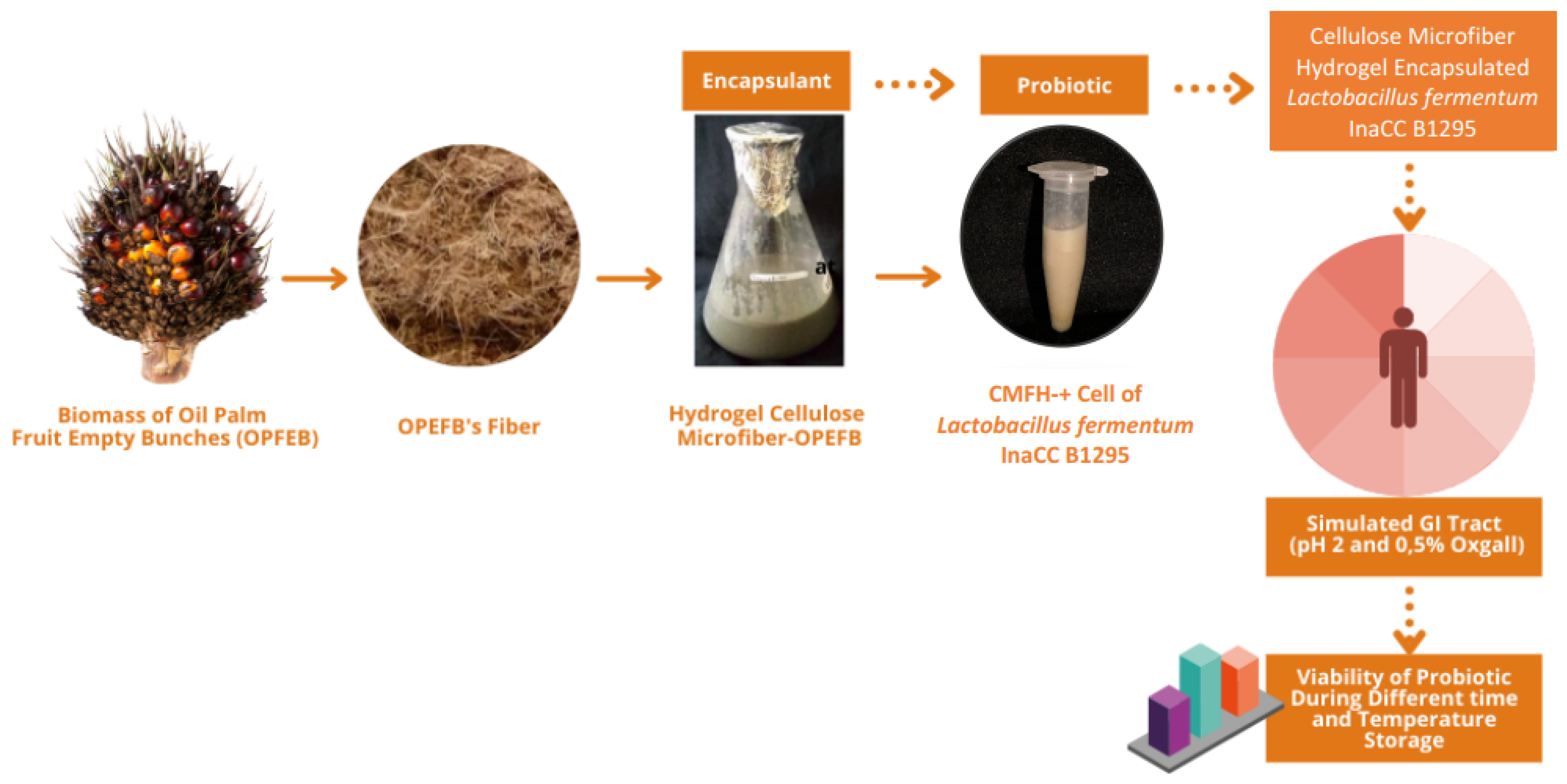
Probiotic Properties of Lactobacillus fermentum InaCC B1295 Encapsulated by Cellulose Microfiber from Oil Palm Empty Fruit Bunches
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of Cellulose Microfibers (CMFs)
2.3. Production of Cellulose Microfiber Hydrogels (CMFHs)
2.4. Preparation of Lactobacillus fermentum InaCC B1295
2.5. Production CMFH-Encapsulated Lactobacillus fermentum InaCC B1295
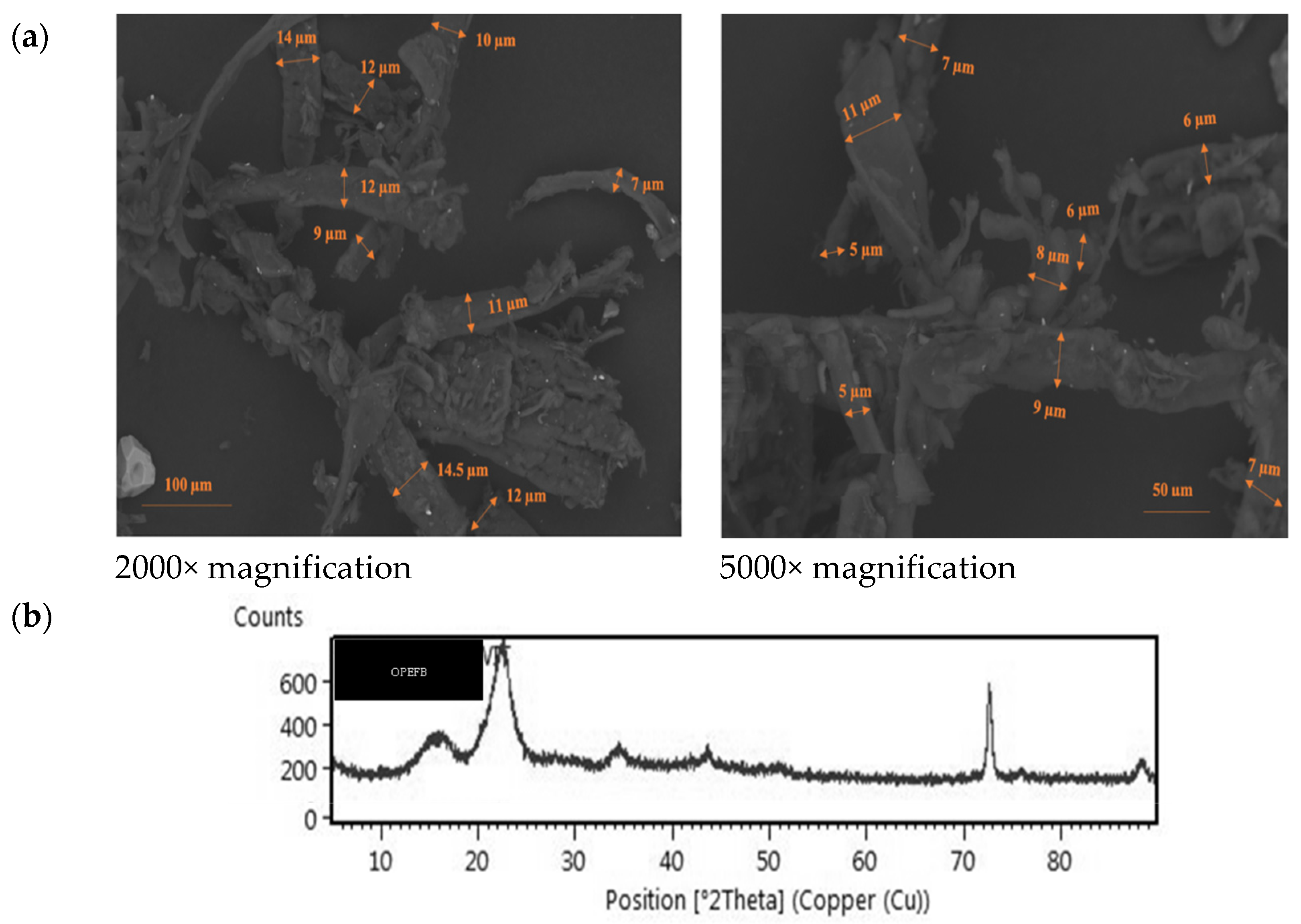
2.6. X-ray Diffraction Analysis of Cellulose Microfiber (CMF)
2.7. The Total Number of Lactobacillus fermentum InaCC B1295 Encapsulated by CMFH-OPEFB during Storage Time
2.8. Resistance to Acid
2.9. Resistance to Bile
2.10. Safety Evaluation of Probiotic
2.10.1. Hemolytic Activity
2.10.2. Production of Biogenic Amine
2.10.3. Cytolysin Activity
2.10.4. Production of Gelatinase
2.11. Antioxidant Activity of Probiotic
2.11.1. Resistance to H2O2
2.11.2. Scavenging of Hydroxyl Radicals
2.11.3. Scavenging for DPPH Radicals
2.12. Experimental Design
2.13. Data Analysis
3. Results
3.1. Evaluation of Cellulose Microfiber-Oil Palm Empty Fruit Bunches
Scanning Electron Microscope of CMF-OPEFB
3.2. Viability and Acid and Bile Tolerance of CMFH-Encapsulated Probiotics during Storage Time
3.3. Safety Evaluation of CMFH-Encapsulated Probiotic
3.4. Antioxidant Activity of CMFH-Encapsulated Probiotic
3.4.1. Resistance to Hydrogen Peroxide
3.4.2. Hydroxyl Radical Scavenging Activity
3.4.3. DPPH Radical Scavenging Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aritonang, S.N.; Roza, E.; Rossi, E.; Purwati, E. Husmaini Isolation and Identification of Lactic Acid Bacteria from Okara and Evaluation of Their Potential as Candidate Probiotics. Pakistan J. Nutr. 2017, 16, 618–628. [Google Scholar] [CrossRef][Green Version]
- Hu, S.; Wang, L.; Jiang, Z. Dietary Additive Probiotics Modulation of the Intestinal Microbiota. Protein Pept. Lett. 2017, 24, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Jaya, B.; Roshan, D. Probiotics Market by Ingredient (Bacteria and Yeast), Function (Regular, Preventive Healthcare, and Therapeutic), Application (Food & Beverage, Dietary Supplements, and Animal Feed), and End User (Human and Animal): Global Opportunity Analysis and Industr. Available online: https://www.alliedmarketresearch.com/probiotics-market (accessed on 19 September 2022).
- Min, M.; Bunt, C.R.; Mason, S.L.; Hussain, M.A. Non-Dairy Probiotic Food Products: An Emerging Group of Functional Foods. Crit. Rev. Food Sci. Nutr. 2019, 59, 2626–2641. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.J.; Cedran, M.F.; Bicas, J.L.; Sato, H.H. Encapsulated Probiotic Cells: Relevant Techniques, Natural Sources as Encapsulating Materials and Food Applications–A Narrative Review. Food Res. Int. 2020, 137, 109682. [Google Scholar] [CrossRef] [PubMed]
- Surono, I.; Verhoeven, J.; Verbruggen, S.; Venema, K. Microencapsulation Increases Survival of the Probiotic Lactobacillus plantarum IS-10506, but Not Enterococcus faecium IS-27526 in a Dynamic, Computer-Controlled in Vitro Model of the Upper Gastrointestinal Tract. J. Appl. Microbiol. 2018, 124, 1604–1609. [Google Scholar] [CrossRef] [PubMed]
- Rathore, S.; Desai, P.M.; Liew, C.V.; Chan, L.W.; Heng, P.W.S. Microencapsulation of Microbial Cells. J. Food Eng. 2013, 116, 369–381. [Google Scholar] [CrossRef]
- Gasperini, L.; Mano, J.F.; Reis, R.L. Natural Polymers for the Microencapsulation of Cells. J. R. Soc. Interface 2014, 11, 20140817. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Q.; Liu, C.-M.; Gong, J. Issues Deserve Attention in Encapsulating Probiotics: Critical Review of Existing Literature. Crit. Rev. Food Sci. Nutr. 2017, 57, 1228–1238. [Google Scholar] [CrossRef]
- Abd El-Salam, M.H.; El-Shibiny, S. Preparation and Properties of Milk Proteins-Based Encapsulated Probiotics: A Review. Dairy Sci. Technol. 2015, 95, 393–412. [Google Scholar] [CrossRef]
- Rossi, E.; Restuhadi, F.; Efendi, R.; Dewi, Y.K. Physicochemical and Microbiological Properties of Yogurt Made with Microencapsulation Probiotic Starter during Cold Storage. Biodiversitas 2021, 22, 2012–2018. [Google Scholar] [CrossRef]
- Oberoi, K.; Tolun, A.; Sharma, K.; Sharma, S. Microencapsulation: An Overview for the Survival of Probiotic Bacteria. J. Microbiol. Biotechnol. Food Sci. 2019, 9, 280–287. [Google Scholar] [CrossRef]
- Pedroso, D.L.; Dogenski, M.; Thomazini, M.; Heinemann, R.J.B.; Favaro-Trindade, C.S. Microencapsulation of Bifidobacterium animalis subsp. lactis and Lactobacillus acidophilus in Cocoa Butter Using Spray Chilling Technology. Braz. J. Microbiol. 2013, 44, 777–783. [Google Scholar] [CrossRef]
- Calinoiu, L.F.; Ştefanescu, B.E.; Pop, I.D.; Muntean, L.; Vodnar, D.C. Chitosan Coating Applications in Probiotic Microencapsulation. Coatings 2019, 9, 194. [Google Scholar] [CrossRef]
- Rosas-Flores, W.; Ramos-Ramírez, E.G.; Salazar-Montoya, J.A. Microencapsulation of Lactobacillus helveticus and Lactobacillus delbrueckii Using Alginate and Gellan Gum. Carbohydr. Polym. 2013, 98, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Padzil, F.N.; Lee, S.H.; Ainun, Z.M.; Lee, C.H.; Abdullah, L.C. Potential of Oil Palm Empty Fruit Bunch Resources in Nanocellulose Hydrogel Production for Versatile Applications: A Review. Materials 2020, 13, 1245. [Google Scholar] [CrossRef]
- Sugiwati, S.; Suaidah, S.; Triwahyuni, E.; Muryanto, M.; Andriani, Y.; Abimanyu, H. Hydrolysis of Cellulose from Oil Palm Empty Fruit Bunch Using Aspergillus niger. E3S Web Conf. 2021, 226, 1–7. [Google Scholar] [CrossRef]
- Dungani, R.; Aditiawati, P.; Aprilia, S.; Yuniarti, K.; Karliati, T.; Suwandhi, I.; Sumardi, I. Biomaterial from Oil Palm Waste: Properties, Characterization and Applications. In Palm Oil; InTech: Milton, QL, Australia, 2018. [Google Scholar]
- Aditiawati, P.; Dungani, R.; Amelia, C. Enzymatic Production of Cellulose Nanofibers from Oil Palm Empty Fruit Bunch (EFB) with Crude Cellulase Of Trichoderma sp. Mater. Res. Express 2018, 5, 34005. [Google Scholar] [CrossRef]
- Galiwango, E.; Abdel Rahman, N.S.; Al-Marzouqi, A.H.; Abu-Omar, M.M.; Khaleel, A.A. Isolation and Characterization of Cellulose and α-Cellulose from Date Palm Biomass Waste. Heliyon 2019, 5, e02937. [Google Scholar] [CrossRef]
- Fahma, F.; Iwamoto, S.; Hori, N.; Iwata, T.; Takemura, A. Isolation, Preparation, and Characterization of Nanofibers from Oil Palm Empty-Fruit-Bunch (OPEFB). Cellulose 2010, 17, 977–985. [Google Scholar] [CrossRef]
- Aditiawati, P.; Dungani, R.; Fikri, R.M.; Hartati, S. Optimization of Cellulose Nanofiber Production from Oil Palm Empty Fruit Bunch Using Trichoderma Sp. with the Solid State Fermentation Method. BioResources 2019, 14, 3688–3700. [Google Scholar] [CrossRef]
- Yunaira, R.; Zulfarina; Pato, U. Hyposensitivity Test of Lactobacillus fermentum InaCC B1295 Probiotic Bacteria on the Growth of Mustard Greens (Brassica Junceae L.). J. Phys. Conf. Ser. 2020, 1655, 12–17. [Google Scholar] [CrossRef]
- Naghmouchi, K.; Belguesmia, Y.; Bendali, F.; Spano, G.; Seal, B.S.; Drider, D. Lactobacillus fermentum: A Bacterial Species with Potential for Food Preservation and Biomedical Applications. Crit. Rev. Food Sci. Nutr. 2019, 60, 3387–3399. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, D.; Mallappa, R.H.; Grover, S. Comprehensive Approaches for Assessing the Safety of Probiotic Bacteria. Food Control 2020, 108, 106872. [Google Scholar] [CrossRef]
- Ikhsani, A.Y.; Riftyan, E.; Safitri, R.A.; Marsono, Y.; Utami, T.; Widada, J.; Rahayu, E.S. Safety Assessment of Indigenous Probiotic Strain Lactobacillus plantarum Mut-7 Using Sprague Dawley Rats as a Model. Am. J. Pharmacol. Toxicol. 2020, 15, 7–16. [Google Scholar] [CrossRef]
- Pisano, M.B.; Viale, S.; Conti, S.; Fadda, M.E.; Deplano, M.; Melis, M.P.; Deiana, M.; Cosentino, S. Preliminary Evaluation of Probiotic Properties of Lactobacillus Strains Isolated from Sardinian Dairy Products. Biomed Res. Int. 2014, 2014, 286390. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.Y.; Jeong, Y.; Kang, C.H. Antioxidant Activity and Probiotic Properties of Lactic Acid Bacteria. Fermentation 2022, 8, 29. [Google Scholar] [CrossRef]
- Pato, U.; Ayu, D.F.; Riftyan, E.; Restuhadi, F.; Pawenang, W.T.; Firdaus, R.; Rahma, A.; Surono, I.S.; Jaswir, I. Physicochemical Property of Oil Palm Leaves and Utilization of Cellulose Microfiber as Probiotic Encapsulant. Biodiversitas J. Biol. Divers. 2021, 22, d220746. [Google Scholar] [CrossRef]
- Pato, U.; Ayu, D.F.; Riftyan, E.; Restuhadi, F.; Pawenang, W.T.; Firdaus, R.; Rahma, A.; Jaswir, I. Cellulose Microfiber Encapsulated Probiotic: Viability, Acid and Bile Tolerance during Storage at Different Temperature. Emerg. Sci. J. 2022, 6, 106–117. [Google Scholar] [CrossRef]
- Fung, W.-Y.; Yuen, K.-H.; Liong, M.-T. Agrowaste-Based Nanofibers as a Probiotic Encapsulant: Fabrication and Characterization. J. Agric. Food Chem. 2011, 59, 8140–8147. [Google Scholar] [CrossRef]
- Yasim-Anuar, T.A.T.; Ariffin, H.; Norrrahim, M.N.F.; Hassan, M.A. Factors Affecting Spinnability of Oil Palm Mesocarp Fiber Cellulose Solution for the Production of Microfiber. BioResources 2017, 12, 715–734. [Google Scholar] [CrossRef][Green Version]
- Nuraida, L.; Winarti, S.; Hana; Prangdimurti, E. In Vitro Evaluation of Cholesterol Assimilation and Bile Salt Deconjugation by Lactic Acid Bacteria Isolated from Breast Milk. J. Teknol. Dan Ind. Pangan 2011, 22, 46–52. [Google Scholar]
- Bover-Cid, S.; Holzapfel, W.H. Improved Screening Procedure for Biogenic Amine Production by Lactic Acid Bacteria. Int. J. Food Microbiol. 1999, 53, 33–41. [Google Scholar] [CrossRef]
- Tan, Q.; Xu, H.; Aguilar, Z.P.; Peng, S.; Dong, S.; Wang, B.; Li, P.; Chen, T.; Xu, F.; Wei, H. Safety Assessment and Probiotic Evaluation of Enterococcus faecium YF5 Isolated from Sourdough. J. Food Sci. 2013, 78, M587–M593. [Google Scholar] [CrossRef] [PubMed]
- Shehata, M.G.; Abu-Serie, M.M.; Abd El-Azi, N.M.; El-Sohaimy, S.A. In Vitro Assessment of Antioxidant, Antimicrobial and Anticancer Properties of Lactic Acid Bacteria. Int. J. Pharmacol. 2019, 15, 651–663. [Google Scholar] [CrossRef]
- Muna, N.; Fauzi, A.A.N.; Setyaningsih, D.; Yuliani, S. Isolation of Microfibrilated Cellulose from Oil Palm Empty Fruit Bunches (EFB) Through Peracetic Acid Delignification and Enzyme Hydrolysis. IOP Conf. Ser. Earth Environ. Sci. 2019, 309, 012063. [Google Scholar] [CrossRef]
- Fatah, I.Y.A.; Abdul Khalil, H.P.S.; Hossain, M.S.; Aziz, A.A.; Davoudpour, Y.; Dungani, R.; Bhat, A. Exploration of a Chemo-Mechanical Technique for the Isolation of Nanofibrillated Cellulosic Fiber from Oil Palm Empty Fruit Bunch as a Reinforcing Agent in Composites Materials. Polymers 2014, 6, 2611–2624. [Google Scholar] [CrossRef]
- Bahloul, A.; Kassab, Z.; Aziz, F.; Hannache, H.; Bouhfid, R.; Qaiss, A.E.K.; Oumam, M.; El Achaby, M. Characteristics of Cellulose Microfibers and Nanocrystals Isolated from Doum Tree (Chamaerops humilis Var. Argentea). Cellulose 2021, 28, 4089–4103. [Google Scholar] [CrossRef]
- Deraman, M.; Zakaria, S.; Murshidi, J.A. Estimation of Crystallinity and Crystallite Size of Cellulose in Benzylated Fibres of Oil Palm Empty Fruit Bunches by X-ray Diffraction. Jpn. J. Appl. Physics Part 1 Regul. Pap. Short Notes Rev. Pap. 2001, 40, 3311–3314. [Google Scholar] [CrossRef]
- Hu, P.-L.; Yuan, Y.-H.; Yue, T.-L.; Guo, C.-F. Bile Acid Patterns in Commercially Available Oxgall Powders Used for the Evaluation of the Bile Tolerance Ability of Potential Probiotics. PLoS ONE 2018, 13, e0168280. [Google Scholar] [CrossRef]
- Goh, K.Y.; Ching, Y.C.; Chuah, C.H.; Abdullah, L.C.; Liou, N.S. Individualization of Microfibrillated Celluloses from Oil Palm Empty Fruit Bunch: Comparative Studies between Acid Hydrolysis and Ammonium Persulfate Oxidation. Cellulose 2016, 23, 379–390. [Google Scholar] [CrossRef]
- Nordin, N.I.A.A.; Ariffin, H.; Andou, Y.; Hassan, M.A.; Shirai, Y.; Nishida, H.; Yunus, W.M.Z.W.; Karuppuchamy, S.; Ibrahim, N.A. Modification of Oil Palm Mesocarp Fiber Characteristics Using Superheated Steam Treatment. Molecules 2013, 18, 9132–9146. [Google Scholar] [CrossRef] [PubMed]
- Yasim-Anuar, T.A.T.; Ariffin, H.; Hassan, M.A. Characterization of Cellulose Nanofiber from Oil Palm Mesocarp Fiber Produced by Ultrasonication. IOP Conf. Ser. Mater. Sci. Eng. 2018, 368, 012033. [Google Scholar] [CrossRef]
- Mettu, S.; Hathi, Z.; Athukoralalage, S.; Priya, A.; Lam, T.N.; Ong, K.L.; Choudhury, R.; Kumar Dutta, N.; Curvello, R.; Garnier, G.; et al. Perspective on Constructing Cellulose-Hydrogel-Based Gut-Like Bioreactors for Growth and Delivery of Multiple-Strain Probiotic Bacteria. J. Agric. Food Chem. 2021, 69, 4959. [Google Scholar] [CrossRef]
- López-Rubio, A.; Sanchez, E.; Sanz, Y.; Lagaron, J.M. Encapsulation of Living Bifidobacteria in Ultrathin PVOH Electrospun Fibers. Biomacromolecules 2009, 10, 2823–2829. [Google Scholar] [CrossRef]
- Çanga, E.M.; Dudak, F.C. Improved Digestive Stability of Probiotics Encapsulated within Poly(Vinyl Alcohol)/Cellulose Acetate Hybrid Fibers. Carbohydr. Polym. 2021, 264, 117990. [Google Scholar] [CrossRef] [PubMed]
- Pato, U. Bile and Acid Tolerance of Lactic Acid Bacteria Isolated from Dadih and Their Antimutagenicity against Mutagenic Heated Tauco. Asian-Australas. J. Anim. Sci. 2003, 16, 1680–1685. [Google Scholar] [CrossRef]
- Borges, S.; Barbosa, J.; Silva, J.; Teixeira, P. Evaluation of Characteristics of Pediococcus Spp. to Be Used as a Vaginal Probiotic. J. Appl. Microbiol. 2013, 115, 527–538. [Google Scholar] [CrossRef]
- Ness, I.F.; Diep, D.B.; Ike, Y. Enterococcal Bacteriocins and Antimicrobial Proteins That Contribute to Niche Control. In Enterococci: From Commensals to Leading Causes of Drug Resistant Infection [Internet]; Massachusetts Eye and Ear Infirmary: Boston, MA, USA, 2014; pp. 1–24. [Google Scholar]
- Villegas, E.; Gilliland, S.E. Hydrogen Peroxide Production by Lactobacillus delbrueckii subsp. lactis I at 5 °C. J. Food Sci. 1998, 63, 1070–1074. [Google Scholar] [CrossRef]
- Calasso, M.; Gobbetti, M. Lactic Acid Bacteria|Lactobacillus Spp.: Other Species. In Encyclopedia of Dairy Sciences; Elsevier: Amsterdam, The Netherlands, 2011; pp. 125–131. ISBN 9780123744074. [Google Scholar]
- Oberg, T.S.; Steele, J.L.; Ingham, S.C.; Smeianov, V.V.; Briczinski, E.P.; Abdalla, A.; Broadbent, J.R. Intrinsic and Inducible Resistance to Hydrogen Peroxide in Bifidobacterium Species. J. Ind. Microbiol. Biotechnol. 2011, 38, 1947–1953. [Google Scholar] [CrossRef]
- Mu, G.; Gao, Y.; Tuo, Y.; Li, H.; Zhang, Y.; Qian, F. Assessing and Comparing Antioxidant Activities of Lactobacilli Strains by Using Different Chemical and Cellular Antioxidant Methods. J. Dairy Sci. 2018, 101, 10792–10806. [Google Scholar] [CrossRef]
| Storage Time (Day) | Storage Temperature | |
|---|---|---|
| Refrigerated Temperature (4 °C) | Room Temperature (25 °C) | |
| Viability (log CFU/g) | ||
| 0 | 9.93 d | 9.80 c |
| 14 | 9.39 b | 9.38 b |
| 28 | 9.13 a | 9.17 a |
| Acid resistance (%) | ||
| 0 | 99.12 | 99.72 |
| 14 | 98.97 | 99.08 |
| 28 | 98.92 | 98.14 |
| Bile resistance (%) | ||
| 0 | 99.72 b | 99.25 b |
| 14 | 98.31 b | 99.13 b |
| 28 | 93.47 a | 92.07 a |
| Probiotic Properties/ Storage Time (Day) | Observation Results | Indications | |
|---|---|---|---|
| Refrigerated Temperature (4 °C) | Room Temperature (25 °C) | ||
| Hemolytic activity | |||
| 0 | None | None | No clear zone around colonies on Columbia Blood Agar medium |
| 14 | None | None | |
| 28 | None | None | |
| Production of biogenic amines from L-histidine, tyrosine, L-ornithine, Lysine | |||
| 0 | None | None | No color change from brown to purple from the colonies on Decarboxylase Agar medium with purple bromocresol as an indicator |
| 14 | None | None | |
| 28 | None | None | |
| Production of cytolysin | |||
| 0 | None | None | No clear zone around colonies on BHI agar medium |
| 14 | None | None | |
| 28 | None | None | |
| Production of gelatinase | |||
| 0 | None | None | No clear zone around colonies on BHI agar medium |
| 14 | None | None | |
| 28 | None | None | |
| Storage Time (Day) | Absorbance at 600 nm | |
|---|---|---|
| Refrigerated Temperature (4 °C) | Room Temperature (25 °C) | |
| Concentration 0.4 mM | ||
| 0 | 1.691 e | 1.691 e |
| 14 | 1.113 c | 1.091 b |
| 28 | 1.139 d | 0.996 a |
| Concentration 0.7 mM | ||
| 0 | 1.582 d | 1.582 d |
| 14 | 1.085 c | 1.034 b |
| 28 | 1.034 b | 0.981 a |
| Concentration 1.0 mM | ||
| 0 | 1.477 d | 1.477 d |
| 14 | 1.047 c | 0.981 b |
| 28 | 0.837 a | 0.837 a |
| Storage Time (Day) | Radical Scavenging Activity (µg/mL) | |
|---|---|---|
| Refrigerated Temperature (4 °C) | Room Temperature (25 °C) | |
| Concentration 1:1 | ||
| 0 | 68.235 a | 68.235 a |
| 14 | 105.426 b | 115.503 b |
| 28 | 111.628 b | 119.380 c |
| Concentration 1:2 | ||
| 0 | 89.803 a | 89.803 a |
| 14 | 120.930 b | 129.457 b |
| 28 | 134.892 b | 170.542 c |
| Storage Time (Day) | DPPH Scavenging Activity (µg/mL) | |
|---|---|---|
| Refrigerated Temperature (4 °C) | Room Temperature (25 °C) | |
| IC50 | ||
| 0 | 36.880 a | 36.880 a |
| 14 | 187.626 b | 189.435 b |
| 28 | 188.773 b | 201.943 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pato, U.; Yusmarini; Riftyan, E.; Rossi, E.; Hidayat, R.; Anjani, S.F.; Riadi, N.; Octaviani, I.N.; Agrina; Syukri, D.; et al. Probiotic Properties of Lactobacillus fermentum InaCC B1295 Encapsulated by Cellulose Microfiber from Oil Palm Empty Fruit Bunches. Fermentation 2022, 8, 602. https://doi.org/10.3390/fermentation8110602
Pato U, Yusmarini, Riftyan E, Rossi E, Hidayat R, Anjani SF, Riadi N, Octaviani IN, Agrina, Syukri D, et al. Probiotic Properties of Lactobacillus fermentum InaCC B1295 Encapsulated by Cellulose Microfiber from Oil Palm Empty Fruit Bunches. Fermentation. 2022; 8(11):602. https://doi.org/10.3390/fermentation8110602
Chicago/Turabian StylePato, Usman, Yusmarini, Emma Riftyan, Evy Rossi, Rahmad Hidayat, Sandra Fitri Anjani, Nabila Riadi, Ika Nur Octaviani, Agrina, Daimon Syukri, and et al. 2022. "Probiotic Properties of Lactobacillus fermentum InaCC B1295 Encapsulated by Cellulose Microfiber from Oil Palm Empty Fruit Bunches" Fermentation 8, no. 11: 602. https://doi.org/10.3390/fermentation8110602
APA StylePato, U., Yusmarini, Riftyan, E., Rossi, E., Hidayat, R., Anjani, S. F., Riadi, N., Octaviani, I. N., Agrina, Syukri, D., & Surono, I. S. (2022). Probiotic Properties of Lactobacillus fermentum InaCC B1295 Encapsulated by Cellulose Microfiber from Oil Palm Empty Fruit Bunches. Fermentation, 8(11), 602. https://doi.org/10.3390/fermentation8110602









