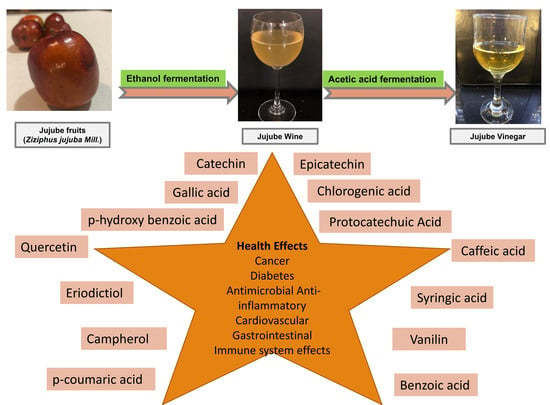
Determination of Changes in Volatile Aroma Components, Antioxidant Activity and Bioactive Compounds in the Production Process of Jujube (Ziziphus jujuba Mill.) Vinegar Produced by Traditional Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Materials
2.3. Preparation of Inoculums
2.4. Production of Jujube Vinegar
2.5. Proximate Composition Analysis
2.6. Organic Acid Compound Analysis
2.7. Phenolic Compound Analysis
2.8. The Total Phenolic Content and Antioxidant Activity Analysis
2.9. Volatile Aroma Component Analysis
2.10. Statistical Analysis
3. Results and Discussion
3.1. Chemical Contents of Jujube Juice, Wine, and Vinegar
3.2. Organic Acid Compound Profiles of Jujube Juice, Wine, and Vinegar
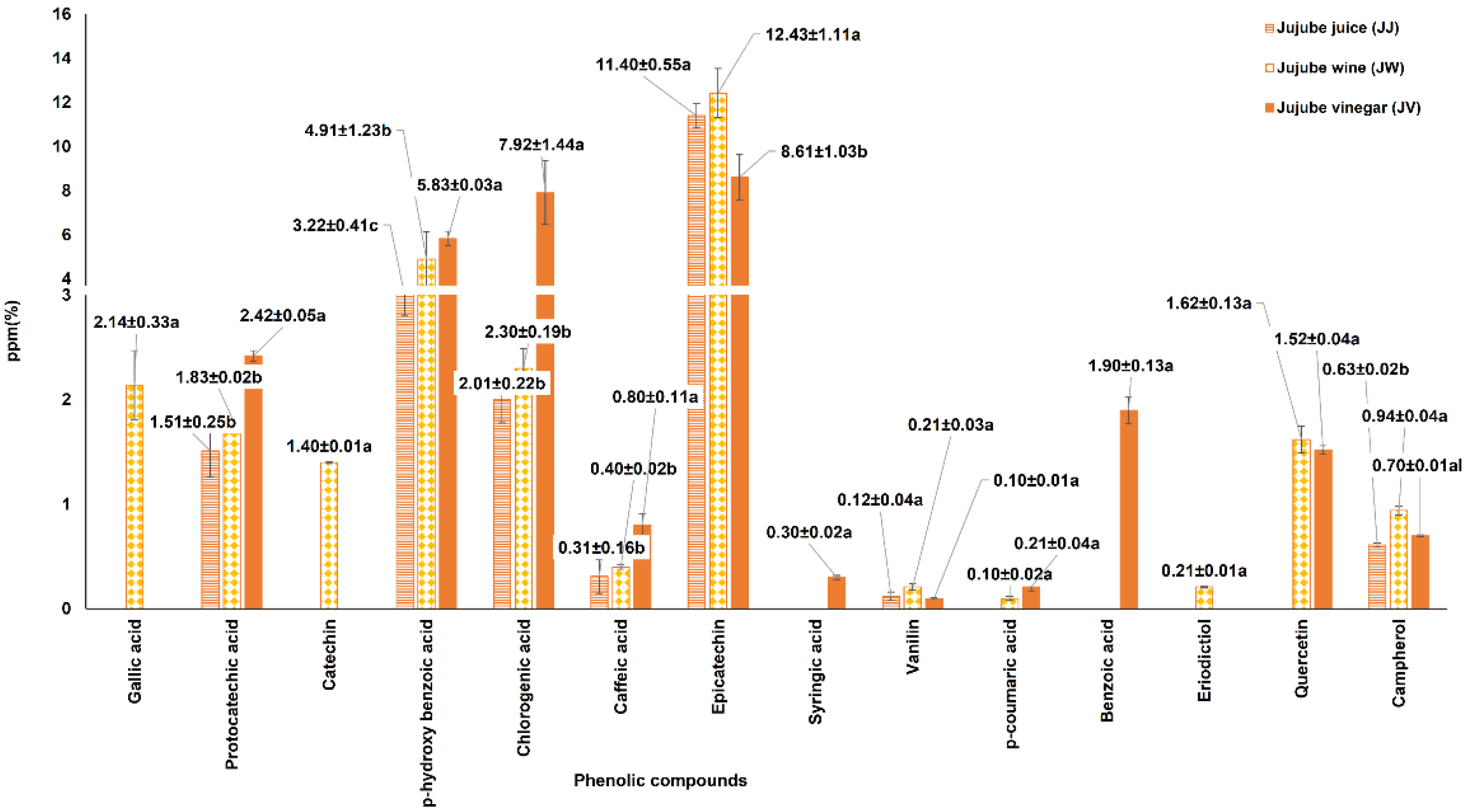
3.3. Phenolic Compound Profiles of the Jujube Juice, Wine, and Vinegar
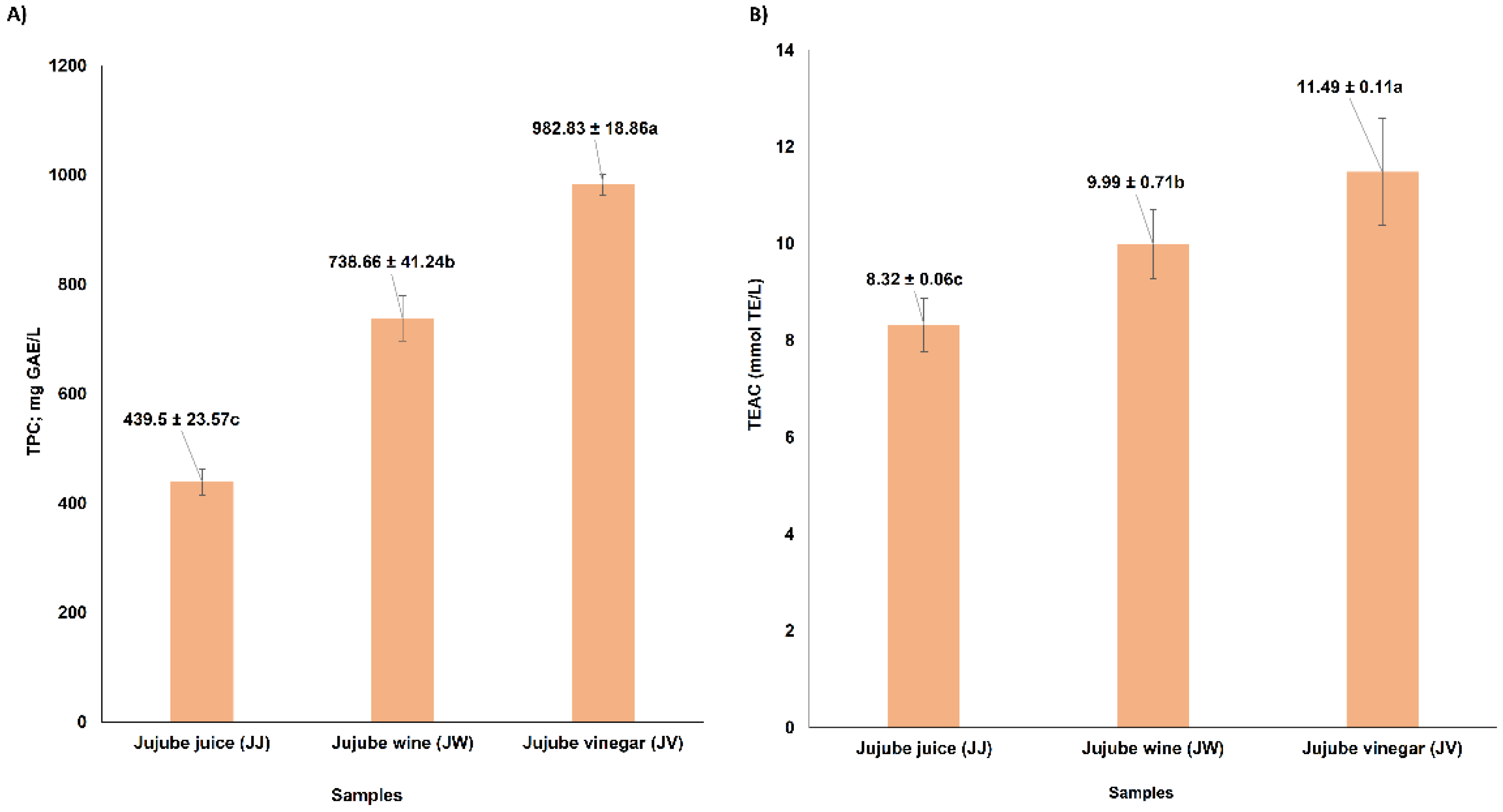
3.4. Total Phenolic Content and Antioxidant Capacity of Jujube Juice, Wine, and Vinegar
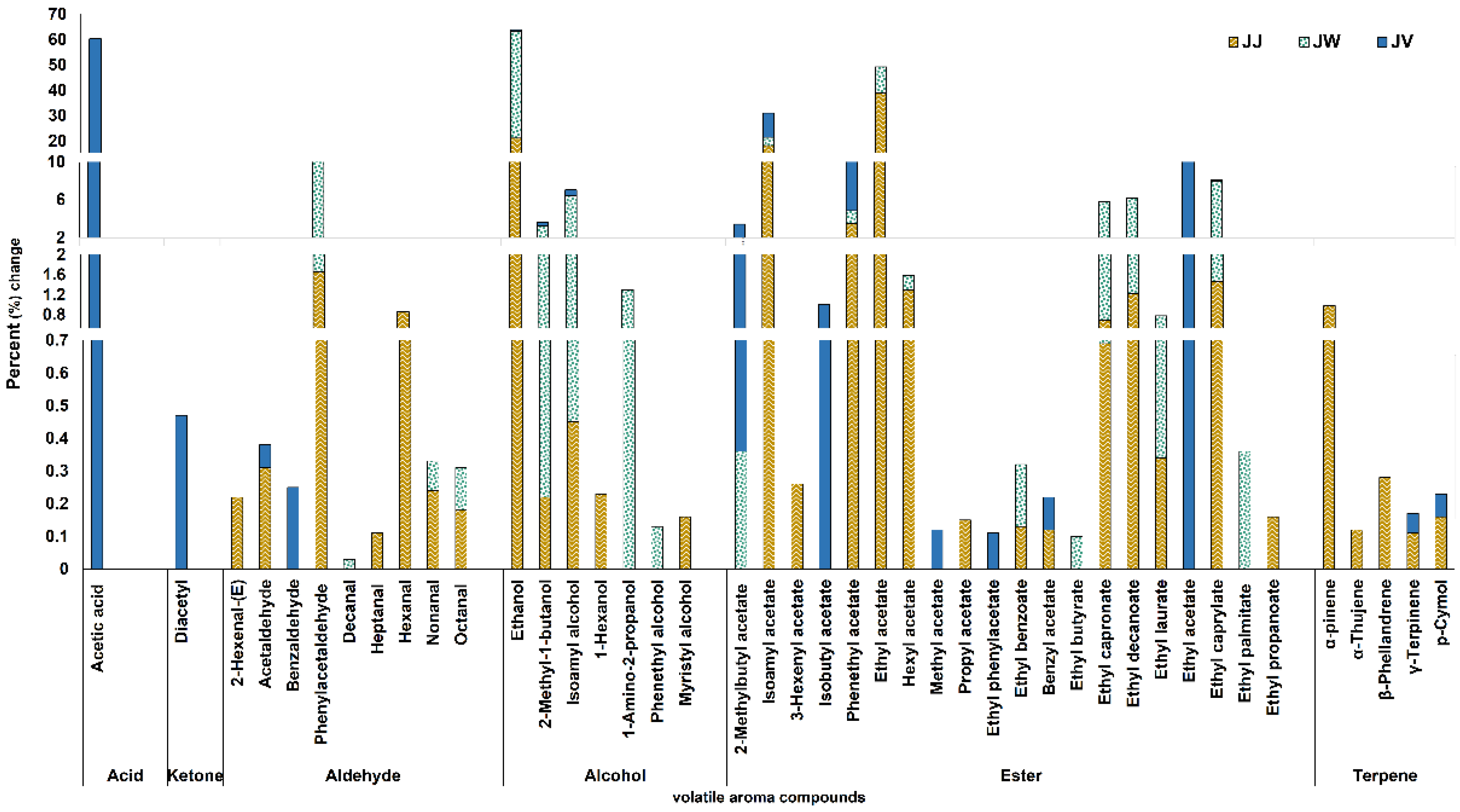
3.5. Volatile Compound Profiles of Jujube Juice, Wine, and Vinegar
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mengjun, L. Genetic Diversity of Chinese Jujube (Ziziphus jujuba Mill.). Acta Hortic. 2003, 623, 351–355. [Google Scholar] [CrossRef]
- Kemeç-Hürkan, Y. Jujuba (Ziziphus jujuba Mill.) Fruit: Medical Importance from Past to Present. J. Inst. Sci. Technol. 2019, 9, 1271–1281. [Google Scholar] [CrossRef]
- Kavas, İ.; Dalkılıç, Z. Determinatıon of Morphological, Phenological Pomological Characteristics in Some Jujube Genotypes and Investigation of Hybridization Possibilities. J. Adnan Menderes Univ. Agric. Fac. 2015, 12, 5–72. [Google Scholar]
- Hamedi, S.; Arian, A.A.; Farzaei, M.H. Gastroprotective Effect of Aqueous Stem Bark Extract of Ziziphus jujuba L. against HCl/Ethanol-Induced Gastric Mucosal Injury in Rats. J. Tradit. Chin. Med. 2015, 35, 666–670. [Google Scholar] [CrossRef]
- Yu, L.; Jiang, B.P.; Luo, D.; Shen, X.C.; Guo, S.; Duan, J.A.; Tang, Y.P. Bioactive Compounds in The Fruits of Ziziphus jujuba Mill. against The İnflammatory İrritant Action of Euphorbia Plants. Phytomedicine 2012, 19, 239–244. [Google Scholar] [CrossRef]
- Ganachari, M.S.; Kumar, S.; Bhat, K.G. Effect of Ziziphus jujuba Leaves Extract on Phagocytosis by Human Neutrophils. J. Nat. Remedies 2004, 4, 47–51. [Google Scholar]
- Choi, S.H.; Ahn, J.B.; Kim, H.J.; Im, N.K.; Kozukue, N.; Levin, C.E.; Friedman, M. Changes in Free Amino Acid, Protein, and Flavonoid Content in Jujube (Ziziphus jujube) Fruit During Eight Stages of Growth and Antioxidative and Cancer Cell Inhibitory Effects by Extracts. J. Agric. Food Chem. 2012, 60, 10245–10255. [Google Scholar] [CrossRef]
- Gülay, S. Investgation of The Relation Between Insulin Release and Several Zizyphus Jujuba Extracts on Pancreatic Beta Cell Lines. Master’s Thesis, Erciyes University, Kayseri, Turkey, 2013. [Google Scholar]
- Özkan, H.İ. The Examination of Some Biochemical Components of Jujube Fruit (Zizyphus jujuba Mill.) and Its Antibacterial, Hypoglycaemic and Total Antioxidant Activities. Master’s Thesis, Balıkesir Üniversitesi, Balıkesir, Turkey, 2017. [Google Scholar]
- Rashwan, A.K.; Karima, N.; Shishira, M.R.I.; Baoa, T.; Lua, Y.; Chena, W. Jujube fruit: A potential nutritious fruit for the development of functional food products. J. Funct. Foods 2020, 75, 104205. [Google Scholar] [CrossRef]
- Li, G.; Yan, N.; Li, G. The Effect of In Vitro Gastrointestinal Digestion on the Antioxidants, Antioxidant Activity, and Hypolipidemic Activity of Green Jujube Vinegar. Foods 2022, 11, 1647. [Google Scholar] [CrossRef]
- Kundi, A.H.K.; Wazir, F.K.; Abdul, G.; Wazir, Z.D.K. Physicochemical Characteristics and Organoleptic Evaluation of Different Ber (Zizyphus jujuba Mill.) Cultivars. Sarhad J. Agric. 1989, 5, 149–155. [Google Scholar]
- Hernández, F.; Noguera, A.L.; Burló, F.; Wojdyło, A.; Carbonell, B.A.A.; Legua, P. PhysicoChemical, Nutritional, and Volatile Composition and Sensory Profile of Spanish Jujube (Ziziphus jujuba Mill.) Fruits. J. Sci. Food Agric. 2016, 96, 2682–2691. [Google Scholar] [CrossRef] [PubMed]
- Afrisham, R.; Aberomand, M.; Ghaffari, M.; Siahpoos, A.; Jamalan, M. Inhibitory Effect of Heracleum persicum and Ziziphus jujuba on Activity of Alpha-Amylase. J. Bot. 2015, 824683. [Google Scholar] [CrossRef]
- San, B.; Yildirim, A.N. Phenolic, AlphaTocopherol, Beta-Carotene and Fatty Acid Composition of Four Promising Jujube (Ziziphus jujuba Miller) Selections. J. Food Compos. Anal. 2010, 23, 706–710. [Google Scholar] [CrossRef]
- Gong, C.; Yanjing, B.; Yuying, Z. Flavonoids from Ziziphus jujuba Mill var. spinasa. Tetrahedron 2000, 56, 8915–8920. [Google Scholar]
- Keleş, H. Changes of some horticultural characteristics in jujube (Ziziphus jujube Mill.) fruit at different ripening stages. Turk. J. Agric. For. 2020, 44, 391–398. [Google Scholar] [CrossRef]
- Vithlani, V.A.; Patel, H.V. Production of functional vinegar from Indian jujube (Zizyphus mauritiana) and its antioxidant properties. J. Food Technol. 2010, 8, 143–149. [Google Scholar] [CrossRef]
- Budak, N.H.; Aykin, E.; Seydim, A.C.; Greene, A.K.; Guzel-Seydim, Z.B. Functional Properties of Vinegar. J. Food Sci. 2014, 79, 757–764. [Google Scholar] [CrossRef]
- Gullo, M.; Verzelloni, E.; Canonico, M. Aerobic Submerged Fermentation by Acetic Acid Bacteria for Vinegar Production: Process and Biotechnological Aspects. Process Biochem. 2014, 49, 1571–1579. [Google Scholar] [CrossRef]
- Budak, H.N.; Guzel-Seydim, Z.B. Antioxidant Activity and Phenolic Content of Wine Vinegars Produced by Two Different Technique. J. Sci. Food Agric. 2010, 90, 2021–2026. [Google Scholar] [CrossRef]
- Özen, M.; Özdemir, N.; Ertekin-Filiz, B.; Budak, H.N.; Kök-Taş, T. Sour Cherry (Prunus cerasus L.) Vinegars Produced from Fresh Fruit or Juice Concentrate: Bioactive Compounds, Volatile Aroma Compounds and Antioxidant Capacities. Food Chem. 2020, 309, 125664. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 15th ed.; AOAC: Washington DC, USA, 1992. [Google Scholar]
- Özdemir, N.; Budak, H.N. Bioactive compounds and volatile aroma compounds in rose (Rosa damascena Mill.) vinegar during the aging period. Food Biosci. 2022, 50 Pt A, 102062. [Google Scholar] [CrossRef]
- Krapez, K.M.; Abram, V.; Kac, M.; Ferjancic, S. Determination of Organic Acids in White Wines by RP-HPLC. Food Technol. Biotechnol. 2001, 39, 93–99. [Google Scholar]
- Caponio, F.; Alloggio, V.; Gomes, T. Phenolic Compounds of Virgin Olive Oil: Influence of Paste Preparation Techniques. Food Chem. 1999, 64, 203–209. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Singh, S.; Singh, R.P. In Vitro Methods of Assay of Antioxidants: An Overview. Food Rev. Int. 2008, 24, 392–415. [Google Scholar] [CrossRef]
- Vichi, S.; Castellote, A.I.; Pizzale, L.; Conte, L.S.; Buxaderas, S.; Lopez-Tamames, E. Analysis of virgin olive oil volatile compounds by headspace solid-phase microextraction coupled to gas chromatography with mass spectrometric and flame ionization detection. J. Chromatogr. A 2003, 983, 19–33. [Google Scholar] [CrossRef]
- Kim, S.-K.; Lee, G.-D.; Chung, S.-K. Monitoring on Fermentation of Persimmon Vinegar from Persimmon Peel. J. Korean Soc. Food Sci. Nutr. 2003, 35, 642–647. [Google Scholar]
- Lee, S.H.; Kim, J.C. A comparative analysis for main compounds change during natural fermentation of persimmon vinegar. J. Korean Soc. Food Sci. Nutr. 2009, 38, 372–376. [Google Scholar] [CrossRef]
- Pashazadeh, H.; Özdemir, N.; Zannou, O.; Koca, I. Antioxidant capacity, phytochemical compounds, and volatile compounds related to aromatic property of vinegar produced from black rosehip (Rosa pimpinellifolia L.) juice. Food Biosci. 2021, 44, 101318. [Google Scholar] [CrossRef]
- Uddin, M.B.; Hussain, I. Development of diversified technology for jujube (Ziziphus jujuba L.) processing and preservation. World J. Dairy Food Sci. 2012, 7, 74–78. [Google Scholar]
- Özdemir, G.B.; Özdemir, N.; Ertekin-Filiz, B.; Gökırmaklı, Ç.; Kök-Taş, T.; Budak, N.H. Volatile aroma compounds and bioactive compounds of hawthorn vinegar produced from hawthorn fruit (Crataegus tanacetifolia (lam.) pers.). J. Food Biochem. 2021, 46, e13676. [Google Scholar] [CrossRef] [PubMed]
- Budak, H.N.; Özdemir, N.; Gökırmaklı, Ç. The changes of physicochemical properties, antioxidants, organic, and key volatile compounds associated with the flavor of peach (Prunus cerasus L. Batsch) vinegar during the fermentation process. J. Food Biochem. 2021, 46, e13978. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.H.; Wu, C.S.; Yu, J.G.; Wang, M.; Ma, Y.J.; Li, C.L. Textural characteristic, antioxidant activity, sugar, organic acid, and phenolic profiles of 10 promising jujube (Ziziphus jujuba Mill. ) selections. J. Food Sci. 2012, 77, 1218–1225. [Google Scholar] [CrossRef] [PubMed]
- Arrigoni, O.; De Tullio, M.C. Ascorbic acid: Much more than just an antioxidant. Biochim. Biophys. Acta 2002, 1569, 1–9. [Google Scholar] [CrossRef]
- Xiang, J.L.; Du, L.; Liu, Z.J.; Li, X.L.; Luo, L.; Zhu, W.X. Changes in biochemical parameters and organic acid content during liquid fermentation of peach vinegar. Mod. Food Sci. Technol. 2015, 31, 193–198. [Google Scholar] [CrossRef]
- Sanarico, D.; Motta, S.; Bertolini, L.; Antonelli, A. HPLC determination of organic acids in traditional balsamic vinegar of reggio emilia. J. Liq. Chromatogr. Relat. 2003, 26, 2177–2187. [Google Scholar] [CrossRef]
- Leejeerajumnean, A.; Duckham, S.C.; Owens, J.D.; Ames, J.M. Volatile compounds in Bacillus-fermented soybeans. J. Sci. Food Agric. 2001, 81, 525–529. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary Phenolics: Chemistry, Bioavailability and Effects on Health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef]
- Yıkmış, S.; Altıner, D.D.; Ozer, H.; Levent, O.; Celik, G.; Çöl, B.G. Modeling and optimization of bioactive compounds from jujube (Ziziphus jujuba Mill.) vinegar using response surface methodology and artificial neural network: Comparison of ultrasound processing and thermal pasteurization. J. Food Process. Preserv. 2022, e17102. [Google Scholar] [CrossRef]
- Verhoef, S.; Ruijssenaars, H.J.; de Bont, J.A.; Wery, J. Bioproduction of p-hydroxybenzoate from renewable feedstock by solvent-tolerant Pseudomonas putida S12. J. Biotechnol. 2007, 132, 49–56. [Google Scholar] [CrossRef]
- Laranjinha, J.A.; Almeida, L.M.; Madeira, V.M. Reactivity of dietary phenolic acids with peroxyl radicals: Antioxidant activity upon low density lipoprotein peroxidation. Biochem. Pharmacol. 1994, 48, 487–494. [Google Scholar] [CrossRef]
- Manuja, R.; Sachdeva, S.; Jain, A.; Chaudhary, J. A Comprehensive Review on Biological Activities of P-Hydroxy Benzoic Acid and Its Derivatives. Int. J. Pharm. Sci. 2013, 22, 109–115. [Google Scholar]
- Kakkar, S.; Bais, S. A Review on Protocatechuic Acid and Its Pharmacological Potential. ISRN Pharmacol. 2014, 12, 952943. [Google Scholar] [CrossRef] [PubMed]
- Espíndola, K.M.M.; Ferreira, R.G.; Narvaez, L.E.M.; Silva Rosario, A.C.R.; da Silva, A.H.M.; Silva, A.G.B.; Vieira, A.P.O.; Monteiro, M.C. Chemical and Pharmacological Aspects of Caffeic Acid and Its Activity in Hepatocarcinoma. Front. Oncol. 2019, 9, 541. [Google Scholar] [CrossRef] [PubMed]
- Madhusoodanan, J. Wildfires Pose a Burning Problem for Wines and Winemakers. Proc. Natl. Acad. Sci. USA 2021, 118, e2113327118. [Google Scholar] [CrossRef]
- Zhao, M.N.; Zhang, F.; Zhang, L.; Liu, B.J.; Meng, X.H. Mixed fermentation of jujube juice (Ziziphus jujuba Mill.) with L. rhamnosus GG and L. plantarum-1: Effects on the quality and stability. Int. J. Food Sci. Technol. 2019, 54, 2624–2631. [Google Scholar] [CrossRef]
- Wang, F.; Song, Y.; Vidyarthi, S.K.; Zhang, R. Physicochemical properties, and volatile compounds of blackened jujube vinegar as prepared by optimized fermentation process. Int. J. Food Prop. 2022, 25, 288–304. [Google Scholar] [CrossRef]
- Aykın, E.; Budak, H.N.; Güzel-Seydim, Z.B. Bioactive Compounds of Mother Vinegar. J. Am. Coll. Nutr. 2015, 34, 80–89. [Google Scholar] [CrossRef]
- Wang, R.; Ding, S.; Zhao, D.; Wang, Z.; Wu, J.; Hu, X. Effect of Dehydration Methods on Antioxidant Activities, Phenolic Contents, Cyclic Nucleotides, and Volatiles of Jujube Fruits. Food Sci. Biotechnol. 2016, 25, 137–143. [Google Scholar] [CrossRef]
- JECFA. The Joint FAO/WHO Expert Committee on Food Additives (JECFA). 2022. Available online: https://www.who.int/groups/joint-fao-who-expert-committee-on-food-additives-(jecfa) (accessed on 10 June 2022).
- Özdemir, N.; Pashazadeh, H.; Zannou, O.; Koca, I. Phytochemical content, and antioxidant activity, and volatile compounds associated with the aromatic property, of the vinegar produced from rosehip fruit (Rosa canina L.). LWT 2022, 154, 112716. [Google Scholar] [CrossRef]
- Silva Ferreira, A.C.; Guedes de Pinho, P.; Rodrigues, P.; Hogg, T. Kinetics of oxidative degradation of white wines and how they are affected by selected technological parameters. J. Agric. Food Chem. 2002, 50, 5919–5924. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Wang, J.; Aubie, E.; Crombleholme, M.; Reynolds, A. Effects of Frozen Materials Other Than Grapes on Red Wine Aroma Compounds. Impacts of Harvest Technologies. Am. J. Enol. Vitic. 2022, 73, 142–155. [Google Scholar] [CrossRef]
- Cai, W.; Tang, F.; Guo, Z.; Guo, X.; Zhang, Q.; Zhao, X.; Ning, M.; Shan, C. Effects of pretreatment methods and leaching methods on jujube wine quality detected by electronic senses and HS-SPME–GC–MS. Food Chem. 2020, 330, 127330. [Google Scholar] [CrossRef] [PubMed]

| Time (Min) | Methanol (%; Pump B) |
|---|---|
| 0.10 | 7 |
| 20.00 | 28 |
| 28.00 | 25 |
| 35.00 | 30 |
| 50.00 | 30 |
| 60.00 | 33 |
| 62.00 | 42 |
| 70.00 | 50 |
| 73.00 | 70 |
| 75.00 | 80 |
| 80.00 | 100 |
| 81.00 | 7 |
| Sample | pH-Values | Total Dry Matter (%) | Total Soluble Solids (°Brix) |
|---|---|---|---|
| JJ | 3.71 ± 0.05 a | 11.98 ± 0.04 a | 12.00 ± 0.01 a |
| JW | 3.55 ± 0.04 b | 4.05 ± 0.66 b | 6.10 ± 1.27 b |
| JV | 3.24 ± 0.01 c | 2.17 ± 0.49 b | 4.75 ± 0.35 b |
| Organic Acid Compounds | Jujube Juice (JJ) | Jujube Wine (JW) | Jujube Vinegar (JV) |
|---|---|---|---|
| Oxalic acid | 56 ± 0.01 b | 57.2 ± 0.02 b | 75.1 ± 0.04 a |
| Tartaric acid | 2232.9 ± 20.4 a | 2075.4 ± 24.2 a | 993.5 ± 18.0 b |
| Formic acid | 865.4 ± 2.40 a | 677.9 ± 3.24 b | 538.5 ± 4.52 c |
| Malic acid | 919.4 ± 5.65 a | 918.8 ± 8.86 a | 655.2 ± 6.42 b |
| Ascorbic acid | 95.5 ± 2.02 b | 323.3 ± 12.4 a | 57.3 ± 1.04 b |
| Lactic acid | 7737.8 ± 64.0 b | 13,231.4 ± 96.2 a | 2030.1 ± 20.4 c |
| Acetic acid | 2198.5 ± 14.6 b | 2651.2 ± 21.4 b | 46,375.3 ± 62.4 a |
| Citric acid | 391.1 ± 5.40 b | 315.2 ± 4.86 b | 530.7 ± 6.20 a |
| Succinic acid | 794.3 ± 4.20 b | 2288.1 ± 16.2 a | 843.4 ± 8.20 b |
| Fumaric acid | n.d. | n.d. | 3.2 ± 0.01 a |
| Compounds | JJ (Jujube Juice) | JW (Jujube Wine) | JV (Jujube Vinegar) | ||||
|---|---|---|---|---|---|---|---|
| Area | Percent (%) | Area | Percent (%) | Area | Percent (%) | ||
| Acid | Acetic acid | n.d. | n.d. | n.d. | n.d. | 45,730,907 | 60.28 ± 4.42 |
| Total | 60.28± 4.42 | ||||||
| Ketone | Diacetyl | n.d. | n.d. | n.d. | n.d. | 359,304 | 0.47 ± 0.06 |
| Total | 0.47± 0.06 | ||||||
| Aldehyde | 2-Hexenal-(E) | 104,820 | 0.22 ± 0.05 | n.d. | n.d. | n.d. | n.d. |
| Acetaldehyde | 152,524 | 0.31 ± 0.07 a* | n.d. | n.d. | 52,781 | 0.07 ± 0.01 b | |
| Benzaldehyde | n.d. | n.d. | n.d. | n.d. | 187,505 | 0.25 ± 0.09 | |
| Phenylacetaldehyde | 806,141 | 1.66 ± 0.42 b | 6,665,457 | 10.92 ± 1.68 a | 118,527 | 0.16 ± 0.08 c | |
| Decanal | n.d. | n.d. | 20,651 | 0.03 ± 0.01 | n.d. | n.d. | |
| Heptanal | 54,258 | 0.11 ± 0.04 | n.d. | n.d. | n.d. | n.d. | |
| Hexanal | 418,309 | 0.86 ± 0.09 | n.d. | n.d. | n.d. | n.d. | |
| Nonanal | 117,790 | 0.24 ± 0.05 a | 57,545 | 0.09 ± 0.02 b | n.d. | n.d. | |
| Octanal | 86,303 | 0.18 ± 0.06 a | 76,958 | 0.13 ± 0.03 a | n.d. | n.d. | |
| Total | 3.58± 0.48 b | 11.17± 0.64 a | 0.48± 0.24 c | ||||
| Alcohol | Ethanol | 10,242,552 | 21.14 ± 6.84 b | 25,782,301 | 42.24 ± 4.62 a | 411,775 | 0.54 ± 0.14 c |
| 2-Methyl-1-butanol | 104,230 | 0.22 ± 0.02 b | 1,812,897 | 2.97 ± 0.86 a | 310,241 | 0.41 ± 0.18 b | |
| Isoamyl alcohol | 216,157 | 0.45 ± 0.12 b | 3,630,717 | 5.95 ± 1.28 a | 488,869 | 0.64 ± 0.34 b | |
| 1-Hexanol | 110,839 | 0.23 ± 0.01 | n.d. | n.d. | n.d. | n.d. | |
| 1-Amino-2-propanol | n.d. | n.d. | 791,642 | 1.30 ± 0.02 | n.d. | n.d. | |
| Phenethyl alcohol | n.d. | n.d. | 80,222 | 0.13 ± 0.01 | n.d. | n.d. | |
| Myristyl alcohol | 75,148 | 0.16 ± 0.03 | n.d. | n.d. | n.d. | n.d. | |
| Total | 22.2± 2.92 b | 52.59± 3.64 a | 1.59± 0.02 c | ||||
| Ester | 2-Methylbutyl acetate | n.d. | n.d. | 218,994 | 0.36 ± 0.06 b | 2,330,967 | 3.07 ± 1.23 a |
| Isoamyl acetate | 8,850,960 | 18.27 ± 3.24 a | 2,014,436 | 3.30 ± 1.24 c | 7,205,463 | 9.50 ± 1.86 b | |
| 3-Hexenyl acetate | 128,237 | 0.26 ± 0.03 | n.d. | n.d. | n.d. | n.d. | |
| Isobutyl acetate | n.d. | n.d. | n.d. | n.d. | 765,346 | 1.01 ± 0.07 | |
| Phenethyl acetate | 1,676,095 | 3.46 ± 0.16 b | 846,233 | 1.39 ± 0.48 c | 6,352,412 | 8.37 ± 2.02 a | |
| Ethyl acetate | 18,808,097 | 38.82 ± 2.86 a | 6,350,651 | 10.40 ± 2.11 b | 8,589,076 | 11.32 ± 4.01 b | |
| Hexyl acetate | 631,438 | 1.30 ± 0.15 a | 178,995 | 0.29 ± 0.04 b | n.d. | n.d. | |
| Methyl acetate | n.d. | n.d. | n.d. | n.d. | 93,706 | 0.12 ± 0.01 | |
| Propyl acetate | 71,829 | 0.15 ± 0.01 | n.d. | n.d. | n.d. | n.d. | |
| Ethyl phenylacetate | n.d. | n.d. | n.d. | n.d. | 77,701 | 0.11 ± 0.02 | |
| Ethyl benzoate | 63,951 | 0.13 ± 0.03 a | 114,907 | 0.19 ± 0.02 a | n.d. | n.d. | |
| Benzyl acetate | 57,490 | 0.12 ± 0.02 a | n.d. | n.d. | 78,551 | 0.10 ± 0.03 a | |
| Ethyl butyrate | n.d. | n.d. | 58,451 | 0.1 ± 0.01 | n.d. | n.d. | |
| Ethyl capronate | 333,686 | 0.69 ± 0.24 b | 3,134,463 | 5.14 ± 2.01 a | n.d. | n.d. | |
| Ethyl decanoate | 597,681 | 1.23 ± 0.14 b | 3,013,764 | 4.94 ± 1.94 a | n.d. | n.d. | |
| Ethyl laurate | 166,749 | 0.34 ± 0.04 a | 270,479 | 0.44 ± 0.21 a | n.d. | n.d. | |
| Ethyl caprylate | 711,830 | 1.47 ± 0.04 b | 3,972,062 | 6.51 ± 0.42 a | 77,700 | 0.10 ± 0.01 c | |
| Ethyl palmitate | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| Ethyl propanoate | 78,836 | 0.16 ± 0.28 | n.d. | n.d. | n.d. | n.d. | |
| Total | 66.4± 1.82 a | 33.42± 1.02 b | 33.70± 1.17 b | ||||
| Terpene | α-pinene | 476,664 | 0.98 ± 0.26 | n.d. | n.d. | n.d. | n.d. |
| α-Thujene | 59,049 | 0.12 ± 0.01 | n.d. | n.d. | n.d. | n.d. | |
| β-Phellandrene | 177,922 | 0.28 ± 0.09 | n.d. | n.d. | n.d. | n.d. | |
| γ-Terpinene | 53,957 | 0.11 ± 0.08 a | n.d. | n.d. | 47,871 | 0.06 ± 0.01 b | |
| p-Cymol | 78,263 | 0.16 ± 0.07 a | n.d. | n.d. | 53,470 | 0.07 ± 0.02 b | |
| Total | 1.65± 0.36 a | n.d. | 0.13± 0.01 b | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Budak, H.N. Determination of Changes in Volatile Aroma Components, Antioxidant Activity and Bioactive Compounds in the Production Process of Jujube (Ziziphus jujuba Mill.) Vinegar Produced by Traditional Methods. Fermentation 2022, 8, 606. https://doi.org/10.3390/fermentation8110606
Budak HN. Determination of Changes in Volatile Aroma Components, Antioxidant Activity and Bioactive Compounds in the Production Process of Jujube (Ziziphus jujuba Mill.) Vinegar Produced by Traditional Methods. Fermentation. 2022; 8(11):606. https://doi.org/10.3390/fermentation8110606
Chicago/Turabian StyleBudak, Havva Nilgün. 2022. "Determination of Changes in Volatile Aroma Components, Antioxidant Activity and Bioactive Compounds in the Production Process of Jujube (Ziziphus jujuba Mill.) Vinegar Produced by Traditional Methods" Fermentation 8, no. 11: 606. https://doi.org/10.3390/fermentation8110606
APA StyleBudak, H. N. (2022). Determination of Changes in Volatile Aroma Components, Antioxidant Activity and Bioactive Compounds in the Production Process of Jujube (Ziziphus jujuba Mill.) Vinegar Produced by Traditional Methods. Fermentation, 8(11), 606. https://doi.org/10.3390/fermentation8110606