Chitosan Production by Fungi: Current State of Knowledge, Future Opportunities and Constraints
Abstract
1. Introduction
2. Physicochemical and Functional Properties of Chitin and Chitosan
3. General Aspects of Chitin and Chitosan Production from Fungal Sources
3.1. Chitin and Chitosan Biosynthesis and Their Biological Functions in Fungi
3.2. Fungal Producers of Chitin and Chitosan
3.3. Production Processes of Fungal Chitin and Chitosan
3.3.1. Solid-State Production of Fungal Chitosan
| Fungal Strain | Solid Substrate | Cultivation Mode | CPY (g/kg) | rP (mg/kg*h) | DD (%) | Viscosity (cP) | References |
|---|---|---|---|---|---|---|---|
| Absidia coerulea CTCC AF 93105 | Non-supplemented cotton seed hulls | Conical flask | 1.62 | 9.64 | 85 | n.r. | [34] |
| Absidia coerulea CTCC AF 93105 | Potato pieces added with sucrose and urea | Conical flask | 6.12 | 36.4 | 85 | n.r. | [34] |
| Aspergillus niger n.s. | Rice straw | Plastic bag with sterile filters | 5.26 | 24.4 | 84.2 | 59 | [105] |
| Aspergillus niger TISTR3245 | Mung bean residues | Conical flask | 1.39 | 19.3 | n.r. | n.r. | [76] |
| Aspergillus niger BBRC 20004 | Soybean residues | Conical flask | 17.03 | 59.1 | n.r. | n.r. | [109] |
| Gongronella butleri USDB0201 | Sweet potato pieces supplemented with urea | Tray reactor | 3.7 | 22.0 | 92–96 | n.r. | [61] |
| Gongronella butleri USDB0201 | Sweet potato pieces supplemented with urea | Tray reactor | 4.31 | 25.7 | n.r. | n.r. | [62] |
| Lentinus edodes SC-495 | Wheat straw | Plastic bag with sterile filters | 6.18 | 21.5 | 87.5 | n.r. | [104] |
| Mucor rouxii ATCC 24905 | Soybean meal | Autoclavable plastic bag with sterile filters | 32.4 | 119.4 | 55–60 | n.r. | [107] |
| Penicillium citrinum n.s. | Rice straw | Plastic bag with sterile filters | 5.12 | 17.8 | 78.5 | 4.6 | [105] |
| Penicillium expansum | Corn straw | Conical flask | 4.31 | n.r. | 80.2 | 4.8 | [110] |
| Rhizopus oryzae n.s. | Rice straw | Plastic bag with sterile filters | 5.63 | 19.6 | 90.2 | 6.8 | [105] |
| Rhizopus oryzae TISTR3189 | Potato peel | Conical flask | 6.6 | 55.0 | 87.5–90 | 3.1–6.1 | [79] |
| Rhizopus oryzae (local isolate) | Corn straw | Conical flask | 8.57 | 29.8 | 91.5 | 7.2 | [110] |
| Rhizopus oryzae TISTR3189 | Soybean residue | Conical flask | 4.3 | 29.9 | n.r. | n.r. | [76] |
3.3.2. Fungal Chitosan Production in Liquid Submerged Bioprocesses
Chitosan Production on Chemically Defined Liquid Media
| Fungal Strain | Culture Condition and Growth Medium | X (g L−1) | CVP (g L−1) | rP (mg L−1 h−1) | References |
|---|---|---|---|---|---|
| Absidia butleri NCIM977 | Shaken cultures on GYT medium | 6.78 | 0.57 | 7.9 | [51] |
| Absidia coerulea ATCC14076 | Aerated shaken cultures on YM broth | 6.2 | 1.86 | 26.0 | [47] |
| Absidia coerulea ATCC14076 | Batch cultures in 2.5 L STR at 250 rpm and 2 vvm with adaptive pH control at 4.5 on GY medium supplemented with (NH4)2SO4 | 20 | 2.33 | 63.8 | [49] |
| Absidia coerulea ATCC14076 | Batch cultures in 20 L STR at 200 rpm and 1 vvm on PGY medium | 13.9 | 0.55 | 11.5 | [113] |
| Absidia coerulea ATCC14076 | Continuous cultures in 2.5 L STR with pH control at 4.5 on GY medium supplemented with (NH4)2SO4 at a dilution rate of 0.05 h−1 | 7.0 | 1.04 | 50.0 | [49] |
| Absidia coerulea ATCC14076 | Continuous cultures in BioFlo C30 chemostat on GYP medium at a dilution rate of 0.025 h−1 | 2.3 | 1.37 | 41.0 | [46] |
| Absidia coerulea CTCC AF 93105 | Shaken cultures on a glucose-based medium added with 0.5 g L−1 as (NH4)2SO4 | 11.4 | 2.86 | 19.9 | [50] |
| Absidia coerulea CCRC 30897 | Batch cultures in airlift with double-net draft tube on GYP medium | 30.8 | 3.16 | 65.8 | [114] |
| Absidia coerulea CCRC 30897 | Batch cultures in bubble column reactor on GYP medium | 11.3 | 1.36 | 28.3 | [114] |
| Absidia glauca (+) | Shaken cultures on GYP medium | 8.8 | 0.65 | 13.5 | [112] |
| Absidia orchidis NCAIM F 00642 | Batch cultures in 5 L STR on GYP medium supplemented with ferrous ions | 45.3 | 1.79 | 37.3 | [31] |
| Absidia orchidis NCAIM F 00642 | Batch cultures in 5 L STR on GYP medium supplemented with Mn2+ ions | 15.2 | 1.05 | 21.9 | [31] |
| Absidia repens CBS 102-32 | Batch cultures in 10 L STR at 350 rpm on a medium made of glucose, yeast extract, and (NH4)2SO4 | 12.9 | 2.8 | 58.3 | [115] |
| Aspergillus niger MTCC 872 | Shaken cultures on a medium made of potato dextrose broth (24 g L−1), glucose (80 g L−1), L-Asparagine (6 g L−1) | 15.9 | 3.35 | 46.5 | [84] |
| Aspergillus niger BBRC 20004 | Shaken cultures on Sabouro dextrose broth added with 2% glucose | 5.17 | 0.84 | 17.5 | [83] |
| Aspergillus nidulans NS | Shaken cultures on peptone-glucose-yeast extract (PGY) salt broth | 5.15 | 0.20 | 4.19 | [112] |
| Benjaminiella poitrasii CSIR isolate | Batch cultures in 2 L STR on medium containing (g L−1): yeast extract, 6.0; peptone, 10.0; soluble starch,10.0 | 10.0 | 0.51 | 10.6 | [66] |
| Cunninghamella bertholletiae IFM 46.114 | Shaken cultures on yeast extract-peptone-dextrose medium | 7.1 | 0.39 | 5.5 | [56] |
| Cunninghamella echinulata | Shaken cultures on glucose-peptone-yeast extract medium added with (NH4)2SO4 | 5.6 | 0.40 | 3.3 | [52] |
| Cunninghamella elegans IFM 46109 | Shaken culture on a medium mainly made of glucose, asparagine, and MgSO4 (60, 3.0, and 0.25 g L−1, respectively) | 11.0 | 0.86 | 8.9 | [53] |
| Cunninghamella elegans UCP 542 | Shaken cultures on Sabouraud-sucrose medium | 12.0 | 0.42 | 8.7 | [57] |
| Gongronella butleri USDB 0201 | Shaken cultures on glucose-peptone-yeast extract medium added with (NH4)2SO4 | 8.2 | 0.47 | 3.9 | [52] |
| Mucor racemosus (soil isolate) | Shaken cultures on Sabouraud dextrose broth | 3.8 | 0.45 | 2.6 | [71] |
| Mucor rouxii ATCC 24905 | Shaken cultures on glucose-peptone-yeast extract medium | 3.8 | 0.28 | 5.8 | [67] |
| Mucor rouxii ATCC 24905 | Shaken cultures on peptone-yeast extract-glucose (PYG) salt broth | 5.6 | 0.21 | 4.4 | [112] |
| Mucor rouxii DSM 1191 | Batch cultures in 30 L STR on glucose-peptone-yeast extract medium | 8.6 | 0.30 | 21.2 | [116] |
| Rhizomucor miehei ATCC 26282 | Shaken cultures on Sabouraud dextrose broth | 4.1 | 0.56 | 3.4 | [71] |
| Rhizopus oryzae USDB 0602 | Shaken cultures on glucose-peptone-yeast extract medium added with (NH4)2SO4 | 5.7 | 0.28 | 2.3 | [52] |
| Syncephalastrum racemosum UCP148 | Shaken cultures on yeast extract-peptone-dextrose medium | 8.0 | 1.26 | 26.1 | [27] |
Chitosan Production on Waste- and Effluent-Based Liquid Media
| Fungal Strain | Culture Condition and Growth Medium | X (g L−1) | CVP (g L−1) | rP (mg L−1 h−1) | References |
|---|---|---|---|---|---|
| Absidia coerulea CTCC AF 93105 | Shaken cultures on a glucose-based medium (20 g L−1) added with 0.5 g L−1 nitrogen as soybean pomace | 15.4 | 4.11 | 28.5 | [50] |
| Aspergillus awamori MTCC6995 | Shaken cultures on thin stillage from rice-based distillery | 5.2 | 0.39 | 4.0 | [122] |
| Aspergillus braziliensis ATCC16404 | Shaken cultures on syrup from date waste | 13.3 | 2.78 | 19.3 | [28] |
| Aspergillus niger NRRL567 | Batch cultures in 7.5 L STR (impeller speed at 200 rpm; adaptive flow rate to ensure 20% dissolved oxygen saturation) on apple pomace sludge | 12.6 | 0.64 | 4.9 | [3] |
| Cunninghamella bertholletiae IFM 46.114 | Shaken cultures on sugarcane juice (10.5 g L−1 sucrose) added with YE (3 g L−1) | 4.2 | 0.53 | 11.1 | [56] |
| Cunninghamella elegans UCP 0542 | Shaken cultures on a medium made of cassava wastewater (CWW, 10%) and corn steep liquor (CSL, 4%) | 5.7 | 0.33 | 4.6 | [54] |
| Gongronella butleri CCT4274 | Shaken cultures on aqueous extract of apple pomace supplemented with NaNO3 (2.5 g L−1) | 5.5 | 1.19 | 16.4 | [64] |
| Gongronella butleri IFO8081 | Shaken cultures on sweet potato shochu distillery wastewater | 6.2 | 0.73 | 6.1 | [121] |
| Gongronella butleri CCT 4274 | Batch cultures in 6.5 L airlift reactor with external loop circulation (aeration rate, 0.6 vvm) on apple pomace extract added with 5 g L−1 (NH4)2SO4 | 6.7 | 0.93 | 62.2 | [65] |
| Lichtheimia hyalospora UCP1266 | Shaken cultures on a medium made of CWW (4%) and CSL (6%) | 11.9 | 0.75 | 6.3 | [119] |
| Mucor rouxii MTCC 386 | Shaken cultures on molasses salt medium added with indole-3-acetic acid (1.0 mg L−1) | 9.1 | 0.95 | 29.7 | [70] |
| Mucor subtilissimus UCP 1262 | Shaken cultures on a medium made of CWW (4%) and CSL (6%) | 4.8 | 0.16 | 1.3 | [119] |
| Penicillium citrinum (local isolate) | Batch cultures in 3 L stirred tank reactor (200 rpm and 2.0 vvm) on paper mill effluent added with 50 mg L−1 acetic acid | n.s. | 0.14 | 2.9 | [90] |
| Rhizopus arrhizus UCP 0402 | Shaken cultures on a medium made of CSL (4%) and honey (13%) | 11.7 | 0.34 | 3.6 | [80] |
| Rhizopus oryzae MTCC262 | Shaken cultures on deproteinized whey added with gibberellic acid (0.1 mg L−1) | 8.3 | 1.13 | 15.7 | [77] |
| Rhizopus oryzae 00.4367 | Batch cultures in 7 L STR (340 rpm, 2.1 vvm) on untreated sugarbeet molasses (45.4 g L−1 total sugars) | 10.7 | 1.06 | 14.7 | [120] |
| Rhizopus oryzae AS 3.819 | Batch cultures in 3 L stirred tank reactor (200 rpm, 1 vvm) on corn stover hydrolysate supplemented with urea (4 g L−1) | 11.0 | 0.99 | 13.8 | [123] |
| Rhizopus oryzae PAS 17 | Shaken cultures on medium made of molasses (7%, v/v) and supplemented with MgSO4 | 10.7 | 1.50 | 7.8 | [81] |
| Rhizopus oryzae ME-F12 | Shaken cultures on corn straw hydrolysate | 5.2 | 0.58 | n.s. | [118] |
| Rhizopus oryzae MTCC262 | Shaken culture on deproteinized whey supplemented with (NH4)2HPO4 (8 g L−1) and YE (2 g L−1) | 6.2 | 0.62 | n.s. | [78] |
| Syncephalastrum racemosum UCP148 | Shaken cultures on sugarcane juice (10.5 g L−1 sucrose) added with YE (3 g L−1) | 8.1 | 0.60 | 5.0 | [82] |
| Syncephalastrum racemosum UCP148 | Batch cultures in 5 L STR on sugarcane juice (10.5 g L−1 sucrose) added with YE (3 g L−1) | 8.0 | 0.96 | 32.0 | [82] |
3.4. Relevant Factors in Chitin and Chitosan Production from Fungi
3.4.1. Fungal Morphology
3.4.2. Harvesting Time
3.4.3. Medium’s Ph
3.4.4. Nitrogen Source and Concentration
3.4.5. Plant Growth Hormones
3.4.6. Organic Stimulators
3.4.7. Inorganic Supplements
4. Integrated Bioprocesses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Islam, S.; Bhuiyan, M.A.R.; Islam, M.N. Chitin and chitosan: Structure, properties and applications in biomedical engineering. J. Polym. Environ. 2017, 25, 854–866. [Google Scholar] [CrossRef]
- Tharanathan, R.N.; Kittur, F.S. Chitin—The undisputed biomolecular of great potential. Crit. Rev. Food Sci. 2003, 43, 61–87. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, G.S.; Kaur, S.; Sarma, S.J.; Brar, S.K. Integrated process for fungal citric acid fermentation using apple processing wastes and sequential extraction of chitosan from waste stream. Ind. Crops Prod. 2013, 50, 346–351. [Google Scholar] [CrossRef]
- Akila, R.M. Fermentative production of fungal chitosan, a versatile biopolymer (perspectives and its applications). Adv. Appl. Sci. Res. 2014, 5, 157–170. [Google Scholar]
- Global Industry Analysts Inc. Chitin and Chitosan Derivatives—Global Market Trajectory & Analytics 2021. Available online: https://www.researchandmarkets.com/reports/338576/chitin_and_chitosan_derivatives_global_market (accessed on 30 December 2021).
- Jang, M.K.; Kong, B.G.; Jeong, Y.I.; Lee, C.H.; Nah, J.W. Physicochemical characterization of α-chitin, β-chitin and γ-chitin separated from natural resources. J. Polym. Sci. Part A 2004, 42, 3423–3432. [Google Scholar] [CrossRef]
- Kurita, K. Chitin and chitosan: Functional biopolymers from marine crustaceans. Mar. Biotechnol. 2006, 8, 203–226. [Google Scholar] [CrossRef]
- Kumari, S.; Kishor, R. Chitin and chitosan: Origin, properties, and applications. In Handbook of Chitin and Chitosan; Gopi, S., Thomas, S., Pius, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 1, pp. 1–33. ISBN 9780128179703. [Google Scholar]
- Younes, S.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef]
- Oyatogun, G.M.; Esan, T.A.; Akpan, E.I.; Adeosun, S.O.; Popoola, A.P.I.; Imasogie, B.I.; Soboyejo, W.O.; Afonja, A.A.; Ibitoye, S.A.; Abere, V.D.; et al. Chitin, chitosan, marine to market. In Handbook of Chitin and Chitosan; Gopi, S., Thomas, S., Pius, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 1, pp. 335–376. ISBN 9780128179703. [Google Scholar]
- Chien, R.C.; Yen, M.T.; Mau, J.L. Antimicrobial and antitumor activities of chitosan from shiitake stipes, compared to commercial chitosan from crab shells. Carbohydr. Polym. 2016, 138, 259–264. [Google Scholar] [CrossRef]
- Teng, W.L.; Khor, E.; Tan, T.K.; Lim, L.Y.; Tan, S.L. Concurrent production of chitin from shrimp shells and fungi. Carbohydr. Res. 2001, 332, 305–316. [Google Scholar] [CrossRef]
- Elsoud, M.M.A.; El Kady, E.M. Current trends in fungal biosynthesis of chitin and chitosan. Bull. Natl. Res. Cent. 2019, 43, 59. [Google Scholar] [CrossRef]
- Chisty, A.H.; Masud, R.A.; Hasan, M.M.; Khan, M.N.; Mallik, A.K.; Rahman, M.M. PEGylated chitin and chitosan derivatives. In Handbook of Chitin and Chitosan; Gopi, S., Thomas, S., Pius, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 1, pp. 59–100. ISBN 9780128179703. [Google Scholar]
- El Knidri, H.; Laajeb, A.; Lahsini, A. Chapter 2—Chitin and chitosan: Chemistry, solubility, fiber formation, and their potential applications. In Handbook of Chitin and Chitosan; Gopi, S., Thomas, S., Pius, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 1, pp. 35–57. ISBN 9780128179703. [Google Scholar]
- Akpan, E.I.; Gbenebor, O.P.; Adeosun, S.O.; Cletus, O. Chapter 5—Solubility, degree of acetylation, and distribution of acetyl groups in chitosan. In Handbook of Chitin and Chitosan; Gopi, S., Thomas, S., Pius, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 1, pp. 131–164. ISBN 9780128179703. [Google Scholar]
- Aranaz, I.; Mengíbar, M.; Harris, R.; Paños, I.; Miralles, B.; Acosta, N.; Galed, G.; Heras, Á. Functional characterization of chitin and chitosan. Curr. Chem. Biol. 2009, 3, 203–230. [Google Scholar] [CrossRef]
- Zheng, L.Y.; Zhu, J.F. Study on antimicrobial activity of chitosan with different molecular weights. Carbohydr. Polym. 2003, 54, 527–530. [Google Scholar] [CrossRef]
- Klaykruayat, B.; Siralertmukul, K.; Srikulkit, K. Chemical modification of chitosan with cationic hyperbranched dendritic polyamidoamine and its antimicrobial activity on cotton fabric. Carbohydr. Polym. 2010, 80, 197–207. [Google Scholar] [CrossRef]
- Hosseinnejad, M.; Jafari, S.M. Evaluation of different factors affecting antimicrobial properties of chitosan. Int. J. Biol. Macromol. 2016, 85, 467–475. [Google Scholar] [CrossRef]
- Duan, C.; Meng, X.; Meng, J.; Khan, M.I.H.; Dai, L.; Khan, A.; An, X.; Zhang, J.; Huq, T.; Ni, Y. Chitosan as a preservative for fruits and vegetables: A review on chemistry and antimicrobial properties. J. Bioresour. Bioprod. 2019, 4, 11–21. [Google Scholar] [CrossRef]
- Goy, R.C.; Britto, D.D.; Assis, O.B. A review of the antimicrobial activity of chitosan. Polímeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Tan, H.; Chu, C.R.; Payne, K.A.; Marra, K.G. Injectable in situ forming biodegradable chitosan-hyaluronic acid based hydrogels for cartilage tissue engineering. Biomaterials 2009, 30, 2499–2506. [Google Scholar] [CrossRef]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- D’Annibale, A.; Stazi, S.R.; Vinciguerra, V.; Di Mattia, E.; Sermanni, G.G. Characterization of immobilized laccase from Lentinula edodes and its use in olive-mill wastewater treatment. Process Biochem. 1999, 34, 697–706. [Google Scholar] [CrossRef]
- Amorim, R.V.S.; Melo, E.S.; Carneiro-da-Cunha, M.G.; Ledingham, W.M.; Campos-Takaki, G.M. Chitosan from Syncephalastrum racemosum used as a film support for lipase immobilization. Bioresour. Technol. 2003, 89, 35–39. [Google Scholar] [CrossRef]
- Tayel, A.A.; Ibrahim, S.I.; Al-Saman, M.A.; Moussa, S.H. Production of fungal chitosan from date wastes and its application as a biopreservative for minced meat. Int. J. Biol. Macromol. 2014, 69, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Lang, X.; Wang, T.; Sun, M.; Chen, X.; Liu, Y. Advances and applications of chitosan-based nanomaterials as oral delivery carriers: A review. Int. J. Biol. Macromol. 2020, 154, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Shafabakhsh, R.; Yousefi, B.; Asemi, Z.; Nikfar, B.; Mansournia, M.A.; Hallajzadeh, J. Chitosan: A compound for drug delivery system in gastric cancer—A review. Carbohydr. Polym. 2020, 242, 116403. [Google Scholar] [CrossRef]
- Jaworska, M.; Konieczna, E. The influence of supplemental components in nutrient medium on chitosan formation by the fungus Absidia orchidis. Appl. Microbiol. Biotechnol. 2001, 56, 220–224. [Google Scholar] [CrossRef]
- Joseph, S.M.; Krishnamoorthy, S.; Paranthaman, R.; Moses, J.A.; Anandharamakrishnan, C. A review on source-specific chemistry, functionality, and applications of chitin and chitosan. Carbohydr. Polym. Technol. Appl. 2021, 2, 100036. [Google Scholar] [CrossRef]
- Arcidiacono, S.; Kaplan, D.L. Molecular weight distribution of chitosan isolated from Mucor rouxii under different culture and processing conditions. Biotechnol. Bioeng. 1992, 39, 281–286. [Google Scholar] [CrossRef]
- Wang, W.; Du, Y.; Qiu, Y.; Wang, X.; Hu, Y.; Yang, J.; Cai, J.; Kennedy, J.F. A new green technology for direct production of low molecular weight chitosan. Carbohydr. Polym. 2008, 74, 127–132. [Google Scholar] [CrossRef]
- Wibowo, S.; Velazquez, G.; Savant, V.; Torres, J.A. Effect of chitosan type on protein and water recovery efficiency from surimi wash water treated with chitosan-alginate complexes. Bioresour. Technol. 2007, 98, 539–545. [Google Scholar] [CrossRef]
- Sumiyoshi, M.; Kimura, Y. Low molecular weight chitosan inhibits obesity induced by feeding a high-fat diet long-term in mice. J. Pharm. Pharmacol. 2006, 58, 201–207. [Google Scholar] [CrossRef]
- Sathiyaseelan, A.; Shajahan, A.; Kalaichelvan, P.T.; Kaviyarasan, V. Fungal chitosan based nanocomposites sponges—An alternative medicine for wound dressing. Int. J. Biol. Macromol. 2017, 104, 1905–1915. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Zivanovic, S.; Draughon, F.A.; Conway, W.S.; Sams, C.E. Physicochemical properties and bioactivity of fungal chitin and chitosan. J. Agric. Food Chem. 2005, 53, 3888–3894. [Google Scholar] [CrossRef] [PubMed]
- Safaei, Z.; Karimi, K.; Zamani, A. Impact of phosphate, potassium, yeast extract, and trace metals on chitosan and metabolite production by Mucor indicus. Int. J. Mol. Sci. 2016, 17, 1429. [Google Scholar] [CrossRef] [PubMed]
- Zininga, J.T.; Puri, A.K.; Govender, A.; Singh, S.; Permaul, K. Concomitant production of chitosan and lipids from a newly isolated Mucor circinelloides ZSKP for biodiesel production. Bioresour. Technol. 2019, 272, 545–551. [Google Scholar] [CrossRef]
- Merzendorfer, H. The cellular basis of chitin synthesis in fungi and insects: Common principles and differences. Eur. J. Cell Biol. 2011, 90, 759–769. [Google Scholar] [CrossRef]
- Sitanggang, A.B.; Sophia, L.; Wu, H.S. Aspects of glucosamine production using microorganisms. Int. Food Res. J. 2012, 19, 393–404. [Google Scholar]
- Batista, A.C.L.; Souza Neto, F.E.; Paiva, W.S. Review of fungal chitosan: Past, present and perspectives in Brazil. Polímeros 2018, 28, 275–283. [Google Scholar] [CrossRef]
- Lenardon, M.D.; Munro, C.A.; Gow, N.A. Chitin synthesis and fungal pathogenesis. Curr. Opin. Microbiol. 2010, 13, 416–423. [Google Scholar] [CrossRef]
- Miyoshi, H.; Shimura, K.; Watanabe, K.; Onodera, K. Characterization of some fungal chitosans. Biosci. Biotechnol. Biochem. 1992, 56, 1901–1905. [Google Scholar] [CrossRef][Green Version]
- Rane, K.D.; Hoover, D.G. Production of chitosan by fungi. Food Biotechnol. 1993, 7, 11–33. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.; Ilari, P.; Tarsi, R.; Dubini, B.; Xia, W. Chitosan from Absidia coerulea. Carbohydr. Polym. 1994, 25, 45–50. [Google Scholar] [CrossRef]
- Hu, K.J.; Yeung, K.W.; Ho, K.P.; Hu, J.L. Rapid extraction of high-quality chitosan from mycelia of Absidia glauca. J. Food Biochem. 1999, 23, 187–196. [Google Scholar] [CrossRef]
- Kim, W.J.; Lee, W.G.; Theodore, K.; Chang, H.N. Optimization of culture conditions and continuous production of chitosan by the fungi, Absidia coerulea. Biotechnol. Bioprocess Eng. 2001, 6, 6–10. [Google Scholar] [CrossRef]
- Jiang, L.; Pan, S.; Kim, J.M. Influence of nitrogen source on chitosan production carried out by Absidia coerulea CTCC AF 93105. Carbohydr. Polym. 2011, 86, 359–361. [Google Scholar] [CrossRef]
- Vaingankar, P.N.; Juvekar, A.R. Fermentative Production of Mycelial Chitosan from Zygomycetes: Media Optimization and Physico-Chemical Characterization. Adv. Biosci. Biotechnol. 2014, 5, 940–956. [Google Scholar] [CrossRef]
- Tan, S.C.; Tan, T.K.; Wong, S.M.; Khor, E. The chitosan yield of zygomycetes at their optimum harvesting time. Carbohydr. Polym. 1996, 30, 239–242. [Google Scholar] [CrossRef]
- Franco, L.D.O.; Maia, R.D.C.C.; Porto, A.L.F.; Messias, A.S.; Fukushima, K.; Campos-Takaki, G.M.D. Heavy metal biosorption by chitin and chitosan isolated from Cunninghamella elegans (IFM 46109). Braz. J. Microbiol. 2004, 35, 243–247. [Google Scholar] [CrossRef]
- Berger, L.R.; Stamford, T.C.; Stamford-Arnaud, T.M.; de Oliveira Franco, L.; do Nascimento, A.E.; Cavalcante, H.M.; Macedo, R.O.; de Campos-Takaki, G.M. Effect of corn steep liquor (CSL) and cassava wastewater (CW) on chitin and chitosan production by Cunninghamella elegans and their physicochemical characteristics and cytotoxicity. Molecules 2014, 19, 2771–2792. [Google Scholar] [CrossRef]
- Berger, L.R.R.; Stamford, T.C.M.; Stamford-Arnaud, T.M.; De Alcântara, S.R.C.; Da Silva, A.C.; Da Silva, A.M.; Do Nascimento, A.E.; Campos-Takaki, D.; Maria, G. Green conversion of agroindustrial wastes into chitin and chitosan by Rhizopus arrhizus and Cunninghamella elegans strains. Int. J. Mol. Sci. 2014, 15, 9082–9102. [Google Scholar] [CrossRef]
- Amorim, R.V.D.S.; Pedrosa, R.P.; Fukushima, K.; Martínez, C.R.; Ledingham, W.M.; Campos-Takaki, D.; Maria, G. Alternative carbon sources from sugar cane process for submerged cultivation of Cunninghamella bertholletiae to produce chitosan. Food Technol. Biotechnol. 2006, 44, 519–523. [Google Scholar]
- Stamford, T.C.M.; Stamford, T.L.M.; Stamford, N.P.; Barros Neto, B.D.; Campos-Takaki, G.M.D. Growth of Cunninghamella elegans UCP 542 and production of chitin and chitosan using yam bean medium. Electr. J. Biotechnol. 2007, 10, 61–68. [Google Scholar] [CrossRef]
- De Oliveira, C.E.V.; Magnani, M.; de Sales, C.V.; de Souza Pontes, A.L.; Campos-Takaki, G.M.; Stamford, T.C.M.; de Souza, E.L. Effects of chitosan from Cunninghamella elegans on virulence of post-harvest pathogenic fungi in table grapes (Vitis labrusca L.). Int. J. Food Microbiol. 2014, 171, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Junior, J.C.V.; Maia, P.S.; Santos, V.P.; Filho, C.C.; Lima, G.S.; do Nascimento, A.E.; de Campos-Takaki, G.M. Microbial and crustacean Chitosan: Isolation, characterization and antimicrobial activity. In Microbes in the Spotlight: Recent Progress in the Understanding of Beneficial and Harmful Microorganisms; Méndez-Vilas, A., Ed.; BrownWalker Press: Boca Raton, FL, USA, 2016; pp. 290–294. ISBN 9781627346122. [Google Scholar]
- Nwe, N.; Chandrkrachang, S.; Stevens, W.F.; Maw, T.; Tan, T.K.; Khor, E.; Wong, S.M. Production of fungal chitosan by solid state and submerged fermentation. Carbohydr. Polym. 2002, 49, 235–237. [Google Scholar] [CrossRef]
- Nwe, N.; Stevens, W.F. Production of fungal chitosan by solid substrate fermentation followed by enzymatic extraction. Biotechnol. Lett. 2002, 24, 131–134. [Google Scholar] [CrossRef]
- Nwe, N.; Stevens, W.F. Effect of urea on fungal chitosan production in solid substrate fermentation. Process Biochem. 2004, 39, 1639–1642. [Google Scholar] [CrossRef]
- Sharifia, M.; Karimi, K.; Taherzadeh, M.J. Production of ethanol by filamentous and yeast-like forms of Mucor indicus from fructose, glucose, sucrose, and molasses. J. Ind. Microbiol. Biotechnol. 2008, 35, 1253–1259. [Google Scholar] [CrossRef]
- Streit, F.; Koch, F.; Laranjeira, M.; Ninow, J.L. Production of fungal chitosan in liquid cultivation using apple pomace as substrate. Braz. J. Microbiol. 2009, 40, 20–25. [Google Scholar] [CrossRef]
- Vendruscolo, F.; Ninow, J.L. Apple pomace as a substrate for fungal chitosan production in an airlift bioreactor. Biocatal. Agric. Biotechnol. 2014, 3, 338–342. [Google Scholar] [CrossRef]
- Mane, S.R.; Pathan, E.K.; Kale, D.; Ghormade, V.; Gadre, R.V.; Rajamohanan, P.R.; Badiger, M.V.; Deshpande, M.V. Optimization for the production of mycelial biomass from Benjaminiella poitrasii to isolate highly deacetylated chitosan. J. Polym. Mater. 2017, 34, 145–156. [Google Scholar]
- Synowiecki, J.; Al-Khateeb, N.A.A.Q. Mycelia of Mucor rouxii as a source of chitin and chitosan. Food Chem. 1997, 60, 605–610. [Google Scholar] [CrossRef]
- Pochanavanich, P.; Suntornsuk, W. Fungal chitosan production and its characterization. Lett. Appl. Microbiol. 2002, 35, 17–21. [Google Scholar] [CrossRef]
- Chatterjee, S.; Adhya, M.; Guha, A.K.; Chatterjee, B.P. Chitosan from Mucor rouxii: Production and physico-chemical characterization. Process Biochem. 2005, 40, 395–400. [Google Scholar] [CrossRef]
- Chatterjee, S.; Chatterjee, S.; Chatterjee, B.P.; Guha, A.K. Influence of plant growth hormones on the growth of Mucor rouxii and chitosan production. Microbiol. Res. 2009, 164, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Tajdini, F.; Amini, M.A.; Nafissi-Varcheh, N.; Faramarzi, M.A. Production, physiochemical and antimicrobial properties of fungal chitosan from Rhizomucor miehei and Mucor racemosus. Int. J. Biol. Macromol. 2010, 47, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Karimi, K.; Zamani, A. Mucor indicus: Biology and industrial application perspectives: A review. Biotechnol. Adv. 2013, 31, 466–481. [Google Scholar] [CrossRef]
- Abasian, L.; Alavijeh, R.S.; Satari, B.; Karimi, K. Sustainable and effective chitosan production by dimorphic fungus Mucor rouxii via replacing yeast extract with fungal extract. Appl. Biochem. Biotechnol. 2020, 191, 666–678. [Google Scholar] [CrossRef]
- Zamani, A.; Edebo, L.; Sjöström, B.; Taherzadeh, M.J. Extraction and precipitation of chitosan from cell wall of zygomycetes fungi by dilute sulfuric acid. Biomacromolecules 2007, 8, 3786–3790. [Google Scholar] [CrossRef]
- Nadarajah, K.; Kader, J.; Mazmira, M.; Paul, D.C. Production of chitosan by fungi. Pak. J. Biol. Sci. 2001, 4, 263–265. [Google Scholar] [CrossRef]
- Suntornsuk, W.; Pochanavanich, P.; Suntornsuk, L. Fungal chitosan production on food processing by-products. Process Biochem. 2002, 37, 727–729. [Google Scholar] [CrossRef]
- Chatterjee, S.; Chatterjee, S.; Chatterjee, B.P.; Guha, A.K. Enhancement of growth and chitosan production by Rhizopus oryzae in whey medium by plant growth hormones. Int. J. Biol. Macromol. 2008, 42, 120–126. [Google Scholar] [CrossRef]
- Chatterjee, S.; Guha, A.K.; Chatterjee, B.P. Evaluation of quantity and quality of chitosan produce from Rhizopus oryzae by utilizing food product processing waste whey and molasses. J. Environ. Manag. 2019, 251, 109565. [Google Scholar] [CrossRef]
- Kleekayai, T.; Suntornsuk, W. Production and characterization of chitosan obtained from Rhizopus oryzae grown on potato chip processing waste. World J. Microbiol. Biotechnol. 2011, 27, 1145–1154. [Google Scholar] [CrossRef]
- Cardoso, A.; Lins, C.I.M.; Dos Santos, E.R.; Silva, M.C.F.; Campos-Takaki, G.M. Microbial enhance of chitosan production by Rhizopus arrhizus using agroindustrial substrates. Molecules 2012, 17, 4904–4914. [Google Scholar] [CrossRef] [PubMed]
- Tasar, O.C.; Erdal, S.; Taskin, M. Chitosan production by psychrotolerant Rhizopus oryzae in non-sterile open fermentation conditions. Int. J. Biol. Macromol. 2016, 89, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Amorim, R.V.S.; Ledingham, W.M.; Kennedy, J.F.; Campos-Takaki, G.M. Chitosan from Syncephalastrum racemosum using sugar cane substrates as inexpensive carbon sources. Food Biotechnol. 2006, 20, 43–53. [Google Scholar] [CrossRef]
- Maghsoodi, V.; Razavi, J.; Yaghmaei, S. Production of chitosan by submerged fermentation from Aspergillus niger. Trans. C Chem. Chem. Eng. 2009, 16, 145–148. [Google Scholar]
- Kumaresapillai, N.; Basha, R.A.; Sathish, R. Production and evaluation of chitosan from Aspergillus niger MTCC strains. Iran. J. Pharm. Res. 2011, 10, 553–558. [Google Scholar]
- Abdel-Gawad, K.M.; Hifney, A.F.; Fawzy, M.A.; Gomaa, M. Technology optimization of chitosan production from Aspergillus niger biomass and its functional activities. Food Hydrocoll. 2017, 63, 593–601. [Google Scholar] [CrossRef]
- Habibi, A.; Karami, S.; Varmira, K.; Hadadi, M. Key parameters optimization of chitosan production from Aspergillus terreus using apple waste extract as sole carbon source. Bioprocess Biosyst. Eng. 2021, 44, 283–295. [Google Scholar] [CrossRef]
- Tianwei, T.; Binwu, W.; Xinyuan, S. Separation of chitosan from Penicillium chrysogenum mycelium and its applications. J. Bioact. Compat. Polym. 2002, 17, 173–182. [Google Scholar] [CrossRef]
- Ebrahimzadeh, M.A.; Chabra, A.; Gharaei-Fathabad, E.; Pourmorad, F. Preparation of chitosan from Penicillium spp. and determination of their degree of deacetylation. Ind. J. Biotechnol. 2013, 12, 231–235. [Google Scholar]
- Aili, D.; Adour, L.; Houali, K.; Amrane, A. Effect of temperature in chitin and chitosan production by solid culture of Penicillium camembertii on YPG medium. Int. J. Biol. Macromol. 2019, 133, 998–1007. [Google Scholar] [CrossRef] [PubMed]
- Namboodiri, M.M.T.; Pakshirajan, K. Sustainable and green approach of chitosan production from Penicillium citrinum biomass using industrial wastewater as a cheap substrate. J. Environ. Manag. 2019, 240, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.J. Fungal cell wall chitinases and glucanases. Microbiology 2004, 150, 2029–2035. [Google Scholar] [CrossRef] [PubMed]
- Banks, I.R.; Specht, C.A.; Donlin, M.J.; Gerik, K.J.; Levitz, S.M.; Lodge, J.K. A chitin synthase and its regulator protein are critical for chitosan production and growth of the fungal pathogen Cryptococcus neoformans. Eukaryot. Cell 2005, 4, 1902–1912. [Google Scholar] [CrossRef]
- Baker, L.G.; Specht, C.A.; Donlin, M.J.; Lodge, J.K. Chitosan, the Deacetylated form of chitin, is necessary for cell wall integrity in Cryptococcus neoformans. Eukaryot. Cell 2007, 6, 855–867. [Google Scholar] [CrossRef]
- Goldman, D.L.; Vicencio, A.G. The chitin connection. MBio 2012, 3, e00056-12. [Google Scholar] [CrossRef]
- El Gueddari, N.E.; Rauchhaus, U.; Moerschbacher, B.M.; Deising, H.B. Developmentally regulated conversion of surface-exposed chitin to chitosan in cell walls of plant pathogenic fungi. New Phytol. 2002, 156, 103–112. [Google Scholar] [CrossRef]
- Kuroki, M.; Okauchi, K.; Yoshida, S.; Ohno, Y.; Murata, S.; Nakajima, Y.; Nozaka, A.; Tanaka, N.; Nakajima, M.; Taguchi, H.; et al. Chitin-deacetylase activity induces appressorium differentiation in the rice blast fungus Magnaporthe oryzae. Sci. Rep. 2017, 7, 9697. [Google Scholar] [CrossRef]
- Gow, N.A.; Latge, J.P.; Munro, C.A. The fungal cell wall: Structure, biosynthesis, and function. Microbiol. Spectr. 2017, 5, 1–25. [Google Scholar] [CrossRef]
- Liao, W.; Liu, Y.; Frear, C.; Chen, S. Co-production of fumaric acid and chitin from a nitrogen-rich lignocellulosic material—dairy manure—Using a pelletized filamentous fungus Rhizopus oryzae ATC 20344. Bioresour. Technol. 2008, 99, 5859–5866. [Google Scholar] [CrossRef] [PubMed]
- Suresh, P.V.; Sachindra, N.M.; Bhaskar, N. Solid state fermentation production of chitin deacetylase by Colletotrichum lindemuthianum ATCC 56676 using different substrates. J. Food Sci. Technol. 2011, 48, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Morales-Aguirre, J.J.; Agüero-Echeverría, W.M.; Ornelas-Carsolio, M.E.; Reséndiz-Sánchez, J.; Gómez-Barreto, D.; Cashat-Cruz, M. Successful treatment of a primary cutaneous zygomycosis caused by Absidia corymbifera in a premature newborn. Pediatric Infect. Dis. J. 2004, 23, 470–472. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, M.M.; Lippmann, N.; Kobelt, L.; Petzold-Quinque, S.; Ritter, L.; Kiess, W.; Siekmeyer, M. Possible pulmonary Rhizopus oryzae infection in a previously healthy child after a near-drowning incident. Infection 2016, 44, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Kapilan, R. Solid state fermentation for microbial products: A review. Arch. Appl. Sci. Res. 2015, 7, 21–25. [Google Scholar]
- Sebastian, J.; Rouissi, T.; Brar, S.K. Fungal chitosan: Prospects and challenges. In Handbook of Chitin and Chitosan; Gopi, S., Thomas, S., Pius, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 1, pp. 419–452. ISBN 9780128179703. [Google Scholar]
- Crestini, C.; Kovac, B.; Giovannozzi-Sermanni, G. Production and isolation of chitosan by submerged and solid-state fermentation from Lentinus edodes. Biotechnol. Bioeng. 1996, 50, 207–210. [Google Scholar] [CrossRef]
- Khalaf, S.A. Production and characterization of fungal chitosan under solid-state fermentation conditions. Int. J. Agric. Biol. 2004, 6, 1033–1036. [Google Scholar] [CrossRef]
- Maghsoodi, V.; Yaghmaei, S.; Beigi, S.M. Influence of different nitrogen sources on amount of chitosan production by Aspergillus niger in solid state fermentation. Iran. J. Chem. Chem. Eng. 2008, 27, 47–52. [Google Scholar] [CrossRef]
- Mondala, A.; Al-Mubarak, R.; Atkinson, J.; Shields, S.; Young, B.; Senger, Y.D.S.; Pekarovic, J. Direct solid-state fermentation of soybean processing residues for the production of fungal chitosan by Mucor rouxii. J. Mater. Sci. Chem. Eng. 2015, 3, 11. [Google Scholar] [CrossRef][Green Version]
- Kannan, M.; Nesakumari, M.; Rajarathinam, K.; Singh, A.J.A.R. Production and characterization of mushroom chitosan under solid-state fermentation conditions. Adv. Biol. Res. 2010, 4, 10–13. [Google Scholar]
- Maghsoodi, V.; Yaghmaei, S. Comparison of solid substrate and submerged fermentation for chitosan production by Aspergillus niger. Sci. Iran. 2010, 17, 153–157. [Google Scholar]
- Omogbai, B.A.; Ikenebomeh, M. Solid-state fermentative production and bioactivity of fungal chitosan. J. Microbiol. Biotechnol. Food Sci. 2021, 3, 172–175. [Google Scholar]
- Nwe, N.; Furuike, T.; Osaka, I.; Fujimori, H.; Kawasaki, H.; Arakawa, R.; Tokura, S.; Stevens, W.F.; Kurozumi, S.; Takamori, Y.; et al. Laboratory scale production of 13C labeled chitosan by fungi Absidia coerulea and Gongronella butleri grown in solid substrate and submerged fermentation. Carbohydr. Polym. 2011, 84, 743–750. [Google Scholar] [CrossRef]
- Hu, K.J.; Hu, J.L.; Ho, K.P.; Yeung, K.W. Screening of fungi for chitosan producers, and copper adsorption capacity of fungal chitosan and chitosanaceous materials. Carbohydr. Polym. 2004, 58, 45–52. [Google Scholar] [CrossRef]
- ElMekawy, A.; El-Baz, A.F.; Soliman, A.E.; Hudson, S. Statistical modeling and optimization of chitosan production from Absidia coerulea using response surface methodology. Curr. Biotechnol. 2013, 2, 125–133. [Google Scholar] [CrossRef]
- Wu, W.T.; Huang, T.K.; Wang, P.M.; Chen, J.W. Cultivation of Absidia coerulea for chitosan production in a modified airlift reactor. J. Chin. Inst. Chem. Eng. 2001, 35, 235–240. [Google Scholar]
- Davoust, N.; Persson, A. Effects of growth morphology and time of harvesting on the chitosan yield of Absidia repens. Appl. Microbiol. Biotechnol. 1992, 37, 572–575. [Google Scholar] [CrossRef]
- Gözke, G.; Kirschhöfer, F.; Heissler, S.; Trutnau, M.; Brenner-Weiss, G.; Ondruschka, J.; Obst, U.; Posten, C. Filtration kinetics of chitosan separation by electrofiltration. Biotechnol. J. 2012, 7, 262–274. [Google Scholar] [CrossRef]
- Shimahara, K.; Takiguchi, Y.; Kobayashi, T.; Uda, K.; Sannan, T. Screening of mucoraceae strains suitable for chitosan production. In Chitin and Chitosan: Sources, Chemistry, Biochemistry, Physical Properties and Applications; Skjak-Braek, G., Anthonsen, T., Sandford, P.A., Eds.; Elsevier Applied Science: London, UK, 1989; pp. 171–178. ISBN 9781851663958. [Google Scholar]
- Tai, C.; Li, S.; Xu, Q.; Ying, H.; Huang, H.; Ouyang, P. Chitosan production from hemicellulose hydrolysate of corn straw: Impact of degradation products on Rhizopus oryzae growth and chitosan fermentation. Lett. Appl. Microbiol. 2010, 51, 278–284. [Google Scholar] [CrossRef]
- De Souza, A.F.; Galindo, H.M.; de Lima, M.A.B.; Ribeaux, D.R.; Rodríguez, D.M.; da Silva Andrade, R.F.; Gusmão, N.B.; de Campos-Takaki, G.M. Biotechnological strategies for chitosan production by mucoralean strains and dimorphism using renewable substrates. Int. J. Mol. Sci. 2020, 21, 4286. [Google Scholar] [CrossRef]
- Göksungur, Y. Optimization of the production of chitosan from beet molasses by response surface methodology. J. Chem. Technol. Biotechnol. 2004, 79, 974–981. [Google Scholar] [CrossRef]
- Yokoi, H.; Aratake, T.; Nishio, S.; Hirose, J.; Hayashi, S.; Takasaki, Y. Chitosan production from shochu distillery wastewater by funguses. J. Ferment. Bioeng. 1998, 85, 246–249. [Google Scholar] [CrossRef]
- Ray, S.G.; Ghangrekar, M.M. Biodegradation kinetics of thin-stillage treatment by Aspergillus awamori and characterization of recovered chitosan. Appl. Microbiol. Biotechnol. 2016, 100, 1955–1965. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, X.; Lai, C.; Fan, Y.; Ouyang, J.; Yong, Q. Fungal chitosan production using xylose rich of corn stover prehydrolysate by Rhizopus oryzae. Biotechnol. Biotechnol. Equip. 2017, 31, 1160–1166. [Google Scholar] [CrossRef]
- Petruccioli, M.; Raviv, M.; Di Silvestro, R.; Dinelli, G. Agriculture and agro-industrial wastes, byproducts and wastewaters: Origin, characteristics and potential in bio-based-compounds production. In Comprehensive Biotechnology, 2nd ed.; Moo-Young, M., Agathos, S.N., Fava, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 6, pp. 531–545. ISBN 9780444533524. [Google Scholar]
- Achi, C.G.; Hassanein, A.; Lansing, S. Enhanced biogas production of cassava wastewater using zeolite and biochar additives and manure co-digestion. Energies 2020, 13, 491. [Google Scholar] [CrossRef]
- Veiter, L.; Rajamanickam, V.; Herwig, C. The filamentous fungal pellet—Relationship between morphology and productivity. Appl. Microbiol. Biotechnol. 2018, 102, 2997–3006. [Google Scholar] [CrossRef] [PubMed]
- Quintanilla, D.; Hagemann, T.; Hansen, K.; Gernaey, K.V. Fungal morphology in industrial enzyme production-modelling and monitoring. Adv. Biochem. Eng. Biotechnol. 2015, 149, 29–54. [Google Scholar]
- Sparringa, R.A.; Owens, J.D. Glucosamine content of tempe mould, Rhizopus oligosporus. Int. J. Food Microbiol. 1999, 47, 153–157. [Google Scholar] [CrossRef]
- Satari, B.; Karimi, K.; Zamani, A. Oil, chitosan, and ethanol production by dimorphic fungus Mucor indicus from different lignocelluloses. J. Chem. Technol. Biotechnol. 2016, 91, 1835–1843. [Google Scholar] [CrossRef]
- Bartnicki-Garcia, S.; Nickerson, W.J. Isolation, composition, and structure of cell walls of filamentous and yeast-like forms of Mucor rouxii. Biochim. Biophys. Acta 1962, 58, 102–119. [Google Scholar] [CrossRef]
- Grifoll-Romero, L.; Pascual, S.; Aragunde, H.; Biarnés, X.; Planas, A. Chitin deacetylases: Structures, specificities, and biotech applications. Polymers 2018, 10, 352. [Google Scholar] [CrossRef] [PubMed]
- Jennings, D.H. Some perspectives on nitrogen and phosphorus metabolism in fungi. In Nitrogen, Phosphorus and Sulphur Utilization by Fungi; Boddy, L., Marchart, R., Read, D.J., Eds.; Cambridge University Press: Cambridge, UK, 1989; pp. 1–31. ISBN 9780521374057. [Google Scholar]
- Gooday, G.W.; Gow, N.A.R.; Brown, J.P.; Schofield, D.; Munro, C.; McCreath, K.; Hunter, L. Dynamics of chitin synthesis and breakdown in fungal walls. In Fungal Cells in Biodefense Mechanism; Suzuki, S., Suzuki, M., Eds.; Saikon: Tokyo, Japan, 1997; pp. 239–245. [Google Scholar]
- Jeihanipour, A.; Zamani, A.; Karimi, K.; Taherzadeh, M.J. Effect of Growing Time on the Chitosan Content of Cell Wall of Zygomycetes Fungi. In Proceedings of the 9th International Conference of the European Chitin Society, Venice, Italy, 23–26 May 2009. [Google Scholar]
- Mohammadi, M.; Zamani, A.; Karimi, K. Effect of phosphate on glucosamine production by ethanolic fungus Mucor indicus. Appl. Biochem. Biotechnol. 2013, 171, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Dzurendova, S.; Zimmermann, B.; Kohler, A.; Tafintseva, V.; Slany, O.; Certik, M.; Shapaval, V. Microcultivation and FTIR spectroscopy-based screening revealed a nutrient-induced co-production of high-value metabolites in oleaginous Mucoromycota fungi. PLoS ONE 2020, 15, e0234870. [Google Scholar] [CrossRef] [PubMed]
- Bignell, E. The molecular basis of pH sensing, signaling, and homeostasis in fungi. Adv. Appl. Microbiol. 2012, 79, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, A. Biorefineries: Current activities and future developments. Energy Conv. Manag. 2009, 50, 2782–2801. [Google Scholar] [CrossRef]
- Cai, J.; Yang, J.; Du, Y.; Fan, L.; Qiu, Y.; Li, J.; Kennedy, J.F. Enzymatic preparation of chitosan from the waste Aspergillus niger mycelium of citric acid production plant. Carbohydr. Polym. 2006, 64, 151–157. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Kaur, S.; Brar, S.K.; Verma, M. Green synthesis approach: Extraction of chitosan from fungus mycelia. Crit. Rev. Biotechnol. 2013, 33, 379–403. [Google Scholar] [CrossRef]
- Kumar, S.J.; Gujjala, L.K.S.; Dash, A.; Talukdar, B.; Banerjee, R. Biodiesel production from lignocellulosic biomass using oleaginous microbes. In Lignocellulosic Biomass Production and Industrial Applications; Scrivener Publishing: Salem, MA, USA, 2017; pp. 65–92. [Google Scholar] [CrossRef]
- Vinche, M.H.; Karimi, K.; Zamani, A.; Asachi, R. Chitosan: A valuable byproduct of ethanolic fermentation by Rhizopus oryzae. J. Biobased Mater. Bioeng. 2012, 6, 552–557. [Google Scholar] [CrossRef]
- Ghormade, V.; Pathan, E.K.; Deshpande, M.V. Can fungi compete with marine sources for chitosan production? Int. J. Biol. Macromol. 2017, 104, 1415–1421. [Google Scholar] [CrossRef]
- Tianqi, W.; Hanxiang, L.; Manyi, W.; Tianwei, T. Integrative extraction of Ergosterol, (1-3)-α-D-glucan and chitosan from Penicillium chrysogenum mycelia. Chin. J. Chem. Eng. 2007, 15, 725–729. [Google Scholar] [CrossRef]
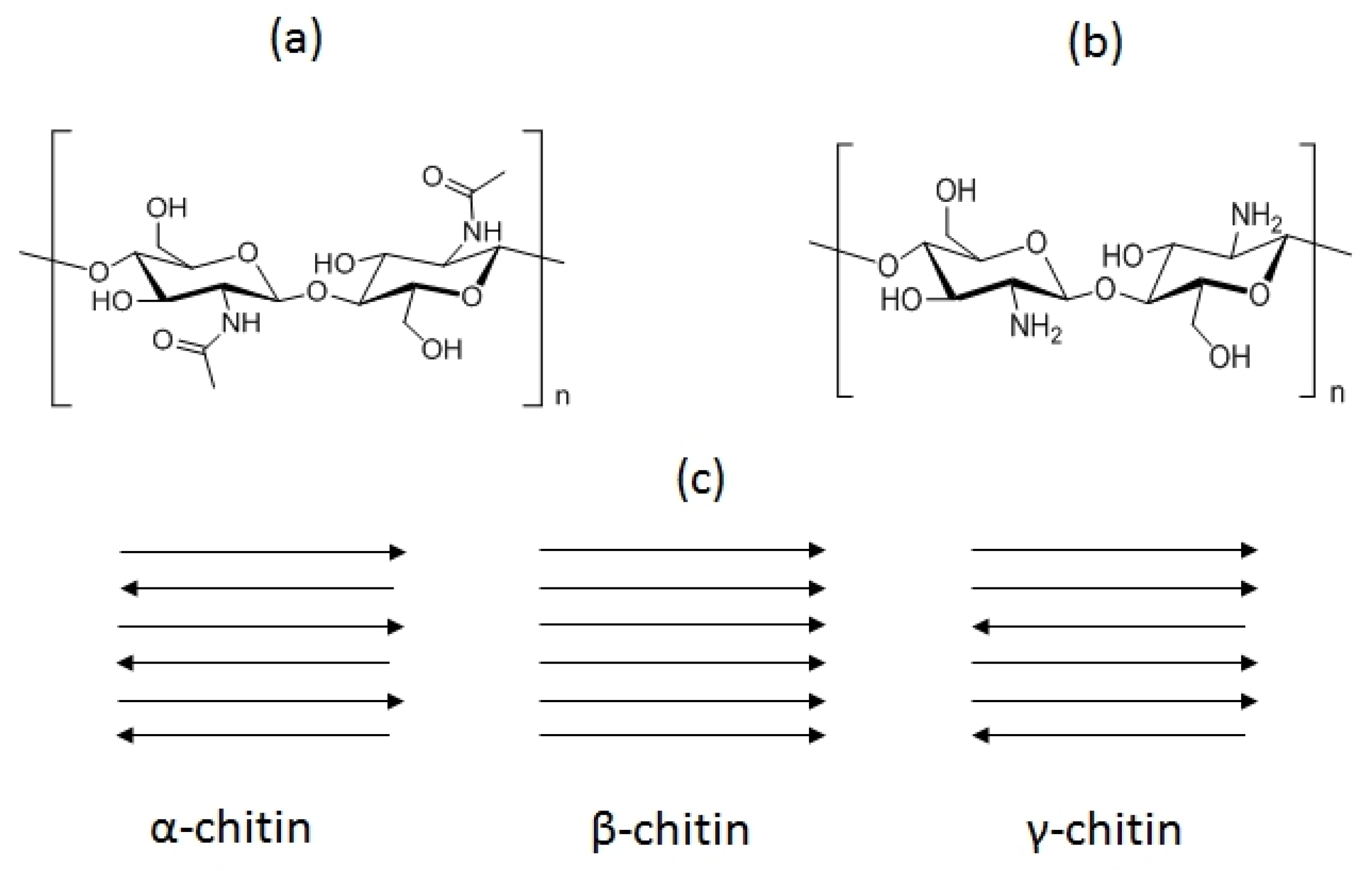
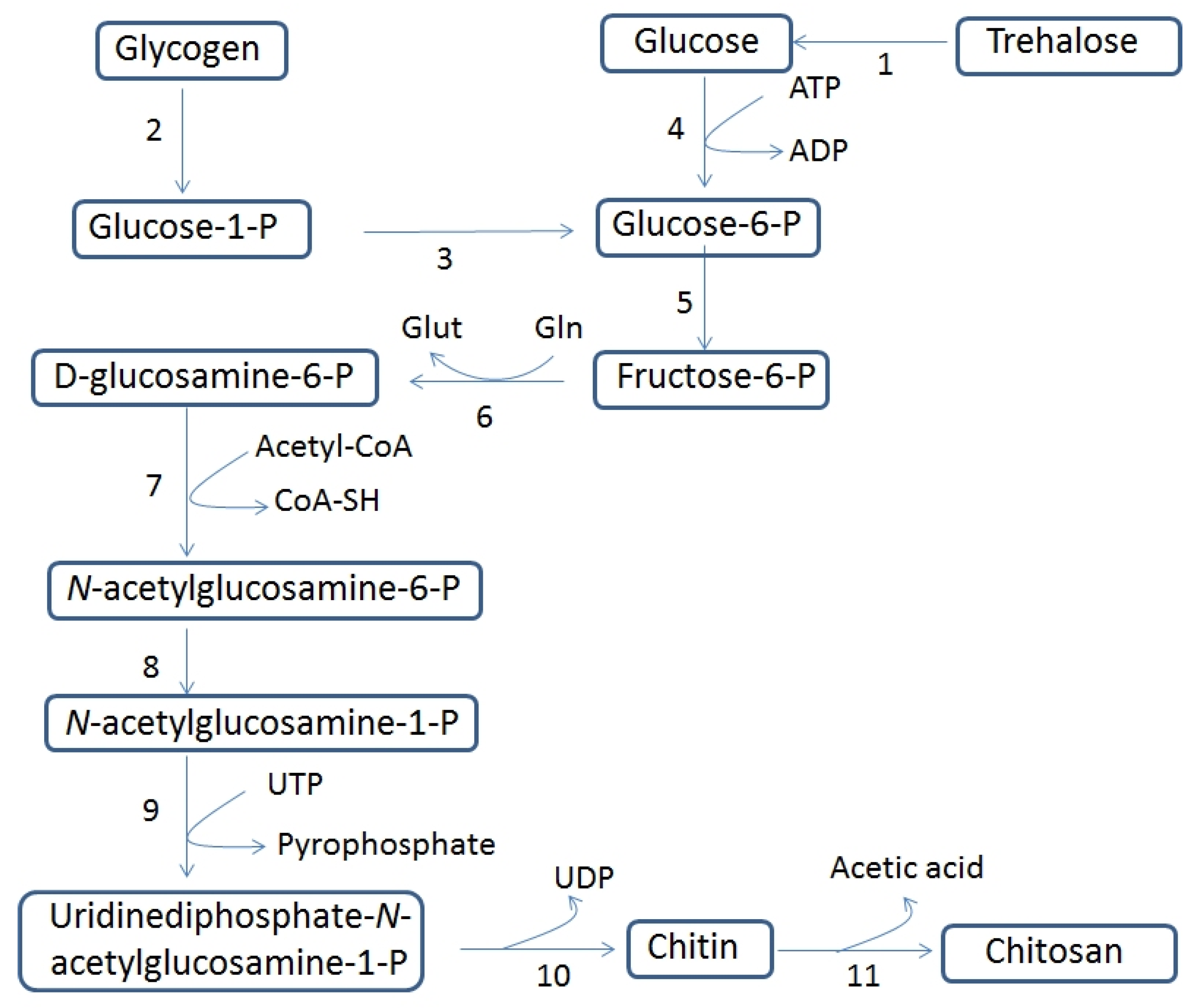
| Evaluation Criterion | Benefits | Drawbacks |
|---|---|---|
| Biomass supply | Not affected by seasonal and geographical factors. Possible biomass supply from pharmaceutical and biotechnological industries. | Lower biomass amounts than those made available from the shellfish industry. |
| Extraction process | Process scheme simpler (no demineralization and decolorization steps) and lower amounts of chemicals employed as compared to those used for crustacean sources. | Less established as compared to that from shellfish waste. |
| Environmental impact and disposal costs of process wastes | More environmentally friendly and lower disposal costs of effluents as compared to the shellfish waste process. | Potential risks of dispersal of pathogenic fungi when dealing with species not satisfying the generally regarded as safe requirements. |
| Inorganic and organic contaminants in the product | Absence of heavy metals and allergenic proteins as opposed to chitosan preparations from shellfish waste. | Some chitosan preparations might contain residual phosphates. |
| Production costs | They can be modulated by the choice of low-cost substrates and low equipment-intensive fermentation techniques, such as solid-state fermentation. | Not yet competitive in terms of production costs compared to the conventional process. |
| Physicochemical properties of the products | Molecular weights and degree of deacetylation of fungal chitosans frequently lower and higher, respectively, than those from conventional sources with ensuing positive impacts on their antimicrobial and antioxidant activities. | The lower MW of fungal chitosans than those from conventional sources make them less suitable as anti-lipidemic and hypocholesterolemic agents. |
| Genus and Species | Class | Order | Family | Chitosan Content (g/kg) | DD (%) | References |
|---|---|---|---|---|---|---|
| Absidia | Zygomycetes | Mucorales | Cunninghamellaceae | [31,45,46,47,48,49,50,51] | ||
| A. blakesleeana | 10–170 | 85 | ||||
| A. coerulea | 30–300 | 93–95 | ||||
| A. glauca | 52–59 | 75–80 | ||||
| A. orchidis | 18–69 | 68–85 | ||||
| Cunninghamella | Zygomycetes | Mucorales | Cunninghamellaceae | [27,51,52,53,54,55,56,57,58,59] | ||
| C. bertholletiae | 55–128 | 87–90 | ||||
| C. echinulata | 50–130 | 85 | ||||
| C. elegans | 35–78 | 72–90 | ||||
| C. ramose | 50–123 | n.r. | ||||
| Gongronella | Zygomycetes | Mucorales | Cunninghamellaceae | [52,60,61,62,63,64,65] | ||
| G. butleri | 58–216 | 89–92 | ||||
| Benjaminiella | Zygomycetes | Mucorales | Mucoraceae | [66] | ||
| B. poitrasii | 51–78 | 94–95 | ||||
| Mucor | Zygomycetes | Mucorales | Mucoraceae | [27,38,40,67,68,69,70,71,72,73] | ||
| M. racemosus | 12–117 | 70–84 | ||||
| M. rouxii | 33–204 | 80–90 | ||||
| M. rouxianus | 181 | 80–90 | ||||
| M. indicus | 94–235 | 72–89 | ||||
| Rhizomucor | Zygomycetes | Mucorales | Mucoraceae | [71,74] | ||
| R. pusillus | 80 | n.r. | ||||
| R. miehei | 14–137 | 81 | ||||
| Rhizopus | Zygomycetes | Mucorales | Mucoraceae | [51,52,55,68,75,76,77,78,79,80,81] | ||
| R. oryzae | 44–138 | 85–89 | ||||
| R. oligosporus | 32 | n.r. | ||||
| R. arrhiizus | 21–58 | 82 | ||||
| Syncephalastrum | Zygomycetes | Mucorales | Syncephalastraceae | [27,82] | ||
| S. racemosum | 74–152 | 72–77 | ||||
| Aspergillus | Eurotiomycetes | Eurotiales | Trichocomaceae | [28,38,68,83,84,85,86] | ||
| A. niger | 70–209 | 81–90 | ||||
| A. terreus | 69–141 | 85–88 | ||||
| Penicillium | Eurotiomycetes | Eurotiales | Trichocomaceae | [87,88,89,90] | ||
| P. chrysogenum | 29–57 | 84–86 | ||||
| P. waksmanii | 297 | 65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crognale, S.; Russo, C.; Petruccioli, M.; D’Annibale, A. Chitosan Production by Fungi: Current State of Knowledge, Future Opportunities and Constraints. Fermentation 2022, 8, 76. https://doi.org/10.3390/fermentation8020076
Crognale S, Russo C, Petruccioli M, D’Annibale A. Chitosan Production by Fungi: Current State of Knowledge, Future Opportunities and Constraints. Fermentation. 2022; 8(2):76. https://doi.org/10.3390/fermentation8020076
Chicago/Turabian StyleCrognale, Silvia, Cristina Russo, Maurizio Petruccioli, and Alessandro D’Annibale. 2022. "Chitosan Production by Fungi: Current State of Knowledge, Future Opportunities and Constraints" Fermentation 8, no. 2: 76. https://doi.org/10.3390/fermentation8020076
APA StyleCrognale, S., Russo, C., Petruccioli, M., & D’Annibale, A. (2022). Chitosan Production by Fungi: Current State of Knowledge, Future Opportunities and Constraints. Fermentation, 8(2), 76. https://doi.org/10.3390/fermentation8020076









