Abstract
Volatile fatty acids (VFAs) have become promising candidates for replacing the conventional expensive carbon sources used to produce polyhydroxyalkanoates (PHAs). Considering the inhibitory effect of VFAs at high concentrations and the influence of VFA mixture composition on bacterial growth and PHA production, a thorough investigation of different cultivation parameters such as VFA concentrations and composition (synthetic and waste-derived VFAs) media, pH, aeration, C/N ratio, and type of nitrogen sources was conducted. Besides common VFAs of acetic, butyric and propionic acids, Cupriavidus necator showed good capability for assimilating longer-chained carboxylate compounds of valeric, isovaleric, isobutyric and caproic acids in feasible concentrations of 2.5–5 g/L. A combination of pH control at 7.0, C/N of 6, and aeration of 1 vvm was found to be the optimal condition for the bacterial growth, yielding a maximum PHA accumulation and PHA yield on biomass of 1.5 g/L and 56%, respectively, regardless of the nitrogen sources. The accumulated PHA was found to be poly(3-hydroxybutyrate-co-3-hydroxyvalerate) with the percentage of hydroxybutyrate in the range 91–96%. Any limitation in the cultivation factors was found to enhance the PHA yield, the promotion of which was a consequence of the reduction in biomass production.
1. Introduction
As a consequence of being versatile, cheap and durable, conventional plastics have become irreplaceable materials with their applications dominating the market from domestic to engineering applications [1]. At the same time, infrastructure for collecting and recycling plastics is not keeping pace with the increasing demands for annual plastic production. Consequently, large volumes of plastics pollute the environment every year. A low recycling rate together with mass production have led to plastic accumulation with numerous serious negative impacts on the environment and ecosystems [2]. In the controversy around addressing plastic pollution, bioplastics have emerged as a promising solution for reducing the dependency on and consumption of fossil-based plastics with the potential for mitigating adverse impacts on the environment [3]. In this regard, biopolymers such as polyhydroxyalkanoates (PHAs), have shown great potential for replacing conventional plastics such as polyethylene (PE) and polypropylene (PP), considering their exceptional biocompatibility, biodegradability and competitive physical properties [4,5].
Large-scale production of PHAs, however, is hindered by their high production cost, mainly due to the expensive fermentation medium of refined sugars or food-grade substrates. Several studies have focused on the utilisation of low-cost industrial and municipal sidestreams, organic wastes and residuals as economical carbon sources to overcome the financial barrier of PHA production [6]. Direct use of waste streams such as fermentation substrate, is in some cases enhanced by the presence of nutrients such as vitamins, minerals and nitrogen sources that can facilitate the growth of PHA-bearing bacteria [7]. However, since waste streams are complex heterogeneous mixtures, they may contain non-fermentable substances and inhibitors such as phenolic compounds and heavy metals that can be detrimental to microbial cultivation [8,9]. Moreover, these negative or low-value heterogeneous carbon sources could be selective for bacterial consumptions, affecting and lowering the production yields [10]. Pseudonomas putida KT 2240, for example, provided a PHA productivity of 0.006 g/L/h when cultivated in glucose derived from grass biomass fermentation [11], while the use of hydrolysed cooking oil waste presented a higher yield of 1.9 g/L/h [12]. Waste streams, therefore, require homogenisation, removal of inhibitory compounds and maximisation of favourable compounds to be used as effective fermentation media.
Acidogenic fermentation, in this regard, is a potential method for managing complex organic waste streams. This biological process is actually anaerobic digestion without the final stage of methanogenesis via which carbohydrates, proteins and lipids in various residues and wastes are converted in a stepwise process to VFAs such as acetic, propionic and butyric acids [13,14]. VFAs have long been known to be highly versatile biochemicals which act as important building blocks in many chemical and biological conversions for the production of biofuels, flavourings, fragrances, preservatives, etc. [15]. Recently, attention has been given to the application of VFAs as a sole carbon source for the biosynthesis of PHAs using different bacterial strains (PHA accumulations up to 80%) [16]. This application is considered to be a potential alternative for the reduction of expensive fermentation medium, ultimately suggesting a solution for the large-scale production of PHAs [17,18]. The valorisation of common organic wastes and residuals into an economical carbon source for PHA production can therefore be a beneficial approach to cost-effective PHA obtainment. The inclusion of these strategies can also open up a possibility to integrate PHA production into the biorefinery concept. Implementation of such a concept has been fully evaluated by a techno-economic analysis and life cycle assessment in a study by Kachrimanidou et al. [19], using sunflower-based biodiesel by-products as substrate for PHA production. A similar scheme can be fully applied with VFAs as core intermediates. For instance, a problematic organic waste such as the food waste generated globally in vast amounts that is normally incinerated, landfilled or converted to biogas and fertiliser [20] could instead be converted to VFAs through acidogenic fermentation, and further to PHAs, thereby addressing problems associated with organic waste generation, inefficient use of resources and fossil-based plastic production.
Cupriavidus necator—formerly known as Ralstoniaeutropha—is a well-studied PHA producer due to its versatile ability to use various waste substrates as a carbon source [21,22]. However, low productivity in both biomass and PHA production has been one of the most critical problems restricting the use of organic wastes as low-cost substrate [4]. To the authors’ best knowledge, while PHA synthesis from VFAs using C. necator has been achieved previously [17,23,24], information on the effect of VFA concentration thresholds and composition and cultivation factors (pH, aeration, agitation, nutrient supplementation, etc.) on C. necator growth and PHA synthesis is scarce. Therefore, optimisation of cultivation factors is essential to increase the conversion efficiency of VFAs, thereby improving the biomass and PHA yields.
The current study has therefore focused on improving the conversion efficiency of VFAs by C. necator through investigating the effects of different cultivation factors on the bacterial growth and PHA production using synthetic VFAs and actual food waste-derived VFAs as the sole carbon source. In this regard, batch cultivation assays were performed using a single VFA or mixture of synthetic and waste derived VFAs, and with consideration to different substrate loadings and composition, pH, aeration rate, agitation, nitrogen source and C/N ratio. The results regarding the changes in the cultivation factors and their combinations were then thoroughly analysed and statistically compared to find an optimal condition for bacterium growth and PHA production.
2. Materials and Methods
2.1. Microorganism
Cupriavidus necator DSM 545—purchased from DSMZ-German collection of microorganisms and cell cultures GmbH (Leibniz Institute, Braunschweig, Germany)—was preserved on a nutrient agar medium containing (g/L) glucose 1, peptone 15, sodium chloride 6, yeast extract 3, and agar 15, then incubated for 48 h at 30 °C, and subsequently kept at 4 °C until use. The bacterium plate was renewed monthly by the streaking method followed by the same incubation conditions.
2.2. Volatile Fatty Acids from Acidogenic Fermentation of Food Waste
In this study, VFAs were produced in an anaerobic fermentation semi-continuous membrane bioreactor (MBR) with a 3.5 L working volume. The MBR media were agitated using nitrogen gas (3 L/min during fermentation and 5 L/min during filtration) with the help of gas diffusers on each side of the Integrated Permeate Channel (IPC)—a backwashable flat-sheet membrane panel supplied by the Flemish Institute of Technological Research (VITO NV, Mol, Belgium). The flat-sheet membrane had an effective area of 137.6 cm2 coated with hydrophilic polyethersulfne (PES) on both sides with an average pore size of 0.3 µm and a clean water permeability of 3000–4000 L/h/m2/bar. The MBR was inoculated with 3.5 L of wastewater sludge as inoculum (4 g VS/L) and thermally treated food waste (4 g VS/L). The wastewater sludge and food waste were obtained from a wastewater treatment plant Gryaab AB (Gothenburg, Sweden) and a solid waste treatment company Renova AB (Gothenburg, Sweden). Detailed composition of the food waste can be found in a study of Parchami et al. [24].
The microorganism- and particle-free VFA effluent was recovered from the membrane module by filtration process. The profile of recovered VFAs from the membrane bioreactor is summarised in Table 1.

Table 1.
The content of volatile fatty acid (VFA) effluent obtained from acidogenic fermentation of food waste.
2.3. Bacterial Cultivation
The batch experiments to find feasible VFA concentrations on which the bacterium thrive were initially performed in 250 mL cotton-plugged Erlenmeyer flasks containing a synthetic nutrient medium according to Vu et al. [25] with the addition of the seeding culture (5% v/v), instead of using a single bacterial colony. Single synthetic VFAs of acetic acid, propionic acid, butyric acid, isobutyric acid, valeric acid, isovaleric acid and caproic acid were sequentially used as the sole carbon source at an initial concentration of 5 g/L. The cultivations were conducted for 48 h in shaking water baths at 120 rpm, 32 °C and without pH control. During the cultivation, parameters of pH, cell density and substrate consumption were measured at different time intervals.
The seeding culture was prepared with the same medium recipe using glucose (10 g/L) as the sole carbon source inoculated with a single bacterial colony. The flask was then placed in a shaking water bath at 120 rpm and 32 °C for 40 h before being harvested as seeding culture. For cultivations in a benchtop bioreactor, a 2.5 L continuous stirred tank reactor (CSTR) with a working volume of 2.0 L was used. Nutrient medium (Na2HPO4·2H2O 4 g/L, KH2PO4 3.6 g/L, (NH4)2SO4 3 g/L and NaCl 0.02 g/L), MgSO4 (0.5 g/L), CaCl2 (0.05 g/L) and VFA solutions were autoclaved separately, and only mixed inside the reactor containing sterilised synthetic medium just before starting the experiment. The seeding culture was prepared at a total volume of 100 mL as described. The pH was maintained at 7.0 by the addition of 2 M HCl and 2 M NaOH. The cultivation was conducted at a temperature of 32 °C, agitating at 150 rpm and under two aeration rates of 0.25 and 1 vvm for 72 h. To prevent foaming, 0.15 mL of fatty acid ester antifoam was added to the reactor. Samples were collected at different time intervals to determine substrate consumption, cell density, biomass production and PHA accumulation.
2.4. Analytical Methods
High performance liquid chromatography (HPLC) (Waters 2695, Waters Corporation, Milford, CT, USA) was used to determine the changes in the carbon source concentration during the fermentation process. The HPLC unit was equipped with a hydrogen-based column (Aminex HPX87, BioRAD Laboratories, Munchen, Germany) and ultraviolet (UV) absorption detector operating at 210 nm wavelength (Waters 2487, Waters Corporation, Milford, CT, USA) working at 60 °C and with a flowing eluent of 5 mM H2SO4 at a rate of 0.6 mL/min.
Cell dry weight (CDW) measurements were conducted at different time intervals by collecting 50 mL of the culture in duplicate from each reactor. The collected samples were centrifuged at 9000× g for 5 min (Megafuge 8, Thermo Fisher Scientific GmbH, Dreieich, Germany). The supernatant was replaced with Mili-Q water and centrifuged again before being dried together with a dried aluminium cup to constant weight at 70 °C for 24 h.
2.5. Kinetics Analysis of Biomass Production and PHA Accumulation
The kinetics of PHA production in this study was measured using the calculations of biomass yield on consumed substrate γX/S (g/g), PHA yield on produced biomass γP/S (g/g), and PHA yield on consumed substrate γP/S (g/g).
2.6. Characterisation of PHA
A Fourier transform infrared (FTIR) spectrometer (Nicolet iS10, Thermo Fisher Scientific, Waltham, MA, USA) was used to analyse functional groups from the extracted samples. The samples were subjected to a scan of 32 times in a spectrum of 400 to 4000 cm−1 by Nicolet OMNIC 4.1 software. The obtained data were then analysed by Essential FTIR (eFTIR, Madison, WI, USA) software.
Thermogravimetric analysis (TGA) (Q500 TA instruments, Waters LLC, New Castle, DE, USA) was performed to determine the thermal stability of the samples. Approximately 5 mg of extracted product was heated from 25 °C to 700 °C at a rate of 20 °C/min under nitrogen atmosphere.
A differential scanning calorimeter (DSC) (QA500 TA instruments, Waters LLC, New Castle, DE, USA) was used to analyse the thermal properties of the extracted samples. Approximately 3–5 mg of each sample was heated from −40 °C to 225 °C at a rate of 10 °C/min under nitrogen atmosphere. The crystallinity of the extracted sample was briefly calculated with the following equation:
where ΔH (J/g) is the specific enthalpy of fusion measured from the peak area taken from the second heating cycle, and (146 J/g) denotes the crystallinity of the extracted sample [26].
13C MAS NMR spectra were recorded on a Bruker AVANCE-II spectrometer at 14.1 T magnetic field (13C resonance frequency 150.9 MHz) using a home-built MAS NMR probe for 25 × 4 mm Si3N4 rotors. In all experiments, the sample spinning frequency was set to 12.5 kHz. The NMR spectra were recorded with proton decoupling (H1 = 100 kHz) using cross polarisation (CP) with a ramped spin locking pulse in the 1H channel together with a pulse in the 13C channel of suitable amplitude. The relaxation delay in the CPMAS NMR experiments was 5 s. The direct excitation experiment, using a single pulse on the 13C channel with 1H decoupling, was conducted with a 90° pulse (3 μs) and a 60 s relaxation delay. In all experiments, 600 transients were acquired.
2.7. Statistical Analysis
The software package MINITAB 17 was used for the statistical analysis of the acquired data. Analysis of variance (ANOVA) was conducted to investigate the significant difference level between the obtained results with the assistance of general linear models with a 95% confidence interval followed by the Tukey’s test for pairwise comparisons. The experiments were conducted in duplicates and the error bars presented two standard deviations with a 95% confidence interval.
3. Results and Discussion
To enhance the utilisation of waste-derived VFAs as an economical carbon source for PHA production, the optimum concentration for each VFAs was defined in shake flask cultivations. The concentrations obtained were then applied in a bioreactor for further investigations of the effects of cultivation parameters such as pH, aeration, agitation, nitrogen sources and C/N ratio on PHAs yield. Lastly, the optimal condition and the influence of cultivation factors when combined were discussed on the basis of the production kinetics and statistical analysis, respectively.
3.1. Effect of Individual VFA Concentrations on Bacterial Growth
Although VFAs have shown their potential as a carbon source for PHA production, at specific concentrations, they have been shown to have an inhibitory effect on bacterial growth [27,28]. Therefore, in this study, the concentration threshold for each VFAs consumed by C. necator was individually determined in batch cultivations. The consumption of each VFA corresponding to the growth of C. necator and the changes in pH are shown in Figure 1.
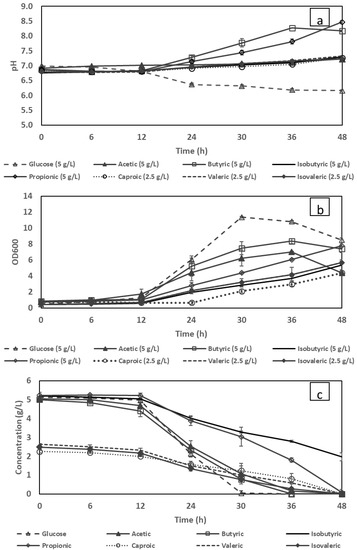
Figure 1.
Changes in the pH (a), cell density (b) and volatile fatty acid (c) concentration during batch cultivation using glucose and individual VFAs as a sole carbon source.
At an initial concentration of 5 g/L, the bacterium could only assimilate four types of VFAs including acetic acid, butyric acid, isobutyric acid and propionic acid. However, only acetic and butyric acids shared a similar consumption pattern, and the media of both were depleted after 36 h of cultivation (Figure 1c). Propionic acid and isobutyric acid, on the other hand, showed a slower consumption rate at which their media were completely consumed, and the latter was left at 2 g/L at the end of the cultivation. The same problem was observed in studies using C. necator where the applied concentrations of each VFA were limited to less than 5 g/L to solve issues with excessive substrate inhibition [17,24]. VFAs that could not be used by the bacterium at 5 g/L were then diluted to 2.5 g/L, enabling feasible growth to be achieved. Regardless of the initial concentrations, the same lag phase of 12 h was observed in all cultivation. This could be attributed to the change in bacterial metabolism (acclimatisation phase) as the seeding culture had been prepared with glucose as the sole carbon source. Although diluted to 2.5 g/L initial concentration, after the lag phase, valeric, isovaleric and caproic VFAs were only consumed gradually, reaching their depletion after 48 h. However, to the best of the authors’ knowledge, the assimilation of long-chained carboxylate compounds (valeric, isovaleric, isobutyric and caproic acids) by C. necator DSM 545 was revealed for the first time in this study.
As expected, the consumption of VFAs resulted in an increase in pH level while for glucose, an opposite trend was observed [25,29]. The bacterial growth was also monitored by the measurement of cell densities which were in compliance with the VFA consumption trends throughout the cultivation (Figure 1b).
Based on the results above, the VFA-rich stream supplied in this study (Table 1) is considered to be favourable for the consumption of the studied bacterium. Consequently, to evaluate the growth and metabolic behaviour of C. necator, another set of batch experiments were conducted using a mixture of synthetic VFAs, the concentration and ratio of which mimicked the content in the real VFA effluent. The changes in consumption of VFAs, cell density and pH during the bacterial cultivation using a mixture of synthetic VFAs are shown in Figure 2a,b.
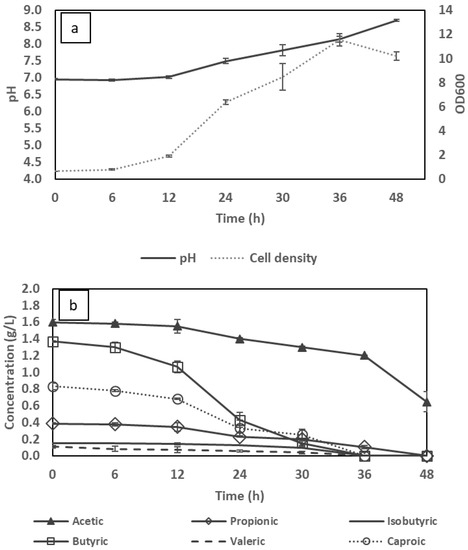
Figure 2.
Changes in pH, cell density (a) and volatile fatty acid (VFA) concentration (b) during flask cultivation in the synthetic VFA mixture.
Compared to cultivations using single VFAs, there is a noticeable change in the consumption behavior. While acetic and butyric acids individually were the most preferred carboxylates, being fully depleted after only 36 h, in the VFA mixture, C. necator tended to initially consume butyric acid followed by caproic acid (originally present at 1.4 and 0.8 g/L, respectively) in the first 36 h. This phenomenon is in line with the studies of Setiadi et al. [30] and Yun [24], where butyrate was shown to be a preferred substrate among other VFAs contributing to bacterial growth in PHA production. In contrast, only 0.4 g/L of acetic acid was consumed in the same period of time, with uptake accelerating only as other VFAs were almost finished. However, in another study of Vu [25], acetic acid appeared to be a preferable carbon source for the production of PHAs using Bacillus megaterium. The utilisation of individual VFAs in a mixture, therefore, is hypothetically dependent on the selective consumption of a particular microorganism. On the other hand, other VFAs were gradually consumed and depleted after 48 h of cultivation, namely, propionic, isobutyric and valeric acids which presented respectively in low concentrations of 0.4, 0.2 and 0.1 g/L. In respect to cell density, a noticeable drop was experienced after 36 h regardless of the existence of acetic acid (0.7 g/L) in the medium at 48 h. This can presumably be explained by the high cell density, lack of supplemented nutrient and increased pH level to 8.7 towards the end of the cultivation in which the residual acetic acid may have been utilised for cell maintenance and prevention of cell lysis rather than for cell growth [31,32].
3.2. Effect of Different Cultivation Factors on Biomass and PHA Production
3.2.1. The Effect of pH
pH is one of the most critical factors in bacterial cultivation, influencing the structure of biological macromolecules (e.g., proteins) and affecting the intracellular chemical reactions and energetic metabolisms, all of which have a direct impact on bacterial growth [33,34]. Microbial growth, conversely, can also change the pH level through resource consumption and metabolite excretion, shifting the pH against the optimal growth [35]. Therefore, in this study, the effect of pH changes on C. necator cultivation for PHA production was studied and compared with the condition of pH control at 7.
As revealed in Section 3.1, the cultivation of C. necator using a mixture of VFAs as the main carbon source results in an increased medium pH level. At the end of the cultivation, the final pH was up to 8.7 (Figure 3b) deviating from the optimal pH for the growth of this bacterium which was found to be between 7 and 7.5 in the studies of Aramvash et al. [36] and Nygaard et al. [37]. This pH change could greatly affect the required energy for bacterial growth, which is instead directed towards cell maintenance, thereby interrupting biomass production. This, in turn, is not favourable for PHA production where high biomass is preferable for the better storage capacity of intracellular PHAs [38].
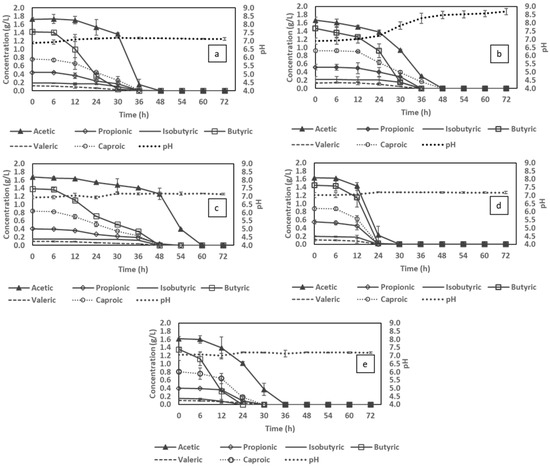
Figure 3.
The effect of different cultivation factors (compared in pairs) on volatile fatty acid (VFA) consumption: pH control (a) vs. no pH control (b); C/N 6 (a) vs. C/N 12 (c); 1 vvm (a) vs. 0.25 vvm (d) and ammonium sulfate (a) vs. urea (e).
As seen in Figure 4b, by comparing the biomass production and PHA accumulation induced by the changes in pH, the difference can be clearly observed from 36 h onwards. At 30 h, the pH in the no control assays reached 8.2 (an 18% offset from the initial pH level) (Figure 3b). This deviation was hypothetically considered to have a strong impact on the bacterial activities, thus lowering the overall VFA consumption. The acetic acid content under no pH control condition remained at 0.9 g/L compared to 0.3 g/L in the pH control counterpart at 36 h. Under unfavourable pH conditions, the microbial metabolism is constrained due to the changes in enzymatic structure which promote destruction and wrecking activity, ultimately leading to the delay in carbon source consumption (VFA assimilation, for instance) [39]. Furthermore, the energy produced from the VFA utilisation, is in this case possibly directed to cell maintenance rather than cell growth, thus preventing cell lysis [40]. Therefore, regardless of reaching the maximum biomass production at 36 h, the biomass obtained in controlled pH condition was 2.8 g/L which was significantly higher than the amount in the culture with no pH control (1.9 g/L). The difference in biomass production, as a consequence, leads to a deviation in PHA accumulation, the concentration of which in fixed pH reactor was higher than no pH control, being respectively 1.5 g/L compared to 1.3 g/L (Figure 4a,b). PHA yield on biomass obtained in the uncontrolled pH assays, on the other hand, was higher as soon as the pH was shifted away from the optimal level. It could be hypothesised that unstable pH is a stress factor which suppresses the bacterial growth, thus triggering PHA accumulation.
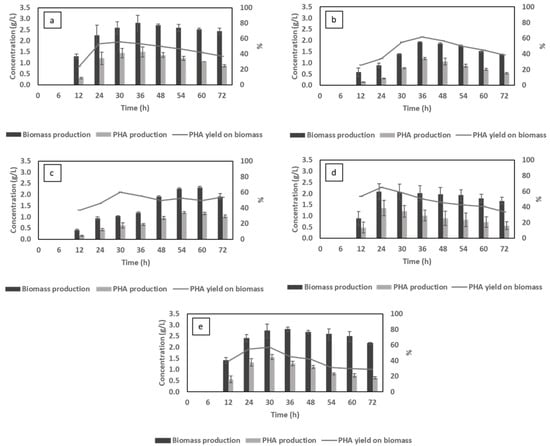
Figure 4.
The effect of different cultivation factors (compared in pairs) on the changes in biomass and polyhydroxyalkanoate (PHA) production: pH control (a) vs. no pH control (b); C/N 6 (a) vs. C/N 12 (c); 1 vvm (a) vs. 0.25 vvm (d) and ammonium sulfate (a) vs. urea (e).
While a rise in the pH resulted in the reduction in biomass after 48 h (decrease of 0.5 g/L), in the pH control assay, the biomass content showed a modest decline with a total decrease of 0.3 g/L (from 48 h to 72 h) followed by a marginal decrease in PHA content which reinforces the role of PHA in lengthening bacterial survival [41].
3.2.2. The Effect of Aeration Rate
Regarding PHA production, substantial biomass production is desirable for maximising the storage capacity of intracellular PHAs. However, growth should be optimised in such a way that it does not jeopardise PHA accumulation through over consumption. One of the critical parameters required to achieve optimum biomass production is satisfying oxygen demand during the bacterial growth. Limited oxygen supply results in the reduction of microbial metabolism by hindering the oxidation of carbohydrates and respiration [42]. Moreover, oxygen deficiency was found to have a negative impact on PHA accumulation [43]. In this regard, the cultivation was performed in an oxygen supply of 0.25 and 1 vvm to evaluate the effect of aeration on the C. necator growth and PHA accumulation under pH control conditions.
As presented in Figure 3a,c, regardless of the aeration rate, the pattern of VFA consumption is similar, where the assimilation of acetic acid only accelerated after other VFAs were completely utilised. A slow uptake of acetic acid was also observed in a study by Wang et al. [44], where mixed microbial cultures were used to produce PHAs using a VFA mixture (acetate, butyrate, propionate and valerate) at low dissolved oxygen. However, when 1 vvm air was applied, the VFA content was taken up fully in 48 h of cultivation, while for aeration at 0.25 vvm, it took about 60 h. In low air provision conditions, the cell density reached its peak corresponding to the biomass production of 2.3 g/L at 60 h (Figure 4c). PHA accumulation, in both cases, gradually increased along with the biomass content, thus sustaining a fluctuating yield on biomass of 49–55% after 30 h. In aspect of 1 vvm, this yield simultaneously reached its peak of 55% and then decreased till the end of the cultivation. These findings, however, are not in line with studies of Lefebvre et al. [45], Shantini et al. [46] and Cavalheiro et al. [47], where it was claimed that PHA yield improved under low dissolved oxygen environments. A low aeration rate of 0.25 vvm in this work did not provide a higher yield but preserved a consistent production rate, which could be attributed to the slow assimilation of the carbon source under a condition of oxygen deficiency. The lack of oxygen could become exacerbated by the increase in cell density resulting in inferior carbohydrate oxidation. Although higher oxygen supplementation could result in better PHA productivity, the energy demand for aeration should be taken into consideration when it comes to PHA production costs, as pointed out by [48].
3.2.3. The Effect of C/N Ratio
Carbon and nitrogen are vital primary building blocks in cell component synthesis. These nutrients should therefore be provided at the balanced ratio favourable to specific microorganisms [49]. PHAs, however, could be considered as a secondary metabolite produced to guarantee the bacterial survival under stress conditions induced by insufficient nitrogen, phosphorus, sulfur and excess carbon sources [50]. There are microbes that can accumulate PHAs during their growth without the need for restricted conditions. C. necator, however, does not belong to this group, where PHA accumulation can only be triggered under stressed circumstances [27]. Major findings have also pointed out that of all the nutrients including phosphorus, sulfur, oxygen, etc., it is the restriction in nitrogen that is the most efficient way to promote PHA accumulation [51]. In general, a low C/N ratio is beneficial to the bacterial growth since it decreases PHA accumulation, and vice versa. In this study, the obtained food-waste based VFA stream contained a chemical oxygen demand to ammonium nitrogen ratio of around 12; therefore, two different ratios of 6 and 12 were applied to evaluate the influence of C/N ratio on PHA accumulation of C. necator with the VFA mixture as the sole carbon source.
As depicted in Figure 4a,d, C/N ratio had a noticeable impact on bacterial growth and PHA accumulation. In particular, excessive nitrogen deficiency (C/N 12) limited biomass production, the maximum amount of which was only 2.1 g/L compared with 2.8 g/L for C/N of 6. This finding is in agreement with the results achieved by Zhou et al. [52], where nitrogen-insufficient conditions are preferred for secondary metabolism of PHA production and concomitantly reducing cell growth. Although the highest PHA content obtained for C/N 12 was 1.4 g/L (approximately 6% lower than that of C/N 6), this nitrogen-insufficient condition provided the highest maximum PHA yield on biomass of 65.1% at 24 h, reaching the maximum 6 h earlier compared to the condition of nitrogen surplus of 55.7%. The higher PHA yield in nitrogen deficient condition could be ascribed to the lower biomass produced while the accumulated PHA was considerably different. Biomass production and PHA accumulation, therefore, should be balanced to achieve a high PHA production per bacterial mass. This approach is consistent with the results of Sangkharak and Prasertsan [53], where a C/N ratio of 6 provided the highest PHA content, the amount of which declined in extreme high or low C/N ratios. The result obtained indicated the influence of C/N ratio on the rate of PHA production since the condition with high nitrogen concentration experienced a long lag before entering the condition of nitrogen deficiency. In the case of nitrogen deficiency, the proton gradient is affected, which inhibits the Acetyl-CoA to TCA cycle for the synthesis of ATP. The excess acetyl-CoA is then directed to PHA synthesis [54]. The time required to achieve maximum PHA content is therefore different depending on the C/N ratio. Together with C/N ratios, fixed concentration of initial carbon and nitrogen is an important factor for the optimisation of PHA accumulation as claimed in a study by Ahn et al. [55].
3.2.4. The Effect of Nitrogen Source
Besides the effort of utilising various waste-based materials as an economical carbon source, provision of a low-cost nitrogen source should also be considered. Scientific studies have previously evaluated inexpensive nitrogen sources of ammonium sulfate, ammonium chloride, ammonium hydroxide or urea for PHA production by C. necator [56,57]. Hydrolysates of cheese whey, chicken feather or silage juice were also studied to provide an alternative to more expensive complex nitrogen [58,59,60]. Urea, in most studies, has shown considerable support for both bacterial growth and PHA biosynthesis, substantially increasing PHA accumulation [61,62,63,64]. However, the main carbon sources used in those studies were common sugars of glucose, fructose and oil-based wastes, while information on the utilisation of VFA mixtures is scarce. In this study, therefore, the influence of urea and ammonium sulfate on PHA accumulation by C. necator with a VFA mixture as the carbon source were investigated.
In general, the uptake trend of VFA content, in both cases, was fairly similar and was consistent with assays for effects of different parameters (excluding C/N ratio). However, the bacterium cultivated in urea as the nitrogen source consumed VFAs faster (36 h) than those cultivated in ammonium sulfate (48 h) (Figure 3e). The swift utilisation, however, did not lead to a significant change in bacterial growth since biomass production in both cases was almost similar throughout the 72 h of cultivation, reaching the same maximum biomass content of 2.8 g/L at 36 h (Figure 4e). This trend was also observed for PHA accumulation, where a concentration of 1.5 g/L was obtained, leading to a maximum PHA yield on biomass of around 55–56% in all instances. The recorded impact of urea in this study was not in line with findings in studies by Arumugam [61] and Dañez [62], where remarkable superiority of urea compared with other inorganic and complex nitrogen sources was reported for PHA accumulation. The presence of urea, on the other hand, is preferable for bacterial assimilation due to its being a small, polar and uncharged organic molecule. These characteristics, altogether, allow the microbes to be less pH-dependent and to consume urea at an accelerated rate [65]. Fast urea consumption can therefore lead to faster C. necator growth as observed in biomass in the first 12 h (Figure 4e). From an economic point of view, if the waste-derived VFA stream lacks the required quantity and quality of nitrogen source for bacterial growth, urea supplementation may be a promising option for shortening the cultivation time and reducing the PHA production cost.
3.3. C. necator Cultivation on VFAs in CSTR
Since it was observed in batch assays that there was no significant difference between ammonium sulfate and urea in respect of biomass (p-value = 0.476) and PHA production (p-value = 1), optimal cultivation factors of pH control, C/N 6, vvm 1 and ammonium sulfate as nitrogen source were applied to determine biomass and PHA production using individual synthetic VFAs and food waste-derived VFAs. The changes in biomass production, PHA accumulation and cell density corresponding to individual VFA consumption are presented in Figure 5.
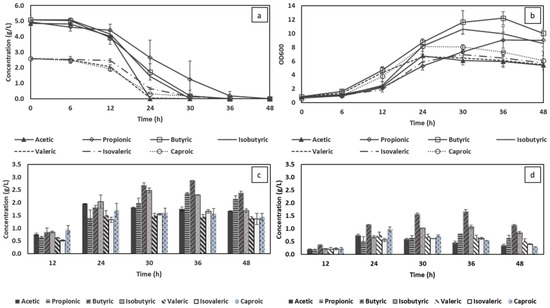
Figure 5.
Changes in individual volatile fatty acid (VFA) consumptions (a) and cell density (b) corresponding to biomass production (c) and PHA accumulation (d) during bioreactor cultivation under optimal conditions.
In the group of individual synthetic VFAs with an initial concentration of 5 g/L, acetic acid was the most preferred carbon source to be assimilated within 24 h, yielding a maximum cell density of 6.7, corresponding to 1.8 g/L bacterial biomass (Figure 5a,c). On the other hand, and regardless of the slower consumption, butyric and isobutyric acids provided better bacterial growth, which can be observed from the maximum cell densities and biomass production of 12.2 and 10.6, and 2.9 g/L and 2.5 g/L, respectively (Figure 5b,c). The higher biomass production consequently led to 30–50% higher PHA accumulation in the case of butyric and isobutyric acid compared to that of acetic acid (0.8 g/L). This can be explained by the assimilation of butyric and isobutyric acids by beta oxidation which forms two molecules of acetyl-CoA and acyl-CoA [66]. The acyl-CoA will ultimately be converted into acetyl-CoA; therefore, butyric and isobutyric acid could have more acetyl-CoA compared with acetic acid. In balanced conditions, acetyl-CoA participates in the Krebs cycle, playing the role of an energy supplier for bacterial growth. However, under conditions of nutrient stress and excess carbon source, acetyl-CoA will be redirected to act as a precursor in PHAs synthesis. The bacterial cultivation in butyric and isobutyric acids, therefore, provided a higher PHA yield. As in the experiments conducted in shake flasks, and compared with acetic, butyric and isobutyric acid, propionic acid utilisation by C. necator took longer (36 h), yielding a maximum biomass and PHA production of 2.4 and 0.8 g/L, respectively. While the initial VFA concentration was set at 2.5 g/L for VFAs acting in an inhibitory fashion at 5 g/L, no significant change in bacterial growth was observed, with the only exception being a slight variation in isovaleric consumption. The latter deviation resulted in the bacterium taking 36 h to achieve the maximum biomass of 1.7 g/L, yielding the highest PHA accumulation of 0.6 g/L cultivated in isovaleric as the sole carbon source.
After cultivations made on a synthetic VFA solution resembling the composition of real VFA effluent, actual food waste-derived VFA effluent was used as a substrate for bacterial cultivation with no additional nutrient supplementation. The C/N ratio of the VFA stream was calculated to be around 12, based on the total COD and nitrogen content. During cultivation, conditions of pH control and aeration of 1 vvm were applied. After 36 h of cultivation, the VFA content was completely assimilated with the same consumption pattern obtained in shake flask batch experiments but at an accelerated rate (Figure 6a). The maximum biomass amount of 2.3 g/L was also achieved at this time, corresponding to the highest PHA accumulation of 1 g/L (Figure 6b). Biomass production was similar, while the accumulated PHA was only about 11% lower than that obtained using synthetic VFAs with the same C/N ratio. However, interestingly, compared to similar cultivation conditions using a synthetic VFA mixture at C/N 6, PHA accumulation for the real effluent was 38% higher.
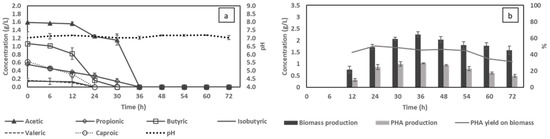
Figure 6.
Changes in volatile fatty acid (VFA) consumption (a), and biomass and PHA production (b) using food waste-derived VFA effluent in a bioreactor using optimal cultivation conditions.
3.3.1. Yields of Biomass Production and PHA Accumulation
The combination of pH control, C/N ratio 6, and aeration at 1 vvm was the optimal condition for maximum biomass production, regardless of the nitrogen source (Table 2). However, only pH control and high C/N ratio showed a significant difference in respect of biomass yield on substrate with p-values of 0.017 and 0.002, respectively. The maximum biomass yield on substrate achieved was up to 82%, indicating the suitability of applied operating parameters and VFAs as a feasible carbon source, while the lower PHA yield on substrate afterwards can be explained by the depletion of VFAs at the end of the cultivation. Although PHA accumulation depends greatly on the microorganisms and substrates used, high biomass production is desirable since bacterial cells act as PHA storage. In comparison to studies of Yun [24] and Agustín Martinez [17] using the same bacterial strain and VFAs (rich in acetic, propionic and butyric acid) as sole carbon, the obtained biomass in this study was higher, 2.8 g/L compared to 1.5 and 2 g/L, respectively. As a consequence, the higher accumulated PHA was also achieved, its content being in a similar range of 50–55%.

Table 2.
Results of kinetic analysis of biomass production and polyhydroxyalkanoate (PHA) accumulation in different cultivation conditions.
Regarding PHA yield on biomass, there is no statistical difference between cultivation factors (p-value > 0.05) excluding the no pH control condition with urea as the nitrogen source (p-value = 0.033). In this study, cultivation factors of no pH control and low aeration were considered to be limiting conditions which hindered bacterial growth, with early triggering of PHA accumulation. In this regard, the most significant difference was observed in a low aeration rate with a p-value of 0.039.
3.3.2. PHA Characterisation
Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
FTIR analysis was conducted to determine the presence of functional groups in extracted samples from C. necator cultivated in CSTR (Figure 7). All spectra obtained revealed typical peaks of 1719 and 1275 cm−1, respectively corresponding to the C=O stretch in the ester carbonyl group and -O-CH group. Those peaks are representative for poly(β-hydroxybutyrate-β-hydroxyvalerate) (PHBV) chemical structures [67,68]. Other stretches in C-O bonding of the ester group ranging from 1000 to 1300 cm−1 were also observed and resemble commercial PHBV.
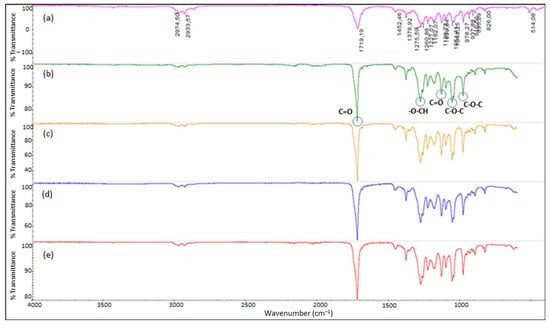
Figure 7.
Fourier Transform Infrared Spectroscopy (FTIR) spectra from extracted polyhydroxyalkanoate (PHA) produced by C. necator DSM 545 using different carbon sources and for poly(β-hydroxybutyrate-β-hydroxyvalerate) (PHBV) commercial pellet: (a) PHBV pellet, (b) glucose, (c) synthetic volatile fatty acids (VFAs) (ammonium sulfate), (d) synthetic VFAs (urea), and (e) real VFA stream.
Nuclear Magnetic Resonance (NMR) Analysis
Figure 8 shows the 13C CPMAS NMR of the four typical samples to determine the effect of different carbon and nitrogen sources used on the accumulated PHA. The main peaks can be assigned to polyhydroxybutyrate (PHB). The smaller peaks are due to the presence of smaller amounts of polyhydroxyvalerate (PHV). The amount of HV in each sample was taken from the ratio of the HV methyl peak area around 10 ppm to the HB methyl peak area around 20 ppm, after scaling by a factor to allow for the cross polarisation dynamics effect. This factor was found by comparing the CPMAS NMR spectrum of the VFA/urea sample with a quantitative single pulse experiment from the same sample (Supplementary Material, Figure S1).
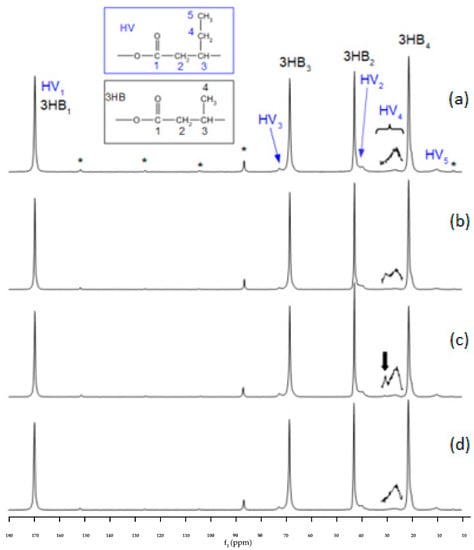
Figure 8.
13C CPMAS NMR spectra of samples produced from different carbon and nitrogen sources: (a) real volatile fatty acid (VFA) stream, (b) glucose/(NH4)2SO4, (c) VFA/urea, (d) VFA/(NH4)2SO4 using a contact time of 2 ms. * indicates peaks due to spinning sidebands.
Figure 9 shows an expansion of the HB methyl peak area from the CPMAS NMR experiment together with the direct excitation MAS NMR experiment. This methyl peak has both a broader and narrower component. The narrow component is assigned to PHB in more ordered areas, presumably the crystalline regions, while the broader component is assigned to PHB in the less ordered, amorphous regions [69,70,71] (see also Supplementary Material). The ratio of these two components found in the CPMAS NMR experiment was corrected for the CP dynamics by comparing the CP spectrum of the VFA/urea sample with the quantitative single pulse experiment shown in Figure 9. This correction factor was used to estimate the degree of crystallinity for the other samples where only CPMAS NMR spectra were measured. The deconvolution of the methyl peaks depends on the peak lineshapes used (Lorentzian/Gaussian) for fitting, but in this case all four samples were fitted with the same lineshapes so the relative amounts of crystalline/amorphous should be comparable.
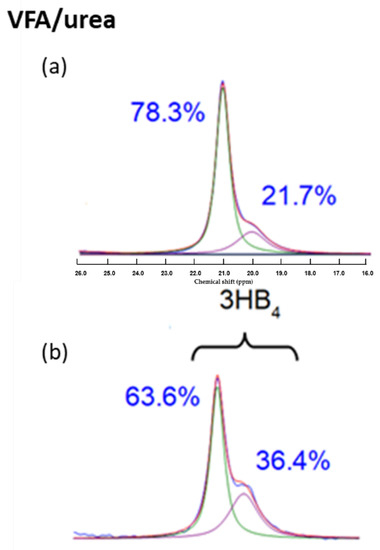
Figure 9.
Comparison of the HB methyl peak area of the volatile fatty acid (VFA)/urea sample from (a) the CPMAS NMR experiment and (b) quantitative direct excitation experiment.
The side chain CH2 carbon peak HV4 from HV, around 27 ppm in the spectra, is frequently seen as asymmetric and, at this low HV content, has been assigned to crystalline HV with different arrangements of the HV side chain in the PHB lattice and amorphous HV [72]. It has also been suggested that at this low level of HV, there is no or little crystalline form of HV present [70,73]. This asymmetric peak is observed for all the samples analysed here. In addition, for two of the samples (VFA/urea and VFA/glucose), there is also a weak peak around 30–31 ppm (indicated by an arrow in Figure 8). When the level of HV is over 30–40%, a peak near this area (29–30 ppm) has been assigned to HV in a crystalline PHV lattice [70]. However, the level of HV in these samples is only 4–8% and at this low level, this crystalline form of HV is not normally observed. In addition, the peak observed here displays a slightly higher chemical shift than normally seen for crystalline HV in a PHV lattice.
To determine the character of this extra peak, a variable contact CPMAS experiment was conducted on the VFA/urea sample (see Supplementary Materials). The CP dynamics of the 31 ppm peak indicated that it is unlikely to be due to any crystalline HV and could be due to some type of amorphous homopolymer of HV. This would agree with the general assumption that crystalline HV in a PHV lattice is not formed at such low HV contents. The asymmetric peak in this area was treated as two overlapping peaks and the variable contact CPMAS indicated that these are most likely due to HV in the amorphous and interfacial regions of the HB/HV copolymer—although due to the poor signal intensity, this conclusion is only tentative (see Supplementary Materials).
The composition and crystallinity of each sample is summarised in Table 3, using the methods and assumptions outlined above. The degrees of crystallinity obtained in Table 3 are in line with those normally observed for HB/HV copolymers [71]. Table 3 could indicate that when the HV content is higher, the crystallinity of the HB polymer is lower. However, this can also be influenced by ageing and thermal history, neither of which were controlled in these samples.

Table 3.
Composition of the copolymers and the total crystallinity found.
3.3.3. Thermogravimetric and Differential Scanning Calorimetry Analysis (TGA and DSC)
Thermal properties of PHA samples were determined by TGA and DSC and the results are presented in Table 4. Compared to extracted samples from a synthetic medium, the polymer obtained from the real VFA stream showed a slight difference in thermal deterioration from both initial decomposition temperature (Tonset) and maximum degradation temperature (Tmax). In particular, the initial and maximum degradation temperatures which were around 201.1 and 308.8 °C, respectively, are both approximately 10 °C lower than those of polymers extracted from bacterium cultivated in synthetic VFAs, indicating a slightly lower thermal stability. This result can be attributed to the surplus impurity content in the polymer recovered from the cultivation in the waste-based stream leading to a total weight loss of only around 80%, corresponding to the difference of 15–16% in the total mass loss. The excessive impurity can also be observed from the decomposition curve being gradual in contrast with the steep slope of extracted samples from the synthetic medium, resembling results from studies of Ntaikou et al. [74] and Rodrigues [75]. Either downstream processing or food waste-based VFA pre-treatment should therefore be developed to achieve purer recovered PHA. Such a development, furthermore, should be conducted in an economical way as the PHA downstream process also accounts for a significant share in PHA production cost [76].

Table 4.
Thermal characteristics of extracted polyhydroxyalkanoate (PHA) obtained from C. necator DSM 545 using different carbon and nitrogen sources.
According to the second heating curves, melting temperatures (Tm) of extracted polymer in this study were in a range of 151.9 to 176.1 °C. Melting enthalpies (∆H), on the other hand, were allocated in a wider range of 15.8 to 66.44 J/g, corresponding to a crystallinity of 10.8 to 45.5%, respectively, and were similar to those in studies by Ntaikou [74], Nygaard et al. [77] and Li et al. [78]. The low degree of crystallinity of synthesised polymers using real VFAs could be an advantage in facilitating various processing windows as suggested by Rosengart et al. [79]. The degrees of crystallinity for the samples by solid state NMR and DSC are different, with the DSC values consistently lower. This is generally seen when comparing crystallinities by DSC and solid state NMR [80]. The DSC degree of crystallinity depends on the heat of fusion used for 100% crystalline PHB. In this case, 146 J/g was used, while some authors have used 109 J/g, which would raise the overall degree of crystallinity, as shown in Table 4. NMR measures crystalline order on a smaller scale and, depending on the lineshapes used for the separation of those components, can also include the interfacial region between the crystalline and amorphous areas. Therefore, it would not be expected that DSC and NMR give the same degrees of crystallinity, rather they should be seen as complimentary methods for probing the crystallinity. Furthermore, the reactions of re-crystallisation and cross-linking isomerization that correspond to the presence of dual meting point were found during the melting process [25,81]. Thermal properties of extracted polymer in this study, moreover, tended to be affected by the carbon sources, where the endothermic peaks of extracted polymer were decreased as the bacterium was cultivated in VFAs (see Supplementary Materials, Figure S3). These findings, however, contrast with the study of Zhila, et al. [82] which used various long chain fatty acids, with the results showing a consistency in thermal properties regardless of the carbon sources.
4. Conclusions
Food waste-based VFAs present the possibility of replacing conventional expensive carbon sources as an economical alternative for tackling the challenging issue of high PHA production costs. VFAs, however, could be toxic and inhibit bacterial growth depending on the tolerance of individual species. In this study, Cupriavidus necator DSM 545 showed the capacity to consume all types of VFAs with a feasible concentration ranging from 2.5 to 5 g/L. Isobutyric, isovaleric and caproic acid are, for the first time, reported to be consumed by C. necator. The cultivation of C. necator, in a mixture of VFAs, presented a preferable consumption in butyric acid, while acetic acid, by contrast, was assimilated at the lowest consumption rate. The accumulated PHAs was confirmed by NMR analysis to be PHBV, with the highest HB content achieved being 96%. Cultivation parameters of pH control, C/N 6, and 1 vvm were found to be favourable for this bacteria’s growth. These conditions can be applied in the feast stage of a two-stage strategy of PHA production, where the biomass production is maximised to facilitate PHA accumulation in the second stage.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/fermentation8110605/s1, Figure S1: Comparison of PHB and PHV methyl areas in the sample “VFA/urea” using cross polarisation (contact time 2 ms) and direct excitation; Figure S2: Fits of the I-I*-S and I-S cross polarisation models to the (a) 3HB4 and 3HB2 peak areas, and (b) the HV peaks. Peak intensities were scaled to 1.0 for the most intense peak. The peaks labelled as HV2 were not used but can be identified in the variable contact experiment; Figure S3: DSC profiles of extracted samples from biomass cultivated in different carbon sources of (a) glucose, (b) synthetic VFAs and (c) real VFA stream; Table S1: Fitted parametersS2 found from the variable contact cross polarisation experiment on the sample VFA/urea. References [83,84] are cited in the supplementary materials.
Author Contributions
Conceptualization, D.H.V. and A.M.; methodology, D.H.V., A.M. and D.Å.; software, D.H.V. and A.R.; validation, A.M., I.H. and D.Å.; writing—original draft preparation, D.H.V.; writing—review and editing, D.H.V., A.M., A.R., D.Å., I.H. and M.J.T.; supervision, A.M., D.Å. and M.J.T.; funding acquisition, A.M., D.Å., M.J.T. and I.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Formas and Sparbanksstiftelsen Sjuhärad. I.H. was funded by the European Regional Development Fund grant number TK 134.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to express their gratitude to Sweden’s Innovation Agency, Formas, Sparbanksstiftelsen Sjuhärad, and the University of Borås for technical and financial support of this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Skoczinski, P.; Krause, L.; Raschka, A.; Dammer, L.; Carus, M. Chapter One-Current Status and Future Development of Plastics: Solutions for a Circular Economy and Limitations of Environmental Degradation. In Methods in Enzymology; Weber, G., Bornscheuer, U.T., Wei, R., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 1–26. [Google Scholar]
- Li, P.; Wang, X.; Su, M.; Zou, X.; Duan, L.; Zhang, H. Characteristics of plastic pollution in the environment: A review. Bull. Environ. Contam. Toxicol. 2021, 107, 577–584. [Google Scholar] [CrossRef]
- Vu, D.H.; Åkesson, D.; Taherzadeh, M.J.; Ferreira, J.A. Recycling strategies for polyhydroxyalkanoate-based waste materials: An overview. Bioresour. Technol. 2020, 298, 122393. [Google Scholar] [CrossRef]
- Adeleye, A.T.; Odoh, C.K.; Enudi, O.C.; Banjoko, O.O.; Osiboye, O.O.; Odediran, E.T.; Louis, H. Sustainable synthesis and applications of polyhydroxyalkanoates (phas) from biomass. Process Biochem. 2020, 96, 174–193. [Google Scholar] [CrossRef]
- Raza, Z.A.; Abid, S.; Banat, I.M. Polyhydroxyalkanoates: Characteristics, production, recent developments and applications. Int. Biodeterior. Biodegrad. 2018, 126, 45–56. [Google Scholar] [CrossRef]
- De Donno Novelli, L.; Sayavedra, S.M.; Rene, E.R. Polyhydroxyalkanoate (pha) production via resource recovery from industrial waste streams: A review of techniques and perspectives. Bioresour. Technol. 2021, 331, 124985. [Google Scholar] [CrossRef]
- Koller, M.; Atlić, A.; Dias, M.; Reiterer, A.; Braunegg, G. Microbial PHA Production from Waste Raw Materials. In Plastics from Bacteria: Natural Functions and Applications; Chen, G.G.-Q., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 85–119. [Google Scholar]
- Silva, L.F.; Taciro, M.K.; Ramos, M.E.M.; Carter, J.M.; Pradella, J.G.; Gomez, J.G. Poly-3-hydroxybutyrate (p3hb) production by bacteria from xylose, glucose and sugarcane bagasse hydrolysate. J. Ind. Microbiol. Biotechnol. 2004, 31, 245–254. [Google Scholar] [CrossRef]
- Solaiman, D.K.Y.; Ashby, R.D.; Foglia, T.A.; Marmer, W.N. Conversion of agricultural feedstock and coproducts into poly(hydroxyalkanoates). Appl. Microbiol. Biotechnol. 2006, 71, 783–789. [Google Scholar] [CrossRef]
- Raza, Z.A.; Tariq, M.R.; Majeed, M.I.; Banat, I.M. Recent developments in bioreactor scale production of bacterial polyhydroxyalkanoates. Bioprocess Biosyst. Eng. 2019, 42, 901–919. [Google Scholar] [CrossRef]
- Davis, R.; Kataria, R.; Cerrone, F.; Woods, T.; Kenny, S.; O’Donovan, A.; Guzik, M.; Shaikh, H.; Duane, G.; Gupta, V.K.; et al. Conversion of grass biomass into fermentable sugars and its utilization for medium chain length polyhydroxyalkanoate (mcl-pha) production by pseudomonas strains. Bioresour. Technol. 2013, 150, 202–209. [Google Scholar] [CrossRef]
- Ruiz, C.; Kenny, S.T.; P, R.B.; Walsh, M.; Narancic, T.; O’Connor, K.E. High cell density conversion of hydrolysed waste cooking oil fatty acids into medium chain length polyhydroxyalkanoate using pseudomonas putida kt2440. Catalysts 2019, 9, 468. [Google Scholar] [CrossRef]
- Harirchi, S.; Wainaina, S.; Sar, T.; Nojoumi, S.A.; Parchami, M.; Parchami, M.; Varjani, S.; Khanal, S.K.; Wong, J.; Awasthi, M.K.; et al. Microbiological insights into anaerobic digestion for biogas, hydrogen or volatile fatty acids (vfas): A review. Bioengineered 2022, 13, 6521–6557. [Google Scholar] [CrossRef]
- Wainaina, S.; Awasthi, M.K.; Sarsaiya, S.; Chen, H.; Singh, E.; Kumar, A.; Ravindran, B.; Awasthi, S.K.; Liu, T.; Duan, Y.; et al. Resource recovery and circular economy from organic solid waste using aerobic and anaerobic digestion technologies. Bioresour. Technol. 2020, 301, 122778. [Google Scholar] [CrossRef]
- Ramos-Suarez, M.; Zhang, Y.; Outram, V. Current perspectives on acidogenic fermentation to produce volatile fatty acids from waste. Rev. Environ. Sci. Bio/Technol. 2021, 20, 439–478. [Google Scholar] [CrossRef]
- Liu, C.C.; Zhang, L.L.; An, J.; Chen, B.; Yang, H. Recent strategies for efficient production of polyhydroxyalkanoates by micro-organisms. Lett. Appl. Microbiol. 2016, 62, 9–15. [Google Scholar] [CrossRef]
- Agustín Martinez, G.; Bertin, L.; Scoma, A.; Rebecchi, S.; Braunegg, G.; Fava, F. Production of polyhydroxyalkanoates from dephenolised and fermented olive mill wastewaters by employing a pure culture of Cupriavidus necator. Biochem. Eng. J. 2015, 97, 92–100. [Google Scholar] [CrossRef]
- Cheah, Y.-K.; Vidal-Antich, C.; Dosta, J.; Mata-Álvarez, J. Volatile fatty acid production from mesophilic acidogenic fermentation of organic fraction of municipal solid waste and food waste under acidic and alkaline ph. Environ. Sci. Pollut. Res. Int. 2019, 26, 35509–35522. [Google Scholar] [CrossRef]
- Kachrimanidou, V.; Ioannidou, S.M.; Ladakis, D.; Papapostolou, H.; Kopsahelis, N.; Koutinas, A.A.; Kookos, I.K. Techno-economic evaluation and life-cycle assessment of poly(3-hydroxybutyrate) production within a biorefinery concept using sunflower-based biodiesel industry by-products. Bioresour. Technol. 2021, 326, 124711. [Google Scholar] [CrossRef]
- Pervez, M.N.; Bilgiç, B.; Mahboubi, A.; Uwineza, C.; Zarra, T.; Belgiorno, V.; Naddeo, V.; Taherzadeh, M.J. Double-stage membrane-assisted anaerobic digestion process intensification for production and recovery of volatile fatty acids from food waste. Sci. Total Environ. 2022, 825, 154084. [Google Scholar] [CrossRef]
- Reddy Prasad, D.M.; Pendyala, R.; Senthilkumar, R.; Azri, M.H.B. Microbial production of poly (3-hydroxybutyrate) (phb) from rubber seed oil using Cupriavidus necator h16. IOP Conf. Ser. Earth Environ. Sci. 2019, 398, 012008. [Google Scholar] [CrossRef]
- Sukruansuwan, V.; Napathorn, S. Use of agro-industrial residue from the canned pineapple industry for polyhydroxybutyrate production by Cupriavidus necator strain a-04. Biotechnol. Biofuels 2018, 11, 202. [Google Scholar] [CrossRef]
- Du, G.; Si, Y.; Yu, J. Inhibitory effect of medium-chain-length fatty acid on synthesis of polyhydroxyalkanoates from volatile fatty acid by ralstonia eutrophus. Biotechnol. Lett. 2001, 23, 1613–1617. [Google Scholar] [CrossRef]
- Yun, J.; Sawant, S.; Kim, B.S. Production of polyhydroxyalkanoates by ralstonia eutropha from volatile fatty acids. Korean J. Chem. Eng. 2013, 30, 2223–2227. [Google Scholar] [CrossRef]
- Vu, D.H.; Wainaina, S.; Taherzadeh, M.J.; Åkesson, D.; Ferreira, J.A. Production of polyhydroxyalkanoates (phas) by bacillus megaterium using food waste acidogenic fermentation-derived volatile fatty acids. Bioengineered 2021, 12, 2480–2498. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.H.; Kummerlöwe, C.; Kammer, H.-W. Crystallization and melting behavior of poly(3-hydroxybutyrate)-based blends. Macromol. Chem. Phys. 2004, 205, 664–675. [Google Scholar] [CrossRef]
- Jaramillo-Sánchez, R.; Alcaraz-Zapata, W. Limitations on production methods for phas obtention: A systematic review. DYNA 2020, 87, 193–203. [Google Scholar]
- Wilbanks, B.; Trinh, C.T. Comprehensive characterization of toxicity of fermentative metabolites on microbial growth. Biotechnol. Biofuels 2017, 10, 262. [Google Scholar] [CrossRef]
- Aremu, M.O.; Ishola, M.M.; Taherzadeh, M.J. Polyhydroxyalkanoates (phas) production from volatile fatty acids (vfas) from organic wastes by pseudomonas oleovorans. Fermentation 2021, 7, 287. [Google Scholar] [CrossRef]
- Setiadi, T.; Aznury, M.; Trianto, A.; Pancoro, A. Production of polyhydroxyalkanoate (pha) by ralstonia eutropha jmp 134 with volatile fatty acids from palm oil mill effluent as precursors. Water Sci. Technol. 2015, 72, 1889–1895. [Google Scholar] [CrossRef]
- Minocha, S.C. PH of the Medium and the Growth and Metabolism of Cells in Culture. In Cell and Tissue Culture in Forestry: General Principles and Biotechnology; Bonga, J.M., Durzan, D.J., Eds.; Springer: Dordrecht, The Netherlands, 1987; pp. 125–141. [Google Scholar]
- Shehadul Islam, M.; Aryasomayajula, A.; Selvaganapathy, P.R. A review on macroscale and microscale cell lysis methods. Micromachines 2017, 8, 83. [Google Scholar] [CrossRef]
- Jin, Q.; Kirk, M.F. PH as a primary control in environmental microbiology: 1. Thermodynamic perspective. Front. Environ. Sci. 2018, 6, 21. [Google Scholar] [CrossRef]
- Sánchez-Clemente, R.; Igeño, M.I.; Población, A.G.; Guijo, M.I.; Merchán, F.; Blasco, R. Study of ph changes in media during bacterial growth of several environmental strains. Proceedings 2018, 2, 1297. [Google Scholar]
- Ratzke, C.; Gore, J. Modifying and reacting to the environmental ph can drive bacterial interactions. PLoS Biol. 2018, 16, e2004248. [Google Scholar] [CrossRef] [PubMed]
- Aramvash, A.; Shahabi, Z.A.; Aghjeh, S.D.; Ghafari, M.D. Statistical physical and nutrient optimization of bioplastic polyhydroxybutyrate production by Cupriavidus necator. Int. J. Environ. Sci. Technol. 2015, 12, 2307–2316. [Google Scholar] [CrossRef]
- Nygaard, D.; Yashchuk, O.; Hermida, É.B. Evaluation of culture medium on poly(3-hydroxybutyrate) production by Cupriavidus necator atcc 17697: Application of the response surface methodology. Heliyon 2019, 5, e01374. [Google Scholar] [CrossRef] [PubMed]
- Cruz, R.A.P.; Oehmen, A.; Reis, M.A.M. The impact of biomass withdrawal strategy on the biomass selection and polyhydroxyalkanoates accumulation of mixed microbial cultures. New Biotechnol. 2022, 66, 8–15. [Google Scholar] [CrossRef]
- Smith, B. How Does PH Level Affect Enzyme Activity? 2017. Available online: https://sciencing.com/role-enzymes-chemical-reactions-5553131.html (accessed on 15 September 2022).
- Foundation, N. Bacterial Responses to PH.; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Catherine, M.-C.; Guwy, A.; Massanet-Nicolau, J. Effect of acetate concentration, temperature, ph and nutrient concentration on polyhydroxyalkanoates (pha) production by glycogen accumulating organisms. Bioresour. Technol. Rep. 2022, 20, 101226. [Google Scholar] [CrossRef]
- Matsakas, A.; Patel, K. Aerobic Metabolism. In Encyclopedia of Exercise Medicine in Health and Disease; Mooren, F.C., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 29–32. [Google Scholar]
- Gao, J.; Ramsay, J.A.; Ramsay, B.A. Fed-batch production of poly-3-hydroxydecanoate from decanoic acid. J. Biotechnol. 2016, 218, 102–107. [Google Scholar] [CrossRef]
- Wang, X.; Oehmen, A.; Freitas, E.B.; Carvalho, G.; Reis, M.A. The link of feast-phase dissolved oxygen (do) with substrate competition and microbial selection in pha production. Water Res. 2017, 112, 269–278. [Google Scholar] [CrossRef]
- Lefebvre, G.; Rocher, M.; Braunegg, G. Effects of low dissolved-oxygen concentrations on poly-(3-hydroxybutyrate-co-3-hydroxyvalerate) production by alcaligenes eutrophus. Appl. Environ. Microbiol. 1997, 63, 827–833. [Google Scholar] [CrossRef]
- Shantini, K.; Yahya, A.R.; Amirul, A.A. Influence of feeding and controlled dissolved oxygen level on the production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) copolymer by cupriavidus sp. Usmaa2-4 and its characterization. Appl. Biochem. Biotechnol. 2015, 176, 1315–1334. [Google Scholar] [CrossRef]
- Cavalheiro, J.M.B.T.; Raposo, R.S.; de Almeida, M.C.M.D.; Cesário, M.T.; Sevrin, C.; Grandfils, C.; da Fonseca, M.M.R. Effect of cultivation parameters on the production of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) and poly(3-hydroxybutyrate-4-hydroxybutyrate-3-hydroxyvalerate) by Cupriavidus necator using waste glycerol. Bioresour. Technol. 2012, 111, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, K.; Kettl, K.-H.; Titz, M.; Koller, M.; Schnitzer, H.; Narodoslawsky, M. Comparison of ecological footprint for biobased pha production from animal residues utilizing different energy resources. Clean Technol. Environ. Policy 2013. 15, 525–536. [CrossRef]
- Zhang, C.C.; Zhou, C.Z.; Burnap, R.L.; Peng, L. Carbon/nitrogen metabolic balance: Lessons from cyanobacteria. Trends Plant Sci. 2018, 23, 1116–1130. [Google Scholar] [CrossRef] [PubMed]
- Muhammadi; Shabina; Afzal, M.; Hameed, S. Bacterial polyhydroxyalkanoates-eco-friendly next generation plastic: Production, biocompatibility, biodegradation, physical properties and applications. Green Chem. Lett. Rev. 2015, 8, 56–77. [Google Scholar] [CrossRef]
- Oliveira, C.S.S.; Silva, C.E.; Carvalho, G.; Reis, M.A. Strategies for efficiently selecting pha producing mixed microbial cultures using complex feedstocks: Feast and famine regime and uncoupled carbon and nitrogen availabilities. New Biotechnol. 2017, 37, 69–79. [Google Scholar] [CrossRef]
- Zhou, W.; Colpa, D.I.; Geurkink, B.; Euverink, G.-J.W.; Krooneman, J. The impact of carbon to nitrogen ratios and ph on the microbial prevalence and polyhydroxybutyrate production levels using a mixed microbial starter culture. Sci. Total Environ. 2022, 811, 152341. [Google Scholar] [CrossRef]
- Sangkharak, K.; Prasertsan, P. Nutrient optimization for production of polyhydroxybutyrate from halotolerant photosynthetic bacteria cultivated under aerobic-dark condition. Electron. J. Biotechnol. 2008, 11, 83–94. [Google Scholar] [CrossRef]
- Mohapatra, S.; Sarkar, B.; Samantaray, D.P.; Daware, A.; Maity, S.; Pattnaik, S.; Bhattacharjee, S. Bioconversion of fish solid waste into phb using bacillus subtilis based submerged fermentation process. Environ. Technol. 2017, 38, 3201–3208. [Google Scholar] [CrossRef]
- Ahn, J.; Jho, E.H.; Nam, K. Effect of c/n ratio on polyhydroxyalkanoates (pha) accumulation by Cupriavidus necator and its implication on the use of rice straw hydrolysates. Environ. Eng. Res. 2015, 20, 246–253. [Google Scholar] [CrossRef]
- Budde, C.F.; Riedel, S.L.; Hübner, F.; Risch, S.; Popović, M.K.; Rha, C.; Sinskey, A.J. Growth and polyhydroxybutyrate production by ralstonia eutropha in emulsified plant oil medium. Appl. Microbiol. Biotechnol. 2011. 89, 1611–1619. [CrossRef]
- da Cruz Pradella, J.G.; Ienczak, J.L.; Delgado, C.R.; Taciro, M.K. Carbon source pulsed feeding to attain high yield and high productivity in poly(3-hydroxybutyrate) (phb) production from soybean oil using Cupriavidus necator. Biotechnol. Lett. 2012, 34, 1003–1007. [Google Scholar] [CrossRef]
- Benesova, P.; Kucera, D.; Marova, I.; Obruca, S. Chicken feather hydrolysate as an inexpensive complex nitrogen source for pha production by Cupriavidus necator on waste frying oils. Lett. Appl. Microbiol. 2017, 65, 182–188. [Google Scholar] [CrossRef]
- Koller, M.; Bona, R.; Hermann, C.; Horvat, P.; Martinz, J.; Neto, J.; Pereira, L.; Varila, P.; Braunegg, G. Biotechnological production of poly(3-hydroxybutyrate) with wautersia eutropha by application of green grass juice and silage juice as additional complex substrates. Biocatal. Biotransformation 2005, 23, 329–337. [Google Scholar] [CrossRef]
- Obruca, S.; Benesova, P.; Oborna, J.; Marova, I. Application of protease-hydrolyzed whey as a complex nitrogen source to increase poly(3-hydroxybutyrate) production from oils by Cupriavidus necator. Biotechnol. Lett. 2014, 36, 775–781. [Google Scholar] [CrossRef]
- Arumugam, A.; Senthamizhan, S.G.; Ponnusami, V.; Sudalai, S. Production and optimization of polyhydroxyalkanoates from non-edible calophyllum inophyllum oil using Cupriavidus necator. Int. J. Biol. Macromol. 2018, 112, 598–607. [Google Scholar] [CrossRef]
- Dañez, J.C.A.; Requiso, P.J.; Alfafara, C.G.; Nayve, F.R.P. Optimization of fermentation factors for polyhydroxybutyrate (phb) production using bacillus megaterium pncm 1890 in simulated glucose-xylose hydrolysates from agricultural residues. Philipp. J. Sci. 2020, 149, 163–175. [Google Scholar]
- Loo, Y.C.; Sudesh, K. Polyhydroxyalkanoates: Bio-based microbial plastics and their properties. Malays. Polym. J. (MPJ) 2007, 2, 31–57. [Google Scholar]
- Riedel, L.S.; Bader, J.; Brigham, C.J.; Budde, C.F.; Yusof, Z.A.; Rha, C.; Sinskey, A.J. Production of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) by ralstonia eutropha in high cell density palm oil fermentations. Biotechnol. Bioeng. 2012, 109, 74–83. [Google Scholar] [CrossRef]
- Kulpreecha, S.; Boonruangthavorn, A.; Meksiriporn, B.; Thongchul, N. Inexpensive fed-batch cultivation for high poly(3-hydroxybutyrate) production by a new isolate of bacillus megaterium. J. Biosci. Bioeng. 2009, 107, 240–245. [Google Scholar] [CrossRef]
- Kumari, A. Chapter 4-beta oxidation of fatty acids. In Sweet Biochemistry; Kumari, A., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 17–19. [Google Scholar]
- Randriamahefa, S.; Renard, E.; Guérin, P.; Langlois, V. Fourier transform infrared spectroscopy for screening and quantifying production of phas by pseudomonas grown on sodium octanoate. Biomacromolecules 2003, 4, 1092–1097. [Google Scholar] [CrossRef]
- Trakunjae, C.; Boondaeng, A.; Apiwatanapiwat, W.; Kosugi, A.; Arai, T.; Sudesh, K.; Vaithanomsat, P. Enhanced polyhydroxybutyrate (phb) production by newly isolated rare actinomycetes rhodococcus sp. Strain bsrt1-1 using response surface methodology. Sci. Rep. 2021, 11, 1896. [Google Scholar]
- Bluhm, T.L.; Hamer, G.K.; Marchessault, R.H.; Fyfe, C.A.; Veregin, R.P. Isodimorphism in bacterial poly(β-hydroxybutyrate-co-β-hydroxyvalerate). Macromolecules 1986. 19, 2871–2876. [CrossRef]
- Kamiya, N.; Sakurai, M.; Inoue, Y.; Chujo, R.; Doi, Y. Study of cocrystallization of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by solid-state high-resolution carbon-13 nmr spectroscopy and differential scanning calorimetry. Macromolecules 1991, 24, 2178–2182. [Google Scholar] [CrossRef]
- William, J.O.; David, L.V.; Terry, L.B.; Robert, H.M. Cocrystallization in random copolymers of poly(β-hydroxybutyrate-co-β-hydroxyvalerate) and its effect on crystalline morphology. Can. J. Chem. 1995, 73, 2094–2100. [Google Scholar]
- VanderHart, D.L.; Orts, W.J.; Marchessault, R.H. 13c nmr determination of the degree of cocrystallization in random copolymers of poly(Beta.-hydroxybutyrate-co-.Beta.-hydroxyvalerate). Macromolecules 1995, 28, 6394–6400. [Google Scholar]
- Bonthrone, K.M.; Clauss, J.; Horowitz, D.M.; Hunter, B.K.; Sanders, J.K.M. The biological and physical chemistry of polyhydroxyalkanoates as seen by nmr spectroscopy. FEMS Microbiol. Lett. 1992, 103, 269–277. [Google Scholar] [CrossRef]
- Ntaikou, I.; Koumelis, I.; Kamilari, M.; Iatridi, Z.; Tsitsilianis, C.; Lyberatos, G. Effect of nitrogen limitation on polyhydroxyalkanoates production efficiency, properties and microbial dynamics using a soil-derived mixed continuous culture. Int. J. Biobased Plast. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Rodrigues, P. Impact of different bacterial strains on the production, composition, and properties of novel polyhydroxyalkanoates using crude palm oil as substrate. Chem. Biochem. Eng. Q. 2018, 32, 141–150. [Google Scholar] [CrossRef]
- Dietrich, K.; Dumont, M.-J.; del Rio, L.F.; Orsat, V. Producing phas in the bioeconomy—towards a sustainable bioplastic. Sustain. Prod. Consum. 2017, 9, 58–70. [Google Scholar] [CrossRef]
- Nygaard, D.; Yashchuk, O.; Noseda, D.G.; Araoz, B.; Hermida, É.B. Improved fermentation strategies in a bioreactor for enhancing poly(3-hydroxybutyrate) (phb) production by wild type Cupriavidus necator from fructose. Heliyon 2021, 7, e05979. [Google Scholar] [CrossRef]
- Li, D.; Li, J.; Ma, X. Accumulation of bioplastic polyhydroxyalkanoate with different substrate forms from pretreated waste lignocellulose hydrolysate. Ind. Crops Prod. 2021, 172, 114061. [Google Scholar] [CrossRef]
- Rosengart, A.; Cesário, M.T.; de Almeida, M.C.M.D.; Raposo, R.S.; Espert, A.; de Apodaca, E.D.; da Fonseca, M.M.R. Efficient p(3hb) extraction from burkholderia sacchari cells using non-chlorinated solvents. Biochem. Eng. J. 2015, 103, 39–46. [Google Scholar] [CrossRef]
- Scattering methods and the properties of polymer materials. Mater. Today 2005, 8, 59. [CrossRef]
- Nair, A.M.; Annamalai, K.; Kannan, S.K.; Kuppusamy, S. Characterization of polyhydroxyalkanoates produced by bacillus subtilis isolated from soil samples. Malaya J. Biosci. 2014, 1, 8–12. [Google Scholar]
- Zhila, N.O.; Sapozhnikova, K.Y.; Kiselev, E.G.; Vasiliev, A.D.; Nemtsev, I.V.; Shishatskaya, E.I.; Volova, T.G. Properties of degradable polyhydroxyalkanoates (phas) synthesized by a new strain, Cupriavidus necator ibp/sfu-1, from various carbon sources. Polymers 2021, 13, 3142. [Google Scholar] [CrossRef] [PubMed]
- Kolodziejski, W.; Klinowski, J. Kinetics of Cross-Polarization in Solid-State NMR: A Guide for Chemists. Chem. Rev. 2002, 102, 613–628. [Google Scholar] [CrossRef]
- Fitted Using the Python curve_fit Routine, Version 2.7, Python Software Foundation. Available online: www.python.org (accessed on 9 October 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).