Hypolipidemic, Antioxidant and Immunomodulatory Effects of Lactobacillus casei ATCC 7469-Fermented Wheat Bran and Spirulina maxima in Rats Fed a High-Fat Diet
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Preparation of the Wheat Bran Fermentation Extracts
2.3. Algae Cultivation
2.4. Experimental Design for Rats Fed Lactobacillus casei ATCC 7469-Fermented Wheat Bran Extract and Spirulina maxima Extract
2.4.1. Animals
2.4.2. Termination of the Experiment
2.4.3. Blood Sampling and Biochemical Parameters
2.4.4. Histopathological Examination
2.5. Statistical Analyses
3. Results and Discussion
3.1. Effect on Lipid Profile
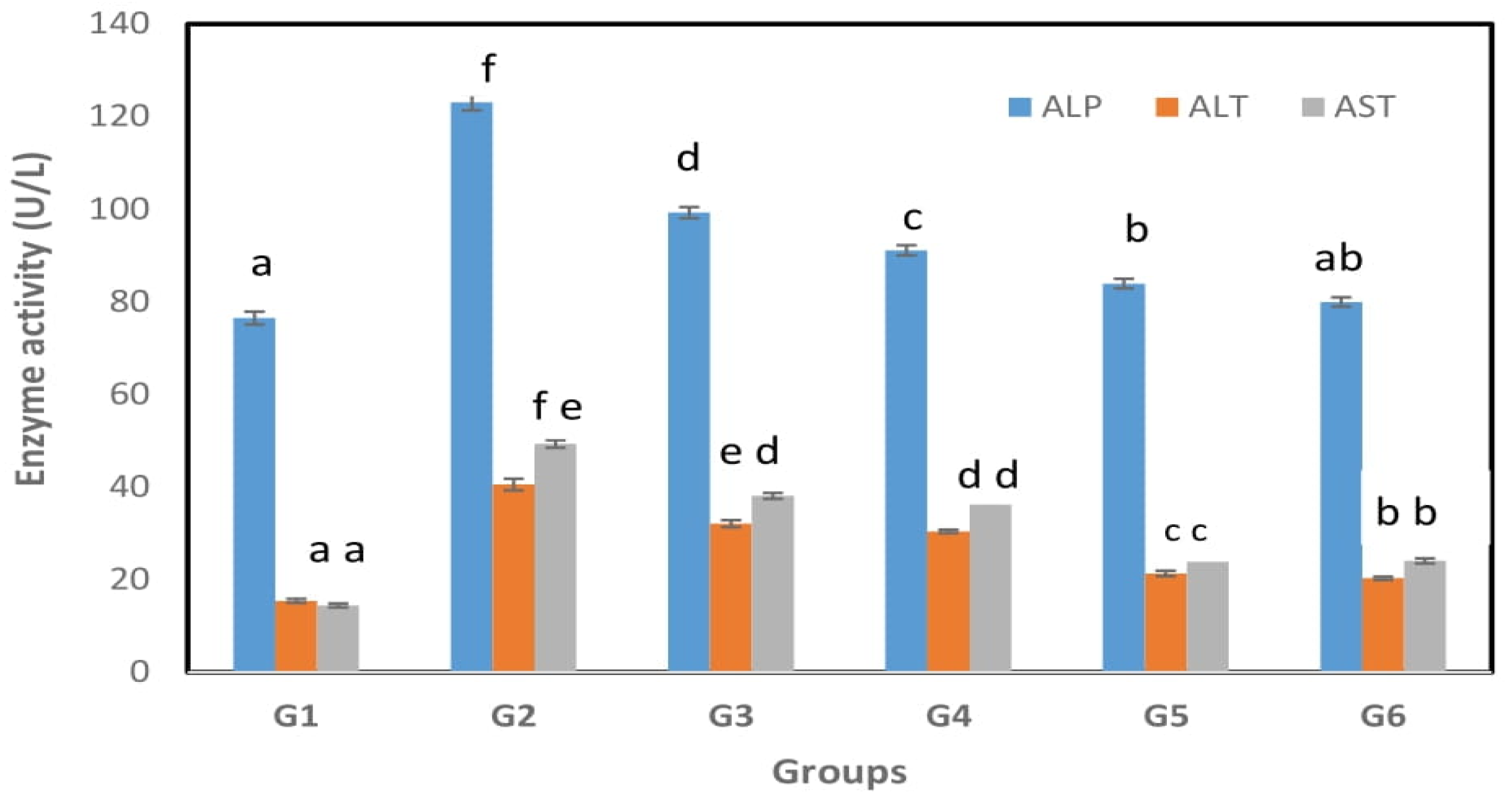
3.2. Effect on Liver Functions
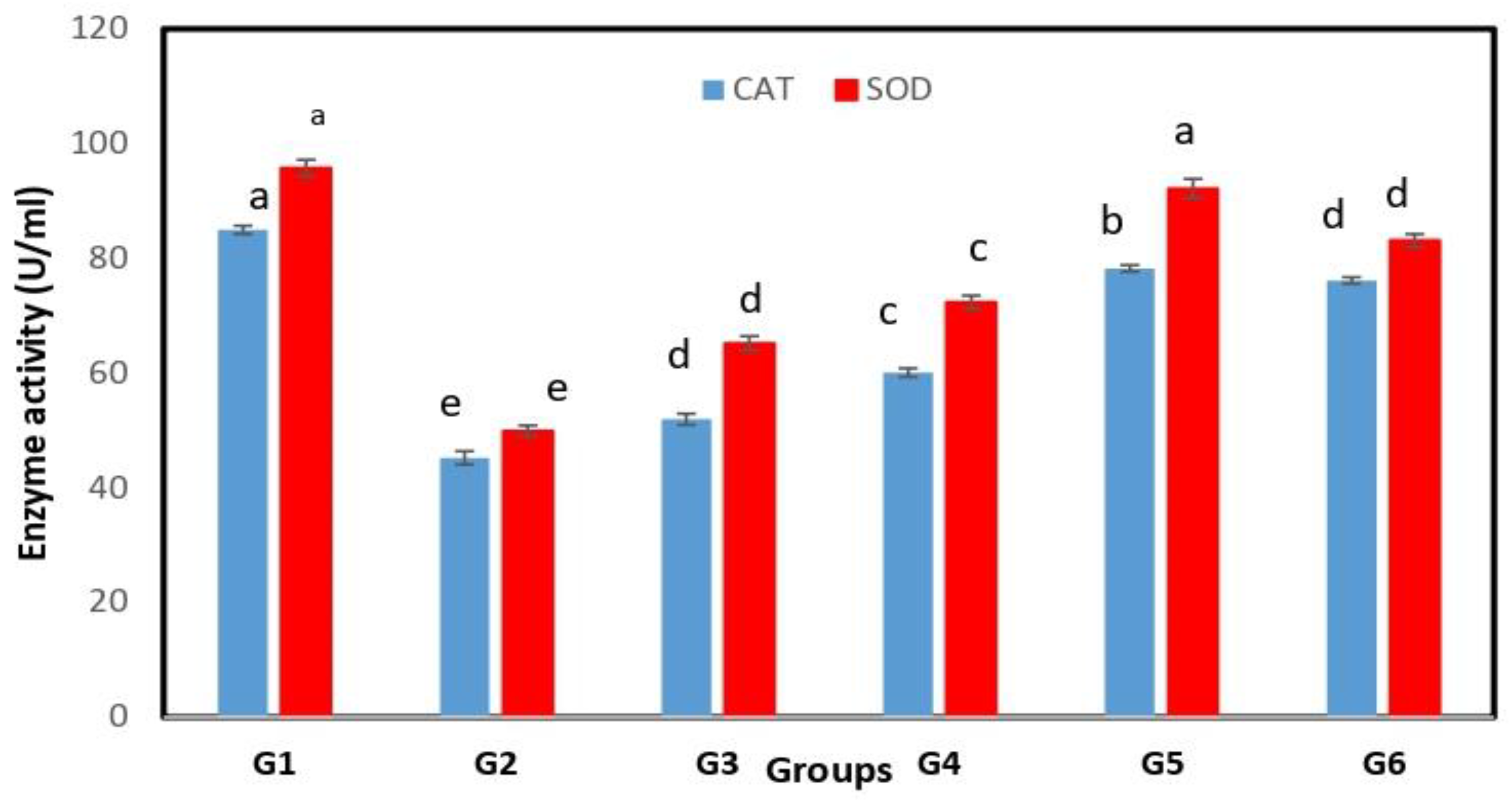
3.3. Antioxidant Activity
3.4. Immunomodulatory Activity
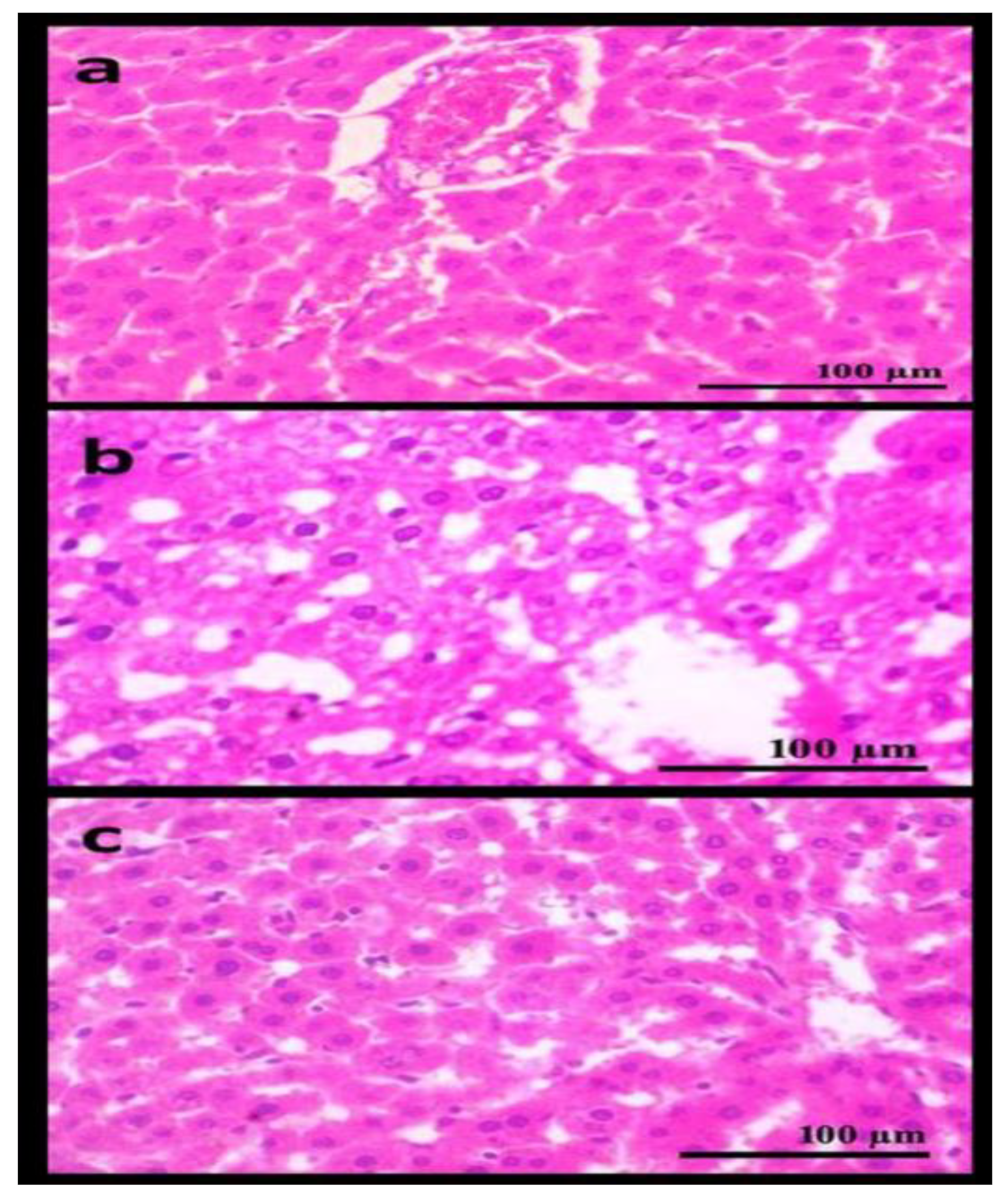
3.5. Histopathological Examination of the Liver
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nelson, R.H. Hyperlipidemia as a Risk Factor for Cardiovascular Disease. Prim. Care 2013, 40, 195–211. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Versmissen, J.; Oosterveer, D.M.; Yazdanpanah, M.; Defesche, J.C.; Basart, D.C.G.; Liem, A.H.; Heeringa, J.; Witteman, J.C.; Lansberg, P.J.; Kastelein, J.J.; et al. Efficacy of statins in familial hypercholesterolaemia: A long term cohort study. BMJ 2008, 337, a2423. [Google Scholar] [CrossRef] [PubMed]
- Davidson, M.H. A systematic review of bile acid sequestrant therapy in children with familial hypercholesterolemia. J. Clin. Lipidol. 2011, 5, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Sashidhara, K.V.; Kumar, M.; Sonkar, R.; Singh, B.S.; Khanna, A.K.; Bhatia, G. Indole-Based Fibrates as Potential Hypolipidemic and Antiobesity Agents. J. Med. Chem. 2012, 55, 2769–2779. [Google Scholar] [CrossRef]
- Drexel, H. Nicotinic acid in the treatment of hyperlipidemia. Fundam. Clin. Pharmacol. 2007, 21, 5–6. [Google Scholar] [CrossRef]
- Golomb, B.A.; Evans, M.A. Statin Adverse Effects: A Review of the Literature and Evidence for a Mitochondrial Mechanism. Am. J. Cardiovasc. Drugs 2008, 8, 373–418. [Google Scholar] [CrossRef]
- Onipe, O.O.; Jideani, A.I.O.; Beswa, D. Composition and functionality of wheat bran and its application in some cereal food products. Int. J. Food Sci. Technol. 2015, 50, 2509–2518. [Google Scholar] [CrossRef]
- Katina, K.; Juvonen, R.; Laitila, A.; Flander, L.; Nordlund, E.; Kariluoto, S.; Piironen, V.; Poutanen, K. Fermented wheat bran as a functional ingredient in baking. Cereal Chem. J. 2012, 89, 126–134. [Google Scholar] [CrossRef]
- Vijayaram, S.; Kannan, S. Probiotics: The Marvelous Factor and Health Benefits. Biomed. Biotechnol. Res. J. 2018, 2, 1–8. [Google Scholar] [CrossRef]
- Ai, X.; Wu, C.; Yin, T.; Zhur, O.; Liu, C.; Yan, X.; Yi, C.; Liu, D.; Xiao, L.; Li, W.; et al. Antidiabetic Function of Lactobacillus fermentum MF423-Fermented Rice Bran and Its Effect on Gut Microbiota Structure in Type 2 Diabetic Mice. Front. Microbiol. 2021, 12, 682290. [Google Scholar] [CrossRef]
- Luana, N.; Rossana, C.; Curiel, J.A.; Poutanen, K.; Marco, G.; Rizzello, C.G. Manufacture and characterization of a yogurt-like beverage made with oat flakes fermented by selected lactic acid bacteria. Int. J. Food Microbiol. 2014, 185, 17–26. [Google Scholar] [CrossRef]
- Shahbazi, R.; Sharifzad, F.; Bagheri, R.; Alsadi, N.; Yasavoli-Sharahi, H.; Matar, C. Anti-Inflammatory and Immunomodulatory Properties of Fermented Plant Foods. Nutrients 2021, 13, 1516. [Google Scholar] [CrossRef]
- Ananya, A.K.; Ahmad, I.Z. Cyanobacteria “the blue green algae” and its novel applications: A brief review. Int. J. Innov. Appl. Res. 2014, 7, 251–261. [Google Scholar]
- Celekli, A.; Alslibi, Z.A.; Bozkurt, H. Use of spirulina in probiotic fermented milk products. Int. J. Adv. Sci. Technol. 2018, 6, 42–48. [Google Scholar]
- Gyenis, B.; Szigeti, J.; Molnar, N.; Varga, L. Use of dried microalgal biomasses to stimulate acid production and growth of Lactobacillus plantarum and Enterococcus faecium in milk. Acta Agrar. Kaposváriensis 2005, 9, 53–59. [Google Scholar]
- Moln’ar, N.; Gyenis, B.; Varga, L. Influence of a powdered Spirulina platensis biomass on acid production of lactococci in milk. Milchwissenschaft 2005, 60, 380–382. [Google Scholar]
- Abdel-Daim, M.M.; Farouk, S.M.; Madkour, F.F.; Azab, S.S. Anti-inflammatory and immunomodulatory effects of Spirulina platensis in comparison to Dunaliella salina in acetic acid-induced rat experimental colitis. Immunopharmacol. Immunotoxicol. 2015, 37, 126–139. [Google Scholar] [CrossRef]
- Zarrouk, C. Contribution a L’etude d’une Cyanobacterie: Influence de Divers Facteurs Physiques et Chimiques sur la Croissance et la Photosynthese de Spirulina maxima (Setchell et Gardner) Geitler. Ph.D. Thesis, University of Paris, Paris, France, 1966. [Google Scholar]
- Assinewe, V.A.; Baum, B.R.; Gagnon, D.; Arnason, J.T. Phytochemistry of Wild Populations of Panax quinquefolius L. (N. Am. Ginseng) J. Agric. Food Chem. 2003, 51, 4549–4553. [Google Scholar] [CrossRef]
- Fossati, P.; Prencipe, L. Serum triglycerides determined colorimetrically with an Nenzyme that produces hydrogen peroxide. Clin. Chem. 1982, 28, 2077–2080. [Google Scholar] [CrossRef]
- Deeg, R.; Ziegenohrm, J. Kinetic enzymatic method for automated determination of total cholesterol in serum. J. Clin. Chem. 1983, 29, 1798–1802. [Google Scholar] [CrossRef]
- Burstein, M.; Selvenick, H.R.; Morfin, R. Rapid method for the isolation of lipoproteins from human serum by precipitation with polyanions. J. Lipid Res. 1970, 11, 583–595. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Norbert, W.T. Clinical Guide to Laboratory Tests, 3rd ed.; W.B. Saunders Company: Philadelphia, PA, USA, 1995. [Google Scholar]
- Reitman, S.; Frankel, S. A colorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [CrossRef]
- Belfield, A.; Goldberg, D.M. Revised assay for serum phenyl phosphatase activity using 4-amino-antipyrine. Enzyme 1971, 12, 561–573. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Cohen, G.; Dembiec, D.; Marcus, J. Measurement of catalase activity in tissue. Anal. Biochem. 1970, 34, 30–38. [Google Scholar] [CrossRef]
- Yan, D.; Kang, P.; Shen, B.; Yang, J.; Zhou, Z.; Duan, L.; Pei, F. Serum levels of IL-1β, IL-6 and TNF-α in rats fed with Kashin-Beck disease-affected diet. Int. J. Rheum. Dis. 2010, 13, 406–411. [Google Scholar] [CrossRef]
- Bancroft, G.D.; Stevens, A.; Turner, D.R. Theory and Practice of Pathological Technique, 4th ed.; Churchill Livingston: New York, NY, USA, 1996. [Google Scholar]
- Snedcor, G.W.; Cochran, W.G. Statistical Methods, 7th ed.; The Iowa State University Press: Ames, IA, USA, 1982; p. 507. [Google Scholar]
- Omole, J.O.; Ighodaro, O.M. Comparative studies of the effects of egg yolk, oats, apple, and wheat bran on serum lipid profile of wistar rats. ISRN Nutr. 2013, 2012, 730479. [Google Scholar] [CrossRef]
- Wang, J.H.; Zhang, H.; Chen, X.; Chen, Y.; Bao, Q. Selection of potential probiotic lactobacilli for cholesterol-lowering properties and their effect on cholesterol metabolism in rats fed a high-lipid diet. J. Dairy Sci. 2012, 95, 1645–1654. [Google Scholar] [CrossRef]
- Tsai, C.C.; Lin, P.P.; Hsieh, Y.M.; Zhang, Z.Y.; Wu, H.C.; Huang, C.C. Cholesterol-Lowering Potentials of Lactic Acid Bacteria Based on Bile-Salt Hydrolase Activity and Effect of Potent Strains on Cholesterol Metabolism In Vitro and In Vivo. Sci. World J. 2014, 2014, 690752. [Google Scholar] [CrossRef]
- Hamouda, R.A.; Hamza, H.A.; Salem, M.L.; Kamal, S.; Alhasani, R.H.; Alsharif, I.; Mahrous, H.; Abdella, A. Synergistic Hypolipidemic and Immunomodulatory Activity of Lactobacillus and Spirulina platensis. Fermentation 2022, 8, 220. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B.; Bajaj, J.S. Bile Acids and the Gut Microbiome. Curr. Opin. Gastroenterol. 2014, 30, 332–338. [Google Scholar] [CrossRef]
- Chiang, J.Y.L. Bile acid metabolism and signaling in liver disease and therapy. Liver Res. 2017, 1, 3–9. [Google Scholar] [CrossRef]
- Jeun, J.; Kim, S.; Cho, S.Y.; Jun, H.J.; Park, H.J.; Seo, J.G.; Chung, N.J.; Lee, S.J. Hypocholesterolemic effects of Lactobacillus plantarum KCTC3928 by increased bile acid excretion in C57BL/6 mice. Nutrition 2010, 26, 321–330. [Google Scholar] [CrossRef]
- Lye, H.S.; Rahmat-Ali, G.R.; Liong, M.T. Mechanisms of cholesterol removal by lactobacilli under conditions that mimic the human gastrointestinal tract. Int. Dairy J. 2010, 20, 169–175. [Google Scholar] [CrossRef]
- Kimoto, H.; Ohmomo, S.; Okamoto, T. Cholesterol removal from media by Lactococci. J. Dairy Sci. 2002, 85, 3182–3188. [Google Scholar] [CrossRef]
- Hassan, A.; Din, A.U.; Zhu, Y.; Zhang, K.; Li, T.; Wang, Y.; Luo, Y.; Wang, G. Updates in understanding the hypocholesterolemia effect. Appl. Microbiol. Biotechnol. 2019, 103, 5993–6006. [Google Scholar] [CrossRef]
- Kitawaki, R.; Nishimura, Y.; Takagi, N.; Iwasaki, M.; Tsuzuki, K.; Fukuda, M. Effects of lactobacillus fermented soymilk and soy yogurt on hepatic lipid accumulation in rats fed on a cholesterol-free diet. Biosci. Biotechnol. Biochem. 2009, 73, 1484–1488. [Google Scholar] [CrossRef]
- Qiu, T.; Ma, X.; Ye, M.; Yuan, R.; Wu, Y. Purification, structure, lipid lowering and liver protecting effects of polysaccharide from Lachnum YM281. Carbohydr. Polym. 2013, 98, 922–930. [Google Scholar] [CrossRef]
- Junejo, S.A.; Geng, H.; Li, S.; Kaka, A.K.; Rashid, A.; Zhou, Y. Superfine wheat bran improves the hyperglycemic and hyperlipidemic properties in a high-fat rat mode. Food Sci. Biotechnol. 2020, 29, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Han, L.K.; Li, D.X.; Xiang, L.; Gong, X.J.; Kondo, Y.; Suzuki, I. Isolation of pancreatic li-paseactivity-inhibitorycomponentof Spirulinaplatensis and it reduce postprandial triacylglycerole-mia. Yakugaku Zasshi 2006, 126, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Franczyk, B.; Rysz, J.; Ławinski, J.; Rysz-Górzynska, M.; Gluba-Brzózka, A. Is a High HDL-Cholesterol Level Always Beneficial? Biomedicines 2021, 9, 1083. [Google Scholar] [CrossRef] [PubMed]
- Dvir, I.; Chayoth, R.; Shany, S.; Stark, A.H.; Arad, S.M. Soluble poysacharides and biomass of red microalgae porphorydium species alter intestinal morphology and reduce serum cholesterol in rats. Br. J. Nutr. 2000, 48, 469–476. [Google Scholar] [CrossRef]
- Nagaoka, S.; Shimizu, K.; Kaneko, H.; Shibayama, F.; Morikawa, K.; Kanamaru, Y.; Hirahashi, T.; Kato, T. A novel protein C-phycocyanin plays a crucial role in the hypocholesterolemic action of Spirulina platensis concentrate in rat. J. Nutr. 2005, 135, 2425–2430. [Google Scholar] [CrossRef]
- Kathak, R.R.; Sumon, A.H.; Molla, N.H.; Hasan, M.; Miah, R.; Tuba, H.R.; Habib, A.; Ali, N. The association between elevated lipid profile and liver enzymes: A study on Bangladeshi adults. Sci. Rep. 2022, 12, 1711. [Google Scholar] [CrossRef]
- Deb, S.; Puthanveetil, P.; Sakharkar, P. A Population-Based Cross-Sectional Study of the Association between Liver Enzymes and Lipid Levels. Int. J. Hepatol. 2018, 2018, 1286170. [Google Scholar] [CrossRef]
- Arauz, J.; Ramos-Tovar, E.; Muriel, P. Redox state and methods to evaluate oxidative stress in liver damage: From bench to bedside. Ann. Hepatol. 2016, 15, 160–173. [Google Scholar]
- Gan, Y.; Tong, J.; Zhou, X.; Long, X.; Pan, Y.; Liu, W.; Zhao, X. Hepatoprotective Effect of Lactobacillus plantarum HFY09 on Ethanol-Induced Liver Injury in Mice. Front. Nutr. 2021, 8, 355. [Google Scholar] [CrossRef]
- Gratz, S.W.; Mykkanen, H.S.; El-Nezami, H.S. Probiotics and gut health: A special focus on liver diseases. World J. Gastroenterol. 2010, 16, 403–410. [Google Scholar] [CrossRef]
- Liang, H.; Lyu, R.; Fu, Y.; Zhou, Z.; Liu, Y.; Zhou, X.; Wang, W.; Liu, M.; Ma, A. Effects of probiotics on alcoholic liver injury in rats and its mechanisms. Chin. Pharmacol. Bull. 2016, 32, 991–997. [Google Scholar]
- Chen, X.; Jing Zhang, J.; Yi, R.; Mu, J.; Zhao, X.; Yang, Z. Hepatoprotective Effects of Lactobacillus on Carbon Tetrachloride-Induced Acute Liver Injury in Mice. Int. J. Mol. Sci. 2018, 19, 2212. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, K.; Lv, L.; Wu, S.; Guo, Z. Ferulic acid ameliorates nonalcoholic fatty liver disease and modulates the gut microbiota composition in high-fat diet fed ApoE−/− mice. Biomed. Pharmacother. 2019, 113, 108753. [Google Scholar] [CrossRef]
- Al-Qayim, M.A.; Abass, D.E. Effects of probiotics (Lactobacillus acidophilus) on liver functions in experimental colitis in rats. Iraqi J. Vet. Med. 2014, 38, 48–54. [Google Scholar] [CrossRef]
- Chen, T.; Li, R.; Chen, P. Gut Microbiota and Chemical-Induced Acute Liver Injury. Front. Physiol. 2021, 12, 688780. [Google Scholar] [CrossRef]
- Al-Homaidan, A.A. Applications of Spirulina to Enhance Liver’s Functions: Effects and Safety. Ph.D. Thesis, College of Science, King Saud University, Riyadh, Saudi Arabia, 2016. [Google Scholar]
- Neyrinck, A.M.; Taminiau, B.; Walgrave, H.; Daube, G.; Cani, P.D.; Bindels, L.B.; Delzenne, N.M. Spirulina protects against hepatic inflammation in aging: An effect related to the modulation of the gut microbiota? Nutrients 2017, 9, 633. [Google Scholar] [CrossRef]
- Mazloomi, S.M.; Samadi, M.; Davarpanah, H.; Babajafari, S.; Clark, C.T.; Ghaemfar, Z.; Rezaiyan, M.; Mosallanezhad, A.; Shafiee, M.; Rostami, H. The effect of Spirulina sauce, as a functional food, on cardiometabolic risk factors, oxidative stress biomarkers, glycemic profile, and liver enzymes in nonalcoholic fatty liver disease patients: A randomized double-blinded clinical trial. Food Sci. Nutr. 2022, 10, 317–328. [Google Scholar] [CrossRef]
- Yang, R.L.; Shi, Y.H.; Hao, G.; Li, W.; Le, G.W. Increasing Oxidative Stress with Progressive Hyperlipidemia in Human: Relation between Malondialdehyde and Atherogenic Index. J. Clin. Biochem. Nutr. 2008, 43, 154–158. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, L.; Su, Y.; Li, H.; Sun, Q.; Liang, X.; Lv, J. Antioxidative activity of lactic acid bacteria in yogurt. Afr. J. Microbiol. Res. 2011, 5, 5194–5201. [Google Scholar]
- Rehman, S.U.; Shah, S.A.; Ali, T.; Chung, J.I.; Kim, M.O. Anthocyanins reversed D-galactose-induced oxidative stress and neuroinflammation mediated cognitive impairment in adult rats. Mol. Neurobiol. 2017, 54, 255–271. [Google Scholar] [CrossRef]
- Wang, A.N.; Yi, X.W.; Yu, H.F.; Dong, B.; Qiao, S.Y. Free radical scavenging activity of Lactobacillus fermentum in vitro and its antioxidative effect on growingfinishing pigs. J. Appl. Microbiol. 2009, 107, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Mei, X. Antioxidant activity of an exopolysaccharide purified from Lactococcus lactis subsp. lactis 12. Carbohydr. Polym. 2010, 80, 908–914. [Google Scholar] [CrossRef]
- Shehu, A.I.; Ma, X.; Venkataramanan, R. Mechanisms of Drug-Induced Hepatotoxicity. Clin. Liver Dis. 2017, 21, 35–54. [Google Scholar] [CrossRef] [PubMed]
- Premkumar, K.; Abraham, S.K.; Santhiya, S.T.; Ramesh, A. Protective effect of Spirulina fusiformis on chemical-induced genotoxicity in mice. Fitoterapia 2004, 75, 24–31. [Google Scholar] [CrossRef]
- Deng, R.; Chow, T.J. Hypolipidemic, antioxidant and antiinflammatory activities of microalgae spirulina. Cardiovasc. Ther. 2010, 28, e33–e45. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, L.; Miron, A.; Klímová, B.; Wan, D.; Kuca, K. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: An overview. Arch. Toxicol. 2016, 90, 1817–1840. [Google Scholar] [CrossRef]
- Ngonda, F. In vitro anti-oxidant activity and free radical scavenging potential of roots of Malawian Trichodesma zeylanicumm (burm. f.). Asian J. Biomed. Pharm. Sci. 2013, 3, 21–25. [Google Scholar]
- Laroui, H.; Ingersoll, S.A.; Liu, H.C.; Baker, M.T.; Ayyadurai, S.; Charania, M.A.; Laroui, F.; Yan, Y.; Sitaraman, S.V.; Merlin, D. Dextran sodium sulfate (DSS) induces colitis in mice by forming nanolipocomplexes with medium-chain-length fatty acids in the colon. PLoS ONE 2012, 7, e32084. [Google Scholar] [CrossRef]
- Zălar, D.M.; Pop, C.; Buzdugan, E.; Kiss, B.; Stefan, M.G.; Ghibu, S.; Crisan, D.; Buruiană-Simic, A. Effects of Colchicine in a Rat Model of Diet-Induced Hyperlipidemia. Antioxidants 2022, 11, 230. [Google Scholar] [CrossRef]
- Tanaka, Y.; Hirose, Y.; Yamamoto, Y.; Yoshikai, Y.; Murosa, S. Daily intake of heat-killed Lactobacillus plantarum L-137 improves infammation and lipid metabolism in overweight healthy adults: A randomized-controlled trial. Eur. J. Nutr. 2020, 59, 2641–2649. [Google Scholar] [CrossRef]
- Kitchens, R.L.; Thompson, P.A.; Munford, R.S.; O’Keefe, G.E. Acute infammation and infection maintain circulating phospholipid levels and enhance lipopolysaccharide binding to plasma lipoproteins. J. Lipid. Res. 2003, 44, 2339–2348. [Google Scholar] [CrossRef]
- Laino, J.; Villena, J.; Kanmani, P.; Kitazawa, H. Immunoregulatory effects triggered by lactic acid bacteria exopolysaccharides: New insights into molecular interactions with host cells. Microorganisms 2016, 4, 27. [Google Scholar] [CrossRef]
- Jianming, L.; Jiamei, W.; Wang, Z.; Liu, G.; Zhang, Q. Antiobesity Effect of Flaxseed Polysaccharide via Inducing Satiety due to Leptin Resistance Removal and Promoting Lipid Metabolism through the AMP-Activated Protein Kinase (AMPK) Signaling Pathway. J. Agric. Food Chem. 2019, 67, 7040–7049. [Google Scholar]
- Sag, D.; Carling, D.; Stout, R.D.; Suttles, J. Adenosine 50-Monophosphate-Activated Protein Kinase Promotes Macrophage Polarization to an Anti-Inflammatory Functional Phenotype. J. Immunol. 2008, 181, 8633–8641. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, C.; Zhang, L.; Zhao, Y.; Duan, C.; Zhang, X.; Gao, L.; Li, S. Lactobacillus plantarum NA136 improves the nonalcoholic fatty liver disease by modulating the AMPK/Nrf2 pathway. Appl. Microbiol. Biotechnol. 2019, 103, 5843–5850. [Google Scholar] [CrossRef]
- Miyauchi, E.; Morita, H.; Tanabe, S. Lactobacillus rhamnosus alleviates intestinal barrier dysfunction in part by increasing expression of zonula occludens-1 and myosin light-chain kinase in vivo. J. Dairy Sci. 2009, 92, 2400–2408. [Google Scholar] [CrossRef]
- Hong, M.; Kim, S.W.; Han, S.H.; Kim, D.J.; Suk, K.T.; Kim, Y.S.; Kim, M.J.; Kim, M.Y.; Baik, S.K.; Ham, Y.L. Probiotics (Lactobacillus rhamnosus R0011 and acidophilus R0052) reduce the expression of toll-like receptor 4 in mice with alcoholic liver disease. PLoS ONE 2015, 10, e0117451. [Google Scholar] [CrossRef]
- Yoda, K.; Miyazawa, M.; Hosoda, M.; Hiramatsu, F.; Yan, F.; He, F. Lactobacillus GG-fermented milk prevents DSSinduced colitis and regulates intestinal epithelial homeostasis through activation of epidermal growth factor receptor. Eur. J. Nutr. 2014, 53, 105–115. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Z.; Liu, Y.; Yang, Y.; Shi, F.; Teng, B. Immunostimulatory Effects of Polysaccharides from Spirulina platensis In Vivo and Vitro and Their Activation Mechanism on RAW246.7 Macrophages. Mar. Drugs 2020, 18, 538. [Google Scholar] [CrossRef]
- Nielsen, C.H.; Balachandran, P.; Christensen, O. Enhancement of natural killer cell activity in healthy subjects by Immulina, a Spirulina extract enriched for Braun-type lipoproteins. Planta Med. 2010, 76, 802–1808. [Google Scholar] [CrossRef]
- Shih, C.M.; Cheng, S.N.; Wong, C.S.; Kuo, Y.L.; Chou, T.C. Antiinflammatory and antihyperalgesic activity of C-phycocyanin. Anesth. Analg. 2009, 108, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.W.; Yang, T.S.; Chen, M.J.; Chang, Y.C.; Wang, E.I.C.; Ho, C.L.; Lai, Y.J.; Yu, C.C.; Chou, J.C.; Chao, L.K.P.; et al. Purification and immunomodulating activity of C-phycocyanin from Spirulina platensis cultured using power plant flue gas. Process Biochem. 2014, 49, 1337–1344. [Google Scholar] [CrossRef]
- Yogianti, F.; Kunisada, M.; Nakano, E.; Ono, R.; Sakumi, K.; Oka, S.; Nakabeppu, Y.; Nishigori, C. Inhibitory effects of dietary Spirulina platensis on UVB-induced skin inflammatory responses and carcinogenesis. J. Investig. Dermatol. 2014, 134, 2610–2619. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, M.F. Physalis peruviana pomace suppresses high-cholesterol diet-induced hypercholesterolemia in rats grasas y aceites. Grasasyaceites 2012, 63, 411–422. [Google Scholar] [CrossRef]
- Yang, M.; Zheng, J.; Zong, X.; Yang, X.; Zhang, Y.; Man, C.; Jiang, Y. Preventive Effect and Molecular Mechanism of Lactobacillus rhamnosus JL1 on Food-Borne Obesity in Mice. Nutrients 2021, 13, 3989. [Google Scholar] [CrossRef]
- Kusumawati, N.R.D.; Panunggal, D.G.; Mexitalia, M.; Sidhartani, M.; Pratiwi, J.; Utari, A. Effect of Probiotic Supplementation on Sprague Dawley Rat Liver Histopathology Fed by High Fat High Fructose Diet. J. Biomed. Transl. Res. 2021, 7, 69–73. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Abuzead, S.M.M.; Halawa, S.M. Protective role of Spirulina platensis against acute deltamethrin-induced toxicity in rats. PLoS ONE 2013, 8, e72991. [Google Scholar] [CrossRef]
- Bae, C.S.; Ahn, T. Albumin infusion ameliorates liver injury in streptozotocin-induced diabetic rats. Vet. Med. 2022, 67, 245–256. [Google Scholar] [CrossRef]
| Variable with Units | G1 | G2 | G3 | G4 | G5 | G6 |
|---|---|---|---|---|---|---|
| TC (mg/dL) | 85.2 ± 1.15 a | 204 ± 2.32 c | 124 ± 1.09 b | 130.64 ± 1.1 b | 92 ± 1.3 a | 88 ± 0.3 a |
| TG (mg/dL) | 67.86 ± 1.15 a | 158.33 ± 1.26 e | 85 ± 1.3 c | 105.28 ± 1.32 d | 73 ± 1.15 b | 69 ± 1.23 ab |
| LDL (mg/dL) | 33.6 ± 0.7 a | 89.06 ± 2.17 e | 64.9 ± 2.8 c | 72.4 ± 2.16 d | 58 ± 1.1 c | 49 ± 1.15 b |
| VLDL (mg/dL) | 12.76 ± 0.3 a | 28 ± 0.51 e | 20.28 ± 0.58 d | 18.36 ± 1.14 c | 14.4 ± 0.24 b | 12.8 ± 0.31 a |
| HDL (mg/dL) | 23.6 ± 0.21 a | 15.12 ± 0.7 e | 17.6 ± 0.16 d | 18.4 ± 0.18 d | 20.52 ± 0.16 c | 22 ± 0.22 b |
| Variable with Units | G1 | G2 | G3 | G4 | G5 | G6 |
|---|---|---|---|---|---|---|
| TNF-α (pg/mL) | 35.4 ± 1.12 a | 105 ± 2.4 f | 69 ± 2.09 d | 72 ± 2.08 e | 52 ± 1.56 c | 42 ± 1.13 b |
| IL-10 (pg/mL) | 5.2 ± 0.2 a | 17.68 ± 0.44 d | 11.23 ± 0.42 c | 13.65 ± 0.4 d | 9.2 ± 0.37 b | 7.9 ± 0.034 b |
| IL-1β (pg/mL) | 3.6 ± 0.1 a | 14.9 ± 0.37 f | 9.6 ± 0.29 e | 8.3 ± 0.22 d | 5.1 ± 0.17 c | 4.7 ± 0.14 b |
| IFN-γ (IU/mL) | 1.53 ± 0.038 a | 5.65 ± 0.14 e | 3.2 ± 0.08 d | 2.9 ± 0.11 c | 1.9 ± 0.04 b | 1.7 ± 0.05 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdella, A.; Elbadawy, M.F.; Irmak, S.; Alamri, E.S. Hypolipidemic, Antioxidant and Immunomodulatory Effects of Lactobacillus casei ATCC 7469-Fermented Wheat Bran and Spirulina maxima in Rats Fed a High-Fat Diet. Fermentation 2022, 8, 610. https://doi.org/10.3390/fermentation8110610
Abdella A, Elbadawy MF, Irmak S, Alamri ES. Hypolipidemic, Antioxidant and Immunomodulatory Effects of Lactobacillus casei ATCC 7469-Fermented Wheat Bran and Spirulina maxima in Rats Fed a High-Fat Diet. Fermentation. 2022; 8(11):610. https://doi.org/10.3390/fermentation8110610
Chicago/Turabian StyleAbdella, Asmaa, Mohamed F. Elbadawy, Sibel Irmak, and Eman S. Alamri. 2022. "Hypolipidemic, Antioxidant and Immunomodulatory Effects of Lactobacillus casei ATCC 7469-Fermented Wheat Bran and Spirulina maxima in Rats Fed a High-Fat Diet" Fermentation 8, no. 11: 610. https://doi.org/10.3390/fermentation8110610
APA StyleAbdella, A., Elbadawy, M. F., Irmak, S., & Alamri, E. S. (2022). Hypolipidemic, Antioxidant and Immunomodulatory Effects of Lactobacillus casei ATCC 7469-Fermented Wheat Bran and Spirulina maxima in Rats Fed a High-Fat Diet. Fermentation, 8(11), 610. https://doi.org/10.3390/fermentation8110610





