Isolation and Molecular Characterization of Processed Soybean Waste for the Development of Synbiotic Yogurt
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Study
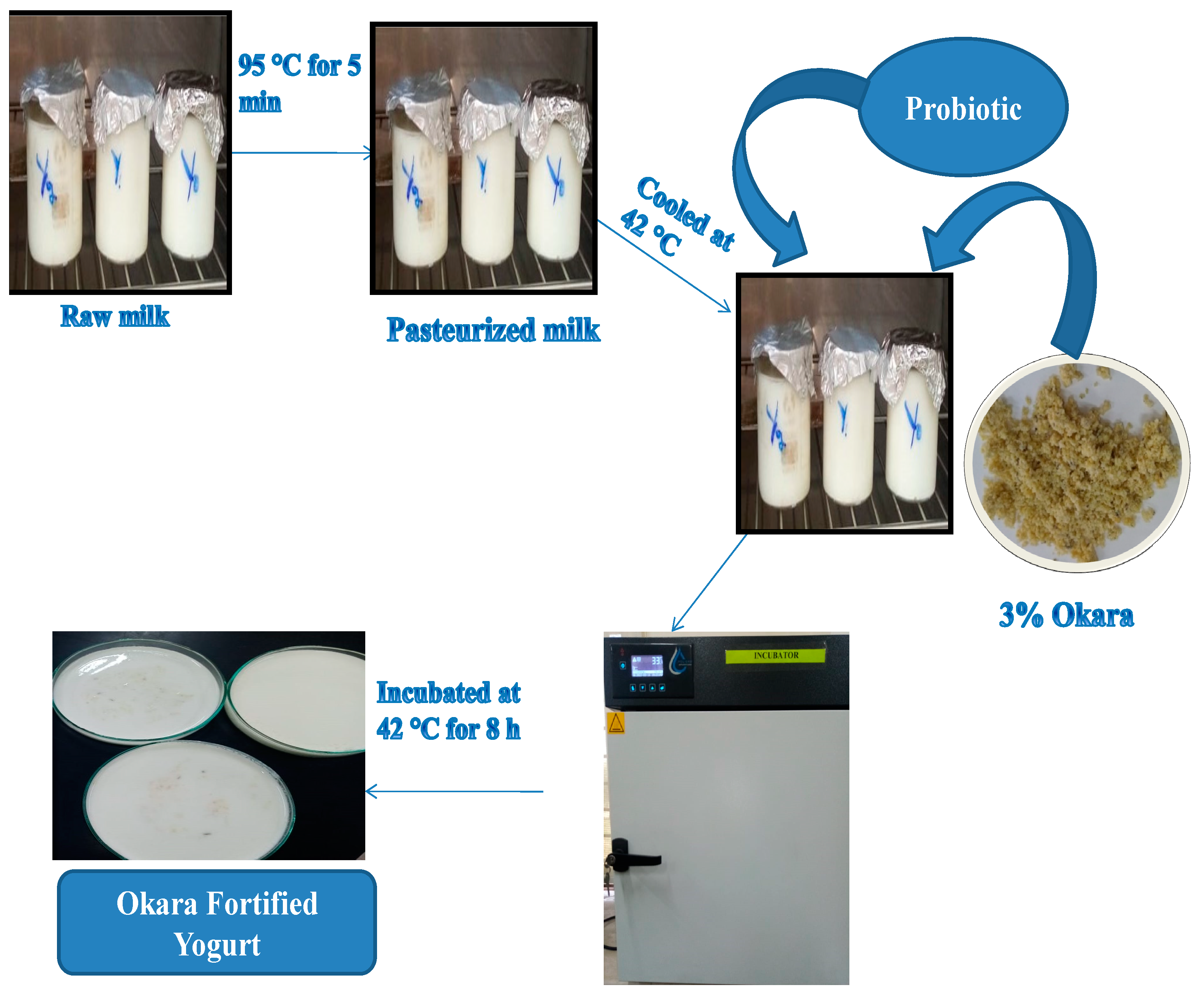
2.2. Isolation of Soybean By-Product (Okara)
2.3. Compositional and Molecular Characterization of Okara Flour
2.4. Preparation of Okara Fortified Yogurt
2.5. Physicochemical Analysis of Yogurt
2.6. Antioxidant Analysis
2.6.1. Total Phenolic Contents
2.6.2. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Activity
2.6.3. 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) Activity
2.7. Microbiological Analysis
2.7.1. Total Viable Count (TVC)
2.7.2. Probiotic Count (PC)
2.8. Sensory Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Compositional Analysis of Okara Flour
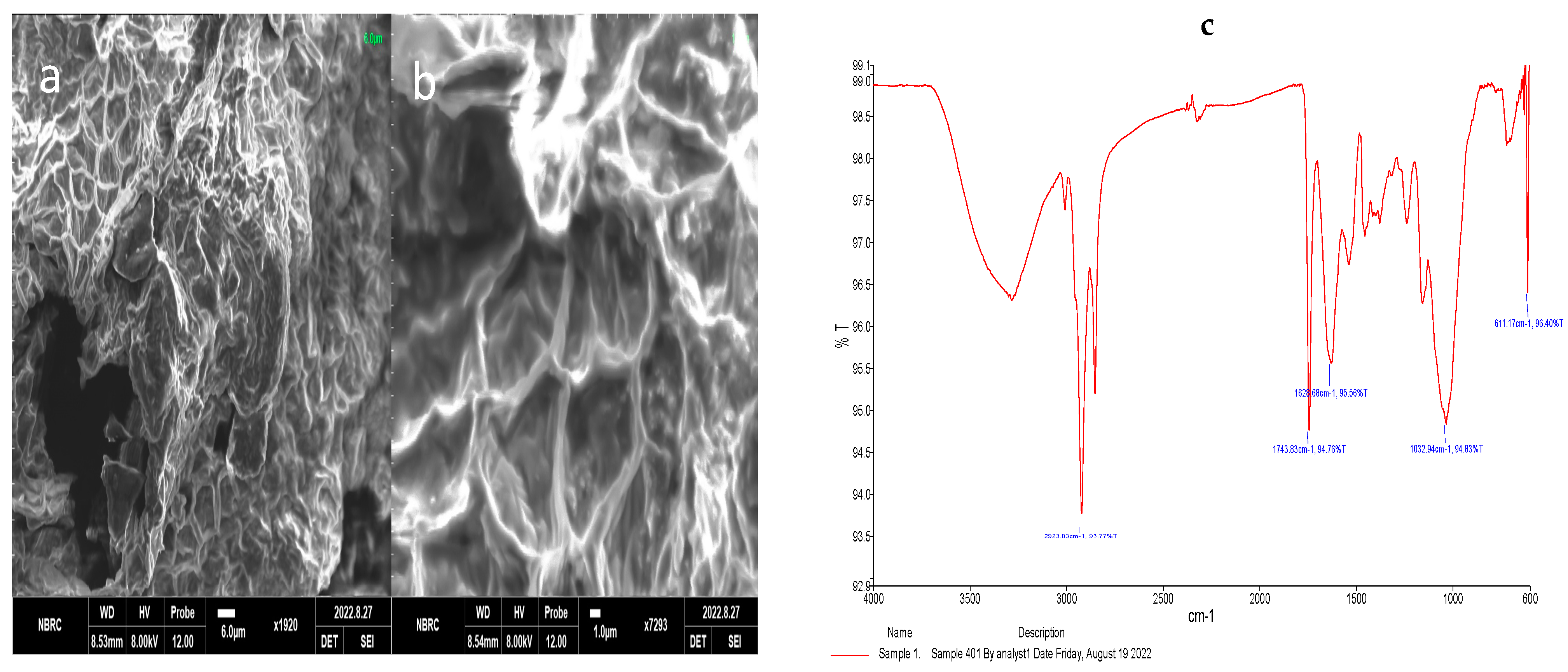
3.2. Scanning Electron Microscope (SEM) of Okara Flour
3.3. Fourier Transform Infrared Radiation (FT-IR) of Okara Flour
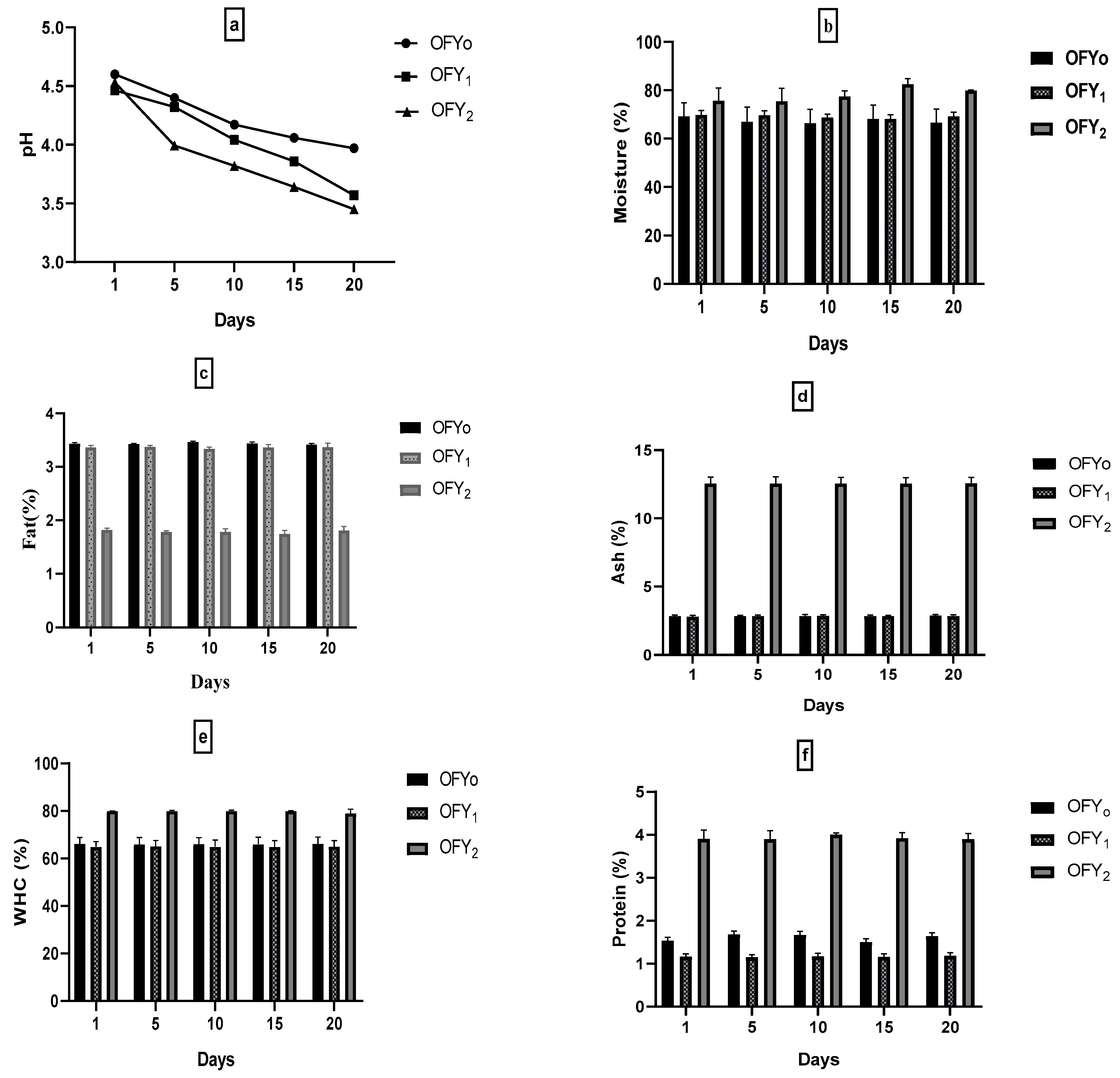
3.4. Physicochemical Analysis of Yogurt
3.5. Colour Analysis
3.6. Antioxidant Potential
3.7. Microbial Analysis
3.8. Sensory Analysis
4. Conclusions
Novelty of Product
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carvalho, M.J.; Perez-Palacios, T.; Ruiz-Carrascal, J. Physico-chemical and sensory characteristics of freeze-dried and air-dehydrated yogurt foam. LWT-Food Sci. Technol. 2017, 80, 328–334. [Google Scholar] [CrossRef]
- Guimaraes, R.M.; Silva, T.E.; Lemes, A.C.; Boldrin, M.C.F.; da Silva, M.A.P.; Silva, F.G.; Egea, M.B. Okara: A soybean by-product as an alternative to enrich vegetable paste. LWT-Food Sci. Technol. 2018, 92, 593–599. [Google Scholar] [CrossRef]
- Kareb, O.; Champagne, C.P.; Jean, J.; Gomaa, A.; Aïder, M. Effect of electro-activated sweet whey on growth of Bifidobacterium, Lactobacillus, and Streptococcus strains under model growth conditions. Food Res. Int. 2018, 103, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.C.; Bae, H.; Seo, H.G.; Han, S.G. Chia seed extract enhances physiochemical and antioxidant properties of yogurt. J. Dairy Sci. 2019, 102, 4870–4876. [Google Scholar] [CrossRef]
- Gahruie, H.H.; Eskandari, M.H.; Mesbahi, G.; Hanifpour, M.A. Scientific and technical aspects of yogurt fortification: A review. Food Sci. Hum. Wellness 2015, 4, 1–8. [Google Scholar] [CrossRef]
- Balthazar, C.F.; Santillo, A.; Guimarães, J.T.; Capozzi, V.; Russo, P.; Caroprese, M.; Marino, R.; Esmerino, E.A.; Raices, R.S.; Silva, M.C.; et al. Novel milk–juice beverage with fermented sheep milk and strawberry (Fragaria× ananassa): Nutritional and functional characterization. J. Dairy Sci. 2019, 102, 10724–10736. [Google Scholar] [CrossRef]
- McCabe, L.; Britton, R.A.; Parameswaran, N. Prebiotic and probiotic regulation of bone health: Role of the intestine and its microbiome. Curr. Osteopor. Rep. 2015, 13, 363–371. [Google Scholar] [CrossRef]
- Voss, G.B.; Monteiro, M.J.P.; Jauregi, P.; Valente, L.M.; Pintado, M.E. Functional characterisation and sensory evaluation of a novel synbiotic okara beverage. Food Chem. 2021, 340, 127793. [Google Scholar] [CrossRef]
- Sharma, M.; Majumdar, P.K. Occupational lifestyle diseases: An emerging issue. Ind. J. Occup. Environ. Med. 2009, 13, 109. [Google Scholar] [CrossRef]
- Satari, B.; Karimi, K. Citrus processing wastes: Environmental impacts, recent advances, and future perspectives in total valorization. Resour. Conser.Recycl. 2018, 129, 153–167. [Google Scholar] [CrossRef]
- Lusembo, J. Development and Characterization of Shelf Stable Cookies from Soy Bean Residues (Okara). Ph.D. Thesis, Makerere University, Kampala, Uganda, 2021. [Google Scholar]
- Villanueva-Suárez, M.J.; Pérez-Cózar, M.L.; Mateos-Aparicio, I.; Redondo-Cuenca, A. Potential fat-lowering and prebiotic effects of enzymatically treated okara in high-cholesterol-fed Wistar rats. Int. J. Food Sci. Nutri. 2016, 67, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Qiao, M.; Lu, F. Composition, nutrition, and utilization of okara (soybean residue). Food Rev. Int. 2012, 28, 231–252. [Google Scholar] [CrossRef]
- Prestamo, G.; Rupérez, P.; Espinosa-Martos, I.; Villanueva, M.J.; Lasunción, M.A. The effects of okara on rat growth, cecal fermentation, and serum lipids. Eur. Food Res. Technol. 2007, 225, 925–928. [Google Scholar] [CrossRef]
- Kamble, D.B.; Rani, S. Bioactive components, in vitro digestibility, microstructure and application of soybean residue (okara): A review. Legume Sci. 2020, 2, e32. [Google Scholar] [CrossRef]
- Ikram, A.; Qasim Raza, S.; Saeed, F.; Afzaal, M.; Munir, H.; Ahmed, A.; Babar Bin Zahid, M.; Muhammad Anjum, F. Effect of adding Aloe vera jell on the quality and sensory properties of yogurt. Food Sci. Nutr. 2021, 9, 480–488. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis, 17th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Lin, D.; Long, X.; Huang, Y.; Yang, Y.; Wu, Z.; Chen, H.; Zhang, Q.; Wu, D.; Qin, W.; Tu, Z. Effects of microbial fermentation and microwave treatment on the composition, structural characteristics, and functional properties of modified okara dietary fiber. LWT Food Sci. Technol. 2020, 123, 109059. [Google Scholar] [CrossRef]
- Jayarathna, K.P.G.N.M.; Prasanna, P.H.P.; Chandramali, D.V.P.; Vidanarachchi, J.K. Effect of fruit processing waste on physicochemical, microbiological and sensory properties of probiotic set-yoghurt. Sri Lanka J. Anim. Prod. 2018, 12, 49–63. [Google Scholar]
- Grasso, N.; Alonso-Miravalles, L.; O’Mahony, J.A. Composition, Physicochemical and Sensorial Properties of Commercial Plant-Based Yogurts. Foods 2020, 9, 252. [Google Scholar] [CrossRef]
- Roslan, I.N.D.; Kamaruding, N.A.; Ismail, N.; Shaharuddin, S. Sensory Attributes and Other Properties of Yogurt Fortified with Immobilized Lactobacillus Plantarum and Soybean Residue (Okara). Int. J. Probiotics Prebiotics 2021, 16, 1–6. [Google Scholar] [CrossRef]
- Peng, D.; Zahid, H.F.; Ajlouni, S.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF/MS Profiling of Australian Mango Peel By-Product Polyphenols and Their Potential Antioxidant Activities. Processes 2019, 7, 764. [Google Scholar] [CrossRef]
- Marafon, A.P.; Sumi, A.; Alcântara, M.R.; Tamime, A.Y.; De Oliveira, M.N. Optimization of the rheological properties of probiotic yoghurts supplemented with milk proteins. LWT-Food Sci. Technol. 2011, 44, 511–519. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Yoshida, B.Y.; Prudencio, S.H. Physical, chemical, and technofunctional properties of okara modified by a carbohydrase mixture. LWT Food Sci. Technol. 2020, 134, 110141. [Google Scholar] [CrossRef]
- Gurbuz, Z.; Erkaya-Kotan, T.; Şengul, M. Evaluation of physicochemical, microbiological, texture and microstructure characteristics of set-style yoghurt supplemented with quince seed mucilage powder as a novel natural stabiliser. Int. Dairy J. 2021, 114, 104938. [Google Scholar] [CrossRef]
- Anjum, S.; Rana, S.; Dasila, K.; Agnihotri, V.; Pandey, A.; Pande, V. Comparative nutritional and antimicrobial analysis of Himalayan black and yellow soybean and their okara. J. Sci. Food Agric. 2022, 102, 5358–5367. [Google Scholar] [CrossRef] [PubMed]
- Quintana, G.; Spínola, V.; Martins, G.N.; Gerbino, E.; Gómez-Zavaglia, A.; Castilho, P.C. Release of health-related compounds during in vitro gastro-intestinal digestion of okara and okara fermented with Lactobacillus plantarum. J. Food Sci. Technol. 2020, 57, 1061–1070. [Google Scholar] [CrossRef]
- Vital, A.C.P.; Croge, C.; da Silva, D.F.; Araújo, P.J.; Gallina, M.Z.; Matumoto-Pintro, P.T. Okara residue as source of antioxidants against lipid oxidation in milk enriched with omega-3 and bioavailability of bioactive compounds after in vitro gastrointestinal digestion. J. Food Sci. Technol. 2018, 55, 1518–1524. [Google Scholar] [CrossRef]
- Ibrahim, I.N.; Kamaruding, N.A.; Ismail, N.; Shaharuddin, S. Value addition to ice cream by fortification with okara and probiotic. J. Food Process. Preser. 2022, 46, e16253. [Google Scholar] [CrossRef]


| Parameter | Okara Flour |
|---|---|
| Protein% | 15.68 ± 0.24 |
| Fat% | 7.54 ± 0.08 |
| Ash% | 10.85 ± 0.15 |
| Insoluble fiber% | 40.76 ± 0.16 |
| Soluble fiber% | 5.97 ± 0.08 |
| Total fiber% | 46.73 ± 0.23 |
| Sample | Lightness (L*) | Redness-Greenness (a*) | Yellowness-Blueness (b*) |
|---|---|---|---|
| OFYo | 97.56 ± 0.4 b | −4.42 ± 0.09 b | 10.75 ± 0.16 b |
| OFY₁ | 97.78 ± 0.2 a | −4.35 ± 0.08 c | 10.70 ± 0.14 c |
| OFY₂ | 97.56 ± 0.2 b | −4.60 ± 0.09 a | 11.35 ± 0.09 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asghar, A.; Afzaal, M.; Nosheen, F.; Saeed, F.; Nayik, G.A.; AL-Farga, A.; Alansari, W.S.; Eskandrani, A.A.; Shamlan, G. Isolation and Molecular Characterization of Processed Soybean Waste for the Development of Synbiotic Yogurt. Fermentation 2022, 8, 622. https://doi.org/10.3390/fermentation8110622
Asghar A, Afzaal M, Nosheen F, Saeed F, Nayik GA, AL-Farga A, Alansari WS, Eskandrani AA, Shamlan G. Isolation and Molecular Characterization of Processed Soybean Waste for the Development of Synbiotic Yogurt. Fermentation. 2022; 8(11):622. https://doi.org/10.3390/fermentation8110622
Chicago/Turabian StyleAsghar, Aasma, Muhammad Afzaal, Farhana Nosheen, Farhan Saeed, Gulzar Ahmad Nayik, Ammar AL-Farga, Wafa S. Alansari, Areej A. Eskandrani, and Ghalia Shamlan. 2022. "Isolation and Molecular Characterization of Processed Soybean Waste for the Development of Synbiotic Yogurt" Fermentation 8, no. 11: 622. https://doi.org/10.3390/fermentation8110622
APA StyleAsghar, A., Afzaal, M., Nosheen, F., Saeed, F., Nayik, G. A., AL-Farga, A., Alansari, W. S., Eskandrani, A. A., & Shamlan, G. (2022). Isolation and Molecular Characterization of Processed Soybean Waste for the Development of Synbiotic Yogurt. Fermentation, 8(11), 622. https://doi.org/10.3390/fermentation8110622









