High-throughput screening of high-yield colonies of R. oryzae for enhanced production of fumaric acid. Ann. Microbiol. 2010, 60, 287–292. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, L.; Yang, S.T. Fumaric acid recovery and purification from fermentation broth by activated carbon adsorption followed with desorption by acetone. Ind. Eng. Chem. Res. 2014, 53, 12802–12808. [Google Scholar] [CrossRef]
- Saganowska, P.; Wesolowski, M. DSC as a screening tool for rapid co-crystal detection in binary mixtures of benzodiazepines with co-formers. J. Therm. Anal. Calorim. 2018, 133, 785–795. [Google Scholar] [CrossRef] [Green Version]


| Carbon Source | Total Sugar (g/L) ns | Fumaric Acid (g/L) ns | Yield (g/g) ns | Productivity (g/L/h) ns |
|---|---|---|---|---|
| Pure glucose | 75.5 ± 0.153 | 5.42 ± 0.072 | 0.07 ± 0.002 | 0.056 ± 0.002 |
| Glucose derived from EFB | 75.5 ± 0.100 | 5.3 ± 0.030 | 0.07 ± 0.002 | 0.055 ± 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boondaeng, A.; Khanoonkon, N.; Vaithanomsat, P.; Apiwatanapiwat, W.; Trakunjae, C.; Janchai, P.; Niyomvong, N. Recovery and Purification of Fumaric Acid from Fermented Oil Palm Empty Fruit Bunches Using a Simple Two-Stage Precipitation Procedure. Fermentation 2022, 8, 121. https://doi.org/10.3390/fermentation8030121
Boondaeng A, Khanoonkon N, Vaithanomsat P, Apiwatanapiwat W, Trakunjae C, Janchai P, Niyomvong N. Recovery and Purification of Fumaric Acid from Fermented Oil Palm Empty Fruit Bunches Using a Simple Two-Stage Precipitation Procedure. Fermentation. 2022; 8(3):121. https://doi.org/10.3390/fermentation8030121
Chicago/Turabian StyleBoondaeng, Antika, Nattaporn Khanoonkon, Pilanee Vaithanomsat, Waraporn Apiwatanapiwat, Chanaporn Trakunjae, Phornphimon Janchai, and Nanthavut Niyomvong. 2022. "Recovery and Purification of Fumaric Acid from Fermented Oil Palm Empty Fruit Bunches Using a Simple Two-Stage Precipitation Procedure" Fermentation 8, no. 3: 121. https://doi.org/10.3390/fermentation8030121
APA StyleBoondaeng, A., Khanoonkon, N., Vaithanomsat, P., Apiwatanapiwat, W., Trakunjae, C., Janchai, P., & Niyomvong, N. (2022). Recovery and Purification of Fumaric Acid from Fermented Oil Palm Empty Fruit Bunches Using a Simple Two-Stage Precipitation Procedure. Fermentation, 8(3), 121. https://doi.org/10.3390/fermentation8030121