Fortification of Cow Milk with Moringa oleifera Extract: Influence on Physicochemical Characteristics, Antioxidant Capacity and Mineral Content of Yoghurt
Abstract
1. Introduction
2. Materials and Methods
2.1. Moringa Extract Production
2.2. Yoghurt Production
2.3. Acidity Measurements
2.4. Microbiological Analyses
2.5. Syneresis, Water Holding Capacity and Rheology Parameters
2.6. Colour Measurements
2.7. Mineral Content Determination
2.8. Total Phenols Content and Antioxidant Capacity
2.9. Sensory Evaluation
2.10. Statistical Analysis
3. Results and Discussion
3.1. Fermentation Time and Acidity
3.2. Microbiological Analysis
3.3. Syneresis and WHC
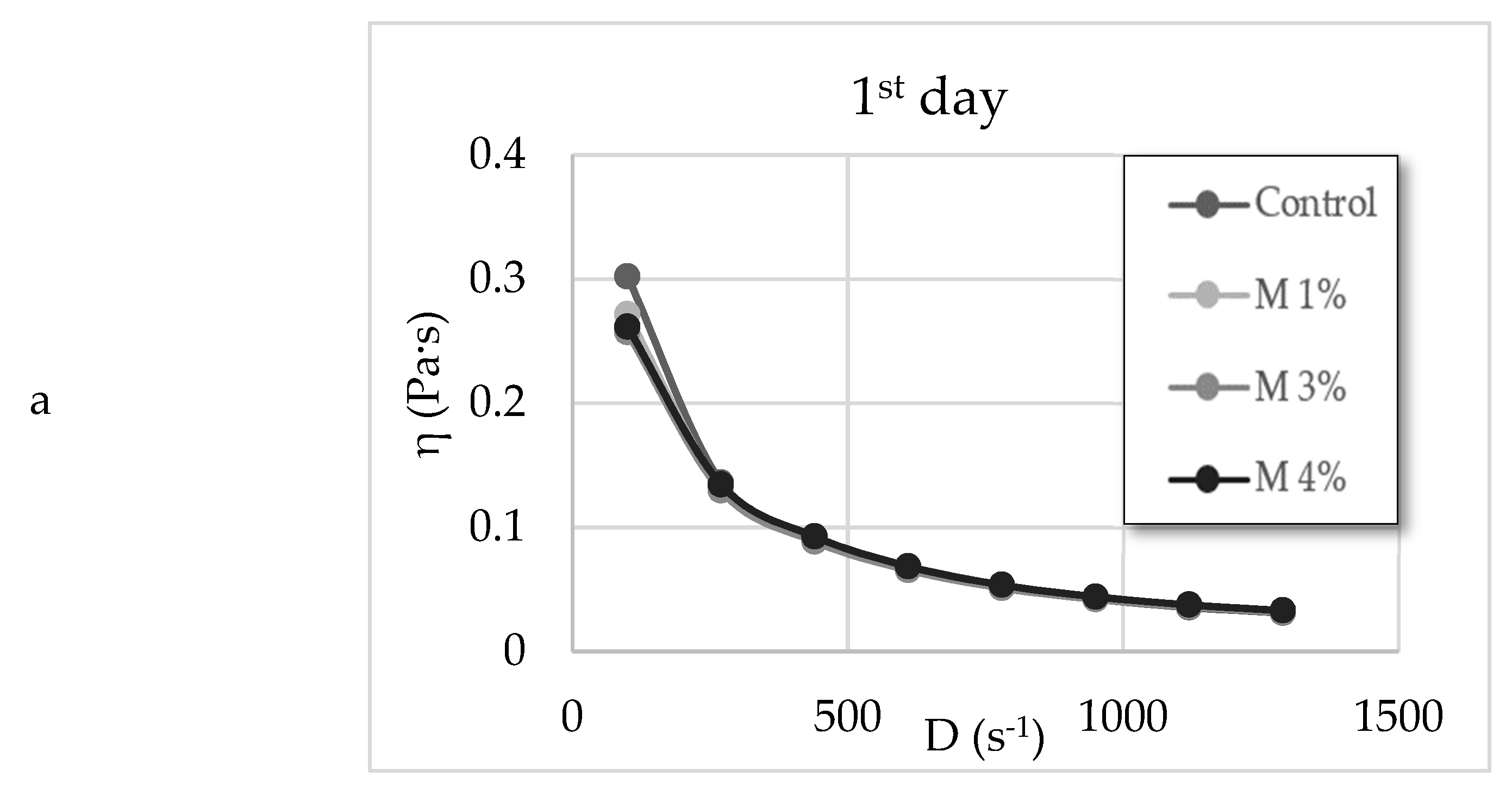
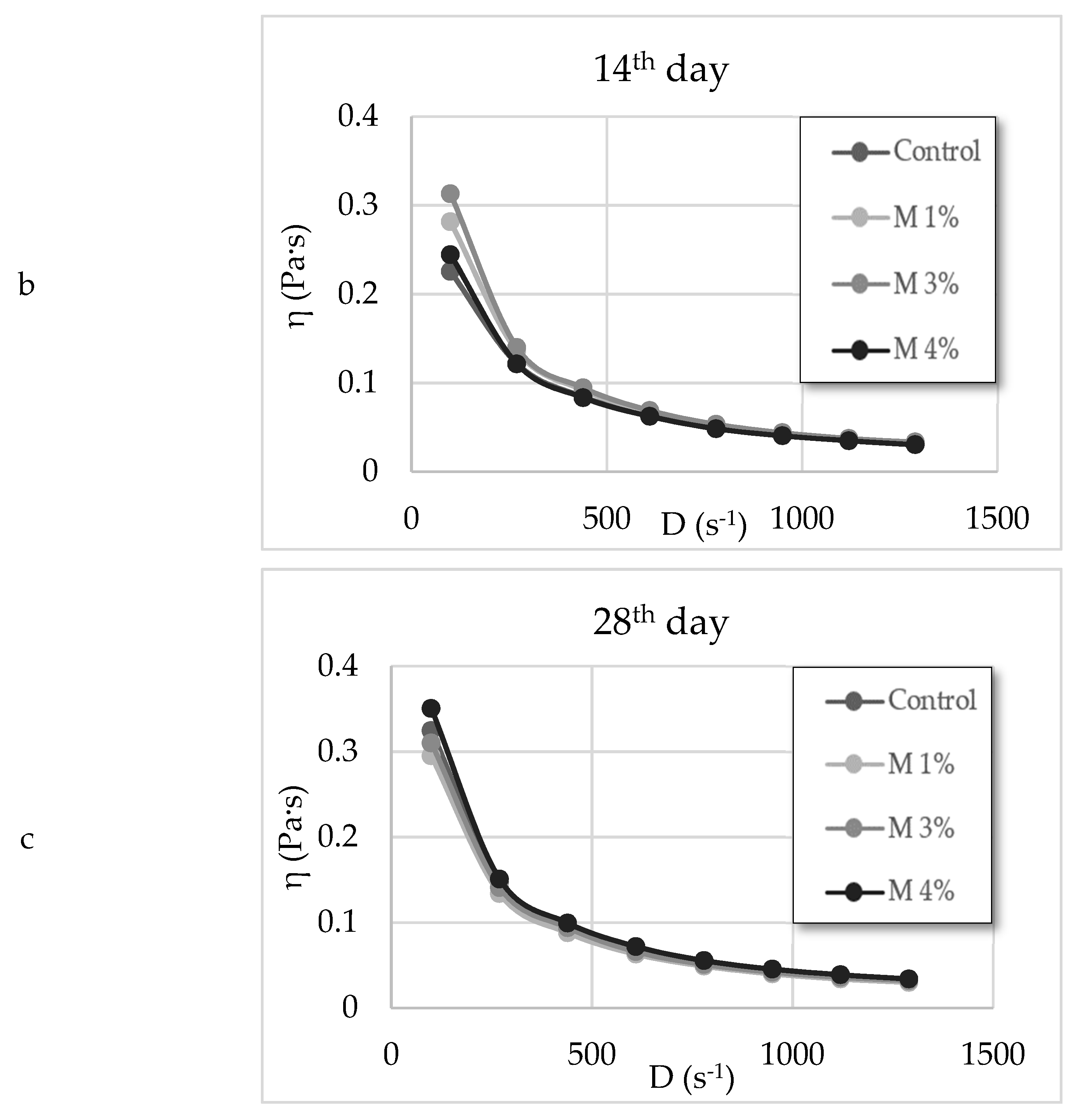
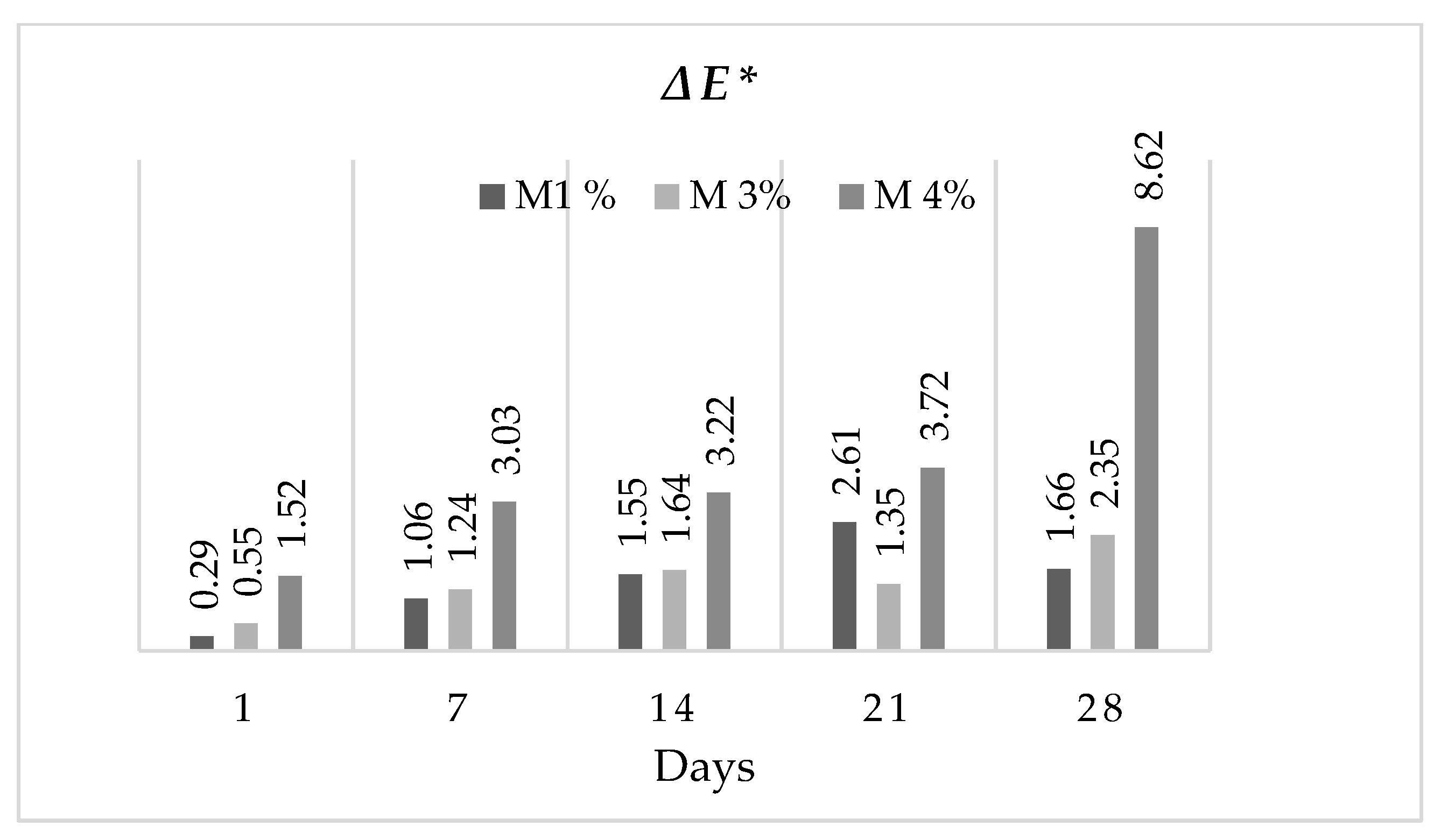
3.4. Rheology Parameters and the Total Colour Difference
3.5. Mineral Content
3.6. TPC and AC
3.7. Sensory Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borgonovi, T.F.; Virgolin, L.B.; Janzantti, N.S.; Casarotti, S.N.; Barretto Penna, A.L. Fruit bioactive compounds: Effect on lactic acid bacteria and on intestinal microbiota. Food Res. Int. 2022, 161, 111809. [Google Scholar] [CrossRef]
- García-Burgos, M.; Moreno-Fernández, J.; Alférez, M.J.M.; Díaz-Castro, J.; López-Aliaga, I. New perspectives in fermented dairy products and their health relevance. J. Funct. Foods 2020, 72, 104059. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, S.; Ramos, I.M.; Seseña, S.; Poveda, J.M.; Palop, M.L. Potential of Lactobacillus strains for health-promotion and flavouring of fermented dairy foods. LWT Food Sci. Technol. 2021, 143, 111102. [Google Scholar] [CrossRef]
- Ozmen-Togay, S.; Gulkun, G.; Degirmencioglu, N.; Guldas, M.; Yildiz, E.; Sahan, Y.; Gurbuz, O. Impact of coffee silverskin on in vitro viability of kefir culture during storage. Mljekarstvo 2022, 72, 22–32. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Behbahani, M. Enhancement of probiotics viability and lactic acid production in yogurts treated with Prangos ferulaceae and Carum copticum plant extracts. Biocatal. Agric. Biotechnol. 2021, 35, 102084. [Google Scholar] [CrossRef]
- Bassogog, C.B.B.; Nyobe, C.E.; Ngui, S.P.; Minka, S.R.; Mune Mune, M.A. Effect of heat treatment on the structure, functional properties and composition of Moringa oleifera seed proteins. Food Chem. 2022, 384, 132546. [Google Scholar] [CrossRef]
- Ao, B.; Lv, J.; Yang, H.; He, F.; Hu, Y.; Hu, B.; Jiang, H.; Huo, X.; Tu, J.; Xia, X. Moringa oleifera extract mediated the synthesis of Bio-SeNPs with antibacterial activity against Listeria monocytogenes and Corynebacterium diphtheria. LWT Food Sci. Technol. 2022, 165, 113751. [Google Scholar] [CrossRef]
- Hassan, F.A.M.; Enab, A.K.; Abd El-Gawad, M.A.M.; Bayoumi, H.M.; Youssef, Y.B. Utilization of Moringa oleifera Leaves Powder in Production of Soft White Cheese. Int. J. Dairy Sci. 2017, 12, 137–142. [Google Scholar] [CrossRef]
- Pandey, A.; Pandey, R.D.; Tripathi, P.; Gupta, P.P.; Haider, J. Moringa Oleifera Lam. (Sahijan)–A Plant with a Plethora of Diverse Therapeutic Benefits: An Updated Retrospection. J. Med. Aromat. Plants. 2012, 1, 1–8. [Google Scholar] [CrossRef]
- Zhang, T.; Jeong, C.H.; Cheng, W.N.; Bae, H.; Geuk Seo, H.; Petriello, M.C.; Han, S.G. Moringa extract enhances the fermentative, textural, and bioactive properties of yogurt. LWT Food Sci. Technol. 2018, 101, 276–284. [Google Scholar] [CrossRef]
- Saad, M.A.; Elkhtab, E.S. Antimicrobial activity of Moringa oleifera leaves extract and its effect on the shelf life and quality of yoghurt. Egypt. J. Dairy Sci. 2019, 47, 91–99. [Google Scholar]
- Hassan, F.A.M.; Bayoumi, H.M.; Abd El-Gawad, M.A.M.; Enab, A.K.; Youssef, Y.B. Utilization of Moringa oleifera Leaves Powder in Production of Yoghurt. Int. J. Dairy Sci. 2016, 11, 69–74. [Google Scholar] [CrossRef][Green Version]
- Nedanovska, E.; Lisak Jakopović, K.; Daniloski, D.; Vaskoska, R.; Vasiljevic, T.; Barukčić, I. Effect of storage time on the microbial, physicochemical and sensory characteristics of ovine whey-based fruit beverages. Int J. Food Sci. Technol. 2022, 57, 5388–5398. [Google Scholar] [CrossRef]
- ISO 6887–5:2010; Microbiology of Food and Animal Feeding Stuffs—Preparation of Test Samples, Initial Suspension and Decimal Dilutions for Microbiological Examination: Specific Rules for the Preparation of Milk and Milk Products. International Organization for Standardization: London, UK, 2010.
- Joung, J.Y.; Lee, J.Y.; Ha, Y.S.; Shin, Y.K.; Kim, Y.; Kim, S.H. Enhanced microbial, functional and sensory properties of herbal yogurt fermented with Korean traditional plant extracts. Korean J. Food Sci. 2016, 36, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Cardines, P.H.F.; Baptista, A.T.A.; Gomes, R.G.; Bergamasco, R.; Vieira, A.M.S. Moringa oleifera seed extracts as promising natural thickening agents for food industry: Study of the thickening action in yogurt production. LWT Food Sci. Technol. 2018, 97, 39–44. [Google Scholar] [CrossRef]
- Feng, C.; Wang, B.; Zhao, A.; Wei, L.; Shao, Y.; Wang, Y.; Cao, B.; Zhang, F. Quality characteristics and antioxidant activities of goat milk yogurt with added jujube pulp. Food Chem. 2018, 277, 238–245. [Google Scholar] [CrossRef]
- Mokrzycki, W.; Tatol, M. Color difference Delta E—A survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- Shortle, E.; O’Grady, M.N.; Gilroy, D.; Furey, A.; Quinn, N.; Kerry, J.P. Influence of etraction technique on anti-oxidative potential of hawthorn (Crataegus monogyna) extracts in bovine muscle homogenates. Meat Sci. 2014, 98, 828–834. [Google Scholar] [CrossRef]
- Benzie, I.F.F. An automated, specific, spectrophotometric method for measuring ascorbic acid in plasma (EFTSA). Clin. Biochem. 1996, 29, 111–116. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- ISO 22935–3:2009 (IDF 99–3:2009); Milk and Milk Products—Sensory Analysis—Part 3: Guidance on a Method for Evaluation of Compliance with Product Specifications for Sensory Properties by Scoring. International Organization for Standardization: London, UK, 2009.
- Molnar, P.; Örsi, F. Determination of weighting factors for the sensory evaluation of food. Nahrung Food 1982, 26, 661–667. [Google Scholar] [CrossRef]
- ISO 8589:2007; Sensory analysis—General Guidance for the Design of Test Rooms. International Organization for Standardization: London, UK, 2007.
- Barukčić, I.; Filipan, K.; Lisak Jakopović, K.; Božanić, R.; Blažić, M.; Repajić, M. The Potential of Olive Leaf Extract as a Functional Ingredient in Yoghurt Production: The Effects on Fermentation, Rheology, Sensory, and Antioxidant Properties of Cow Milk Yoghurt. Foods 2022, 11, 701. [Google Scholar] [CrossRef] [PubMed]
- Al- Ahwal, R.; Saleh, A.; Moussa, M. The Importance of Using Moringa Oleifera Extract on the Quality and Nutritive Value of Yoghurt. J. Food Dairy Sci. 2017, 8, 237–241. [Google Scholar] [CrossRef]
- El–Gammal, R.E.; Abdel–Aziz, M.E.; Darwish, M.S. Utilization of Aqueous Extract of Moringa oleifera for Production of Functional Yogurt. J. Food Dairy Sci. 2017, 8, 45–53. [Google Scholar] [CrossRef]
- Charlton, A.J.; Baxter, N.J.; Khan, M.L.; Moir, A.J.G.; Haslam, E.; Davies, A.P. Polyphenol/peptide binding and precipitation. J. Agric. Food Chem. 2002, 50, 1593–1601. [Google Scholar] [CrossRef]
- Shori, A.B. Storage quality and antioxidant properties of yogurt fortified with polyphenol extract from nutmeg, black pepper, and white pepper. Electron. J. Biotechnol. 2022, 57, 24–30. [Google Scholar] [CrossRef]
- Oliveira, M.N.; Sodini, I.; Remeuf, F.; Corrieu, G. Effect of milk supplementation and culture composition on acidification, textural properties, and microbiological stability of fermented milks containing probiotic bacteria. Int. Dairy J. 2001, 11, 935–942. [Google Scholar] [CrossRef]
- Bikheet, M.M.; Yasien, E.E.; Galal, S.M. Preparation of Functional Yoghrt Drink Fortified with Moringa oleifera Leaves. J. Food Dairy Sci. 2021, 12, 217–223. [Google Scholar] [CrossRef]
- Mthiyane, F.T.; Dludla, P.V.; Ziqubu, K.; Mthembu, S.; Muvhulawa, N.; Hlengwa, N.; Nkambule, B.; Mazibuko-Mbeje, S. A Review on the Antidiabetic Properties of Moringa oleifera Extracts: Focusing on Oxidative Stress and Inflammation as Main Therapeutic Targets. Front. Pharmacol. 2022, 13, 940572. [Google Scholar] [CrossRef]
- Anwar, F.; Latif, S.; Ashraf, M.; Gilani, A.H. Moringa oleifera: A food plant with multiple medicinal uses. Phytother. Res. 2007, 21, 17–25. [Google Scholar] [CrossRef]
- Vajravelu, K.; Prasad, K.V.; Datti, P.S.; Raju, B.T. Convective flow, heat and mass transfer of Ostwald-de Waele fluid over a vertical stretching sheet. J. King Saud Univ. Eng. Sci. 2017, 29, 57–67. [Google Scholar] [CrossRef]
- Babar, H.; Rizwan, B.; Babar, A.; Nazia, H.; Noreen, S.; Naeem, N.; Raza, F.; Seed, Z.; Imran, S. Therapeutic Effect of Moringa Oleifera: A Review. Pakistan Biomed. J. 2022, 5, 10–13. [Google Scholar] [CrossRef]
- Tratnik, L.; Božanić, R. Milk and Dairy Products, 1st ed.; Croatian Dairy Union: Zagreb, Croatia, 2008. [Google Scholar]


| Time (Days) | pH Value | |||
|---|---|---|---|---|
| Control | M 1% | M 3% | M 4% | |
| 1 | 4.53 ± 0.12 | 4.51 ± 0.12 | 4.43 ± 0.09 | 4.40 ± 0.11 |
| 7 | 4.32 ± 0.08 | 4.22 ± 0.02 | 4.21 ± 0.10 | 4.15 ± 0.09 |
| 14 | 4.27 ± 0.10 | 4.12 ± 0.10 | 4.24 ± 0.12 | 4.21 ± 0.11 |
| 21 | 4.34 ± 0.11 | 4.35 ± 0.13 | 4.36 ± 0.09 | 4.29 ± 0.10 |
| 28 | 4.37 ± 0.13 | 4.32 ± 0.11 | 4.36 ± 0.11 | 4.32 ± 0.04 |
| Source of Variation | pH | Total Phenols (mg GAE L−1) | FRAP (µmol TE L−1) | Flow Index (n) | Consistency (K, mPas) |
|---|---|---|---|---|---|
| Extract content (%) | p = 0.001 * | p < 0.001 * | p < 0.001 * | p = 0.524 | p = 0.166 |
| 0 | 4.37 ± 0.03 b | 77.4 ± 1.6 a | 503.3 ± 1.6 a | 0.72 ± 0.04 a | 5.77 ± 0.31 a |
| 1 | 4.29 ± 0.04 a | 86.5 ± 1.8 b | 529.8 ± 1.8 a | 0.66 ± 0.02 a | 5.39 ± 0.18 a |
| 3 | 4.32 ± 0.03 ab | 89.2 ± 4.0 b | 636.3 ± 4.0 b | 0.70 ± 0.03 a | 5.74 ± 0.20 a |
| 4 | 4.28 ± 0.03 a | 100.5 ± 2.9 c | 633.2 ± 2.9 b | 0.69 ± 0.01 a | 5.81 ± 0.20 a |
| Storage time (day) | p < 0.001 * | p = 0.214 | p = 0.757 | p < 0.001 * | p = 0.001 * |
| 1 | 4.46 ± 0.02 c | 85.0 ± 3.0 a | 587.8 ± 26.4 a | 0.60 ± 0.01 a | 4.64 ± 0.23 a |
| 7 | 4.23 ± 0.03 a | 84.7 ± 1.9 a | 593.5 ± 21.2 a | 0.66 ± 0.01 ab | 5.92 ± 0.02 ab |
| 14 | 4.21 ± 0.02 a | 91.0 ± 5.1 a | 570.5 ± 15.8 a | 0.68 ± 0.01 bc | 5.69 ± 0.09 ab |
| 21 | 4.33 ± 0.01 b | 97.0 ± 3.6 a | 558.4 ± 27.3 a | 0.74 ± 0.03 cd | 5.95 ± 0.19 b |
| 28 | 4.34 ± 0.01 b | 84.2 ± 5.3 a | 568.1 ± 32.2 a | 0.80 ± 0.02 d | 6.18 ± 0.21 b |
| Grand mean | 4.31 ± 0.02 | 88.4 ± 1.9 | 575.7 ± 1.9 | 0.69 ± 0.01 | 5.68 ± 0.11 |
| Lactobacillus sp., log (CFU mL−1) | |||
| Sample | Beginning | End | 28th day of cold storage |
| Control | 8.77 ± 0.30 | 10.48 ± 0.08 | 7.55 ± 0.06 |
| M 1% | 8.87 ± 0.16 | 8.70 ± 0.07 | 3.98 ± 0.01 |
| M 3% | 8.99 ± 0.01 | 10.43 ± 0.35 | 4.32 ± 0.08 |
| M 4% | 8.98 ± 0.01 | 9.23 ± 0.07 | 5.79 ± 0.29 |
| Streptococcus sp., log (CFU mL−1) | |||
| Sample | Beginning | End | 28th day of cold storage |
| Control | 6.97 ± 0.03 | 9.89 ± 0.06 | 9.12 ± 0.15 |
| M 1% | 6.92 ± 0.04 | 9.15 ± 0.08 | 9.34 ± 0.16 |
| M 3% | 6.93 ± 0.03 | 11.60 ± 0.08 | 8.84 ± 0.05 |
| M 4% | 6.92 ± 0.05 | 9.17 ± 0.01 | 9.30 ± 0.06 |
| Source of Variation | Lactobacillus sp. (log CFU mL−1) | Streptococcus sp. (log CFU mL−1) |
| Extract content (%) | p = 0.464 | p = 0.950 |
| 0 | 8.9 ± 0.5 a | 8.7 ± 0.6 a |
| 1 | 7.2 ± 1.0 a | 8.5 ± 0.5 a |
| 3 | 7.9 ± 1.2 a | 9.1 ± 0.9 a |
| 4 | 8.0 ± 0.7 a | 8.5 ± 0.5 a |
| Storage time | p < 0.001 * | p < 0.001 * |
| Fermentation start (0 day) | 8.9 ± 0.1 b | 6.9 ± 0.0 a |
| Fermentation end (0 day) | 9.7 ± 0.3 b | 10.0 ± 0.4 b |
| 28th day | 5.4 ± 0.5 a | 9.1 ± 0.1 b |
| Grand mean | 8.0 ± 0.4 | 8.7 ± 0.3 |
| Syneresis (%) | WHC (%) | |||
|---|---|---|---|---|
| Sample | 1 Day | 28 Day | 1 Day | 28 Day |
| Control | 62.72 ± 0.49 | 50.48 ± 0.66 | 34.38 ± 0.57 | 46.55 ± 0.42 |
| M 1% | 58.36 ± 0.28 | 50.14 ± 0.35 | 38.50 ± 0.40 | 47.33 ± 0.30 |
| M 3% | 58.56 ± 0.39 | 49.94 ± 0.33 | 39.47 ± 0.49 | 48.34 ± 0.51 |
| M 4% | 56.95 ± 0.06 | 51.93 ± 0.19 | 42.24 ± 0.35 | 46.68 ± 0.30 |
| Source of Variation | Syneresis (%) | WHC (%) |
| Extract content (%) | p = 0.897 | p = 0.544 |
| 0 | 56.6 ± 3.5 a | 40.5 ± 3.5 a |
| 1 | 54.3 ± 2.4 a | 42.9 ± 2.6 a |
| 3 | 54.3 ± 2.5 a | 43.9 ± 2.6 a |
| 4 | 54.4 ± 1.5 a | 44.5 ± 1.3 a |
| Storage time (day) | p < 0.001 * | p < 0.001 * |
| 1 | 59.1 ± 0.8 b | 38.6 ± 1.1 a |
| 28 | 50.6 ± 0.3 a | 47.2 ± 0.3 b |
| Grand mean | 54.9 ± 1.2 | 42.9 ± 1.2 |
| Days of Storage | 1 | 7 | 14 | 21 | 28 |
|---|---|---|---|---|---|
| Sample | Flow index (n) | ||||
| Control | 0.56 ± 0.06 | 0.68 ± 0.14 | 0.70 ± 0.07 | 0.79 ± 0.05 | 0.89 ± 0.01 |
| M 1% | 0.58 ± 0.14 | 0.65 ± 0.06 | 0.68 ± 0.06 | 0.65 ± 0.06 | 0.75 ± 0.02 |
| M 3% | 0.62 ± 0.01 | 0.63 ± 0.14 | 0.65 ± 0.01 | 0.79 ± 0.01 | 0.81 ± 0.01 |
| M 4% | 0.64 ± 0.14 | 0.68 ± 0.01 | 0.68 ± 0.00 | 0.71 ± 0.01 | 0.75 ± 0.05 |
| Sample | Consistency coefficient (K)/mPas | ||||
| Control | 4.14 ± 0.01 | 5.89 ± 0.07 | 5.6 ± 0.07 | 6.27 ± 0.01 | 6.93 ± 0.05 |
| M 1% | 4.15 ± 0.02 | 5.89 ± 0.01 | 5.44 ± 0.04 | 5.08 ± 0.07 | 5.39 ± 0.01 |
| M 3% | 4.62 ± 0.07 | 5.97 ± 0.01 | 5.66 ± 0.03 | 6.26 ± 0.07 | 6.22 ± 0.01 |
| M 4% | 4.65 ± 0.04 | 5.95 ± 0.02 | 6.08 ± 0.07 | 6.18 ± 0.02 | 6.20 ± 0.07 |
| Sample | Coefficient of regression (R2) | ||||
| Control | 0.9965 ± 0.0007 | 0.9675 ± 0.0007 | 0.9965 ± 0.0007 | 0.9955 ± 0.0014 | 0.9875 ± 0.0014 |
| M 1% | 0.9970 ± 0.0000 | 0.9907 ± 0.014 | 0.9945 ± 0.0007 | 0.9845 ± 0.0055 | 0.9955 ± 0.0007 |
| M 3% | 0.9985 ± 0.0007 | 0.9935 ± 0.0007 | 0.9935 ± 0.0007 | 0.9675 ± 0.0021 | 0.9905 ± 0.0014 |
| M 4% | 0.999 ± 0.0000 | 0.9875 ± 0.0007 | 0.9905 ± 0.0007 | 0.9990 ± 0.0000 | 0.9925 ± 0.0007 |
| Mineral Elements (mg kg−1) | |||||
|---|---|---|---|---|---|
| Sample | Mg | Ca | Fe | Cu | Zn |
| Control | 83.0 ± 1.4 ab | 888.5 ± 3.5 a | 0.172 ± 0.002 a | 0.038 ± 0.000 a | 0.003 ± 0.000 a |
| M 1% | 101.0 ± 1.4 b | 1092.0 ± 7.1 a | 0.245 ± 0.007 b | 0.051 ± 0.000 b | 0.004 ± 0.000 a |
| M 3% | 102.0 ± 4.2 b | 1047.0 ± 97.6 a | 0.290 ± 0.001 c | 0.047 ± 0.000 b | 0.004 ± 0.000 a |
| M 4% | 167.0 ± 1.4 c | 1753.5 ± 20.5 b | 0.420 ± 0.014 d | 0.071 ± 0.004 c | 0.007 ± 0.000 b |
| Days of Storage | 1 | 7 | 14 | 21 | 28 |
|---|---|---|---|---|---|
| Sample | TPC (mg GAE L−1) | ||||
| Control | 73.85 ± 0.73 | 76.09 ± 0.05 | 76.36 ± 0.78 | 86.89 ± 0.04 | 73.67 ± 0.14 |
| M 1% | 82.22 ± 0.71 | 87.78 ± 0.49 | 78.64 ± 0.49 | 91.31 ± 0.64 | 92.50 ± 0.52 |
| M 3% | 89.56 ± 0.59 | 87.78 ± 0.49 | 102.74 ± 0.57 | 98.05 ± 0.67 | 67.79 ± 0.71 |
| M 4% | 94.45 ± 0.63 | 87.27 ± 0.57 | 106.09 ± 0.93 | 111.93 ± 1.02 | 102.88 ± 0.63 |
| Sample | FRAP (µmol TE L−1) | ||||
| Control | 495.79 ± 0.61 | 532.41 ± 7.20 | 499.10 ± 0.24 | 464.20 ± 2.40 | 524.92 ± 1.39 |
| M 1% | 545.64 ± 1.41 | 543.26 ± 7.14 | 594.00 ± 3.16 | 516.52 ± 1.63 | 449.79 ± 7.28 |
| M 3% | 644.87 ± 3.34 | 656.77 ± 3.01 | 584.67 ± 7.04 | 648.49 ± 1.40 | 646.75 ± 7.13 |
| M 4% | 664.96 ± 1.45 | 641.45 ± 6.58 | 604.20 ± 1.40 | 604.31 ± 1.49 | 650.93 ± 1.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lisak Jakopović, K.; Repajić, M.; Rumora Samarin, I.; Božanić, R.; Blažić, M.; Barukčić Jurina, I. Fortification of Cow Milk with Moringa oleifera Extract: Influence on Physicochemical Characteristics, Antioxidant Capacity and Mineral Content of Yoghurt. Fermentation 2022, 8, 545. https://doi.org/10.3390/fermentation8100545
Lisak Jakopović K, Repajić M, Rumora Samarin I, Božanić R, Blažić M, Barukčić Jurina I. Fortification of Cow Milk with Moringa oleifera Extract: Influence on Physicochemical Characteristics, Antioxidant Capacity and Mineral Content of Yoghurt. Fermentation. 2022; 8(10):545. https://doi.org/10.3390/fermentation8100545
Chicago/Turabian StyleLisak Jakopović, Katarina, Maja Repajić, Ivana Rumora Samarin, Rajka Božanić, Marijana Blažić, and Irena Barukčić Jurina. 2022. "Fortification of Cow Milk with Moringa oleifera Extract: Influence on Physicochemical Characteristics, Antioxidant Capacity and Mineral Content of Yoghurt" Fermentation 8, no. 10: 545. https://doi.org/10.3390/fermentation8100545
APA StyleLisak Jakopović, K., Repajić, M., Rumora Samarin, I., Božanić, R., Blažić, M., & Barukčić Jurina, I. (2022). Fortification of Cow Milk with Moringa oleifera Extract: Influence on Physicochemical Characteristics, Antioxidant Capacity and Mineral Content of Yoghurt. Fermentation, 8(10), 545. https://doi.org/10.3390/fermentation8100545










