Production of Fumaric Acid by Rhizopus arrhizus NRRL 1526: A Simple Production Medium and the Kinetic Modelling of the Bioprocess
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism and Stock Elaboration
2.2. Media and Culture Conditions
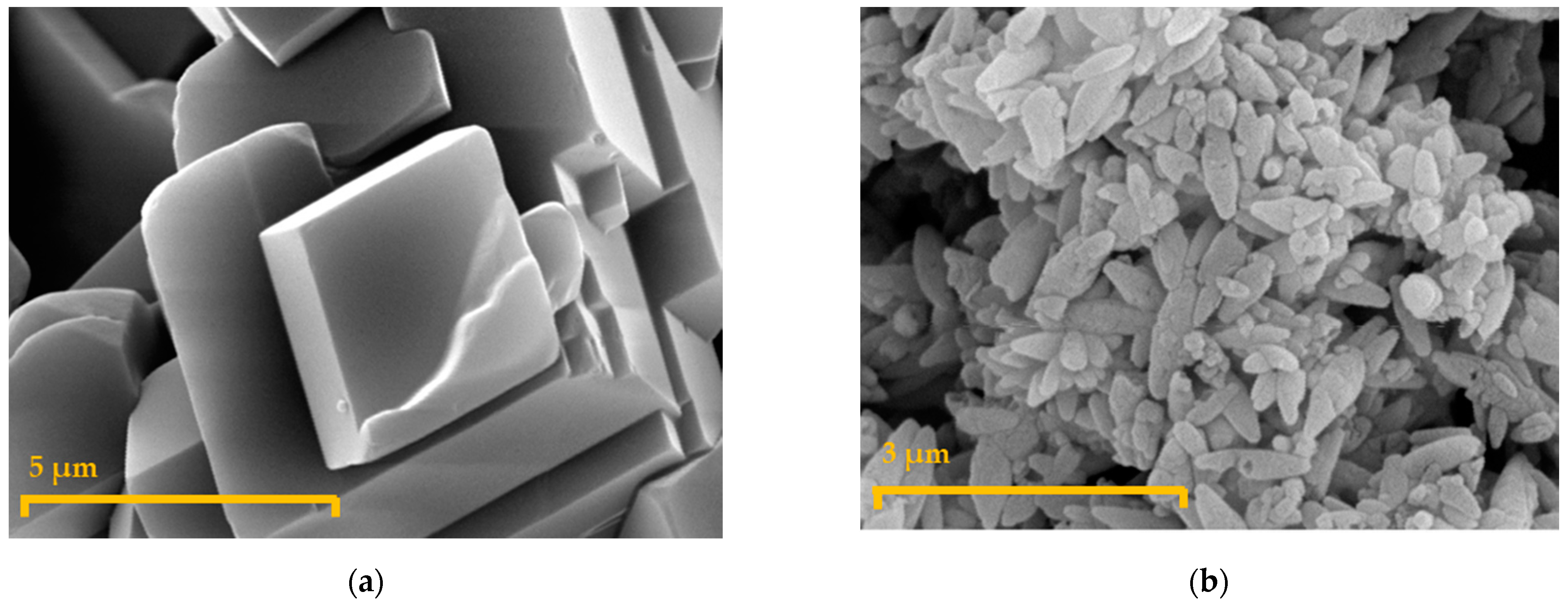
2.3. Carbonate Characterization
2.4. Biomass Characterization
2.5. Sample Analysis
2.6. Mathematical Procedures
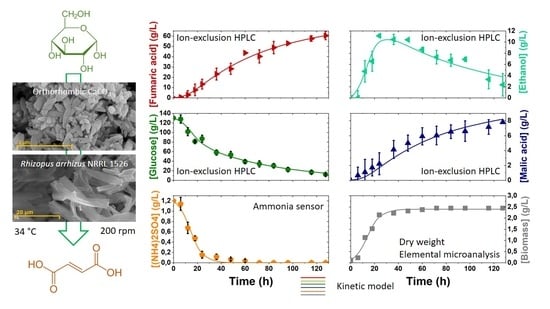
3. Results and Discussion
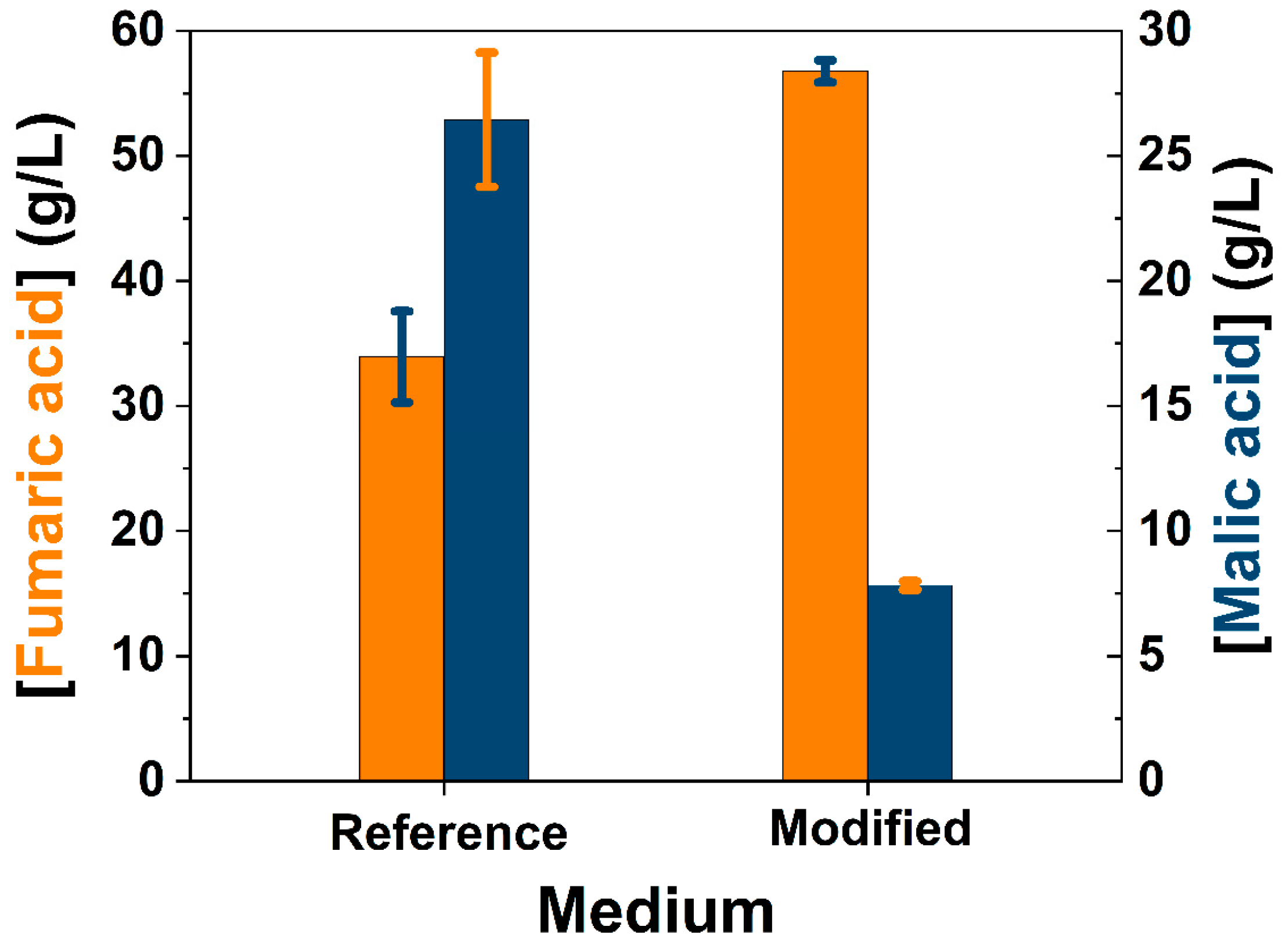
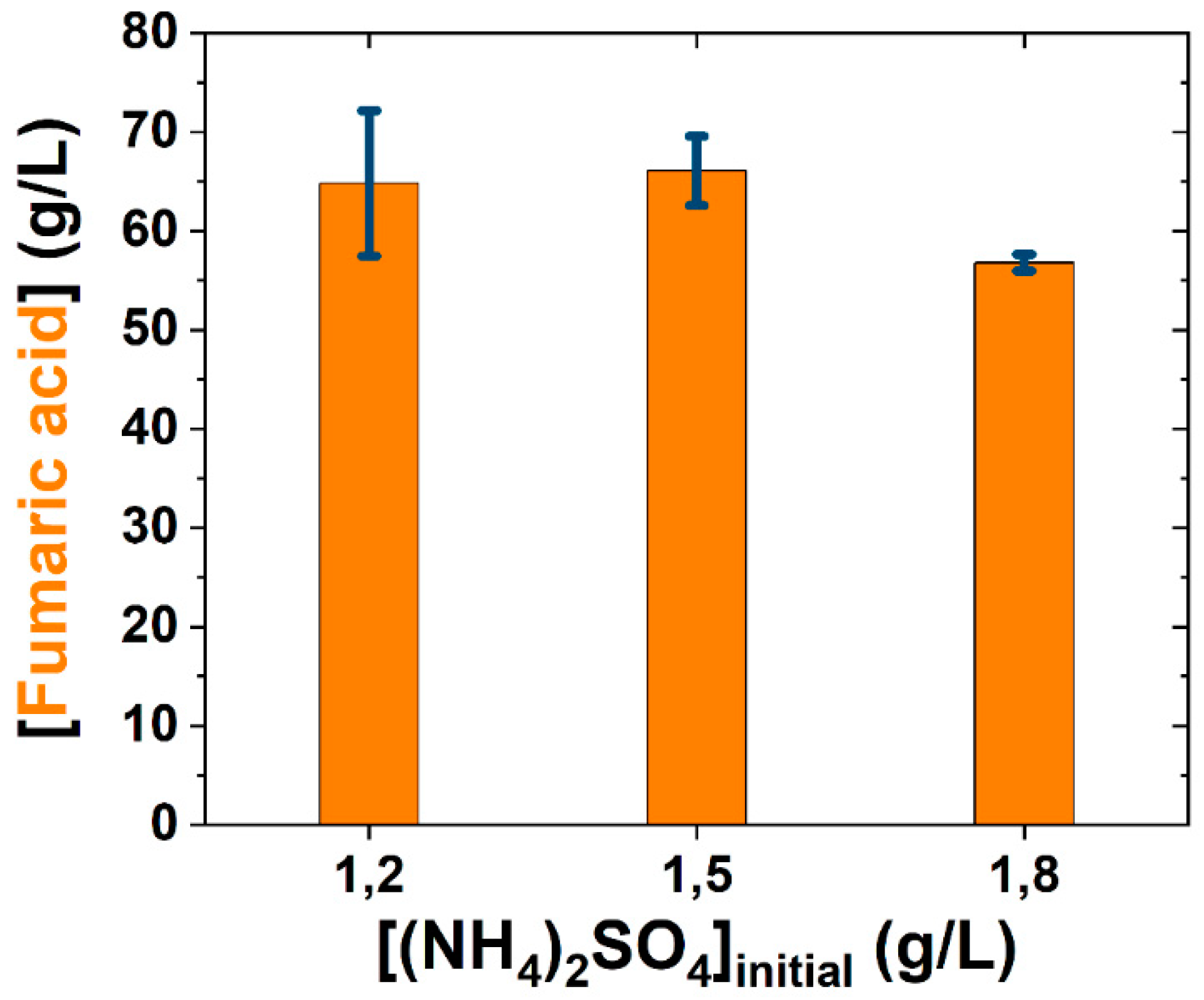
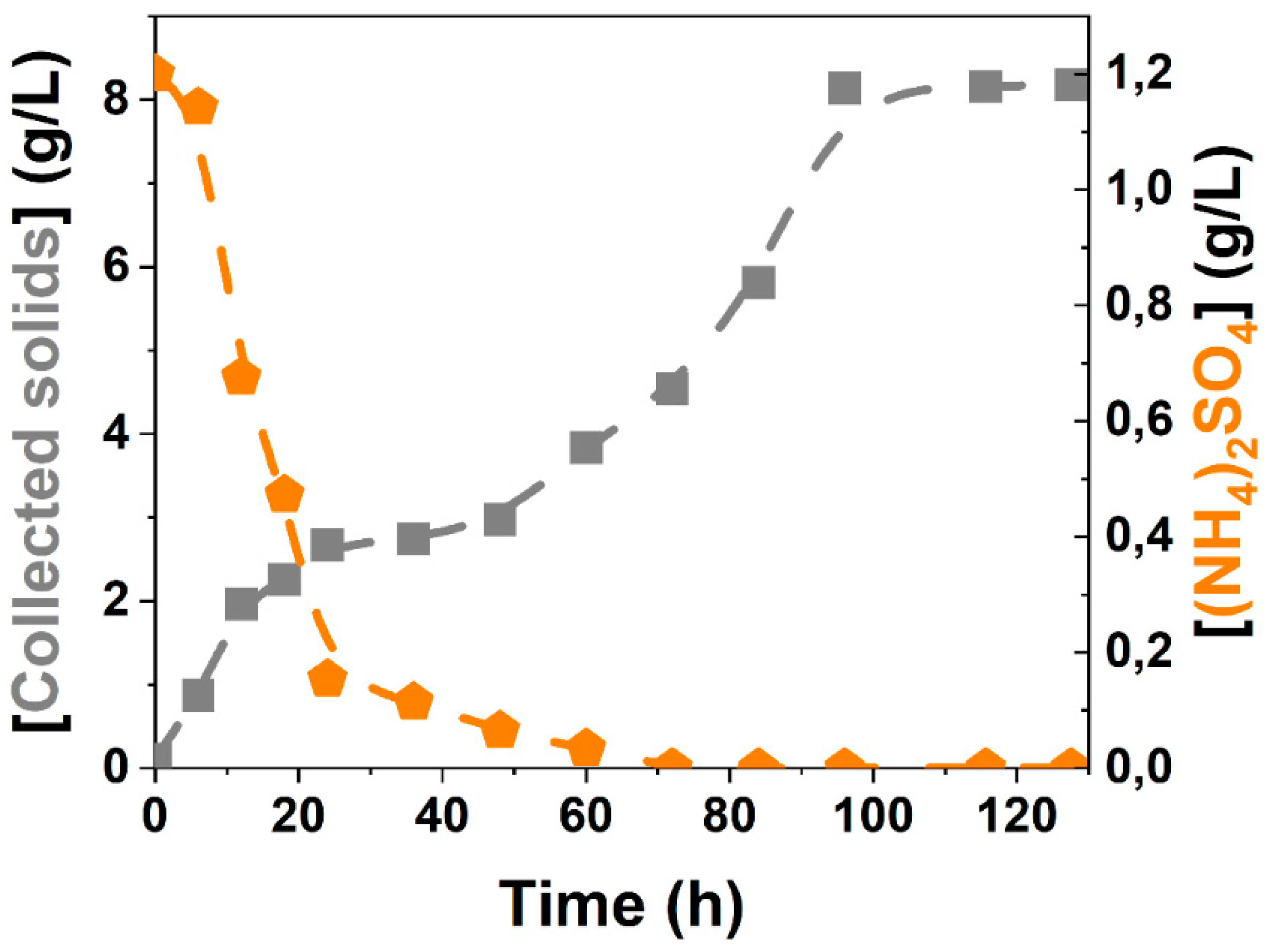
3.1. Medium Simplification
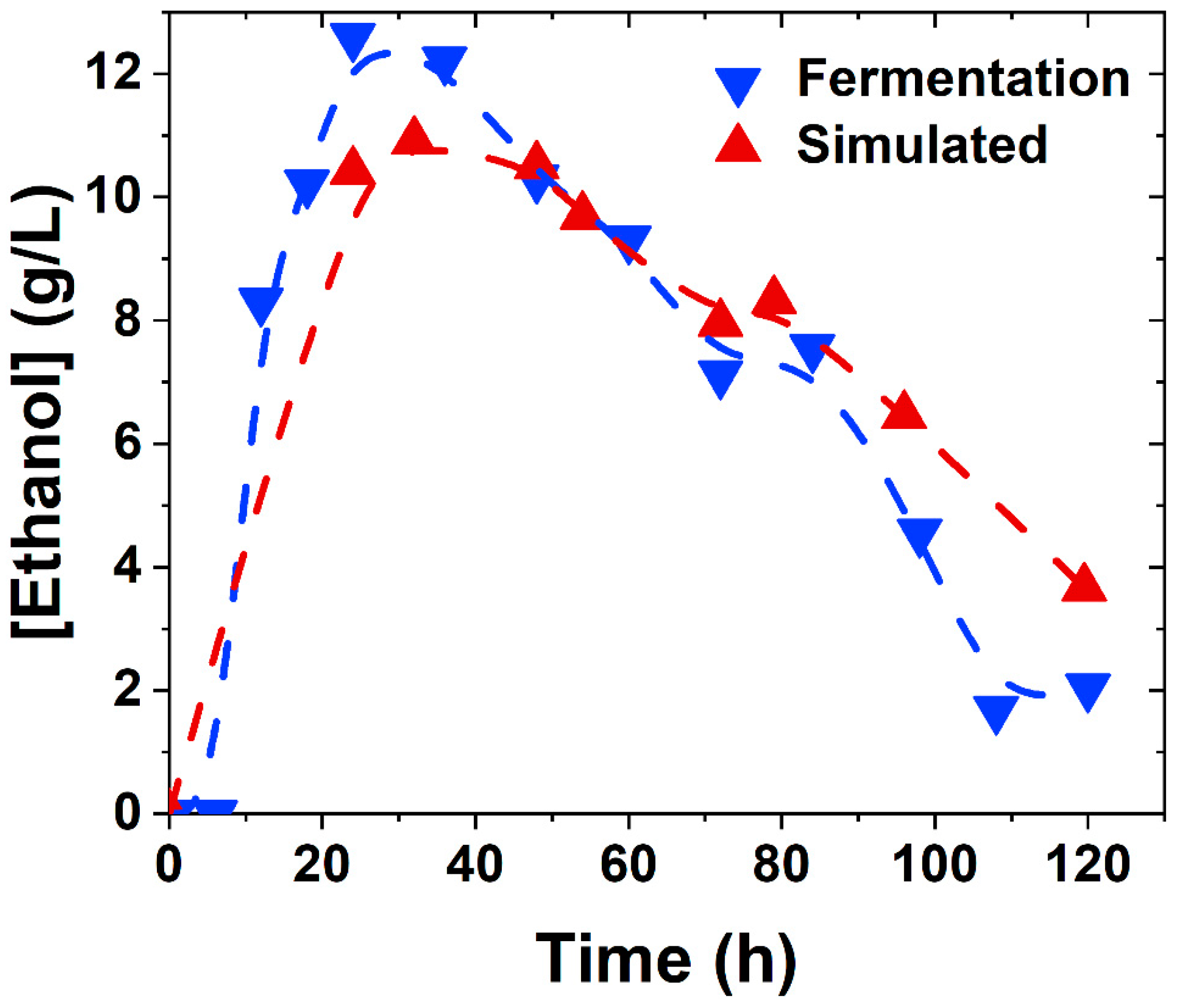
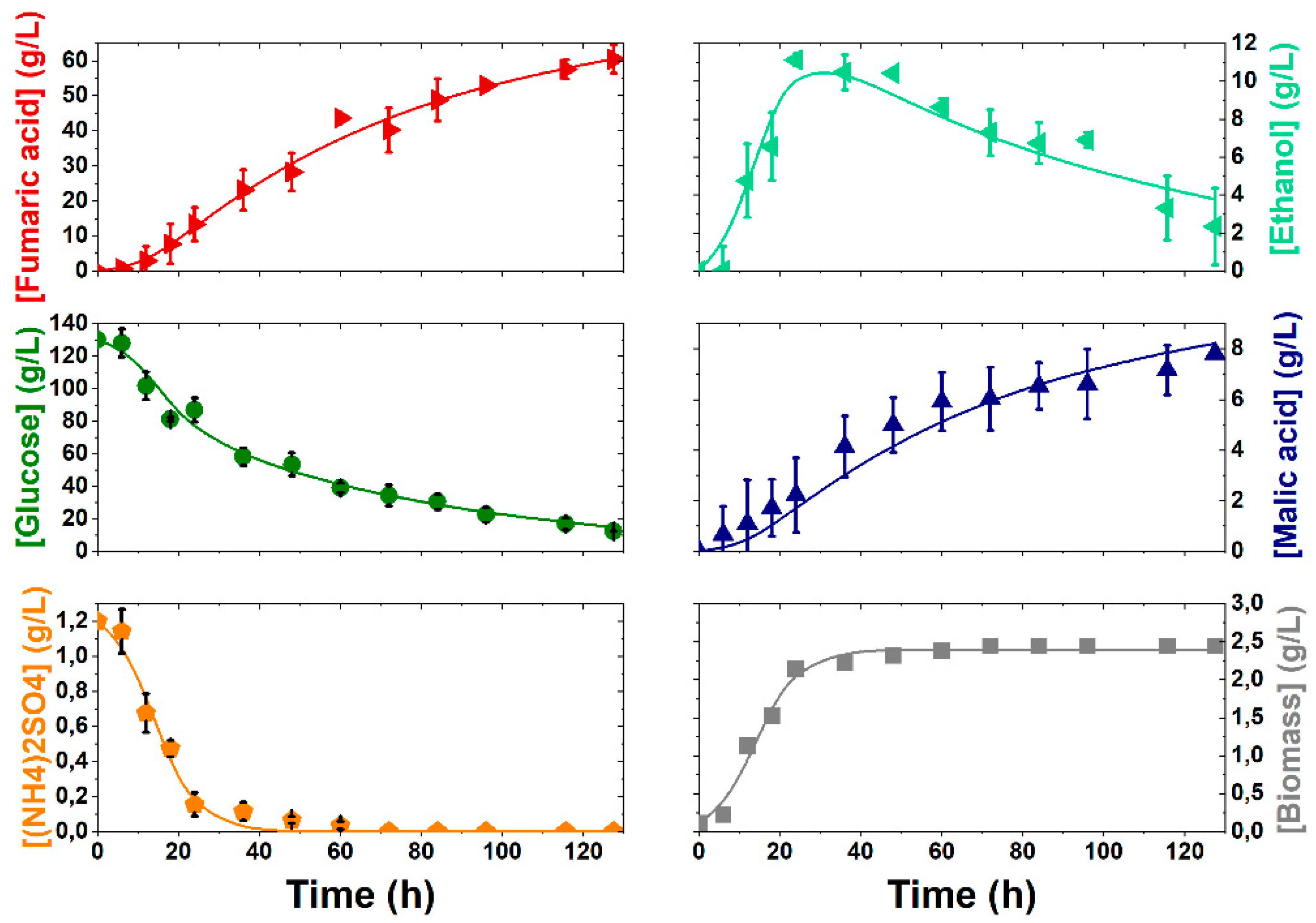
3.2. Kinetic Modelling
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Components | |
| CSL | Corn Steep Liquor |
| E | Ethanol |
| F | Fumaric acid |
| G | Glucose |
| M | Malic acid |
| N | Ammonium sulphate |
| PDA | Potato Dextrose Agar medium |
| X | Biomass |
| Nomenclature | |
| %VE | Percentage of variation explained |
| DOE | U. S. Departement Of Energy |
| dp | Particle diameter |
| F95 | Parameter of F-test at 95% confident |
| kE | Ethanol evaporation parameter |
| kF | Fumaric acid production kinetic parameter |
| RMSE | Root Main Square Error |
| RE | Ethanol evolution rate |
| RF | Fumaric acid production rate |
| RG | Glucose consumption rate |
| RM | Malic acid production rate |
| RN | Ammonium sulphate consumption rate |
| RX | Biomass production rate |
| TCA | Tri-Carboxylic Acid cycle |
| yCO2F | Carbon dioxide-fumaric acid stoichiometric yield |
| yEX | Ethanol-biomass stoichiometric yield |
| YEX | Ethanol-biomass yield |
| yGF | Glucose-fumaric acid stoichiometric yield |
| YGF | Glucose- fumaric acid yield |
| yGX | Glucose-biomass stoichiometric yield |
| YGX | Glucose-biomass yield |
| yMF | Malic acid-fumaric acid stoichiometric yield |
| YMF | Malic acid-fumaric acid yield |
| YNX | Ammonium sulphate-biomass yield |
| yNX | Ammonium sulphate-biomass stoichiometric yield |
| μm | Biomass specific growth rate |
References
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas Energy Efficiency and Renewable Energy; PNLL: Richland, WA, USA, 1992. [Google Scholar]
- Jiménez-Quero, A.; Pollet, E.; Zhao, M.; Marchioni, E.; Avérous, L.; Phalip, V. Itaconic and fumaric acid production from biomass hydrolysates by Aspergillus strains. J. Microbiol. Biotechnol. 2016, 26, 1557–1565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esteban, J.; Ladero, M. Food waste as a source of value-added chemicals and materials: A biorefinery perspective. Int. J. Food Sci. Technol. 2018, 53, 1095–1108. [Google Scholar] [CrossRef]
- Martin-Dominguez, V.; Estevez, J.; De Borja Ojembarrena, F.; Santos, V.E.; Ladero, M. Fumaric acid production: A biorefinery perspective. Fermentation 2018, 4, 33. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Yu, C.; Yang, S.T. Effects of soybean meal hydrolysate as the nitrogen source on seed culture morphology and fumaric acid production by Rhizopus oryzae. Process. Biochem. 2015, 50, 173–179. [Google Scholar] [CrossRef]
- Das, R.K.; Brar, S.K. Enhanced fumaric acid production from brewery wastewater and insight into the morphology of Rhizopus oryzae 1526. Appl. Biochem. Biotechnol. 2014, 172, 2974–2988. [Google Scholar] [CrossRef]
- Martin-Dominguez, V.; Bouzas-Santiso, L.; Martinez-Peinado, N.; Santos, V.E.; Ladero, M. Kinetic Modelling of the coproduction process of fumaric and malic acids by Rhizopus arrhizus NRRL 1526. Processes 2020, 8, 188. [Google Scholar] [CrossRef] [Green Version]
- Magalhães Júnior, A.I.; Soccol, C.R.; Camara, M.C.; Molina Aulestia, D.T.; de Souza Vandenberghe, L.P.; Cesar de Carvalho, J. Challenges in the production of second-generation organic acids (potential monomers for application in biopolymers). Biomass Bioenergy 2021, 149, 106092. [Google Scholar] [CrossRef]
- Das, R.K.; Brar, S.K.; Verma, M. Fumaric Acid; Elsevier Inc.: Amsterdam, The Netherlands, 2016; ISBN 9780128029800. [Google Scholar]
- Weißert, R. Multiple Sklerose-Risiken und Nutzen der neuen antiinflammatorischen Substanzen. J. Neurol. Neurochir. Psychiatr. 2014, 16, 95–101. [Google Scholar]
- Rhodes, R.A.; Moyer, A.J.; Smith, M.L.; Kelley, S.E. Production of fumaric acid by Rhizopus arrhizus. Appl. Microbiol. 1959, 7, 74–80. [Google Scholar] [CrossRef]
- Sebastian, J.; Dominguez, K.V.; Brar, S.K.; Rouissi, T. Fumaric acid production using alternate fermentation mode by immobilized Rhizopus oryzae-a greener production strategy. Chemosphere 2021, 281, 130858. [Google Scholar] [CrossRef]
- De la Torre, I.; Martin-Dominguez, V.; Acedos, M.G.; Esteban, J.; Santos, V.E.; Ladero, M. Utilisation/upgrading of orange peel waste from a biological biorefinery perspective. Appl. Microbiol. Biotechnol. 2019, 103, 5975–5991. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.; Moideen, S.K.; Mathew, A.K.; Sr, A.R.; Sindhu, R.; Pandey, A.; Sang, B.-I.; Sukumaran, R.K. Fumaric acid production from sugarcane trash hydrolysate using Rhizopus oryzae NIIST 1. Indian J. Exp. Biol. 2020, 58, 548–556. [Google Scholar]
- Papadaki, A.; Papapostolou, H.; Alexandri, M.; Kopsahelis, N.; Papanikolaou, S.; de Castro, A.M.; Freire, D.M.G.; Koutinas, A.A. Fumaric acid production using renewable resources from biodiesel and cane sugar production processes. Environ. Sci. Pollut. Res. 2018, 25, 35960–35970. [Google Scholar] [CrossRef] [PubMed]
- Wakai, S.; Arazoe, T.; Ogino, C.; Kondo, A.; Song, P.; Li, S.; Ding, Y.; Xu, Q.; Huang, H.; Peleg, Y.; et al. Future insights in fungal metabolic engineering. Appl. Microbiol. Biotechnol. 2017, 115, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, A.; Androutsopoulos, N.; Patsalou, M.; Koutinas, M.; Kopsahelis, N.; de Castro, A.M.; Papanikolaou, S.; Koutinas, A.A. Biotechnological production of fumaric acid: The effect of morphology of Rhizopus arrhizus NRRL 2582. Fermentation 2017, 3, 33. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.; Huang, D.; Wen, J.; Li, S.; Chen, Y.; Jia, X. Metabolic profiling of a Rhizopus oryzae fumaric acid production mutant generated by femtosecond laser irradiation. Bioresour. Technol. 2012, 114, 610–615. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, Q.; Lv, C.; Yan, C.; Li, S.; Jiang, L.; Huang, H.; Ouyang, P. Study of metabolic profile of Rhizopus oryzae to enhance fumaric acid production under low pH condition. Appl. Biochem. Biotechnol. 2015, 177, 1508–1519. [Google Scholar] [CrossRef]
- Naude, A.; Nicol, W. Improved continuous fumaric acid production with immobilised Rhizopus oryzae by implementation of a revised nitrogen control strategy. N. Biotechnol. 2018, 44, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Zhao, S.; Jin, Y.; Yue, X.; Deng, L.; Wang, F.; Tan, T. Production of fumaric acid by immobilized Rhizopus arrhizus RH 7-13-9# on loofah fiber in a stirred-tank reactor. Bioresour. Technol. 2017, 244, 929–933. [Google Scholar] [CrossRef]
- Troiano, D.; Orsat, V.; Dumont, M.J. Status of filamentous fungi in integrated biorefineries. Renew. Sustain. Energy Rev. 2020, 117, 109472. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Lennartsson, P.R.; Edebo, L.; Taherzadeh, M.J. Zygomycetes-based biorefinery: Present status and future prospects. Bioresour. Technol. 2013, 135, 523–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, X.; Liu, H.; Liu, J.; Wang, C.; Wen, J. Omics-based approaches reveal phospholipids remodeling of Rhizopus oryzae responding to furfural stress for fumaric acid-production from xylose. Bioresour. Technol. 2016, 222, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Roa Engel, C.A.; Van Gulik, W.M.; Marang, L.; van der Wielen, L.A.M.; Straathof, A.J.J. Development of a low pH fermentation strategy for fumaric acid production by Rhizopus oryzae. Enzyme Microb. Technol. 2011, 48, 39–47. [Google Scholar] [CrossRef]
- Eidt, L. Nutzung Nachwachsender Rohstoffe für die Biotechnologische Produktion von Fumarsäure. Ph.D. Thesis, Technical University of Braunschweig, Braunschweig, Germany, 2021. [Google Scholar]
- Fu, Y.; Xu, Q.; Li, S.; Huang, H.; Chen, Y. A novel multi-stage preculture strategy of Rhizopus oryzae ME-F12 for fumaric acid production in a stirred-tank reactor. World J. Microbiol. Biotechnol. 2009, 25, 1871–1876. [Google Scholar] [CrossRef]
- Ling, L.; Ng, T. Fermentation Process for Carboxylic Acids. U.S. Patent 4,877,731, 31 October 1989. [Google Scholar]
- Naude, A.; Nicol, W. Fumaric acid fermentation with immobilised Rhizopus oryzae: Quantifying time-dependent variations in catabolic flux. Process Biochem. 2017, 56, 8–20. [Google Scholar] [CrossRef]
- Yang, P.B.; Tian, Y.; Wang, Q.; Cong, W. Effect of different types of calcium carbonate on the lactic acid fermentation performance of Lactobacillus lactis. Biochem. Eng. J. 2015, 98, 38–46. [Google Scholar] [CrossRef]
- Lopez-Exposito, P.; Negro, C.; Blanco, A. Direct estimation of microalgal flocs fractal dimension through laser reflectance and machine learning. Algal Res. 2019, 37, 240–247. [Google Scholar] [CrossRef]
- López Expósito, P.; Blanco Suárez, A.; Negro Álvarez, C. Laser reflectance measurement for the online monitoring of Chlorella sorokiniana biomass concentration. J. Biotechnol. 2017, 243, 10–15. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Esteban, J.; Ladero, M.; García-Ochoa, F. Kinetic modelling of the solventless synthesis of solketal with a sulphonic ion exchange resin. Chem. Eng. J. 2015, 269, 194–202. [Google Scholar] [CrossRef]
- De la Torre, I.; Ladero, M.; Santos, V.E. Production of d-lactic acid by Lactobacillus delbrueckii ssp. delbrueckii from orange peel waste: Techno-economical assessment of nitrogen sources. Appl. Microbiol. Biotechnol. 2018, 102, 10511–10521. [Google Scholar] [CrossRef] [PubMed]
- Esteban, J.; Fuente, E.; Blanco, A.; Ladero, M.; Garcia-Ochoa, F. Phenomenological kinetic model of the synthesis of glycerol carbonate assisted by focused beam reflectance measurements. Chem. Eng. J. 2015, 260, 434–443. [Google Scholar] [CrossRef]
- Xu, G.; Chen, X.; Liu, L.; Jiang, L. Fumaric acid production in Saccharomyces cerevisiae by simultaneous use of oxidative and reductive routes. Bioresour. Technol. 2013, 148, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Kenealy, W.; Zaady, E.; du Preez, J.C. Biochemical aspects of fumaric acid accumulation by Rhizopus arrhizus. Appl. Environ. Microbiol. 1986, 52, 128–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.; Zhang, B.; Yang, S.-T. Production of citric, itaconic, fumaric, and malic acids in filamentous fungal fermentations. In Bioprocessing Technologies in Biorefinery for Sustainable Production of Fuels, Chemicals, and Polymers; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 375–398. [Google Scholar] [CrossRef]
- Riscaldati, E.; Moresi, M.; Federici, F.; Petruccioli, M. Direct ammonium fumarate production by Rhizopus arrhizus under phosphorous limitation. Biotechnol. Lett. 2000, 22, 1043–1047. [Google Scholar] [CrossRef]
- Kang, S.W.; Lee, H.; Kim, D.; Lee, D.; Kim, S.; Chun, G.T.; Lee, J.; Kim, S.W.; Park, C. Strain development and medium optimization for fumaric acid production. Biotechnol. Bioprocess Eng. 2010, 15, 761–769. [Google Scholar] [CrossRef]
- El-Enshasy, H.A. Filamentous fungal cultures-process characteristics, products, and applications. In Bioprocessing for Value-Added Products from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2007; pp. 225–261. [Google Scholar] [CrossRef]
- Fu, Y.Q.; Li, S.; Chen, Y.; Xu, Q.; Huang, H.; Sheng, X.Y. Enhancement of fumaric acid production by Rhizopus oryzae using a two-stage dissolved oxygen control strategy. Appl. Biochem. Biotechnol. 2010, 162, 1031–1038. [Google Scholar] [CrossRef]



| Component | Inoculum Medium (g/L) [7] | Production Medium (g/L) [28] |
|---|---|---|
| Glucose | 40 | 130 |
| (NH4)2SO4 | 4 | 1.8 1 |
| MgSO4 × 7 H2O | 0.4 | 0.4 |
| ZnSO4 × 7 H2O | 0.044 | 0.44 |
| KH2PO4 | 1.6 | 0.3 |
| FeCl3 × 6 H2O | 0.0075 | 0.0075 |
| Tartaric acid | - | 0.0075 2 |
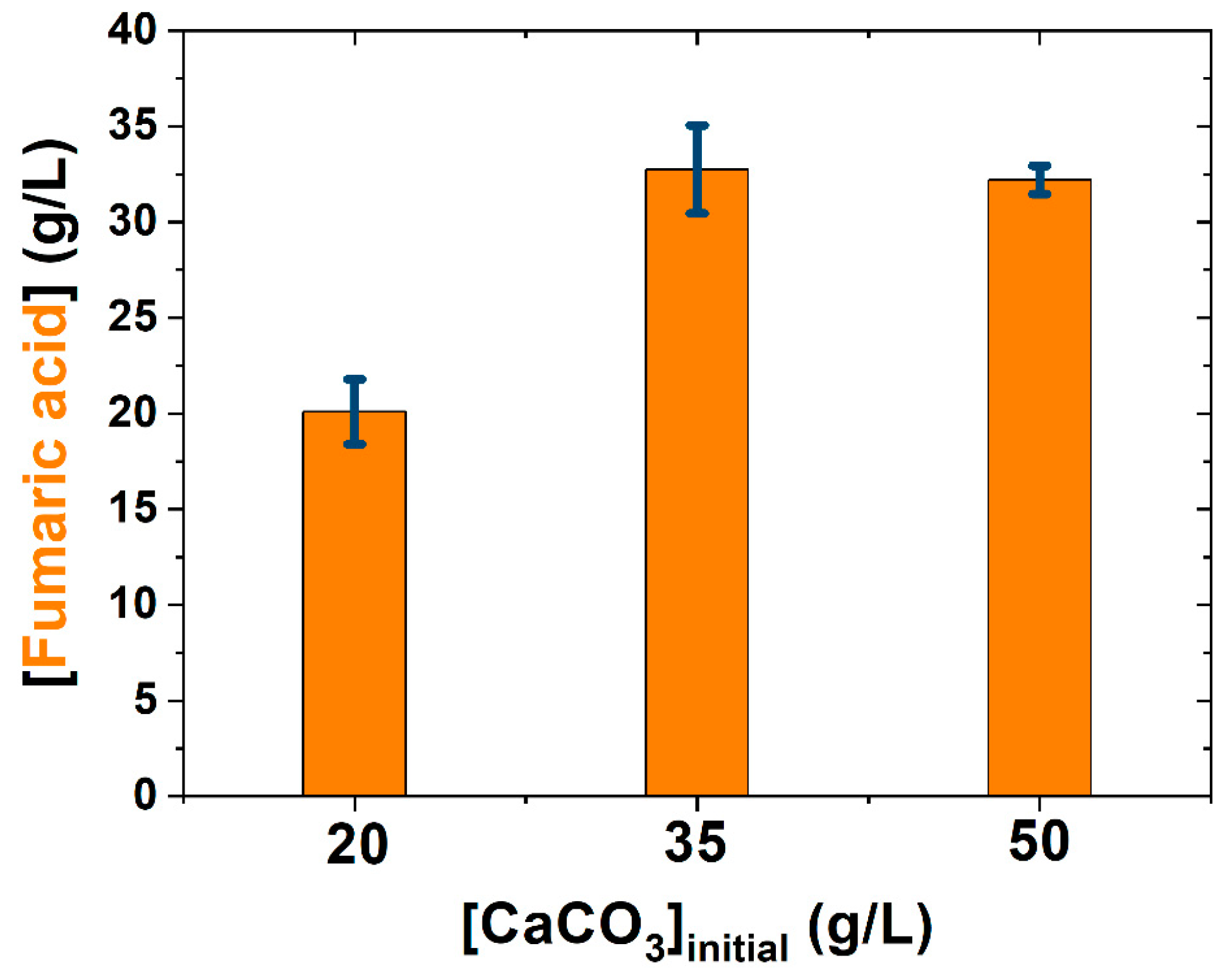
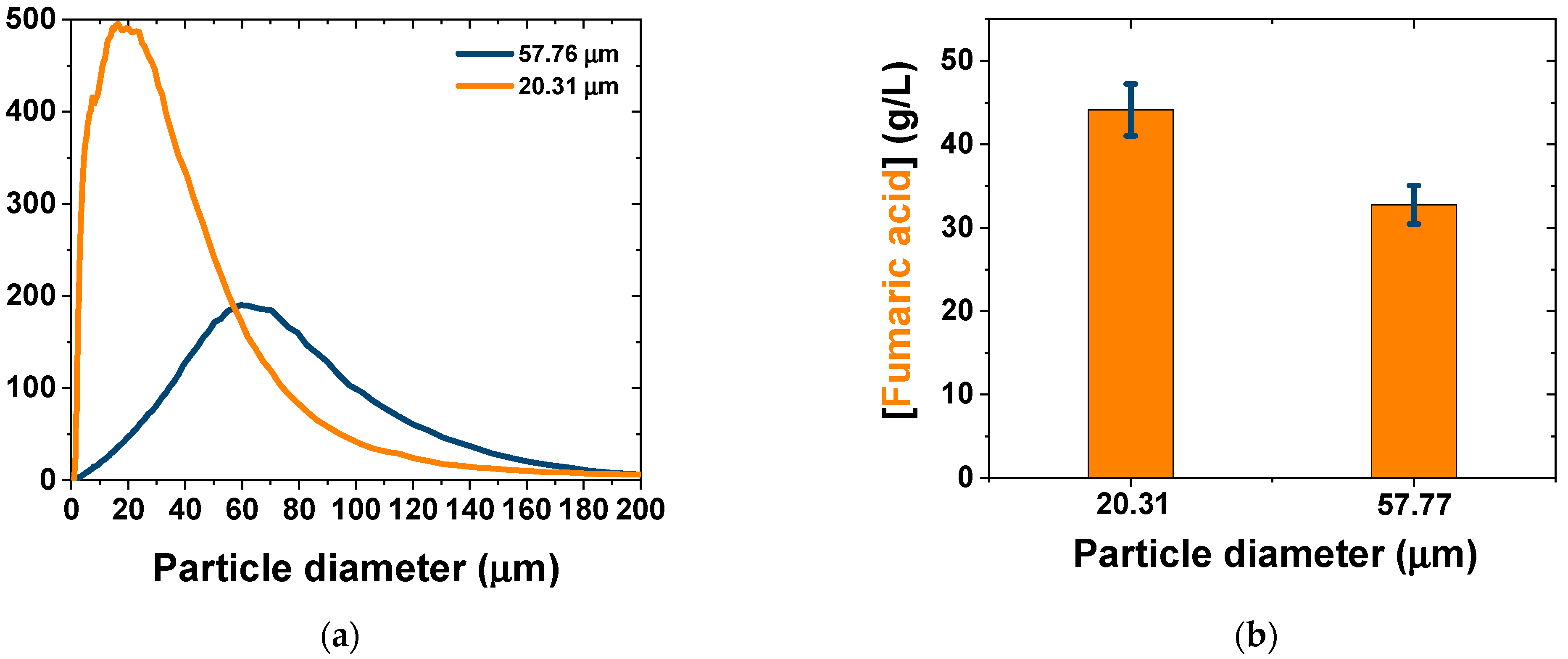
| CaCO3 | - | 50 1 |
| Corn steep liquor (CSL) | 0.5 mL/L | 0.5 mL/L 2 |
| Reference | N Source | Yield (gFumaric acid/gconsumed glucose) | Process Conditions | pH Control | Inoculum | Operation Time (h) |
|---|---|---|---|---|---|---|
| [7] | (NH4)2SO4: 1.8 g/L | 0.26 | [Glucose]0 = 130 g/L 200 rpm; 34 °C; pH ≈ 5.5 | CaCO3: 50 g/L (dp = 57.77 μm) | 12 h, pH free, 106 spores/mL | 120 |
| [40] | (NH4)2SO4: 0.8 g/L | 0.33 | [Glucose]0 = 130 g/L 220 rpm; 32 °C; pH ≈ 5.5 | (NH4)2SO4: 2 M (dp: not specified) | 8–10 h, pH controlled, 107 spores/mL | 120 |
| [41] | CSL: 0.5% | 0.43 | [Glucose]0 = 100 g/L 250 rpm; 35 °C; pH ≈ 6.5 | CaCO3: 2% w/w (dp: not specified) | 12 h, pH controlled, 107 spores/mL | 120 |
| Present study | (NH4)2SO4: 1.2 g/L | 0.46 | [Glucose]0 = 130 g/L 200 rpm; 34 °C, pH ≈ 5.5 | CaCO3: 35 g/L (dp = 20.31 μm) | 12 h, pH free, 106 spores/mL | 120 |
| t (h) | C % (±0.35%) | H % (±0.30%) | N % (±0.30%) | S % (±0.35%) | O % 1 | C:N |
|---|---|---|---|---|---|---|
| 6 | 43.04 | 6.47 | 10.89 | 0.54 | 39.06 | 3.95 |
| 12 | 44.23 | 6.66 | 9.77 | 0.50 | 38.84 | 4.53 |
| 18 | 45.05 | 6.77 | 8.85 | 0.46 | 38.87 | 5.09 |
| 24 | 44.37 | 6.77 | 8.17 | 0.40 | 40.29 | 5.43 |
| 36 | 45.27 | 6.86 | 7.31 | 0.34 | 40.22 | 6.19 |
| 48 | 44.94 | 6.45 | 5.57 | 0.25 | 42.79 | 8.07 |
| 60 | 47.96 | 7.04 | 6.09 | 0.28 | 38.63 | 7.87 |
| 72 | 46.71 | 6.42 | 4.55 | 0.22 | 42.10 | 10.27 |
| 84 | 46.68 | 6.32 | 4.49 | 0.20 | 42.31 | 10.39 |
| 96 | 45.38 | 5.74 | 3.30 | 0.15 | 45.43 | 13.75 |
| 108 | 44.96 | 5.57 | 2.95 | 0.13 | 46.39 | 15.24 |
| 120 | 44.89 | 5.37 | 2.39 | 0.12 | 47.23 | 18.78 |
| Parameter | Units | Value |
|---|---|---|
| µm | 1.69∙10−1 ± 3.04∙10−3 | |
| kF | 5.45∙10−3 ± 2.07∙10−4 | |
| YMF | 1.36∙10−1 ± 2.81∙10−2 | |
| YEX | 5.87 ± 9.87∙10−1 | |
| kE | h−1 | 1.13∙10−2 ± 3.9210−3 |
| YNX | 5.26∙10−1 ± 5.65∙10−3 | |
| YGX | 19.75 ± 1.59 | |
| YGF | 1.16 ± 9.19∙10−2 |
| Parameter | Value |
|---|---|
| Degrees of freedom | 82 |
| RMSE | 3.48 |
| F95 | 823.74 |
| %VE | 98.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin-Dominguez, V.; Cabrera, P.I.A.; Eidt, L.; Pruesse, U.; Kuenz, A.; Ladero, M.; Santos, V.E. Production of Fumaric Acid by Rhizopus arrhizus NRRL 1526: A Simple Production Medium and the Kinetic Modelling of the Bioprocess. Fermentation 2022, 8, 64. https://doi.org/10.3390/fermentation8020064
Martin-Dominguez V, Cabrera PIA, Eidt L, Pruesse U, Kuenz A, Ladero M, Santos VE. Production of Fumaric Acid by Rhizopus arrhizus NRRL 1526: A Simple Production Medium and the Kinetic Modelling of the Bioprocess. Fermentation. 2022; 8(2):64. https://doi.org/10.3390/fermentation8020064
Chicago/Turabian StyleMartin-Dominguez, Victor, Paula I. Aleman Cabrera, Laslo Eidt, Ulf Pruesse, Anja Kuenz, Miguel Ladero, and Victoria E. Santos. 2022. "Production of Fumaric Acid by Rhizopus arrhizus NRRL 1526: A Simple Production Medium and the Kinetic Modelling of the Bioprocess" Fermentation 8, no. 2: 64. https://doi.org/10.3390/fermentation8020064
APA StyleMartin-Dominguez, V., Cabrera, P. I. A., Eidt, L., Pruesse, U., Kuenz, A., Ladero, M., & Santos, V. E. (2022). Production of Fumaric Acid by Rhizopus arrhizus NRRL 1526: A Simple Production Medium and the Kinetic Modelling of the Bioprocess. Fermentation, 8(2), 64. https://doi.org/10.3390/fermentation8020064