Changes in Phytochemical Compounds and Antioxidant Activity of Two Irradiated Sorghum (Sorghum bicolor (L.) Monech) Cultivars during the Fermentation and Cooking of Traditional Sudanese Asida
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Radiation Process
2.3. Fermentation
2.4. Cooking of the Traditional ASIDA
2.5. Extraction
2.6. Determination of Total Phenolic Content
2.7. Determination of Total Flavonoid Content
2.8. Tannin Determination
2.9. Antioxidant Activity Assays
2.9.1. DPPH Scavenging Assay
2.9.2. Reducing Power Assay
2.9.3. Hydrogen Peroxide Scavenging Assay
2.10. Statistical Analysis
3. Results and Discussion
3.1. Total Phenolic Content
3.2. Total Flavonoid Content
3.3. Tannin Content
3.4. Antioxidant Activity
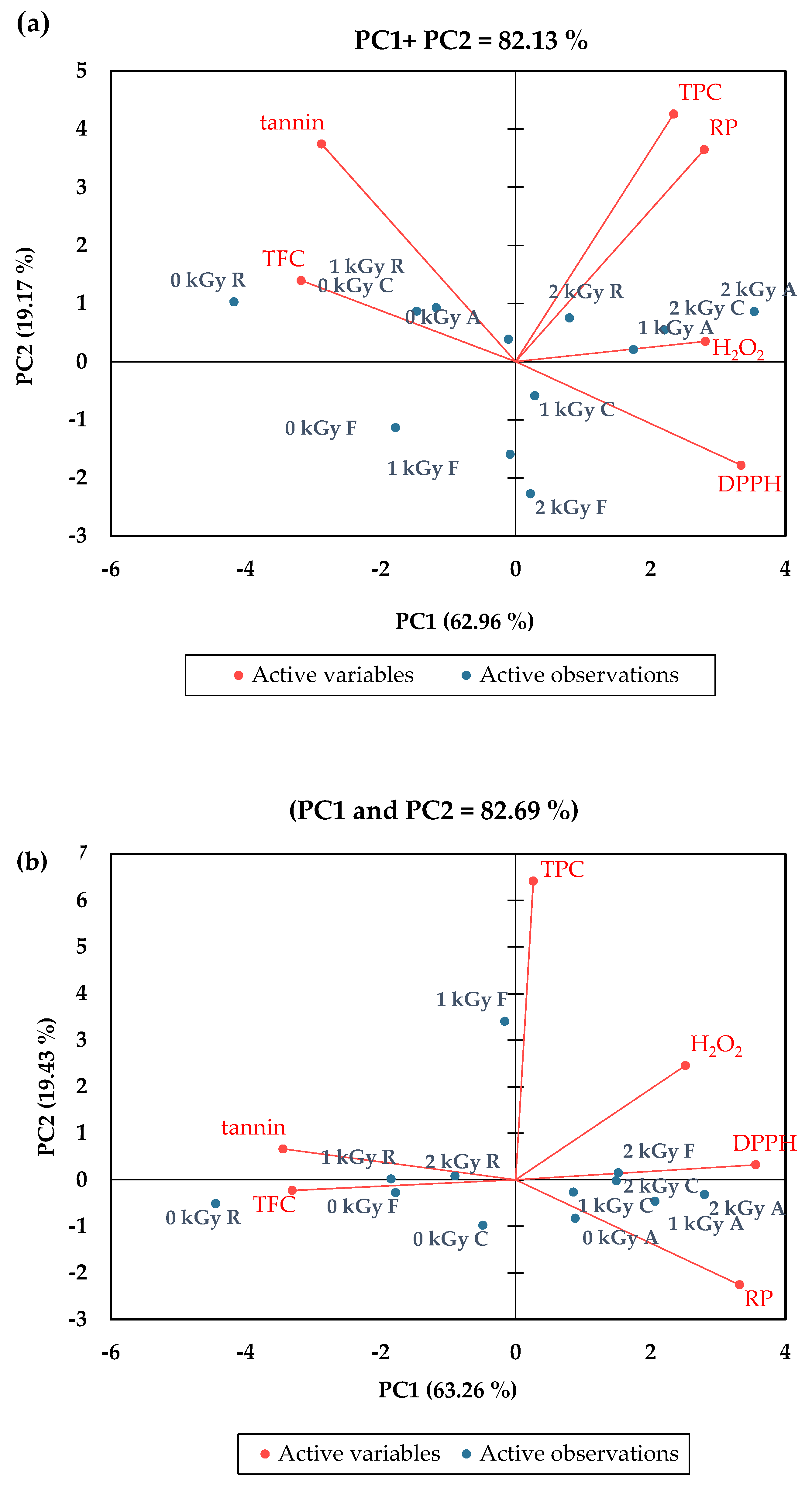
3.5. Principle Component Analysis (PCA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramatoulaye, F.; Mady, C.; Fallou, S.; Amadou, K.; Cyril, D.; Massamba, D. Production and Use Sorghum: A Literature Review. J. Nutr. Health Food Sci. 2016, 4, 1–4. [Google Scholar]
- Awika, J.M.; Rooney, L.W. Sorghum phytochemicals and their potential impact on human health. Phytochemistry 2004, 65, 1199–1221. [Google Scholar] [CrossRef] [PubMed]
- Dykes, L.; Rooney, L.W. Sorghum and millet phenols and antioxidants. J. Cereal Sci. 2006, 44, 236–251. [Google Scholar] [CrossRef]
- Fardet, A. New hypotheses for the health-protective mechanisms of whole-grain cereals: What is beyond fibre? Nutr. Res. Rev. 2010, 23, 65–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Datta, A.; Schmeits, J.L.; Amin, N.S.; Lau, P.J.; Myung, K.; Kolodner, R.D. Checkpoint-dependent activation of mutagenic repair in Saccharomyces cerevisiae pol3-01 mutants. Mol. Cell 2000, 6, 593–603. [Google Scholar] [CrossRef]
- Halliwell, B.; Zentella, A.; Gomez, E.O.; Kershenobich, D. Antioxidants and human disease: A general introduction. Nutr. Rev. 1997, 55, S44. [Google Scholar] [CrossRef]
- Chung, K.-T.; Wong, T.Y.; Wei, C.-I.; Huang, Y.-W.; Lin, Y. Tannins and human health: A review. Crit. Rev. Food Sci. Nutr. 1998, 38, 421–464. [Google Scholar] [CrossRef]
- Pietta, P.-G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Mahmoud, N.S.; Awad, S.H.; Madani, R.M.A.; Osman, F.A.; Elmamoun, K.; Hassan, A.B. Effect of γ radiation processing on fungal growth and quality characteristics of millet grains. Food Sci. Nutr. 2016, 4, 342–347. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.M.; Abdalla, I.G.; Salih, A.; Hassan, A.B. Effect of gamma radiation on storability and functional properties of sorghum grains (Sorghum bicolor L.). Food Sci. Nutr. 2018, 6, 1933–1939. [Google Scholar] [CrossRef]
- Mahgoub, S.E.; Ahmed, B.M.; Ahmed, M.M.; El Nazeer, A. Effect of traditional Sudanese processing of kisra bread andhulu-mur drink on their thiamine, riboflavin and mineral contents. Food Chem. 1999, 67, 129–133. [Google Scholar] [CrossRef]
- Talhaoui, N.; Gómez-Caravaca, A.M.; León, L.; De la Rosa, R.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Determination of phenolic compounds of ‘Sikitita’ olive leaves by HPLC-DAD-TOF-MS. Comparison with its parents ‘Arbequina’ and ‘Picual’olive leaves. LWT Food Sci. Technol. 2014, 58, 28–34. [Google Scholar] [CrossRef]
- Waterhouse, A.L. Determination of total phenolics. Curr. Protoc. Food Anal. Chem. 2002, 6, I1-1. [Google Scholar]
- Kim, D.-O.; Jeong, S.W.; Lee, C.Y. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- Price, M.L.; Van Scoyoc, S.; Butler, L.G. A critical evaluation of the vanillin reaction as an assay for tannin in sorghum grain. J. Agric. Food Chem. 1978, 26, 1214–1218. [Google Scholar] [CrossRef]
- Chang, S.-T.; Wu, J.-H.; Wang, S.-Y.; Kang, P.-L.; Yang, N.-S.; Shyur, L.-F. Antioxidant activity of extracts from Acacia confusa bark and heartwood. J. Agric. Food Chem. 2001, 49, 3420–3424. [Google Scholar] [CrossRef]
- Gülçin, İ.; Oktay, M.; Küfrevioğlu, Ö.İ.; Aslan, A. Determination of antioxidant activity of lichen Cetraria islandica (L.) Ach. J. Ethnopharmacol. 2002, 79, 325–329. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Rao, L.J.; Sakariah, K.K. Antioxidant activities of flavidin in different in vitro model systems. Bioorg. Med. Chem. 2004, 12, 5141–5146. [Google Scholar] [CrossRef]
- Vidal, N.P.; Manful, C.F.; Pham, T.H.; Stewart, P.; Keough, D.; Thomas, R. The use of XLSTAT in conducting principal component analysis (PCA) when evaluating the relationships between sensory and quality attributes in grilled foods. MethodsX 2020, 7, 100835. [Google Scholar] [CrossRef]
- Variyar, P.S.; Gholap, A.; Sharma, A. Changes in flavor components in γ-irradiated fresh ginger (Zingiber officinale) rhizomes during storage. J. Herbs Spices Med. Plants 2007, 12, 25–35. [Google Scholar] [CrossRef]
- Zaroug, M.; Orhan, I.E.; Senol, F.S.; Yagi, S. Comparative antioxidant activity appraisal of traditional Sudanese kisra prepared from two sorghum cultivars. Food Chem. 2014, 156, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Harrison, K.; Were, L. Effect of gamma irradiation on total phenolic content yield and antioxidant capacity of almond skin extracts. Food Chem. 2007, 102, 932–937. [Google Scholar] [CrossRef]
- Adamo, M.; Capitani, D.; Mannina, L.; Cristinzio, M.; Ragni, P.; Tata, A.; Coppola, R. Truffles decontamination treatment by ionizing radiation. Rad. Phys. Chem. 2004, 71, 167–170. [Google Scholar] [CrossRef]
- Mun’im, A.; Ramadhani, F.; Chaerani, K.; Amelia, L.; Arrahman, A. Effects of gamma irradiation on microbiological, phytochemical content, antioxidant activity and inhibition of angiotensin converting enzyme (ACE) activity of Peperomia pellucida (L.) Kunth. J. Young Pharm. 2017, 9, S65. [Google Scholar] [CrossRef] [Green Version]
- Hassan, A.B.; Ahmed, S.M.; Elkhatim, K.A.S.; Abdelhalim, T.S.; Fawale, S.O.; Adiamo, O.Q.; Ahmed, I.A.M. Effect of gamma irradiation and microwave heating treatments on microbial load and antioxidant potentials in cinnamon, fennel and hot pepper. J. Food Meas. Charact. 2019, 13, 1130–1138. [Google Scholar] [CrossRef]
- Capasso, A. Antioxidant action and therapeutic efficacy of Allium sativum L. Molecules 2013, 18, 690–700. [Google Scholar] [CrossRef]
- Topuz, A.; Ozdemir, F. Influences of gamma irradiation and storage on the capsaicinoids of sun-dried and dehydrated paprika. Food Chem. 2004, 86, 509–515. [Google Scholar] [CrossRef]
- Hassan, A.B.; Osman, G.A.; Rushdi, M.A.; Eltayeb, M.M.; Diab, E. Effect of gamma irradiation on the nutritional quality of maize cultivars (Zea mays) and sorghum (Sorghum bicolor) grains. Pak. J. Nutr. 2009, 8, 167–171. [Google Scholar]
- Amol, B.M.; Kalpana, K.; Smita, S.L. Effect of gamma irradiation on total phenolic content and in vitro antioxidant activity of pomegranate (Punica granatum L.) peels. Food Nutr. Sci. 2011, 2, 5756. [Google Scholar]
- Variyar, P.S.; Limaye, A.; Sharma, A. Radiation-induced enhancement of antioxidant contents of soybean (Glycine max Merrill). J. Agric. Food Chem. 2004, 52, 3385–3388. [Google Scholar] [CrossRef]
- Moosavi, K.S.; Hosseini, S.; Dehghan, G.; Jahanban-Esfahlan, A. The effect of gamma irradiation on phytochemical content and antioxidant activity of stored and none stored almond (Amygdalus communis L.) hull. Pharm. Sci. 2014, 20, 102–106. [Google Scholar]
- Bhat, R.; Sridhar, K.R.; Tomita-Yokotani, K. Effect of ionizing radiation on antinutritional features of velvet bean seeds (Mucuna pruriens). Food Chem. 2007, 103, 860–866. [Google Scholar] [CrossRef]
- Hassan, A.B.; Al Maiman, S.A.; Mohammed, M.A.; Alshammari, G.M.; Alkhudhayri, D.A.; Alhuthayli, H.F.; Alfawaz, M.A.; Osman, M.A. Effect of Natural Fermentation on the Chemical Composition, Mineral Content, Phytochemical Compounds, and Antioxidant Activity of Ziziphus spina-christi (L.) “Nabag” Seeds. Processes 2021, 9, 1228. [Google Scholar] [CrossRef]
- Mutwali, N.I.; Mustafa, A.I.; Gorafi, Y.S.; Mohamed Ahmed, I.A. Effect of environment and genotypes on the physicochemical quality of the grains of newly developed wheat inbred lines. Food Sci. Nutr. 2016, 4, 508–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Cultivar | Processing | Radiation Dose | ||
|---|---|---|---|---|
| 0 kGy | 1.0 kGy | 2.0 kGy | ||
| Tabat | Raw flour | 8.9 ± 0.42 Bb | 9.0 ± 0.24 Bb | 9.4 ± 0.36 Aa |
| Cooked | 7.6 ± 0.23 Cc | 8.6 ± 0.24 Bc | 9.6 ± 0.32 Aa | |
| Fermented | 8.7 ± 0.82 Ab | 8.5 ± 0.24 Ac | 7.7 ± 0.12 Bc | |
| Asida | 9.8 ± 0.19 Aa | 9.5 ± 0.39 Ba | 8.8 ± 0.40 Cb | |
| Wad Ahmed | Raw flour | 9.7 ± 0.80 Bb | 9.8 ± 0.83 Bc | 10.2 ± 0.94 Ab |
| Cooked | 7.1 ± 0.01 Cc | 9.8 ± 0.30 Bc | 12.1 ± 0.01 Aa | |
| Fermented | 9.5 ± 0.69 Bb | 10.2 ± 0.54 Ab | 9.2 ± 0.57 Bd | |
| Asida | 11.2 ± 0.42 Aa | 11.1 ± 0.17 Aa | 9.8 ± 0.19 Bc | |
| Cultivar | Processing | Radiation Dose | ||
|---|---|---|---|---|
| 0 kGy | 1.0 kGy | 2.0 kGy | ||
| Tabat | Raw flour | 60.4 ± 1.52 Aa | 52.4 ± 2.78 Ba | 50.5 ± 2.53 Ca |
| Cooked | 45.4 ± 0.84 Ac | 34.7 ± 1.10 Bc | 31.8 ± 0.51 Cc | |
| Fermented | 49.6 ± 2.42 Ab | 38.4 ± 0.42 Bb | 36.2 ± 0.23 Cb | |
| Asida | 34.1 ± 3.79 Ad | 31.8 ± 0.51 Bd | 25.7 ± 1.82 Cd | |
| Wad Ahmed | Raw flour | 52.5 ± 0.05 Aa | 50.2 ± 0.51 Ba | 49.0 ± 0.51 Ba |
| Cooked | 41.7 ± 1.33 Ab | 37.5 ± 1.51 Bb | 34.0 ± 1.91 Cb | |
| Fermented | 37.3 ± 1.33 Ac | 37.0 ± 0.75 Ab | 31.9 ± 1.23 Bc | |
| Asida | 33.8 ± 1.01 Ad | 29.8 ± 3.03 Bc | 28.3 ± 0.51 Bd | |
| Cultivar | Processing | Radiation Dose | ||
|---|---|---|---|---|
| 0 kGy | 1.0 kGy | 2.0 kGy | ||
| Tabat | Raw flour | 1.4 ± 0.00 Aa | 1.2 ± 0.03 Aa | 0.8 ± 0.07 Ba |
| Cooked | 1.0 ± 0.07 Ab | 0.5 ± 0.09 Bb | 0.5 ± 0.00 Bb | |
| Fermented | 0.6 ± 0.02 Ac | 0.5 ± 0.09 Abb | 0.4 ± 0.00 Bb | |
| Asida | 0.9 ± 0.09 Ab | 0.4 ± 0.07 Bb | 0.4 ± 0.02 Bb | |
| Wad Ahmed | Raw flour | 5.0 ± 0.07 Aa | 4.8 ± 0.07 Aa | 4.3 ± 0.14 Ba |
| Cooked | 3.3 ± 0.51 Ac | 3.2 ± 0.06 ABc | 3.1 ± 0.07 Bb | |
| Fermented | 3.8 ± 0.43 Ab | 3.5 ± 0.27 Bb | 3.1 ± 0.00 Cb | |
| Asida | 2.7 ± 0.07 Ad | 2.4 ± 0.17 Bd | 2.3 ± 0.14 Bc | |
| Antioxidant Method | Cultivar | Processing | Radiation Dose | ||
|---|---|---|---|---|---|
| 0 kGy | 1.0 kGy | 2.0 kGy | |||
| DPPH scavenging (%) | Tabat | Raw flour | 56.0 ± 1.00 Cc | 70.0 ± 2.61 Bc | 74.0 ± 1.01 Ad |
| Cooked | 69.9 ± 1.47 Cb | 75.9 ± 1.50 Bb | 78.9 ± 3.35 Ac | ||
| Fermented | 71.1 ± 1.48 Cb | 80.4 ± 1.50 Ba | 82.9 ± 1.09 Ab | ||
| Asida | 77.5 ± 0.52 Ca | 81.9 ± 3.48 Ba | 85.0 ± 2.43 Aa | ||
| Wad Ahmed | Raw flour | 45.5 ± 0.98 Cd | 67.9 ± 2.54 Bc | 81.1 ± 0.06 Ac | |
| Cooked | 67.9 ± 2.22 Cb | 73.3 ± 2.54 Bb | 82.2 ± 2.31 Abc | ||
| Fermented | 61.1 ± 1.39 Cc | 73.3 ± 1.67 Bb | 83.5 ± 0.75 Ab | ||
| Asida | 76.4 ± 2.08 Ca | 81.7 ± 1.50 Ba | 89.6 ± 0.46 Aa | ||
| RP (mg AAE/g) | Tabat | Raw flour | 1.0 ± 0.03 Bb | 2.7 ± 0.19 Aa | 3.2 ± 0.08 Ab |
| Cooked | 2.4 ± 0.03 Aa | 2.7 ± 0.01 Aa | 3.2 ± 0.06 Ab | ||
| Fermented | 1.1 ± 0.02 Ab | 1.4 ± 0.02 Ab | 1.5 ± 0.03 Ac | ||
| Asida | 2.5 ± 0.01 Ba | 2.8 ± 0.04 ABa | 3.8 ± 0.02 Aa | ||
| Wad Ahmed | Raw flour | 1.5 ± 0.00 Bc | 2.5 ± 0.03 ABb | 2.9 ± 0.05 Ac | |
| Cooked | 3.3 ± 0.03 Aa | 3.4 ± 0.02 Aa | 3.4 ± 0.02 Ab | ||
| Fermented | 2.1 ± 0.02 Ab | 2.2 ± 0.00 Ab | 2.9 ± 0.02 Ac | ||
| Asida | 3.5 ± 0.03 Aa | 3.6 ± 0.04 Aa | 3.9 ± 0.02 Aa | ||
| H2 O2 scavenging (%) | Tabat | Raw flour | 79.1 ± 1.09 Ca | 81.5 ± 0.29 Bab | 84.4 ± 0.65 Aa |
| Cooked | 79.3 ± 0.49 Ca | 80.6 ± 1.09 Bb | 83.8 ± 0.31 Aab | ||
| Fermented | 78.4 ± 0.78 Ca | 81.7 ± 0.00 Bab | 82.8 ± 0.00 Ab | ||
| Asida | 78.9 ± 0.78 Ca | 82.1 ± 1.68 Ba | 84.6 ± 0.00 Aa | ||
| Wad Ahmed | Raw flour | 76.9 ± 1.65 Cb | 81.8 ± 0.49 Ba | 82.8 ± 0. 43 Aa | |
| Cooked | 79.0 ± 1.23 Ca | 82.7 ± 0.78 Ba | 83.6 ± 0.93 Aa | ||
| Fermented | 79.2 ± 1.82 Ca | 82.4 ± 1.17 Ba | 83.8 ± 0.74 Aa | ||
| Asida | 78.9 ± 2.14 Ca | 81.6 ± 0.22 Ba | 83.1 ± 0.36 Aa | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, L.K.; Sulieman, M.A.; Yagoub, A.E.A.; Mohammed, M.A.; Alhuthayli, H.F.; Mohamed Ahmed, I.A.; Almaiman, S.A.; Alfawaz, M.A.; Osman, M.A.; Hassan, A.B. Changes in Phytochemical Compounds and Antioxidant Activity of Two Irradiated Sorghum (Sorghum bicolor (L.) Monech) Cultivars during the Fermentation and Cooking of Traditional Sudanese Asida. Fermentation 2022, 8, 60. https://doi.org/10.3390/fermentation8020060
Mohamed LK, Sulieman MA, Yagoub AEA, Mohammed MA, Alhuthayli HF, Mohamed Ahmed IA, Almaiman SA, Alfawaz MA, Osman MA, Hassan AB. Changes in Phytochemical Compounds and Antioxidant Activity of Two Irradiated Sorghum (Sorghum bicolor (L.) Monech) Cultivars during the Fermentation and Cooking of Traditional Sudanese Asida. Fermentation. 2022; 8(2):60. https://doi.org/10.3390/fermentation8020060
Chicago/Turabian StyleMohamed, Layla K., Mashair A. Sulieman, Abu ElGasim A. Yagoub, Mohammed A. Mohammed, Haya F. Alhuthayli, Isam A. Mohamed Ahmed, Salah A. Almaiman, Mohammed A. Alfawaz, Magdi A. Osman, and Amro B. Hassan. 2022. "Changes in Phytochemical Compounds and Antioxidant Activity of Two Irradiated Sorghum (Sorghum bicolor (L.) Monech) Cultivars during the Fermentation and Cooking of Traditional Sudanese Asida" Fermentation 8, no. 2: 60. https://doi.org/10.3390/fermentation8020060
APA StyleMohamed, L. K., Sulieman, M. A., Yagoub, A. E. A., Mohammed, M. A., Alhuthayli, H. F., Mohamed Ahmed, I. A., Almaiman, S. A., Alfawaz, M. A., Osman, M. A., & Hassan, A. B. (2022). Changes in Phytochemical Compounds and Antioxidant Activity of Two Irradiated Sorghum (Sorghum bicolor (L.) Monech) Cultivars during the Fermentation and Cooking of Traditional Sudanese Asida. Fermentation, 8(2), 60. https://doi.org/10.3390/fermentation8020060







