Enrichment of Pistachio Shell with Olive Mill Waste or Lathyrus clymenum Pericarp Mixtures via Solid State Fermentation with Pleurotus ostreatus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Microorganisms
2.2. Inoculation and Solid-State Fermentation
2.3. Analytical Methods
2.4. Statistical Analysis
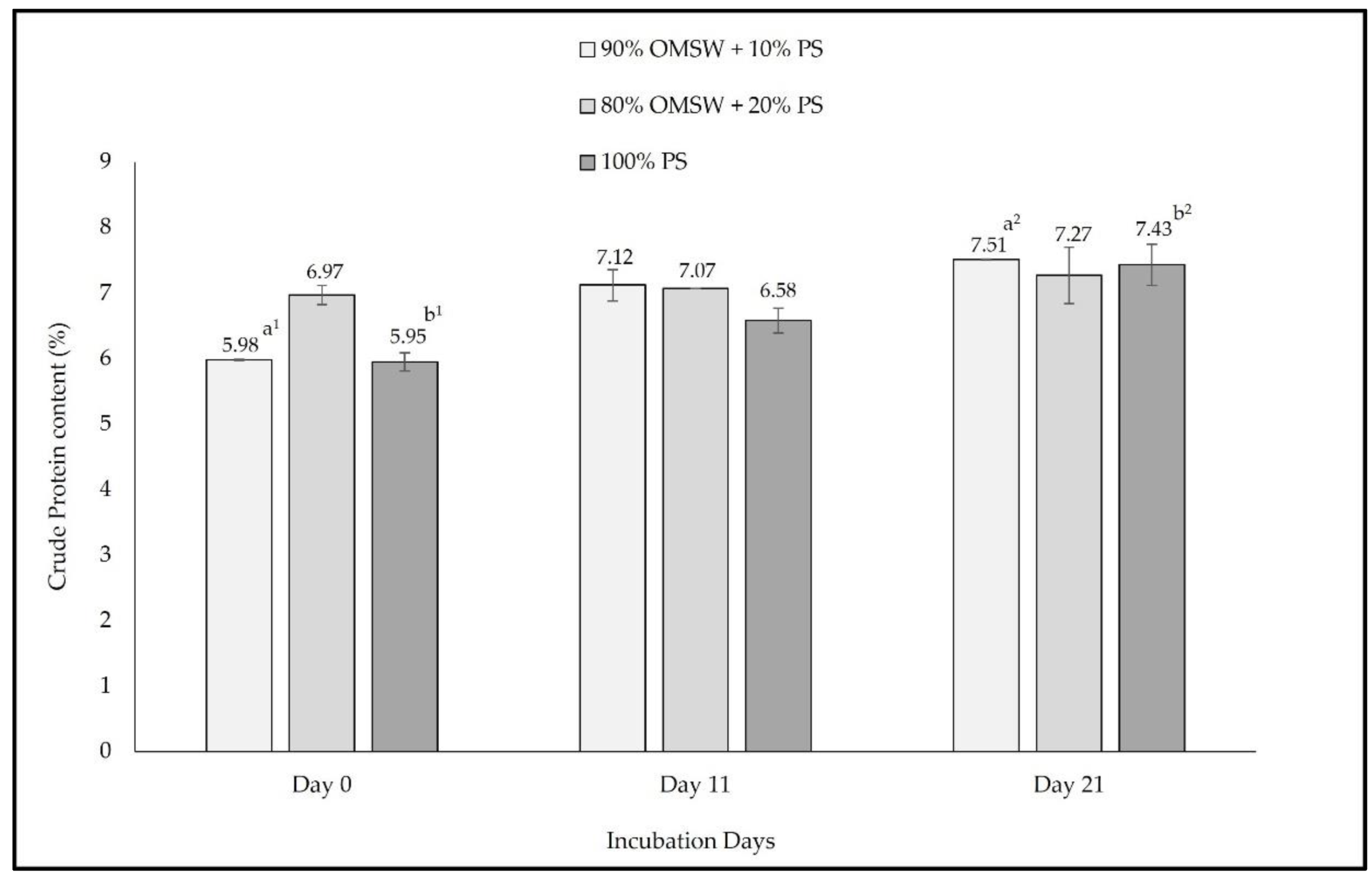
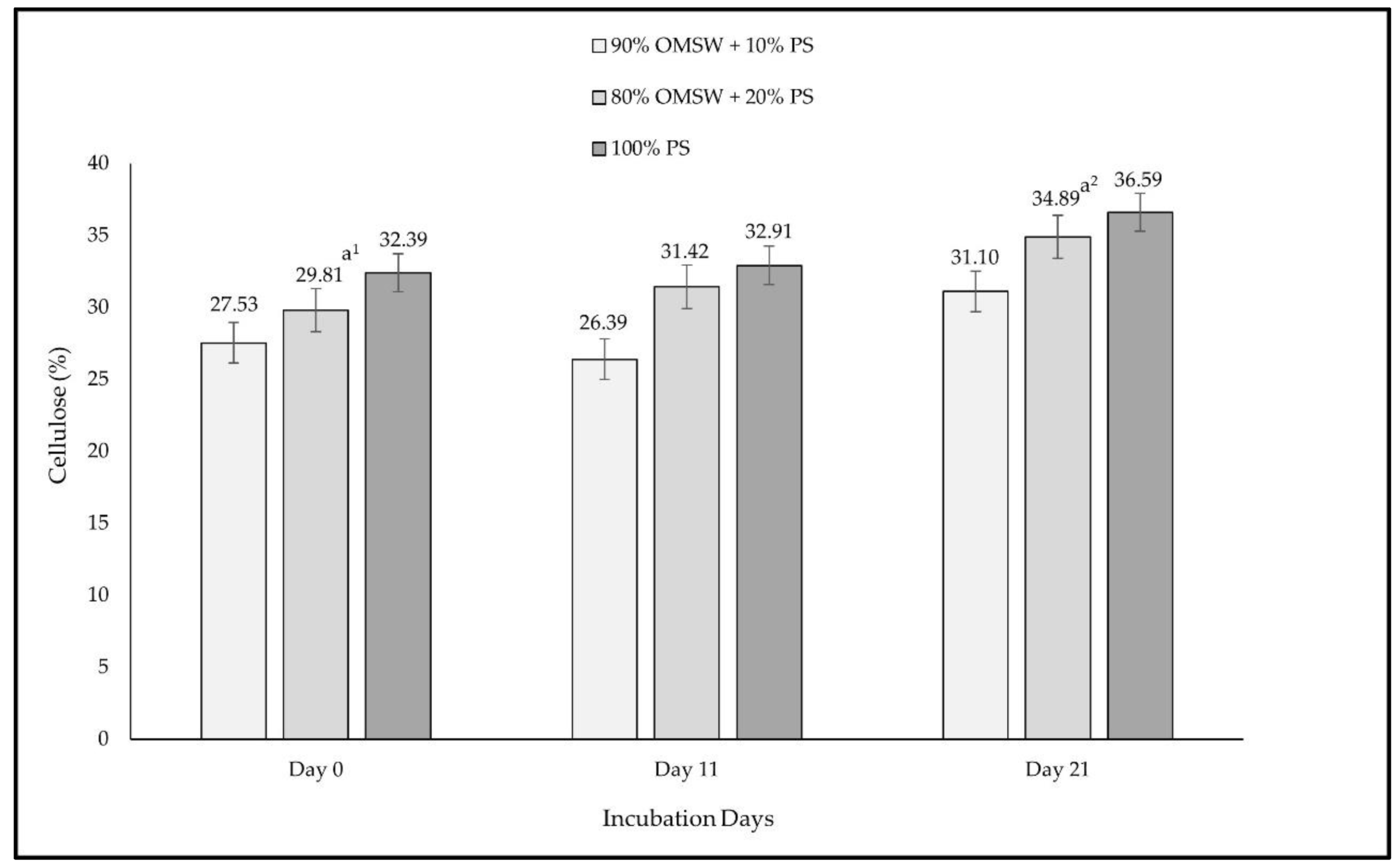
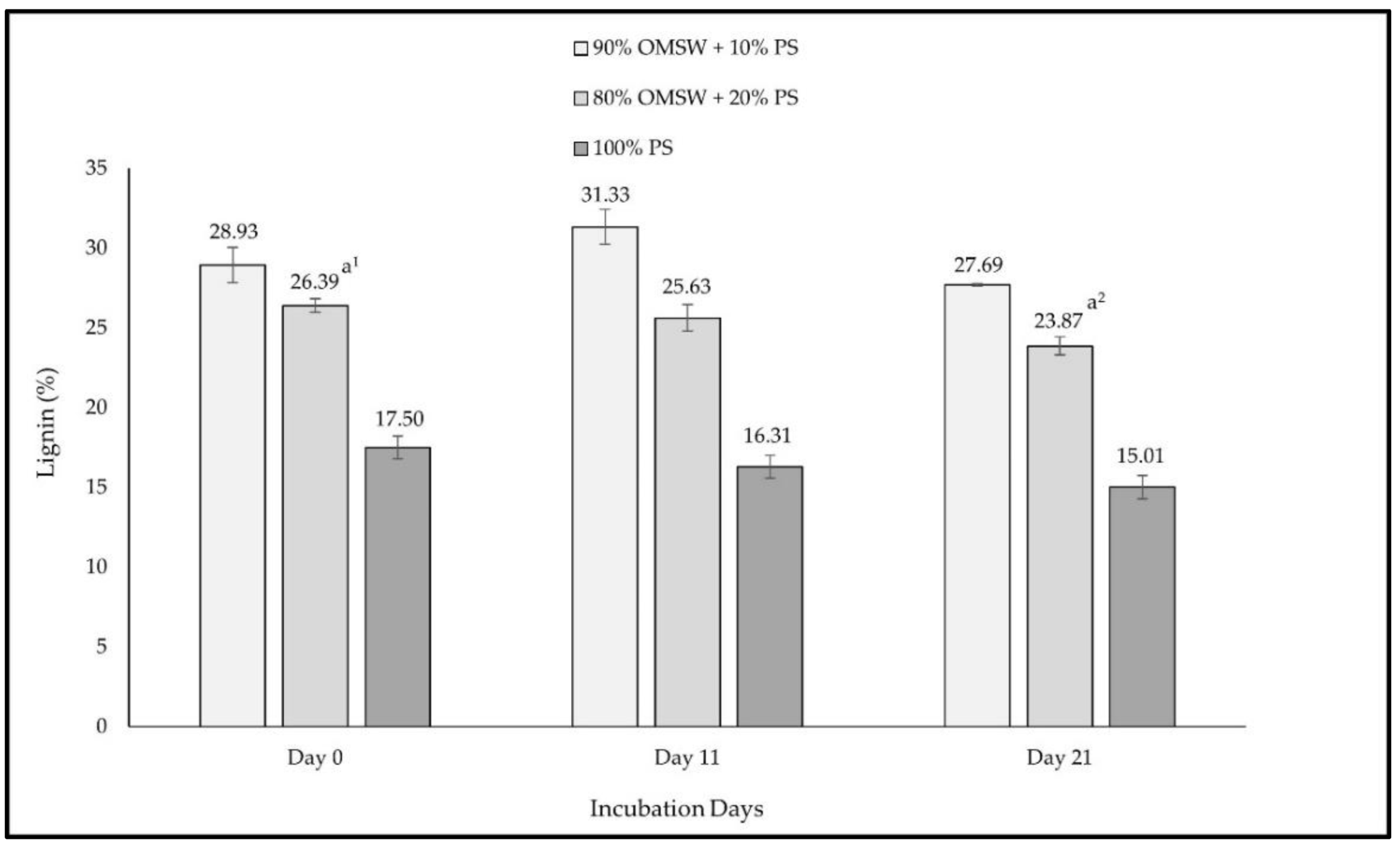
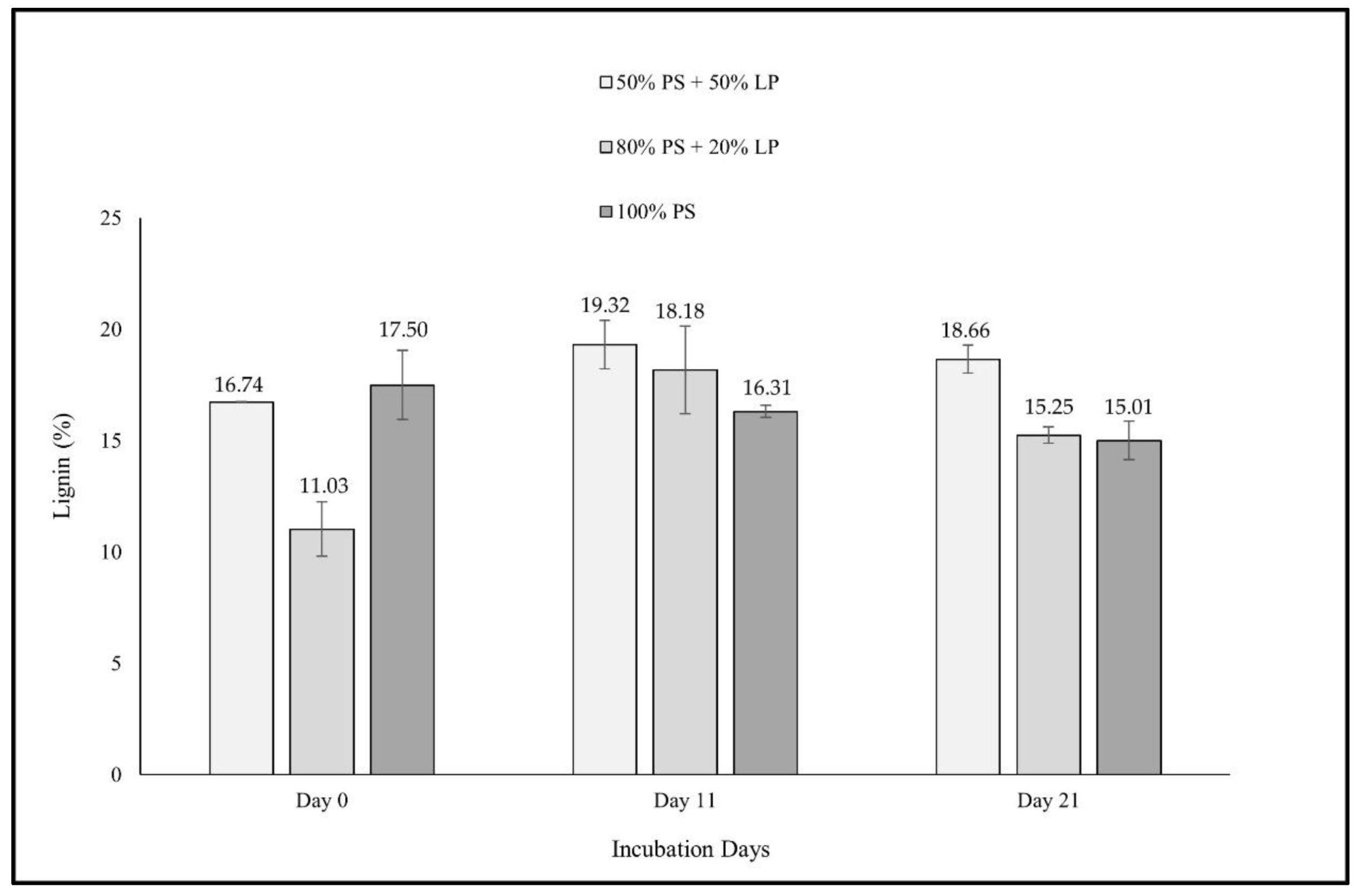
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leite, P.; Silva, C.; Salgado, J.M.; Belo, I. Simultaneous production of lignocellulolytic enzymes and extraction of antioxidant compounds by solid-state fermentation of agro-industrial wastes. Ind. Crop. Prod. 2019, 137, 315–322. [Google Scholar] [CrossRef] [Green Version]
- Ravindran, R.; Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. A Review on Bioconversion of Agro-Industrial Wastes to Industrially Important Enzymes. Bioengineering 2018, 5, 93. [Google Scholar] [CrossRef] [Green Version]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-industrial wastes and their utilization using solid state fermentation: A review. Bioresour. Bioprocess. 2018, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Marzo, C.; Diaz, A.B.; Caro, I.; Blandino, A. Valorization of agro-industrial wastes to produce hydrolytic enzymes by fungal solid-state fermentation. Waste Manag. Res. J. Sustain. Circ. Econ. 2018, 37, 149–156. [Google Scholar] [CrossRef]
- Abu Yazid, N.; Barrena, R.; Komilis, D.; Sánchez, A. Solid-State Fermentation as a Novel Paradigm for Organic Waste Valorization: A Review. Sustainability 2017, 9, 224. [Google Scholar] [CrossRef] [Green Version]
- Leite, P.; Sousa, D.; Fernandes, H.; Ferreira, M.; Costa, A.R.; Filipe, D.; Gonçalves, M.; Peres, H.; Belo, I.; Salgado, J.M. Recent advances in production of lignocellulolytic enzymes by solid-state fermentation of agro-industrial wastes. Curr. Opin. Green Sustain. Chem. 2020, 27, 100407. [Google Scholar] [CrossRef]
- Grau, A.; Lerma, J.; Heredia, A.; Andrés, A. Enhancing the Nutritional Profile and Digestibility of Lentil Flour by Solid State Fermentation with Pleurotus ostreatus. Food Funct. 2020, 11, 7905–7912. [Google Scholar] [CrossRef]
- Han, M.-L.; An, Q.; He, S.-F.; Zhang, X.-L.; Zhang, M.-H.; Gao, X.-H.; Wu, Q.; Bian, L.-S. Solid-state fermentation on poplar sawdust and corncob wastes for lignocellulolytic enzymes by different Pleurotus ostreatus strains. BioResources 2020, 15, 4982–4995. [Google Scholar] [CrossRef]
- Anele, U.Y.; Anike, F.N.; Davis-Mitchell, A.; Isikhuemhen, O.S. Solid-state fermentation with Pleurotus ostreatus improves the nutritive value of corn stover-kudzu biomass. Folia Microbiol. 2020, 66, 41–48. [Google Scholar] [CrossRef]
- Nunes, M.A.; Pimentel, F.B.; Costa, A.S.; Alves, R.C.; Oliveira, M.B.P. Olive by-products for functional and food applications: Challenging opportunities to face environmental constraints. Innov. Food Sci. Emerg. Technol. 2016, 35, 139–148. [Google Scholar] [CrossRef]
- Ruiz-Rodriguez, A.; Soler-Rivas, C.; Polonia, I.; Wichers, H.J. Effect of olive mill waste (OMW) supplementation to Oyster mushrooms substrates on the cultivation parameters and fruiting bodies quality. Int. Biodeterior. Biodegrad. 2010, 64, 638–645. [Google Scholar] [CrossRef] [Green Version]
- Arjeh, E.; Akhavan, H.-R.; Barzegar, M.; Carbonell-Barrachina, A.A. Bio-active compounds and functional properties of pistachio hull: A review. Trends Food Sci. Technol. 2020, 97, 55–64. [Google Scholar] [CrossRef]
- Özbek, H.N.; Yanık, D.K.; Fadıloğlu, S.; Göğüş, F. Effect of microwave-assisted alkali pre-treatment on fractionation of pistachio shell and enzymatic hydrolysis of cellulose-rich residues. J. Chem. Technol. Biotechnol. 2020, 96, 521–531. [Google Scholar] [CrossRef]
- Sadeghian-Abadi, S.; Rezaei, S.; Yousefi-Mokri, M.; Faramarzi, M.A. Enhanced production, one-step affinity purification, and characterization of laccase from solid-state culture of Lentinus tigrinus and delignification of pistachio shell by free and immobilized enzyme. J. Environ. Manag. 2019, 244, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Cavada, E.; Juan, R.; Pastor, J.E.; Alaiz, M.; Vioque, J. Nutritional characteristics of seed proteins in 15 Lathyrus species (fabaceae) from Southern Spain. LWT 2011, 44, 1059–1064. [Google Scholar] [CrossRef]
- Zawieja, B.; Rybiński, W.; Nowosad, K.; Bocianowski, J. Assessment of Lathyrus Species Accession Variability Using Visual and Statistical Methods. Pak. J. Bot. 2018, 50, 2277–2284. [Google Scholar]
- Ralli, P.; Koutis, K.; Drosou, I.; Alexandris, C. The in situ Conservation of ‘Arakas’ (Lathyrus clymenum L.) in Thera Island throughout the Centuries. Landraces 2020, 20, 34. [Google Scholar]
- Karim, M.R.; Mahal, Z.; Iqbal, S.; Perves, H.O.R.; Jamal, M.; Karim, M.R.; Rahman, M. Solid State Fermentation of Lathyrus sativus and Sugarcane Bagasse by Pleurotus Sajor-Caju. Int. J. Agron. Agric. Res. 2014, 4, 1–9. [Google Scholar]
- Fan, L.; Pandey, A.; Mohan, R.; Soccol, C.R. Use of various coffee industry residues for the cultivation of Pleurotus ostreatus in solid state fermentation. Acta Biotechnol. 2000, 20, 41–52. [Google Scholar] [CrossRef]
- Yoon, L.W.; Ang, T.N.; Ngoh, G.C.; Chua, A.S.M. Fungal solid-state fermentation and various methods of enhancement in cellulase production. Biomass Bioenergy 2014, 67, 319–338. [Google Scholar] [CrossRef]
- Abdullah, J.J.; Greetham, D.; Pensupa, N.; Tucker, G.A.; Du, C. Optimizing Cellulase Production from Municipal Solid Waste (MSW) using Solid State Fermentation (SSF). J. Fundam. Renew. Energy Appl. 2016, 6, 206. [Google Scholar] [CrossRef] [Green Version]
- AOAC. Official Methods of Analysis, 14th ed.; Association of Official Analytical Chemists: Washigton, DC, USA, 1995. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F.G. A Colorimetric Method for the Determination of Sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Nagel, F.-J.J.; Tramper, J.; Bakker, M.S.; Rinzema, A. Model for on-line moisture-content control during solid-state fermentation. Biotechnol. Bioeng. 2000, 72, 231–243. [Google Scholar] [CrossRef]
- Darwish, G.A.; Bakr, A.; Abdallah, M. Nutritional value upgrading of maize stalk by using Pleurotus ostreatus and Saccharomyces cerevisiae in solid state fermentation. Ann. Agric. Sci. 2012, 57, 47–51. [Google Scholar] [CrossRef] [Green Version]
- Terrasan, C.R.F.; Carmona, E.C. Solid-state fermentation of brewer’s spent grain for xylanolytic enzymes production by Penicillium janczewskii and analyses of the fermented substrate. Biosci. J. 2015, 31, 1826–1836. [Google Scholar] [CrossRef] [Green Version]
- Akinfemi, A.; Adu, O.A.; Doherty, F. Conversion of sorghum stover into animal feed with white-rot fungi: Pleurotus ostreatus and Pleurotus pulmonarius. Afr. J. Biotechnol. 2010, 9, 1706–1712. [Google Scholar] [CrossRef] [Green Version]
- Oseni, O.; Akindahunsi, A.A. Some Phytochemical Properties and Effect of Fermentation on the Seed of Jatropha curcas L. Am. J. Food Technol. 2011, 6, 158–165. [Google Scholar] [CrossRef] [Green Version]
- Nasehi, M.; Torbatinejad, N.M.; Zerehdaran, S.; Safaie, A.R. Effect of solid-state fermentation by oyster mushroom (Pleurotus florida) on nutritive value of some agro by-products. J. Appl. Anim. Res. 2016, 45, 221–226. [Google Scholar] [CrossRef] [Green Version]
- Parani, K.; Eyini, M. Biodegradation of Coffee Pulp Waste by Different Fungal Associations. Biosci. Discov. 2012, 3, 222–228. [Google Scholar]
- Dairo, F.; Ogunlade, S.; Oluwasola, T. Proximate Composition and Amino Acid Profile of Rice Husk Biodegraded with Pleurotus ostreatus for Different Periods. Afr. J. Food Agric. Nutr. Dev. 2017, 17, 12244–12255. [Google Scholar] [CrossRef]
- Eliopoulos, C.; Arapoglou, D.; Chorianopoulos, N.; Markou, G.; Haroutounian, S.A. Conversion of brewers’ spent grain into proteinaceous animal feed using solid state fermentation. Environ. Sci. Pollut. Res. 2021, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chebaibi, S.; Grandchamp, M.L.; Burgé, G.; Clément, T.; Allais, F.; Laziri, F. Improvement of protein content and decrease of anti-nutritional factors in olive cake by solid-state fermentation: A way to valorize this industrial by-product in animal feed. J. Biosci. Bioeng. 2019, 128, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C. Lignocellulosic residues: Biodegradation and bioconversion by fungi. Biotechnol. Adv. 2009, 27, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Ergun, S.O.; Urek, R.O. Production of ligninolytic enzymes by solid state fermentation using Pleurotus ostreatus. Ann. Agrar. Sci. 2017, 15, 273–277. [Google Scholar] [CrossRef]
- Ritota, M.; Manzi, P. Pleurotus spp. Cultivation on Different Agri-Food By-Products: Example of Biotechnological Application. Sustainability 2019, 11, 5049. [Google Scholar] [CrossRef] [Green Version]
- Nigam, P.S.-N.; Pandey, A. Biotechnology for Agro-Industrial Residues Utilisation: Utilisation of Agro-Residues; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2009; ISBN 1-4020-9942-8. [Google Scholar]
- Hanczakowska, E.; Świa̧tkiewicz, M.; Białecka, A. Pure Cellulose as a Feed Supplement for Piglets. Med. Weter. 2008, 64, 45–48. [Google Scholar]
- Pascoal, L.A.F.; Thomaz, M.C.; Watanabe, P.H.; Ruiz, U.D.S.; Ezequiel, J.M.B.; Amorim, A.B.; Daniel, E.; Masson, G.C.I. Fiber sources in diets for newly weaned piglets. Rev. Bras. Zootec. 2012, 41, 636–642. [Google Scholar] [CrossRef] [Green Version]
- Van Hees, H.M.J.; Davids, M.; Maes, D.; Millet, S.; Possemiers, S.; Hartog, L.A.D.; Van Kempen, T.A.T.G.; Janssens, G.P.J. Dietary fibre enrichment of supplemental feed modulates the development of the intestinal tract in suckling piglets. J. Anim. Sci. Biotechnol. 2019, 10, 83. [Google Scholar] [CrossRef] [Green Version]
- Peace, O.; Aladesanmi, A. Effect of Fermentation on Some Chemical and Nutritive Properties of Berlandier Nettle Spurge (Jatropha cathartica) and Physic Nut (Jatropha curcas) Seeds. Pak. J. Nutr. 2008, 7, 292–296. [Google Scholar]
- Rajesh, N.; Joseph, I.; Raj, R.P. Value addition of vegetable wastes by solid-state fermentation using Aspergillus niger for use in aquafeed industry. Waste Manag. 2010, 30, 2223–2227. [Google Scholar] [CrossRef] [PubMed]
- Okpako, C.; Ntui, V.; Osuagwu, A.; Obasi, F. Proximate Composition and Cyanide Content of Cassava Peels Fermented with Aspergillus Niger and Lactobacillus Rhamnosus. J. Food Agric. Environ. 2008, 6, 251–255. [Google Scholar]
- Zhu, F.; Du, B.; Bian, Z.; Xu, B. Beta-glucans from edible and medicinal mushrooms: Characteristics, physicochemical and biological activities. J. Food Compos. Anal. 2015, 41, 165–173. [Google Scholar] [CrossRef]
- Chiozzi, V.; Eliopoulos, C.; Markou, G.; Arapoglou, D.; Agriopoulou, S.; El Enshasy, H.A.; Varzakas, T. Biotechnological Addition of β-Glucans from Cereals, Mushrooms and Yeasts in Foods and Animal Feed. Processes 2021, 9, 1889. [Google Scholar] [CrossRef]
- Seo, G.; Hyun, C.; Choi, S.; Kim, Y.M.; Cho, M. The wound healing effect of four types of beta-glucan. Appl. Biol. Chem. 2019, 62, 20. [Google Scholar] [CrossRef] [Green Version]





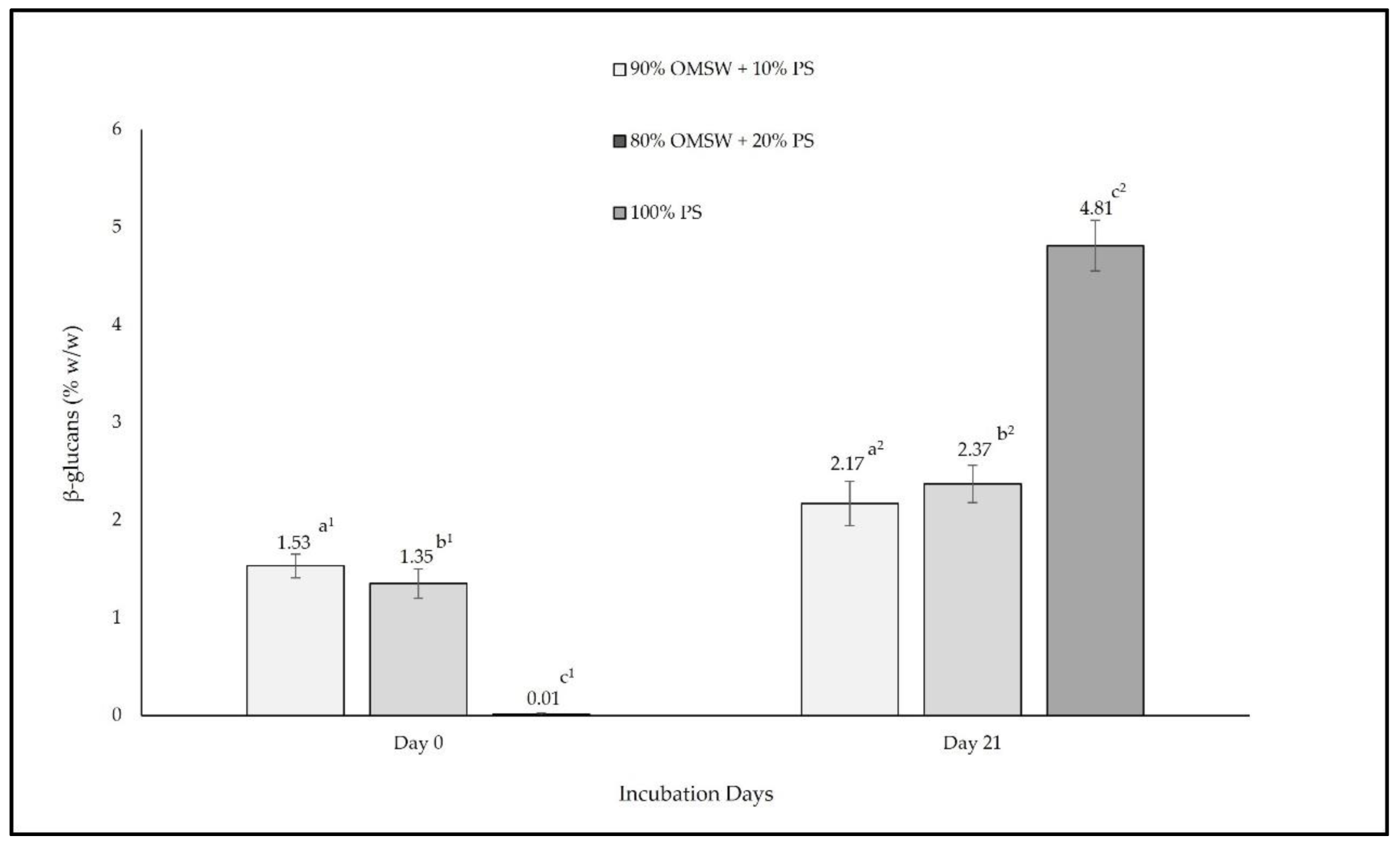
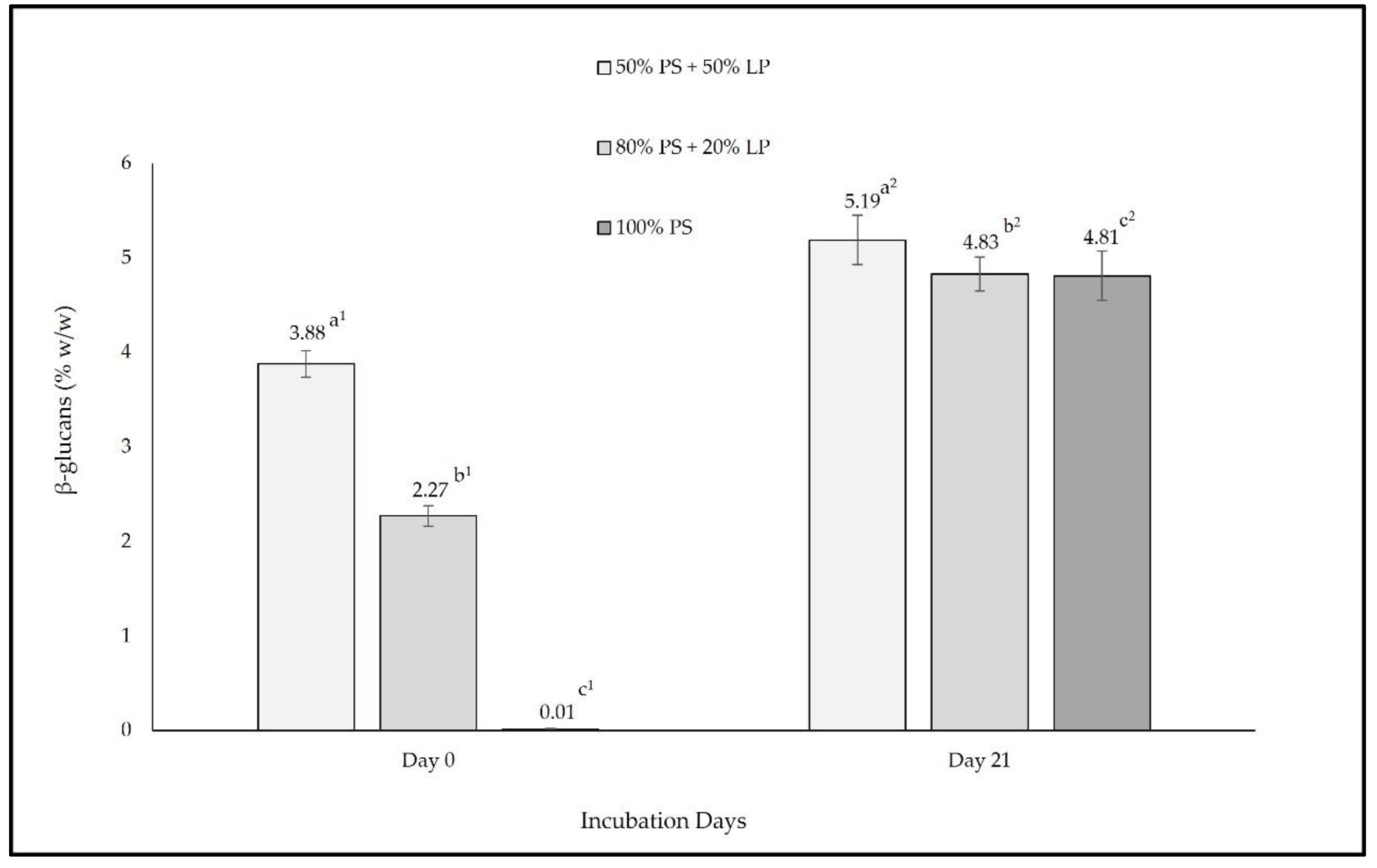
| Ratios (% w/w) | Moisture (%) | TSS (%) | RSS (%) | Ash (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 21 | Day 0 | Day 21 | Day 0 | Day 21 | Day 0 | Day 21 | |
| OMSW + PS | ||||||||
| 90–10 | 41.81 ± 1.11 | 47.96 ± 0.95 | 6.64 ± 0.19 a1 | 13.65 ± 0.29 a2 | 0.66 ± 0.02 b1 | 2.04 ± 0.17 b2 | 3.63 ± 0.18 | 3.63 ± 0.19 |
| 80–20 | 48.68 ± 0.02 | 51.32 ± 3.03 | 10.74 ± 0.27 | 8.16 ± 0.96 | 0.77 ± 0.05 | 0.75 ± 0.15 | 3.59 ± 0.19 | 3.68 ± 0.37 |
| PS | ||||||||
| 100 | 51.97 ± 0.60 | 59.09 ± 5.71 | 13.47 ± 0.84 | 19.32 ± 0.74 | 1.56 ± 0.09 c1 | 0.38 ± 0.02 c2 | 1.42 ± 0.02 | 1.57 ± 0.06 |
| PS + LP | ||||||||
| 50–50 | 64.51 ± 0.12 | 66.00 ± 2.98 | 22.48 ± 1.40 d1 | 10.90 ± 1.58 d2 | 1.91 ± 0.28 | 1.78 ± 0.18 | 1.56 ± 0.22 | 1.81 ± 0.13 |
| 80–20 | 55.24 ± 1.14 | 62.29 ± 9.12 | 27.92 ± 1.67 | 19.60 ± 0.24 | 2.54 ± 0.98 e1 | 1.64 ± 0.01 e2 | 0.93 ± 0.13 f1 | 1.62 ± 0.10 f2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eliopoulos, C.; Markou, G.; Kremmyda, A.; Haroutounian, S.A.; Arapoglou, D. Enrichment of Pistachio Shell with Olive Mill Waste or Lathyrus clymenum Pericarp Mixtures via Solid State Fermentation with Pleurotus ostreatus. Fermentation 2022, 8, 59. https://doi.org/10.3390/fermentation8020059
Eliopoulos C, Markou G, Kremmyda A, Haroutounian SA, Arapoglou D. Enrichment of Pistachio Shell with Olive Mill Waste or Lathyrus clymenum Pericarp Mixtures via Solid State Fermentation with Pleurotus ostreatus. Fermentation. 2022; 8(2):59. https://doi.org/10.3390/fermentation8020059
Chicago/Turabian StyleEliopoulos, Christos, Giorgos Markou, Alexandra Kremmyda, Serkos A. Haroutounian, and Dimitrios Arapoglou. 2022. "Enrichment of Pistachio Shell with Olive Mill Waste or Lathyrus clymenum Pericarp Mixtures via Solid State Fermentation with Pleurotus ostreatus" Fermentation 8, no. 2: 59. https://doi.org/10.3390/fermentation8020059
APA StyleEliopoulos, C., Markou, G., Kremmyda, A., Haroutounian, S. A., & Arapoglou, D. (2022). Enrichment of Pistachio Shell with Olive Mill Waste or Lathyrus clymenum Pericarp Mixtures via Solid State Fermentation with Pleurotus ostreatus. Fermentation, 8(2), 59. https://doi.org/10.3390/fermentation8020059









