Influence of Biochar on Physico-Chemical, Microbial Community and Maturity during Biogas Residue Aerobic Composting Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Feedstock
2.2. Composting Process
2.3. Analytical Methods
2.4. Data Analysis
3. Results and Discussion
3.1. Variations of Physico-Chemical Parameters during the Composting Process
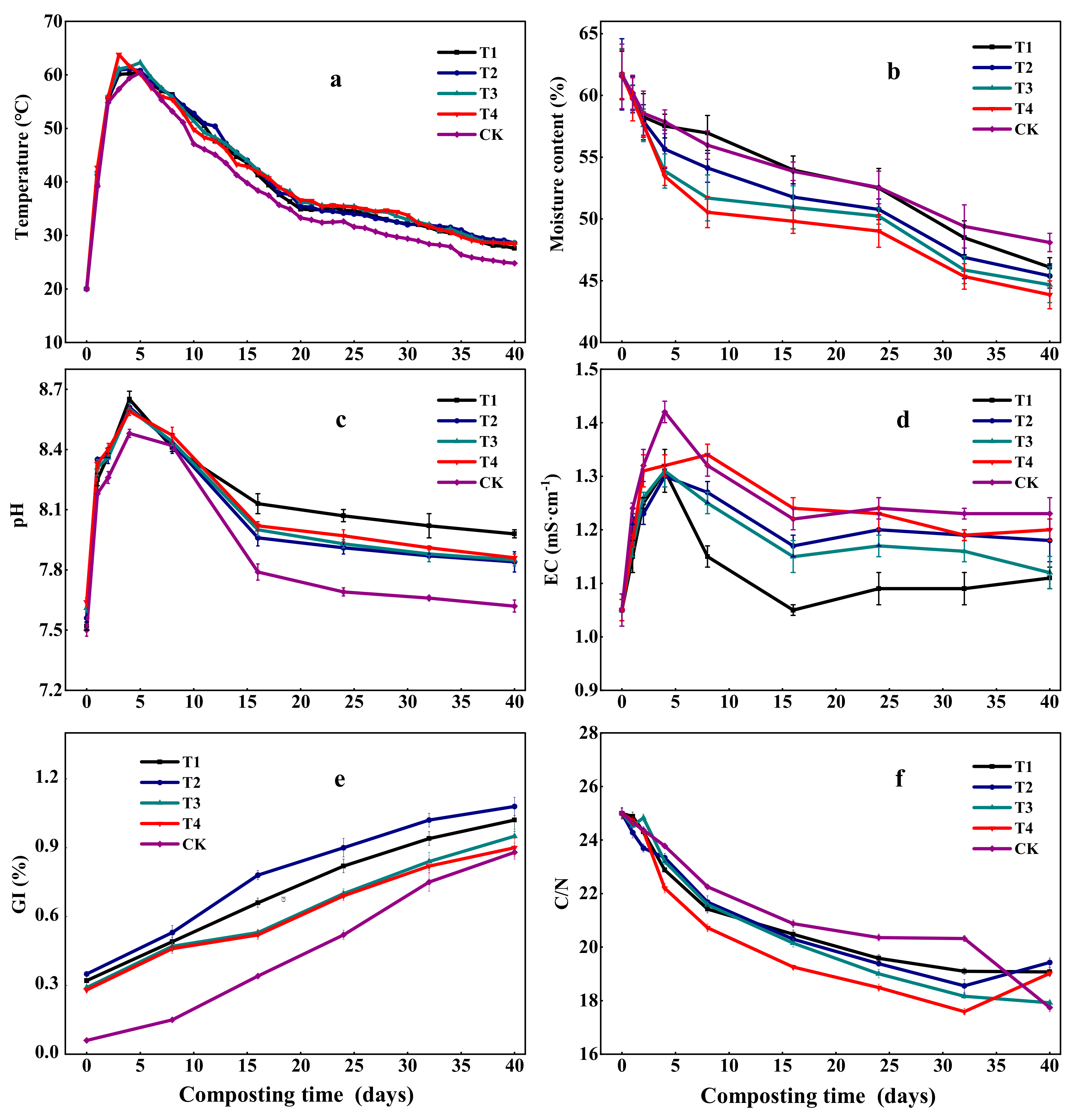
3.1.1. Temperature
3.1.2. Moisture Content
3.1.3. pH
3.1.4. EC
3.1.5. GI
3.1.6. C/N
3.2. Variations of Physico-Chemical Parameters during the Composting Process
3.2.1. Nitrogen Variations
3.2.2. DOC Variations
3.3. Microbiological Analysis
3.3.1. Bacteria
3.3.2. Actinomycete
3.3.3. Fungi
3.4. Correlation Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GI | Germination index |
| EC | Electrical conductivity |
| BS | Biogas residue |
| HAs | Humic acids |
| OM | Organic matter |
| LM | Livestock manure |
| PS | Particle size |
| BET | Specific surface area |
| CS | Corn straw |
| MC | Moisture content |
| TC | Total carbon |
| TN | Total nitrogen |
| DOC | Dissolved organic carbon |
| VFAs | Volatile fatty acids |
References
- Arthurson, V. Closing the global energy and nutrient cycles through application of biogas residue to agricultural land–potential benefits and drawbacks. Energies 2009, 2, 226–242. [Google Scholar] [CrossRef]
- Nkoa, R. Agricultural benefits and environmental risks of soil fertilization with anaerobic digestates: A review. Agron. Sustain. Dev. 2014, 34, 473–492. [Google Scholar] [CrossRef]
- Zorpas, A.A. Recycle and reuse of natural zeolites from composting process: A 7-year project. Desalin. Water Treat. 2014, 52, 6847–6857. [Google Scholar] [CrossRef]
- Tsapekos, P.; Alvarado-Morales, M.; Kougias, P.G.; Treu, L.; Angelidaki, I. Enhancing anaerobic digestion of agricultural residues by microaerobic conditions. Biomass Convers. Biorefin. 2021, 11, 2325–2333. [Google Scholar] [CrossRef]
- Song, C.; Li, M.; Wei, Z.; Jia, X.; Xi, B.; Liu, D.; Zhu, C.; Pan, H. Effect of inoculation with multiple composite microorganisms on characteristics of humic fractions and bacterial community structure during biogas residue and livestock manure co-composting. J. Chem. Technol. Biotechnol. 2016, 91, 155–164. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, Y.; Xu, X.; Meng, X.; Qu, J.; Wang, Z.; Liu, C.; Qu, B. Preparation, characterization and application of activated carbon from corn cob by KOH activation for removal of Hg (II) from aqueous solution. Bioresour. Technol. 2020, 306, 123154. [Google Scholar] [CrossRef]
- Dong, X.; Singh, B.P.; Li, G.; Lin, Q.; Zhao, X. Biochar application constrained native soil organic carbon accumulation from wheat residue inputs in a long-term wheat-maize cropping system. Agric. Ecosyst. Environ. 2018, 252, 200–207. [Google Scholar] [CrossRef]
- Meng, X.; Dai, J.; Zhang, Y.; Wang, X.; Zhu, W.; Yuan, X.; Yuan, H.; Cui, Z. Composted biogas residue and spent mushroom substrate as a growth medium for tomato and pepper seedlings. J. Environ. Manag. 2018, 216, 62–69. [Google Scholar] [CrossRef]
- Chen, W.; Liao, X.; Wu, Y.; Liang, J.B.; Mi, J.; Huang, J.; Zhang, H.; Wu, Y.; Qiao, Z.; Li, X.; et al. Effects of different types of biochar on methane and ammonia mitigation during layer manure composting. Waste Manag. 2017, 61, 506–515. [Google Scholar] [CrossRef]
- Taghizadeh-Toosi, A.; Clough, T.J.; Condron, L.M.; Sherlock, R.R.; Anderson, C.R.; Craigie, R.A. Biochar incorporation into pasture soil suppresses in situ nitrous oxide emissions from ruminant urine patches. J. Environ. Qual. 2011, 40, 468–476. [Google Scholar] [CrossRef]
- Zhang, J.; Lü, F.; Shao, L.; He, P. The use of biochar-amended composting to improve the humification and degradation of sewage sludge. Bioresour. Technol. 2014, 168, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Stirling, E.; Smernik, R.; Macdonald, L.; Cavagnaro, T. Fire influences needle decomposition: Tipping point in Pinus radiata carbon chemistry and soil nitrogen transformations. Soil Biol. Biochem. 2019, 135, 361–368. [Google Scholar] [CrossRef]
- Qu, J.; Sun, Y.; Awasthi, M.K.; Liu, Y.; Xu, X.; Meng, X.; Zhang, H. Effect of different aerobic hydrolysis time on the anaerobic digestion characteristics and energy consumption analysis. Bioresour. Technol. 2021, 320, 124332. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, B.; Nguyen, D.D.; Chaudhary, D.K.; Chang, S.W.; Kim, J.; Lee, S.R.; Shin, J.; Jeon, B.H.; Chung, S.; Lee, J. Influence of biochar on physico-chemical and microbial community during swine manure composting process. J. Environ. Manag. 2019, 232, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Shutao, W.; Jin, Z.; Tong, X. Biochar influences the microbial community structure during tomato stalk composting with chicken manure. Bioresour. Technol. 2014, 154, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Xu, X.; Qiu, X.; Zhang, J. Biochar influences the succession of microbial communities and the metabolic functions during rice straw composting with pig manure. Bioresour. Technol. 2019, 272, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Liu, L.; Ge, S.; Xue, Q.; Li, J.; Wan, Y.; Hui, X. Coupling model of aerobic waste degradation considering temperature, initial moisture content and air injection volume. Waste Manag. Res. 2018, 36, 277–287. [Google Scholar] [CrossRef]
- Shangguan, H.; Fu, T.; Wu, J.; Tang, J.; Zeng, R.J.; Zhou, S. Use of an in situ thermoelectric generator for electric field-assisted aerobic composting. Sci. Total Environ. 2020, 742, 140618. [Google Scholar] [CrossRef]
- Janczak, D.; Malińska, K.; Czekała, W.; Cáceres, R.; Lewicki, A.; Dach, J. Biochar to reduce ammonia emissions in gaseous and liquid phase during composting of poultry manure with wheat straw. Waste Manag. 2017, 66, 36–45. [Google Scholar] [CrossRef]
- Jain, M.S.; Jambhulkar, R.; Kalamdhad, A.S. Biochar amendment for batch composting of nitrogen rich organic waste: Effect on degradation kinetics, composting physics and nutritional properties. Bioresour. Technol. 2018, 253, 204–213. [Google Scholar] [CrossRef]
- Yu, H.; Xie, B.; Khan, R.; Shen, G. The changes in carbon, nitrogen components and humic substances during organic-inorganic aerobic co-composting. Bioresour. Technol. 2019, 271, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, M.K.; Wang, Q.; Huang, H.; Li, R.; Shen, F.; Lahori, A.H.; Wang, P.; Guo, D.; Guo, Z.; Jiang, S.; et al. Effect of biochar amendment on greenhouse gas emission and bio-availability of heavy metals during sewage sludge co-composting. J. Clean. Prod. 2016, 135, 829–835. [Google Scholar] [CrossRef]
- Meng, X.; Liu, B.; Zhang, H.; Wu, J.; Yuan, X.; Cui, Z. Co-composting of the biogas residues and spent mushroom substrate: Physicochemical properties and maturity assessment. Bioresour. Technol. 2019, 276, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, Z.; Sun, Y.; Yang, G. One-pot pyrolysis route to Fe− N-Doped carbon nanosheets with outstanding electrochemical performance as cathode materials for microbial fuel cell. Int. J. Agric. Biol. Eng. 2020, 13, 207–214. [Google Scholar] [CrossRef]
- Ginebra, M.; Muñoz, C.; Calvelo-Pereira, R.; Doussoulin, M.; Zagal, E. Biochar impacts on soil chemical properties, greenhouse gas emissions and forage productivity: A field experiment. Sci. Total Environ. 2022, 806, 150465. [Google Scholar] [CrossRef]
- Meng, X.; Yan, J.; Zuo, B.; Wang, Y.; Yuan, X.; Cui, Z. Full-scale of composting process of biogas residues from corn stover anaerobic digestion: Physical-chemical, biology parameters and maturity indexes during whole process. Bioresour. Technol. 2020, 302, 122742. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, G.; Sun, H.; Zhou, S.; Zou, G. Straw biochar hastens organic matter degradation and produces nutrient-rich compost. Bioresour. Technol. 2016, 200, 876–883. [Google Scholar] [CrossRef]
- Du, J.; Zhang, Y.; Hu, B.; Qv, M.; Ma, C.; Wei, M.; Zhang, H. Insight into the potentiality of big biochar particle as an amendment in aerobic composting of sewage sludge. Bioresour. Technol. 2019, 288, 121469. [Google Scholar] [CrossRef]
- Khan, N.; Clark, I.; Sánchez-Monedero, M.A.; Shea, S.; Meier, S.; Bolan, N. Maturity indices in co-composting of chicken manure and sawdust with biochar. Bioresour. Technol. 2014, 168, 245–251. [Google Scholar] [CrossRef]
- Luo, Y.; Liang, J.; Zeng, G.; Chen, M.; Mo, D.; Li, G.; Zhang, D. Seed germination test for toxicity evaluation of compost: Its roles, problems and prospects. Waste Manag. 2018, 71, 109–114. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Liu, J.; Ishii, M.; Igarashi, Y.; Cui, Z. Effect of oxygen concentration on the composting process and maturity. Compost Sci. Util. 2007, 15, 184–190. [Google Scholar] [CrossRef]
- Ma, S.; Xiong, J.; Cui, R.; Sun, X.; Han, L.; Xu, Y.; Kan, Z.; Gong, X.; Huang, G. Effects of intermittent aeration on greenhouse gas emissions and bacterial community succession during large-scale membrane-covered aerobic composting. J. Clean. Prod. 2020, 266, 121551. [Google Scholar] [CrossRef]
- Santos, A.; Bustamante, M.; Tortosa, G.; Moral, R.; Bernal, M. Gaseous emissions and process development during composting of pig slurry: The influence of the proportion of cotton gin waste. J. Clean. Prod. 2016, 112, 81–90. [Google Scholar] [CrossRef]
- Vuorinen, A.H.; Saharinen, M.H. Evolution of microbiological and chemical parameters during manure and straw co-composting in a drum composting system. Agric. Ecosyst. Environ. 1997, 66, 19–29. [Google Scholar] [CrossRef]
- Bernal, M.P.; Alburquerque, J.A.; Moral, R. Composting of animal manures and chemical criteria for compost maturity assessment. A review. Bioresour. Technol. 2009, 100, 5444–5453. [Google Scholar] [CrossRef]
- Li, Y.; Shao, J.; Wang, X.; Deng, Y.; Yang, H.; Chen, H. Characterization of modified biochars derived from bamboo pyrolysis and their utilization for target component (furfural) adsorption. Energy Fuels 2014, 28, 5119–5127. [Google Scholar] [CrossRef]
- Peng, P.; Lang, Y.H.; Wang, X.M. Adsorption behavior and mechanism of pentachlorophenol on reed biochars: pH effect, pyrolysis temperature, hydrochloric acid treatment and isotherms. Ecol. Eng. 2016, 90, 225–233. [Google Scholar] [CrossRef]
- Yan, H.; Niu, Q.; Zhu, Q.; Wang, S.; Meng, Q.; Li, G.; Li, X.; Ma, C.; Li, Q. Biochar reinforced the populations of cbbL-containing autotrophic microbes and humic substance formation via sequestrating CO2 in composting process. J. Biotechnol. 2021, 333, 39–48. [Google Scholar] [CrossRef]
- Liu, N.; Zhou, J.; Han, L.; Huang, G. Characterization of lignocellulosic compositions’ degradation during chicken manure composting with added biochar by phospholipid fatty acid (PLFA) and correlation analysis. Sci. Total Environ. 2017, 586, 1003–1011. [Google Scholar] [CrossRef]
- Ren, G.; Xu, X.; Qu, J.; Zhu, L.; Wang, T. Evaluation of microbial population dynamics in the co-composting of cow manure and rice straw using high throughput sequencing analysis. World J. Microbiol. Biotechnol. 2016, 32, 101. [Google Scholar] [CrossRef]
- Chan, M.T.; Selvam, A.; Wong JW, C. Reducing nitrogen loss and salinity during ‘struvite’food waste composting by zeolite amendment. Bioresour. Technol. 2016, 200, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Kawase, Y. Aerobic composting of waste activated sludge: Kinetic analysis for microbiological reaction and oxygen consumption. Waste Manag. 2006, 26, 49–61. [Google Scholar] [CrossRef]
- Ko, H.G.; Park, S.H.; Kim, S.H.; Park, H.G.; Park, W.M. Detection and recovery of hydrolytic enzymes from spent compost of four mushroom species. Folia Microbiol. 2005, 50, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Fang, C.; Sun, X.; Han, L.; He, X.; Huang, G. Bacterial community succession during pig manure and wheat straw aerobic composting covered with a semi-permeable membrane under slight positive pressure. Bioresour. Technol. 2018, 259, 221–227. [Google Scholar] [CrossRef]
- Ravindran, B.; Mnkeni, P.N.S. Bio-optimization of the carbon-to-nitrogen ratio for efficient vermicomposting of chicken manure and waste paper using Eisenia fetida. Environ. Sci. Pollut. Res. 2016, 23, 16965–16976. [Google Scholar] [CrossRef] [PubMed]
- Ryckeboer, J.; Mergaert, J.; Vaes, K.; Klammer, S.; De Clercq, D.; Coosemans, J.; Insam, H.; Swings, J. A survey of bacteria and fungi occurring during composting and self-heating processes. Ann. Microbiol. 2003, 53, 349–410. [Google Scholar]
- Feng, L.; Li, X.; Zhen, X.; Dong, H.; Zheng, J.; Wang, Y. Study of enzyme activity changing pattern in livestock manures composting. Appl. Ecol. Env. Res. 2019, 17, 6581–6593. [Google Scholar] [CrossRef]
- Fang, Y.; Jia, X.; Chen, L.; Lin, C.; Zhang, H.; Chen, J. Effect of thermotolerant bacterial inoculation on the microbial community during sludge composting. Can. J. Microbiol. 2019, 65, 750–761. [Google Scholar] [CrossRef]
- Prost, K.; Borchard, N.; Siemens, J.; Kautz, T.; Séquaris, J.M.; Möller, A.; Amelung, W. Biochar affected by composting with farmyard manure. J. Environ. Qual. 2013, 42, 164–172. [Google Scholar] [CrossRef]
- Okamoto, K.; Sugita, Y.; Nishikori, N.; Nitta, Y.; Yanase, H. Characterization of two acidic β-glucosidases and ethanol fermentation in the brown rot fungus Fomitopsis palustris. Enzyme Microb. Technol. 2011, 48, 359–364. [Google Scholar] [CrossRef]
- Straathof, A.L.; Comans RN, J. Input materials and processing conditions control compost dissolved organic carbon quality. Bioresour. Technol. 2015, 179, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Schmidt, S.; Qin, W.; Li, J.; Li, G.; Zhang, W. Towards the circular nitrogen economy–A global meta-analysis of composting technologies reveals much potential for mitigating nitrogen losses. Sci. Total Environ. 2020, 704, 135401. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, M.K.; Duan, Y.; Awasthi, S.K.; Liu, T.; Zhang, Z. Effect of biochar and bacterial inoculum additions on cow dung composting. Bioresour. Technol. 2020, 297, 122407. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, X. Changes in physical, chemical, and microbiological properties during the two-stage co-composting of green waste with spent mushroom compost and biochar. Bioresour. Technol. 2014, 171, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Li, B.; Chen, K.; Zhao, C.; Xu, L.; Yang, Z.; Sun, Q.; Chandio, F.A.; Wu, G. Rice straw addition and biological inoculation promote the maturation of aerobic compost of rice straw biogas residue. Biomass Convers. Biorefin. 2021, 11, 1885–1896. [Google Scholar] [CrossRef]
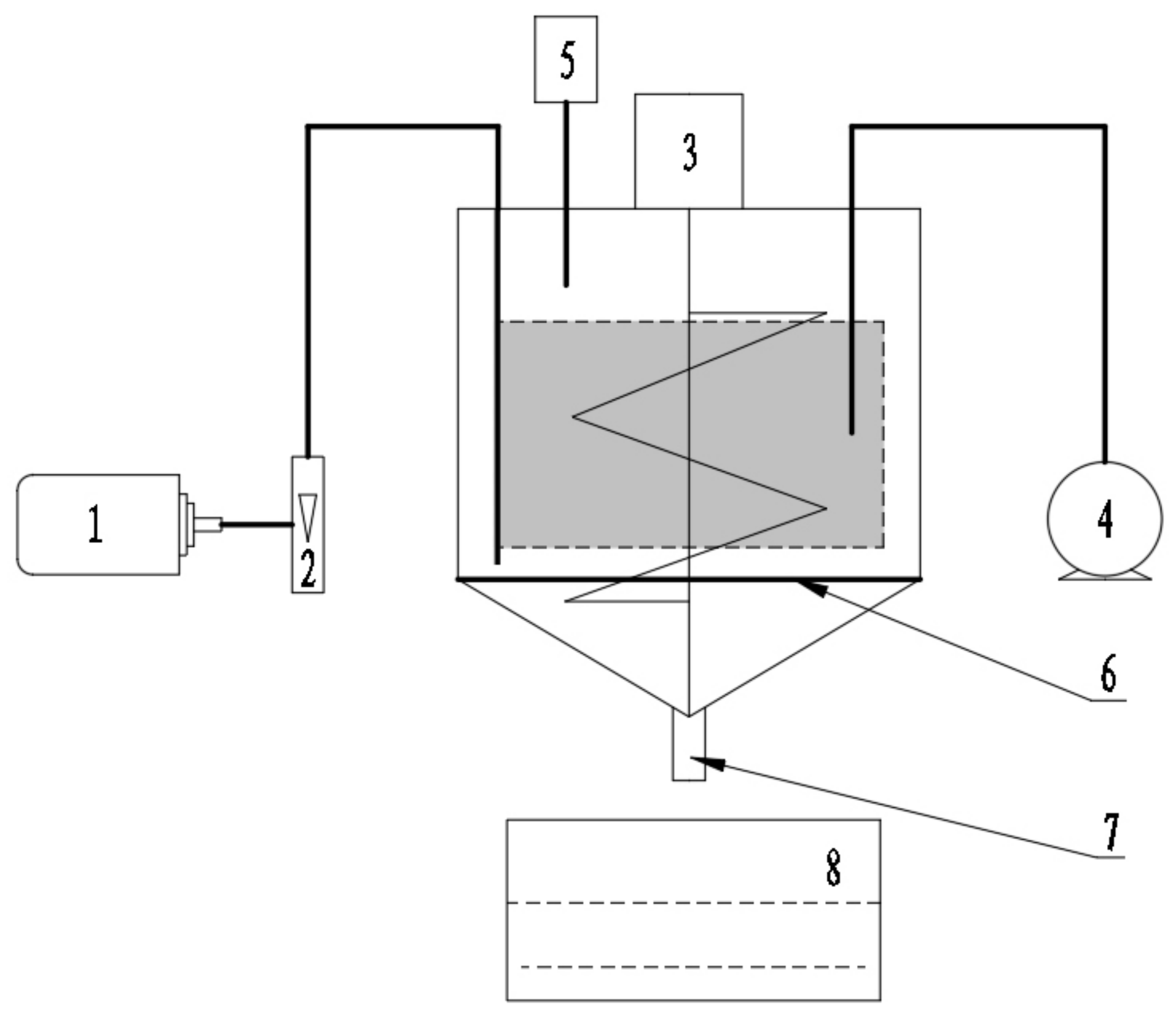


| Parameters | Biogas Residue | Pig Manure | Corn Stalk | Biochar |
|---|---|---|---|---|
| TC (%) | 29.92 ± 0.58 | 8.52 ± 0.58 | 41.53 ± 2.18 | 46.14 ± 1.76 |
| TN (%) | 1.53 ± 0.03 | 0.54 ± 0.03 | 0.78 ± 0.06 | 0.94 ± 0.03 |
| Moisture content (%) | 68.20 ± 1.35 | 69.34 ± 2.86 | 10.44 ± 0.51 | 7.42 ± 0.17 |
| pH | 7.46 ± 0.37 | 7.48 ± 0.32 | 6.96 ± 0.35 | 9.90 ± 0.37 |
| EC (mS · cm−1) | 12.19 ± 0.48 | 5.26 ± 0.15 | 2.58 ± 0.20 | 12.14 ± 0.48 |
| OM (%) | 59.58 ± 1.26 | 46.05 ± 1.48 | 90.82 ± 3.56 | 64.23 ± 2.87 |
| BET (m2/g) | NA | NA | NA | 120.24 ± 6.11 |
| Treatment | Biogas Residue (kg) | Corn Stalk (kg) | Pig Manure (kg) | Biochar (kg) | Biochar Ratio (%) |
|---|---|---|---|---|---|
| T1 | 8.00 | 1.05 | 0.85 | 0.10 | 2.5 |
| T2 | 8.00 | 0.93 | 0.87 | 0.20 | 5 |
| T3 | 8.00 | 0.83 | 0.88 | 0.29 | 7.5 |
| T4 | 8.00 | 0.74 | 0.88 | 0.38 | 10 |
| CK | 8.00 | 1.17 | 0.83 | - | - |
| Nitrogen Variations | Composting Time (Days) | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 4 | 8 | 16 | 32 | 40 | ||
| NH4+-N (g · kg−1) | T1 | 2.11 ± 0.09 b | 2.48 ± 0.12 b | 3.09 ± 0.14 b | 3.27 ± 0.16 c | 2.58 ± 0.11 c | 1.86 ± 0.06 b | 1.01 ± 0.04 a |
| T2 | 2.33 ± 0.11 a | 2.44 ± 0.08 b | 3.11 ± 0.17 b | 4.76 ± 0.22 b | 3.49 ± 0.18 ab | 2.12 ± 0.09 a | 1.15 ± 0.06 a | |
| T3 | 2.46 ± 0.08 a | 2.43 ± 0.10 b | 3.11 ± 0.18 b | 5.04 ± 0.24 a | 3.54 ± 0.09 b | 2.14 ± 0.10 a | 1.07 ± 0.08 a | |
| T4 | 2.44 ± 0.14 a | 2.83 ± 0.11 a | 3.61 ± 0.13 a | 5.29 ± 0.23 a | 4.01 ± 0.19 a | 2.35 ± 0.07 a | 1.17 ± 0.03 a | |
| NO3−-N (mg · kg−1) | T1 | 2.12 ± 0.07 b | 2.47 ± 0.13 b | 3.13 ± 0.15 b | 3.32 ± 0.17 c | 2.42 ± 0.13 c | 1.76 ± 0.08 b | 0.96 ± 0.03 a |
| T2 | 16.32 ± 0.87 c | 18.06 ± 0.90 c | 11.38 ± 0.44 d | 11.19 ± 0.55 c | 112.11 ± 4.78 a | 237.80 ± 10.58 c | 264.77 ± 10.28 b | |
| T3 | 17.14 ± 0.64 b | 19.88 ± 0.81 b | 14.26 ± 0.59 b | 16.70 ± 0.62 b | 89.55 ± 3.64 c | 265.32 ± 17.62 b | 283.49 ± 13.74 b | |
| T4 | 17.26 ± 0.95 ab | 20.17 ± 1.21 a | 14.70 ± 0.86 b | 9.02 ± 0.47 d | 101.66 ± 5.02 b | 286.79 ± 14.37 a | 296.87 ± 15.93 ab | |
| TN(%) | T1 | 17.58 ± 0.54 a | 20.32 ± 0.78 a | 15.78 ± 0.64 a | 13.95 ± 0.59 c | 86.79 ± 2.95 c | 298.35 ± 16.68 a | 318.36 ± 16.71 a |
| T2 | 16.55 ± 0.62 c | 9.55 ± 0.60 d | 13.59 ± 0.41 c | 39.27 ± 2.37 a | 51.02 ± 3.77 d | 93.58 ± 4.60 d | 155.42 ± 7.55 c | |
| T3 | 1.22 ± 0.06 a | 1.13 ± 0.05 a | 1.16 ± 0.05 a | 1.27 ± 0.04 a | 1.39 ± 0.07 a | 1.48 ± 0.07 a | 1.61 ± 0.08 a | |
| T4 | 2.33 ± 0.11 a | 2.44 ± 0.08 b | 3.11 ± 0.17 b | 4.76 ± 0.22 b | 3.49 ± 0.18 ab | 2.12 ± 0.09 a | 1.15 ± 0.06 a | |
| EC | TN | NH4+-N | NH4+-N/NO3−-N | DOC | DOC/TN | C/N | T | GI | |
|---|---|---|---|---|---|---|---|---|---|
| EC | 1 | ||||||||
| TN | −0.656 | 1 | |||||||
| NH4+-N | 0.909 * | −0.903 * | 1 | ||||||
| NH4+-N/NO3−-N | 0.686 | −0.981 ** | 0.892 * | 1 | |||||
| DOC | −0.261 | 0.905 * | −0.625 | −0.884 * | 1 | ||||
| DOC/TN | 0.012 | 0.554 | −0.417 | −0.606 | 0.940 * | 1 | |||
| C/N | 0.700 | −0.600 | 0.325 | 0.503 | −0.255 | −0.009 | 1 | ||
| T | 0.763 | −0.549 | 0.343 | 0.458 | −0.121 | 0.149 | 0.982 ** | 1 | |
| GI | −0.989 ** | 0.807 * | −0.882 * | −0.961 ** | 0.907 * | −0.408 | 0.929 ** | −0.993 ** | 1 |
| GI | Biochar Content | Composting Raw Materials | Periods | References |
|---|---|---|---|---|
| 1.08 | 5% | BS + CS + pig manure | 40 days | NA |
| 0.89 | 5% | Rice straw + pig manure | 42 days | (Zhou et al., 2019) [16] |
| 1.05 | 12% | Cow manure + wheat straw + bacterial consortium | 42 days | (Awasthi et al., 2020) [53] |
| 1.1 | 10% | Swine manure + sawdust | 50 days | (Ravindran et al., 2019) [14] |
| 0.85 | 0 | Biogas residues | 80 days | (Meng et al., 2020) [26] |
| 0.65 | 0 | Green waste | 30 days | (Zhang et al., 2014) [54] |
| 0.86 | 0 | Rice straw + BS | 84 days | (Du et al., 2019) [55] |
| 0.86 | 0 | Food waste | 56 days | (Chan et al., 2016) [41] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, Y.; Qu, J.; Yan, W.; Yue, T.; Zhang, Q.; Yi, W.; Liu, X.; Sun, Y. Influence of Biochar on Physico-Chemical, Microbial Community and Maturity during Biogas Residue Aerobic Composting Process. Fermentation 2022, 8, 623. https://doi.org/10.3390/fermentation8110623
Qu Y, Qu J, Yan W, Yue T, Zhang Q, Yi W, Liu X, Sun Y. Influence of Biochar on Physico-Chemical, Microbial Community and Maturity during Biogas Residue Aerobic Composting Process. Fermentation. 2022; 8(11):623. https://doi.org/10.3390/fermentation8110623
Chicago/Turabian StyleQu, Youpei, Jingbo Qu, Wencong Yan, Tian Yue, Quanguo Zhang, Weiming Yi, Xiaofeng Liu, and Yong Sun. 2022. "Influence of Biochar on Physico-Chemical, Microbial Community and Maturity during Biogas Residue Aerobic Composting Process" Fermentation 8, no. 11: 623. https://doi.org/10.3390/fermentation8110623
APA StyleQu, Y., Qu, J., Yan, W., Yue, T., Zhang, Q., Yi, W., Liu, X., & Sun, Y. (2022). Influence of Biochar on Physico-Chemical, Microbial Community and Maturity during Biogas Residue Aerobic Composting Process. Fermentation, 8(11), 623. https://doi.org/10.3390/fermentation8110623






