Fermentation of Dairy-Relevant Sugars by Saccharomyces, Kluyveromyces, and Brettanomyces: An Exploratory Study with Implications for the Utilization of Acid Whey, Part II
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fermentation Media
2.3. Yeast Cultures
2.4. Fermentation Setup
2.5. Data Collection
2.5.1. Density, pH, and Microbial Enumeration
2.5.2. Analyses of Sugars, Organic Acids, and Ethanol
2.6. Statistical Analysis
2.6.1. General Analyses
2.6.2. Nonlinear Density Modeling
3. Results and Discussion
3.1. Fermentation Characterization
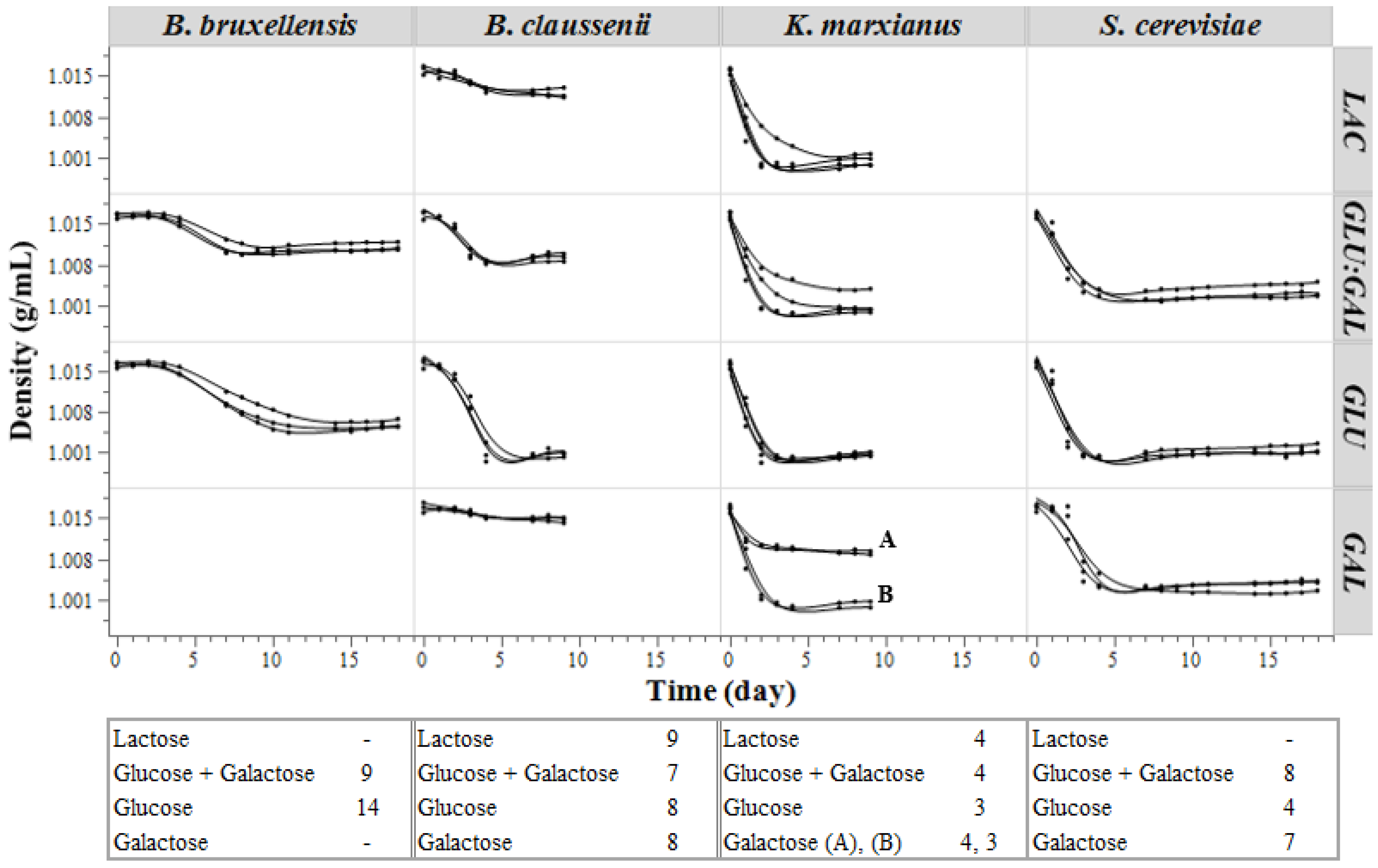
3.1.1. Density
Nonlinear Density Modeling
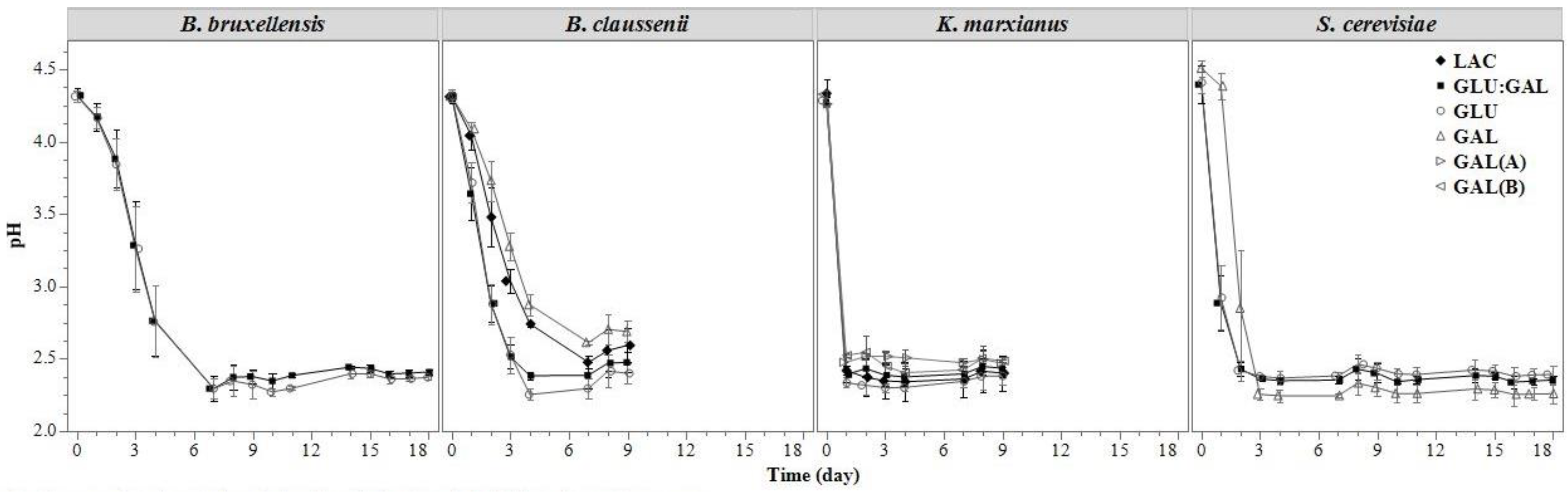
3.1.2. pH
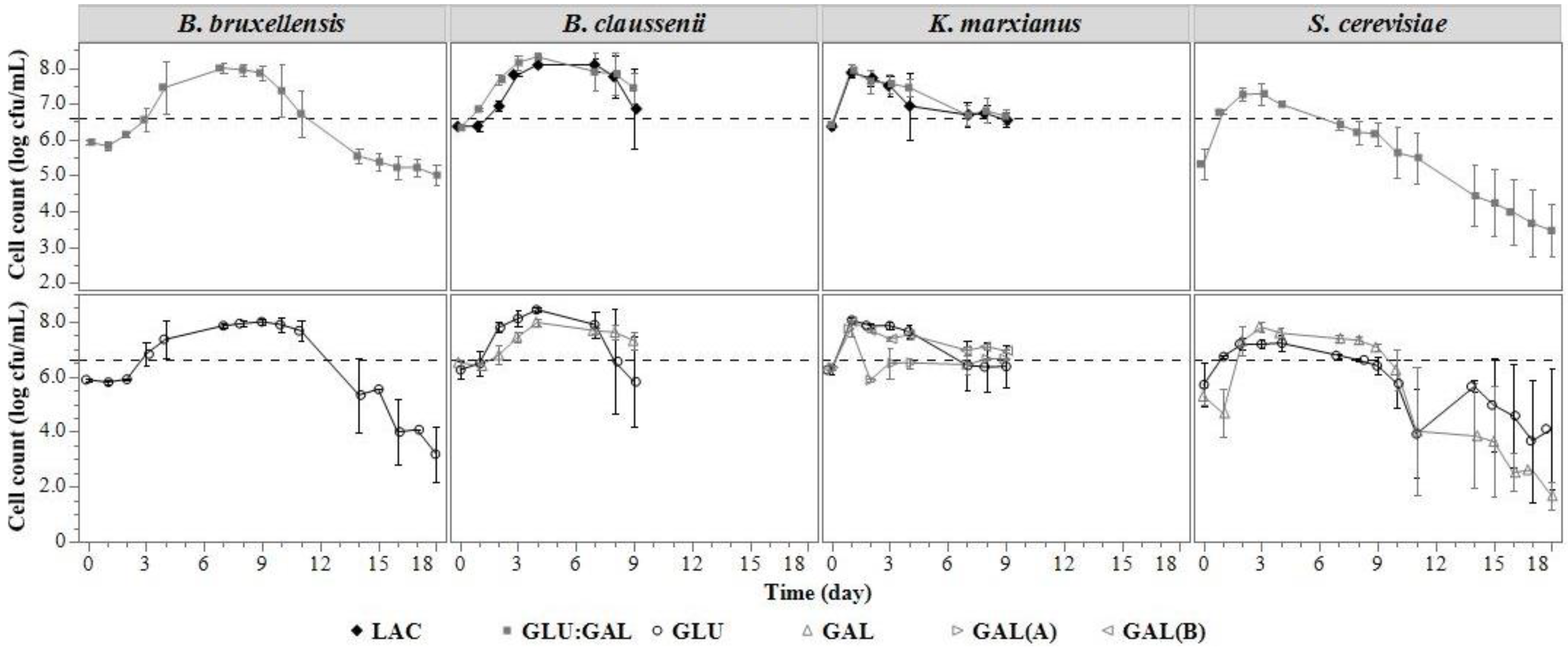
3.1.3. Microbial Concentration
3.2. Sugar Concentration
3.3. Production of Ethanol and Organic Acids
3.3.1. Ethanol
3.3.2. Organic Acids
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erickson, B. Acid Whey: Is the Waste Product an Untapped Goldmine? Available online: https://cen.acs.org/articles/95/i6/Acid-whey-waste-product-untapped.html (accessed on 18 March 2022).
- Statista. Statista Dossier on Greek Yogurt in the U.S.; Statista: Hamburg, Germany, 2021; Available online: https://www-statista-com.proxy.library.cornell.edu/study/25622/greek-yogurt-statista-dossier/ (accessed on 18 March 2022).
- Shahbandeh, M. Annual Domestic Consumption of Yogurt in the United States from 2000 to 2020 (in Million Pounds)*; Statista: Hamburg, Germany, 2021; Available online: https://www.statista.com/statistics/1066974/us-yogurt-consumption/ (accessed on 18 March 2022).
- Jelen, P. WHEY PROCESSING|Utilization and Products. In Encyclopedia of Dairy Sciences, 2nd ed.; Fuquay, J.W., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 731–737. [Google Scholar]
- Ketterings, Q.; Czymmek, K.; Gami, S.; Godwin, G.; Ganoe, K. Guidelines for land application of acid whey. Dep. Anim. Sci. Publ. Ser. 2017, 247, 1–18. [Google Scholar]
- Rocha-Mendoza, D.; Kosmerl, E.; Krentz, A.; Zhang, L.; Badiger, S.; Miyagusuku-Cruzado, G.; Mayta-Apaza, A.; Giusti, M.; Jiménez-Flores, R.; García-Cano, I. Invited review: Acid whey trends and health benefits. J. Dairy Sci. 2021, 104, 1262–1275. [Google Scholar] [CrossRef] [PubMed]
- Lawton, M.R.; deRiancho, D.L.; Alcaine, S.D. Lactose utilization by Brettanomyces claussenii expands potential for valorization of dairy by-products to functional beverages through fermentation. Curr. Opin. Food Sci. 2021, 42, 93–101. [Google Scholar] [CrossRef]
- Rivera Flores, V.K.; DeMarsh, T.A.; Gibney, P.A.; Alcaine, S.D. Fermentation of dairy-relevant sugars by Saccharomyces, Kluyveromyces, and Brettanomyces: An exploratory study with implications for the utilization of acid whey, Part I. Fermentation 2021, 7, 266. [Google Scholar] [CrossRef]
- Menchik, P.; Zuber, T.; Zuber, A.; Moraru, C.I. Short communication: Composition of coproduct streams from dairy processing: Acid whey and milk permeate. J. Dairy Sci. 2019, 102, 3978–3984. [Google Scholar] [CrossRef]
- Comerford, K.B.; Miller, G.D.; Boileau, A.C.; Masiello Schuette, S.N.; Giddens, J.C.; Brown, K.A. Global Review of Dairy Recommendations in Food-Based Dietary Guidelines. Front. Nutr. 2021, 8, 671999. [Google Scholar] [CrossRef]
- Harvard Medical School. Listing of Vitamins. Available online: https://www.health.harvard.edu/staying-healthy/listing_of_vitamins (accessed on 25 October 2021).
- Ferruzzi, M.G.; Tanprasertsuk, J.; Kris-Etherton, P.; Weaver, C.M.; Johnson, E.J. Perspective: The Role of Beverages as a Source of Nutrients and Phytonutrients. Adv. Nutr. 2020, 11, 507–523. [Google Scholar] [CrossRef]
- Fior Markets. Global Functional Beverages Market Is Expected to Reach USD 216.7 Billion by 2028: Fior Markets. Available online: https://www.globenewswire.com/en/news-release/2021/05/24/2234940/0/en/Global-Functional-Beverages-Market-Is-Expected-to-Reach-USD-216-7-billion-by-2028-Fior-Markets.html (accessed on 13 August 2021).
- Istrati, D.I.; Pricop, E.M.; Profir, A.G.; Vizireanu, C. Fermented Functional Beverages. In Functional Foods; Lagouri, V., Ed.; IntechOpen: London, UK, 2019. [Google Scholar]
- Valdes, D.S.; So, D.; Gill, P.A.; Kellow, N.J. Effect of Dietary Acetic Acid Supplementation on Plasma Glucose, Lipid Profiles, and Body Mass Index in Human Adults: A Systematic Review and Meta-analysis. J. Acad. Nutr. Diet 2021, 121, 895–914. [Google Scholar] [CrossRef]
- Marcus, J.F.; DeMarsh, T.A.; Alcaine, S.D. Upcycling of Whey Permeate through Yeast- and Mold-Driven Fermentations under Anoxic and Oxic Conditions. Fermentation 2021, 7, 16. [Google Scholar] [CrossRef]
- Luo, S.; DeMarsh, T.A.; deRiancho, D.; Stelick, A.; Alcaine, S.D. Characterization of the Fermentation and Sensory Profiles of Novel Yeast-Fermented Acid Whey Beverages. Foods 2021, 10, 1204. [Google Scholar] [CrossRef]
- Freer, S.N. Acetic acid production by Dekkera/Brettanomyces yeasts. World J. Microbiol. Biotechnol. 2002, 18, 271–275. [Google Scholar] [CrossRef]
- Freer, S.N.; Dien, B.; Matsuda, S. Production of acetic acid by Dekkera/Brettanomyces yeasts under conditions of constant pH. World J. Microbiol. Biotechnol. 2003, 19, 101–105. [Google Scholar] [CrossRef]
- Buehler, A.J.; Evanowski, R.L.; Martin, N.H.; Boor, K.J.; Wiedmann, M. Internal transcribed spacer (ITS) sequencing reveals considerable fungal diversity in dairy products. J. Dairy Sci. 2017, 100, 8814–8825. [Google Scholar] [CrossRef] [PubMed]
- Painting, K.; Kirsop, B. A quick method for estimating the percentage of viable cells in a yeast population, using methylene blue staining. World J. Microbiol. Biotechnol. 1990, 6, 346–347. [Google Scholar] [CrossRef] [PubMed]
- Roman, W. Yeasts, 1st ed.; Junk: The Hague, The Netherlands, 1957. [Google Scholar]
- Michel, M.; Meier-Dörnberg, T.; Jacob, F.; Methner, F.J.; Wagner, R.S.; Hutzler, M. Review: Pure non-Saccharomyces starter cultures for beer fermentation with a focus on secondary metabolites and practical applications. J. Inst. Brew. 2016, 122, 569–587. [Google Scholar] [CrossRef]
- Burini, J.A.; Eizaguirre, J.I.; Loviso, C.; Libkind, D. Levaduras no convencionales como herramientas de innovación y diferenciación en la producción de cerveza. Rev. Argent. Microbiol. 2021, 53, 359–377. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.; Ludovico, P.; Leão, C. Sugar Metabolism in Yeasts: An Overview of Aerobic and Anaerobic Glucose Catabolism. In Biodiversity and Ecophysiology of Yeasts; Springer: Berlin/Heidelberg, Germany, 2006; Volume 101–121. [Google Scholar] [CrossRef]
- Lukondeh, T.; Ashbolt, N.J.; Rogers, P.L. Fed-batch fermentation for production of Kluyveromyces marxianus FII 510700 cultivated on a lactose-based medium. J. Ind. Microbiol. Biotechnol. 2005, 32, 284–288. [Google Scholar] [CrossRef]
- Aguilar Uscanga, M.G.; Délia, M.L.; Strehaiano, P. Brettanomyces bruxellensis: Effect of oxygen on growth and acetic acid production. Appl. Microbiol. Biotechnol. 2003, 61, 157–162. [Google Scholar] [CrossRef]
- Pampulha, M.E.; Loureiro-Dias, M.C. Combined effect of acetic acid, pH and ethanol on intracellular pH of fermenting yeast. Appl. Microbiol. Biotechnol. 1989, 31–31, 547–550. [Google Scholar] [CrossRef]
- Rasmussen, J.E.; Schultz, E.E.; Snyder, R.E.; Jones, R.S.; Smith, C.R.J. Acetic Acid as a Causative Agent in Producing Stuck Fermentations. Am. J. Enol. Vitic. 1995, 46, 278–280. [Google Scholar]
- Taherzadeh, M.J.; Niklasson, C.; Lidén, G. Acetic acid—friend or foe in anaerobic batch conversion of glucose to ethanol by Saccharomyces cerevisiae? Chem. Eng. Sci. 1997, 52, 2653–2659. [Google Scholar] [CrossRef]
- Lawton, M. Biotechnological Approaches for Combating Food Waste in the Dairy Industry; Cornell University: Ithaca, NY, USA, 2021. [Google Scholar]
- Torres, D.P.M.; Gonçalves, M.D.P.F.; Teixeira, J.A.; Rodrigues, L.R. Galacto-Oligosaccharides: Production, Properties, Applications, and Significance as Prebiotics. Compr. Rev. Food Sci. Food Saf. 2010, 9, 438–454. [Google Scholar] [CrossRef] [PubMed]
- Levy, S.F.; Ziv, N.; Siegal, M.L. Bet hedging in yeast by heterogeneous, age-correlated expression of a stress protectant. PLoS Biol. 2012, 10, e1001325. [Google Scholar] [CrossRef] [PubMed]
- Tuite, M.F.; Serio, T.R. The prion hypothesis: From biological anomaly to basic regulatory mechanism. Nat. Rev. Mol. Cell Biol. 2010, 11, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, D.; Lancaster, A.; Brown, J.; Lindquist, S. An Evolutionarily Conserved Prion-like Element Converts Wild Fungi from Metabolic Specialists to Generalists. Cell 2014, 158, 1072–1082. [Google Scholar] [CrossRef][Green Version]
- Dawson, C.C.; Intapa, C.; Jabra-Rizk, M.A. “Persisters”: Survival at the cellular level. PLoS Pathog. 2011, 7, e1002121. [Google Scholar] [CrossRef]
- Lewis, K. Multidrug Tolerance of Biofilms and Persister Cells. In Current Topics in Microbiology and Immunology; Ahmed, R., Akira, S., Casadevall, A., Galan, J.E., Garcia-Sastre, A., Malissen, B., Rappuoli, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 107–131. [Google Scholar]
- Roberts, M.E.; Stewart, P.S. Modelling protection from antimicrobial agents in biofilms through the formation of persister cells. Microbiology 2005, 151, 75–80. [Google Scholar] [CrossRef]
- Van Heerden, J.H.; Wortel, M.T.; Bruggeman, F.J.; Heijnen, J.J.; Bollen, Y.J.M.; Planqué, R.; Hulshof, J.; O’Toole, T.G.; Wahl, S.A.; Teusink, B. Lost in Transition: Start-Up of Glycolysis Yields Subpopulations of Nongrowing Cells. Science 2014, 343, 1245114. [Google Scholar] [CrossRef]
- Gibney, P.A.; Chen, A.; Schieler, A.; Chen, J.C.; Xu, Y.; Hendrickson, D.G.; McIsaac, R.S.; Rabinowitz, J.D.; Botstein, D. A tps1Δ persister-like state in Saccharomyces cerevisiae is regulated by MKT1. PLoS ONE 2020, 15, e0233779. [Google Scholar] [CrossRef]
- Margaritis, A.; Bajpai, P. Direct Fermentation of D-Xylose to Ethanol by Kluyveromyces marxianus Strains. Appl. Environ. Microbiol. 1982, 44, 1039–1041. [Google Scholar] [CrossRef]
- Silveira, W.B.; Passos, F.J.V.; Mantovani, H.C.; Passos, F.M.L. Ethanol production from cheese whey permeate by Kluyveromyces marxianus UFV-3: A flux analysis of oxido-reductive metabolism as a function of lactose concentration and oxygen levels. Enzyme Microb. Technol. 2005, 36, 930–936. [Google Scholar] [CrossRef]
- Steensels, J.; Daenen, L.; Malcorps, P.; Derdelinckx, G.; Verachtert, H.; Verstrepen, K.J. Brettanomyces yeasts—From spoilage organisms to valuable contributors to industrial fermentations. Int. J. Food Microbiol. 2015, 206, 24–38. [Google Scholar] [CrossRef] [PubMed]
| Supplemented Medium | ||||||
|---|---|---|---|---|---|---|
| Lactose * | Glucose + Galactose | Glucose | Galactose | |||
| Species | Timepoint | Lactose (g/L) | Glucose (g/L) | Galactose (g/L) | Glucose (g/L) | Galactose (g/L) |
| B. bruxellensis | Day 0 | - | 18.96 ± 0.13 | 18.77 ± 0.22 | 38.16 ± 0.54 | - |
| Day 18 | - | ND | 18.94 ± 0.61 | 7.26 ± 0.50 | - | |
| B. claussenii | Day 0 | 34.84 ± 1.20 | 18.74 ± 0.56 | 18.57 ± 0.76 | 38.08 ± 0.87 | 37.21 ± 0.42 |
| Day 9 | 19.38 ± 4.18 | ND | 14.79 ± 0.60 | ND | 28.26 ± 1.60 | |
| K. marxianus | Day 0 | 32.76 ± 3.22 | 18.94 ± 0.14 | 18.73 ± 0.69 | 38.04 ± 0.92 | 36.94 ± 0.81 |
| Day 9 | 0.43 ± 0.75 | ND | 0.6 ± 1.04 | ND | A: 18.61 ± 0.93 B: ND | |
| S. cerevisiae | Day 0 | - | 18.55 ± 0.57 | 18.46 ± 0.69 | 37.6 ± 1.27 | 35.89 ± 1.74 |
| Day 18 | - | ND | 3.24 ± 2.21 | ND | 2.09 ± 0.86 | |
| Ethanol (% v/v) | |||||
|---|---|---|---|---|---|
| Species | Timepoint | Lactose | Glucose + Galactose | Glucose | Galactose |
| B. bruxellensis | Day 18 | - | 0.20 ± 0.02 Bc | 0.59 ± 0.05 Ac | - |
| B. claussenii | Day 9 | 0.11 ± 0.08 Bb | 0.42 ± 0.13 Bbc | 1.47 ± 0.18 Aa | ND |
| K. marxianus | Day 9 | 1.48 ± 0.32 Aa | 1.52 ± 0.19 Aa | 1.43 ± 0.09 Aa | A: 0.600 ± 0.033 Bb B: 1.576 ± 0.922 Aa |
| S. cerevisiae | Day 18 | - | 0.63 ± 0.08 Ab | 0.95 ± 0.19 Ab | 0.62 ± 0.35 Ab |
| Lactic Acid (g/L) | |||||
|---|---|---|---|---|---|
| Species | Timepoint | Lactose | Glucose + Galactose | Glucose | Galactose |
| B. bruxellensis | Day 0 | - | 0.109 ± 0.001 | 0.108 ± 0.002 | - |
| Day 18 | - | 0.076 ± 0.004 A | 0.082 ± 0.006 A | - | |
| B. claussenii | Day 0 | 0.103 ± 0.002 | 0.107 ± 0.002 | 0.084 ± 0.056 | 0.109 ± 0.001 |
| Day 9 | ND | ND | ND | 0.020 ± 0.035 | |
| K. marxianus | Day 0 | 0.102 ± 0.002 | 0.106 ± 0.004 | 0.108 ± 0.001 | 0.109 ± 0.001 |
| Day 9 | ND | 0.018 ± 0.035 | ND | A: ND B: ND | |
| S. cerevisiae | Day 0 | - | 0.104 ± 0.003 | 0.103 ± 0.009 | 0.102 ± 0.008 |
| Day 18 | - | 0.104 ± 0.011 A | 0.091 ± 0.009 AB | 0.076 ± 0.005 B | |
| Acetic Acid (g/L) | |||||
| Species | Timepoint | Lactose | Glucose + Galactose | Glucose | Galactose |
| B. bruxellensis | Day 0 | - | ND | ND | - |
| Day 18 | - | 6.069 ± 0.275 Ba | 7.696 ± 0.275 Aa | - | |
| B. claussenii | Day 0 | ND | ND | ND | ND |
| Day 9 | 4.672 ± 0.500 Aa | 4.575 ± 1.911 Aa | 4.029 ± 0.72 Ab | 2.803 ± 0.421 Aa | |
| K. marxianus | Day 0 | ND | ND | ND | ND |
| Day 9 | 0.021 ± 0.042 Ab | 0.022 ± 0.026 Ab | 0.156 ± 0.271 Ad | A: 0.062 ± 0.036 Ab B: 0.023 ± 0.026 Ab | |
| S. cerevisiae | Day 0 | - | ND | ND | ND |
| Day 18 | - | 1.612 ± 0.155 Bb | 1.893 ± 0.309 Bc | 2.586 ± 0.261 Aa | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera Flores, V.K.; DeMarsh, T.A.; Gibney, P.A.; Alcaine, S.D. Fermentation of Dairy-Relevant Sugars by Saccharomyces, Kluyveromyces, and Brettanomyces: An Exploratory Study with Implications for the Utilization of Acid Whey, Part II. Fermentation 2022, 8, 257. https://doi.org/10.3390/fermentation8060257
Rivera Flores VK, DeMarsh TA, Gibney PA, Alcaine SD. Fermentation of Dairy-Relevant Sugars by Saccharomyces, Kluyveromyces, and Brettanomyces: An Exploratory Study with Implications for the Utilization of Acid Whey, Part II. Fermentation. 2022; 8(6):257. https://doi.org/10.3390/fermentation8060257
Chicago/Turabian StyleRivera Flores, Viviana K., Timothy A. DeMarsh, Patrick A. Gibney, and Samuel D. Alcaine. 2022. "Fermentation of Dairy-Relevant Sugars by Saccharomyces, Kluyveromyces, and Brettanomyces: An Exploratory Study with Implications for the Utilization of Acid Whey, Part II" Fermentation 8, no. 6: 257. https://doi.org/10.3390/fermentation8060257
APA StyleRivera Flores, V. K., DeMarsh, T. A., Gibney, P. A., & Alcaine, S. D. (2022). Fermentation of Dairy-Relevant Sugars by Saccharomyces, Kluyveromyces, and Brettanomyces: An Exploratory Study with Implications for the Utilization of Acid Whey, Part II. Fermentation, 8(6), 257. https://doi.org/10.3390/fermentation8060257






