Plant Proteins as an Alternative Nitrogen Source for Chiral Purity L-Lactic Acid Fermentation from Lignocellulose Feedstock
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Feedstock
2.2. Reagents and Enzymes
2.3. Microorganisms and Medium
2.4. Pretreatment and Biodetoxification
2.5. Preparation of Cottonseed Meal Hydrolysate
2.6. Cellulosic L-Lactic Acid SSCF
2.7. Analysis
3. Results and Discussions
3.1. Screening Alternative Nitrogen Sources for High Chiral Purity Lactic Acid Fermentation
3.2. Hydrolysis of Cottonseed Meal and Consequent Cellulosic L-Lactic Acid Fermentation
3.3. Cellulose L-Lactic Acid Production by SSCF
3.4. Preliminary Techno-Economic Evaluations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, Z.; He, F.; Shi, Y.; Lu, M.; Yu, L. Fermentative production of L(+)-lactic acid using hydrolyzed acorn starch, persimmon juice and wheat bran hydrolysate as nutrients. Bioresour. Technol. 2010, 101, 3642–3648. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, M.A.; Xiao, Y.; Tashiro, Y.; Wang, Y.; Zendo, T.; Sakai, K.; Sonomoto, K. Fed-batch fermentation for enhanced lactic acid production from glucose/xylose mixture without carbon catabolite repression. J. Biosci. Bioeng. 2015, 119, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Inkinen, S.; Hakkarainen, M.; Albertsson, A.C.; Sodergard, A. From lactic acid to poly(lactic acid) (PLA): Characterization and analysis of PLA and its precursors. Biomacromolecules 2011, 12, 523–532. [Google Scholar] [CrossRef]
- Ahmad, A.; Banat, F.; Taher, H. A review on the lactic acid fermentation from low-cost renewable materials: Recent developments and challenges. Environ. Technol. Innov. 2020, 20, 101138. [Google Scholar] [CrossRef]
- Tejayadi, S.; Cheryan, M. Lactic acid from cheese whey permeate. Productivity and economics of a continuous membrane bioreactor. Appl. Microbio. Biotechnol. 1995, 43, 242–248. [Google Scholar] [CrossRef]
- Altaf, M.; Venkateshwar, M.; Srijana, M.; Reddy, G. An economic approach for L-(+) lactic acid fermentation by Lactobacillus amylophilus GV6 using inexpensive carbon and nitrogen sources. J. Appl. Microbiol. 2007, 103, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Juturu, V.; Wu, J.C. Microbial production of lactic acid: The latest development. Crit. Rev. Biotechnol. 2016, 36, 967–977. [Google Scholar] [CrossRef]
- Zhang, Y.; Yoshida, M.; Vadlani, P.V. Biosynthesis of D-lactic acid from lignocellulosic biomass. Biotechnol. Lett. 2018, 40, 1167–1179. [Google Scholar] [CrossRef]
- Pauli, T.; Fitzpatrick, J.J. Malt combing nuts as a nutrient supplement to whey permeate for producing lactic by fermentation with Lactobacillus casei. Process Biochem. 2002, 38, 1–6. [Google Scholar] [CrossRef]
- Wee, Y.J.; Ryu, H.W. Lactic acid production by Lactobacillus sp. RKY2 in a cell-recycle continuous fermentation using lignocellulosic hydrolyzates as inexpensive raw materials. Bioresour. Technol. 2009, 100, 4262–4270. [Google Scholar] [CrossRef]
- Sikder, J.; Roy, M.; Dey, P.; Pal, P. Techno-economic analysis of a membrane-integrated bioreactor system for production of lactic acid from sugarcane juice. Biochem. Eng. J. 2012, 63, 81–87. [Google Scholar] [CrossRef]
- Rivas, B.; Moldes, A.B.; Domínguez, J.M.; Parajó, J.C. Development of culture media containing spent yeast cells of Debaryomyces hansenii and corn steep liquor for lactic acid production with Lactobacillus rhamnosus. Int. J. Food Microbiol. 2004, 97, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.; Fontes Coelho, L.; Blanco, K.; Contiero, J. Response surface optimization of D(-)-lactic acid production by Lactobacillus SMI 8 using corn steep liquor and yeast autolysate as an alternative nitrogen source. Afr. J. Biotechnol. 2009, 8, 5842–5846. [Google Scholar]
- Yu, L.; Lei, T.; Ren, X.; Pei, X.; Feng, Y. Response surface optimization of L-(+)-lactic acid production using corn steep liquor as an alternative nitrogen source by Lactobacillus rhamnosus CGMCC 1466. Biochem. Eng. J. 2008, 39, 496–502. [Google Scholar] [CrossRef]
- Pejin, J.; Radosavljevic, M.; Pribic, M.; Kocic-Tanackov, S.; Mladenovic, D.; Djukic-Vukovic, A.; Mojovic, L. Possibility of L-(+)-lactic acid fermentation using malting, brewing, and oil production by-products. Waste Manage. 2018, 79, 153–163. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Ju, J.; Yu, B.; Ma, Y. Efficient production of polymer-grade D-lactate by Sporolactobacillus laevolacticus DSM442 with agricultural waste cottonseed as the sole nitrogen source. Bioresour. Technol. 2013, 142, 186–191. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, B.; Li, F.; Xu, K.; Ma, C.; Tao, F.; Li, Q.; Xu, P. Highly efficient production of D-lactate by Sporolactobacillus sp. CASD with simultaneous enzymatic hydrolysis of peanut meal. Appl. Microbiol. Biotechnol. 2011, 89, 1009–1017. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C. Determination of Sugars, Byproducts, and Degradation Products in Liquid Fraction Process Samples; NREL/TP-510–42623; National Renewable Energy Laboratory: Golden, CO, USA, 2008.
- Sluiter, A.; Hames, B.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Structural Carbohydrates and Lignin in Biomass National Renewable; NREL/TP-510–42623; National Renewable Energy Laboratory: Golden, CO, USA, 2012.
- Zhang, B.; Khushik, F.A.; Zhan, B.; Bao, J. Transformation of lignocellulose to starch-like carbohydrates by organic acid-catalyzed pretreatment and biological detoxification. Biotechnol. Bioeng. 2021, 118, 4105–4118. [Google Scholar] [CrossRef]
- Qiu, Z.; Gao, Q.; Bao, J. Engineering Pediococcus acidilactici with xylose assimilation pathway for high titer cellulosic l-lactic acid fermentation. Bioresour. Technol. 2018, 249, 9–15. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, Q.; Li, H.; Qureshi, A.S.; Zhang, J.; Bao, X.; Bao, J. Dry biorefining maximizes the potentials of simultaneous saccharification and co-fermentation for cellulosic ethanol production. Biotechnol. Bioeng. 2018, 115, 60–69. [Google Scholar] [CrossRef]
- Liu, G.; Sun, J.; Zhang, J.; Tu, Y.; Bao, J. High titer L-lactic acid production from corn stover with minimum wastewater generation and techno-economic evaluation based on Aspen plus modeling. Bioresour. Technol. 2015, 198, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ding, S.; Li, Z.; Tan, T. L-lactic acid production by Lactobacillus casei fermentation with corn steep liquor-supplemented acid-hydrolysate of soybean meal. Biotechnol. J. 2006, 1, 1453–1458. [Google Scholar] [CrossRef] [PubMed]
- John, R.P.; Nampoothiri, K.M.; Pandey, A. Fermentative production of lactic acid from biomass: An overview on process developments and future perspectives. Appl. Microbiol. Biotechnol. 2007, 74, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Alves de Oliveira, R.; Komesu, A.; Vaz Rossell, C.E.; Maciel Filho, R. Challenges and opportunities in lactic acid bioprocess design—From economic to production aspects. Biochem. Eng. J. 2018, 133, 219–239. [Google Scholar] [CrossRef]
- Edwinoliver, N.G.; Thirunavukarasu, K.; Purushothaman, S.; Rose, C.; Gowthaman, M.K.; Kamini, N.R. Corn steep liquor as a nutrition adjunct for the production of Aspergillus niger lipase and hydrolysis of oils thereof. J. Agric. Food Chem. 2009, 57, 10658–10663. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yuan, Y.J. Comprehensive quality evaluation of corn steep liquor in 2-keto-L-gluonic acid fermentation. J. Agric. Food Chem. 2011, 59, 9845–9853. [Google Scholar] [CrossRef]
- Selling, G.W.; Hojilla-Evangelista, M.P.; Hay, W.T.; Utt, K.D.; Grose, G.D. Preparation and properties of solution cast films from pilot-scale cottonseed protein isolate. Ind. Crop. Prod. 2022, 178, 114615. [Google Scholar] [CrossRef]
- Brock, S.; Kuenz, A.; Prüße, U. Impact of hydrolysis methods on the utilization of agricultural residues as nutrient source for D-lactic acid production by Sporolactobacillus inulinus. Fermentation 2019, 5, 12. [Google Scholar] [CrossRef]
- Tanksley, T.D., Jr.; Knabe, D.A.; Purser, K.; Zebrowska, T.; Corley, J.R. Apparent digestibility of amino acids and nitrogen in three cottonseed meals and one soybean meal. J. Anim. Sci. 1981, 52, 769–777. [Google Scholar] [CrossRef]
- Gao, M.T.; Hirata, M.; Toorisaka, E.; Hano, T. Study on acid-hydrolysis of spent cells for lactic acid fermentation. Biochem. Eng. J. 2006, 28, 87–91. [Google Scholar] [CrossRef]
- Nielsen, H.P.; Frandsen, F.J.; Dam-Johansen, K.; Baxter, L.L. The implications of chlorine-associated corrosion on the operation of biomass-fired boilers. Prog. Energy Combust. Sci. 2000, 26, 283–298. [Google Scholar] [CrossRef]
- He, N.; Jia, J.; Qiu, Z.; Fang, C.; Liden, G.; Liu, X.; Bao, J. Cyclic L-lactide synthesis from lignocellulose biomass by biorefining with complete inhibitor removal and highly simultaneous sugars assimilation. Biotechnol. Bioeng. 2022, 119, 1903–1915. [Google Scholar] [CrossRef] [PubMed]
- Åkerberg, C.; Zacchi, G. An economic evaluation of the fermentative production of lactic acid from wheat flour. Bioresour. Technol. 2000, 75, 119–126. [Google Scholar] [CrossRef]
- González, M.I.; Álvarez, S.; Riera, F.; Álvarez, R. Economic evaluation of an integrated process for lactic acid production from ultrafiltered whey. J. Food Eng. 2007, 80, 553–561. [Google Scholar] [CrossRef]
- Humbird, D.; Davis, R.; Tao, L.; Kinchin, C.; Hsu, D.; Aden, A.; Schoen, P.; Lukas, J.; Olthof, B.; Worley, M.; et al. Process Design and Economics for Biochemical Conversion of Lignocellulosic Biomass to Ethanol: Dilute-Acid Pretreatment and Enzymatic Hydrolysis of Corn Stover; NREL/TP-5100–47764; National Renewable Energy Laboratory: Golden, CO, USA, 2011. [CrossRef]


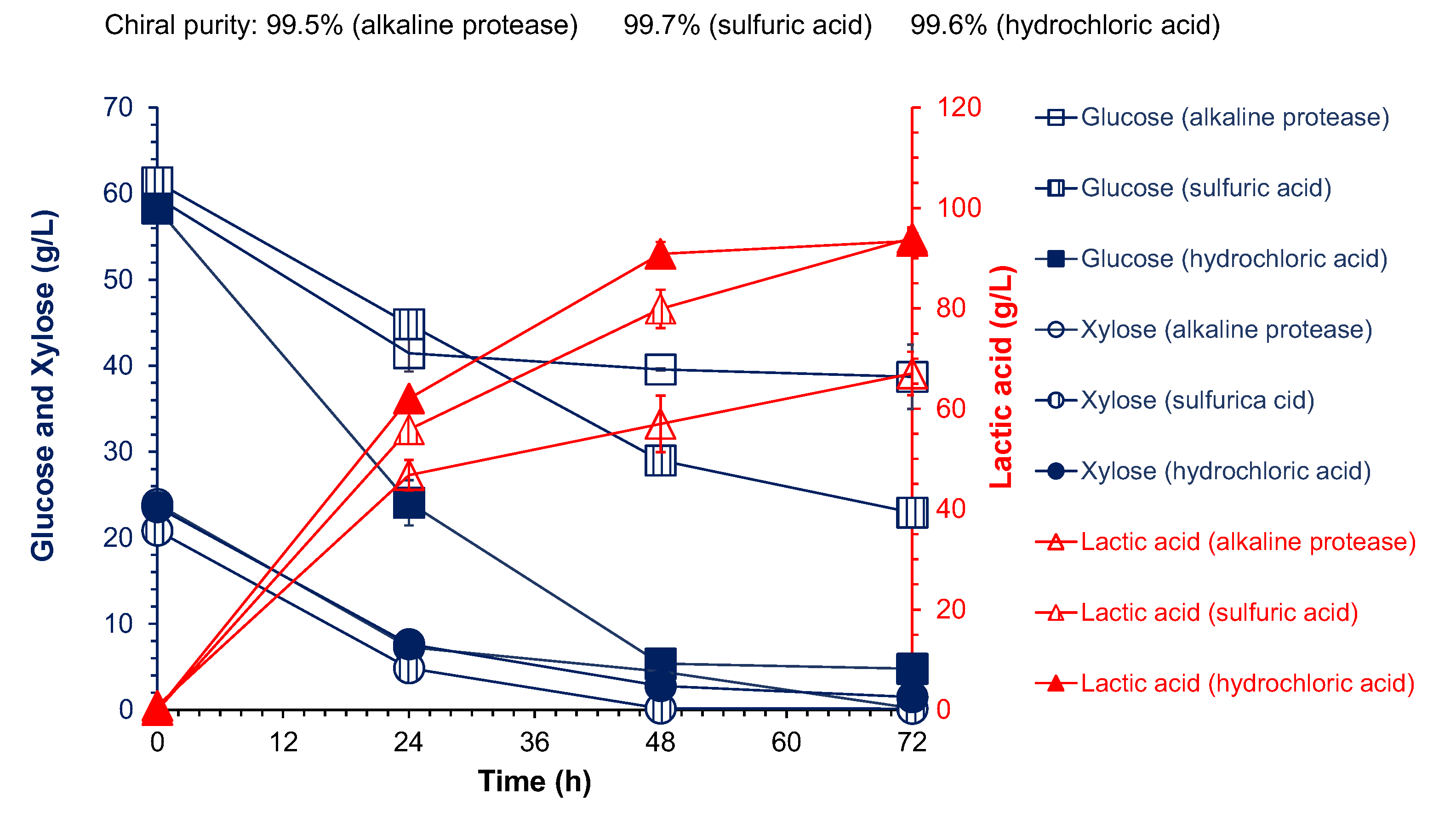
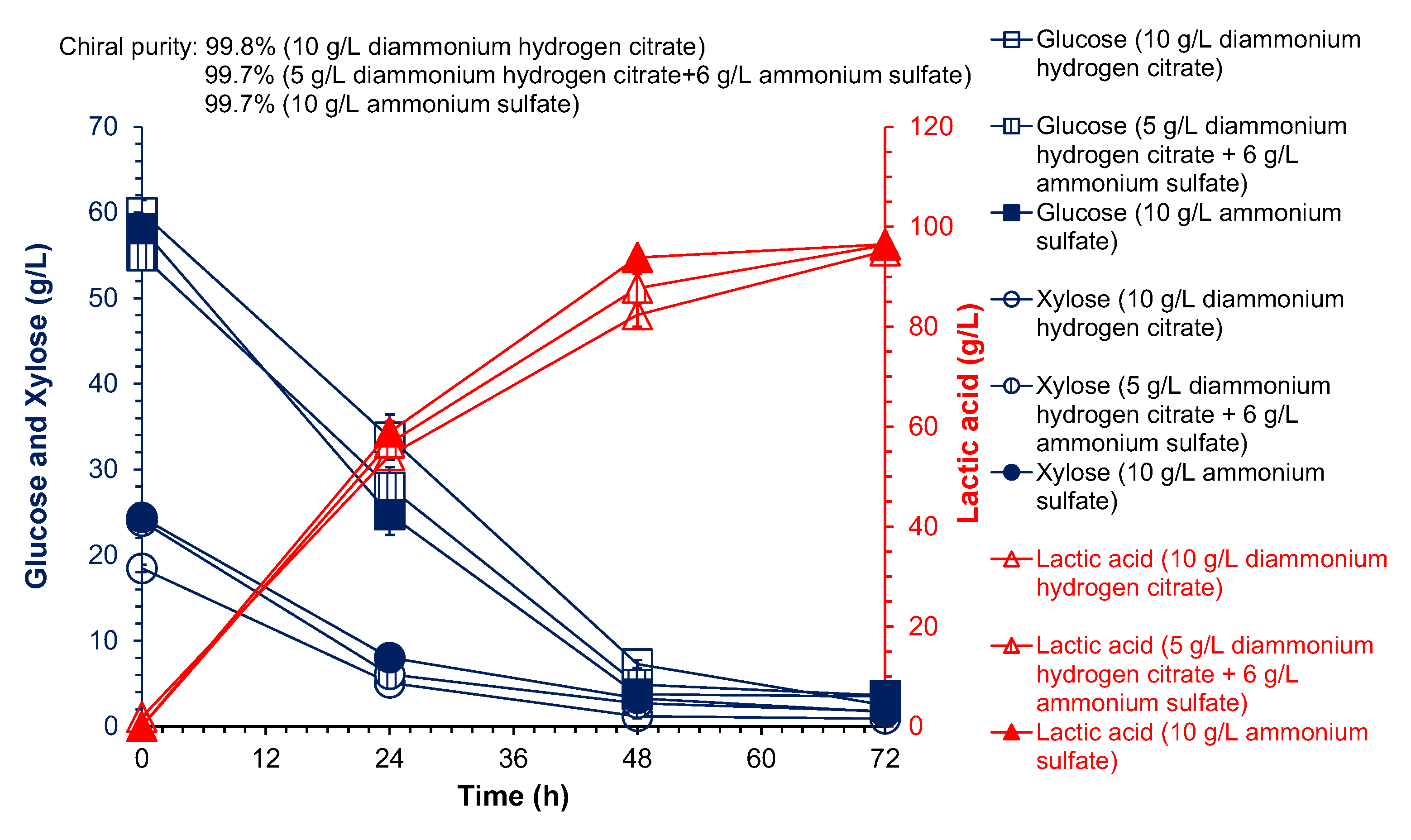
| Nitrogen Sources a | Dosage (g/L) | Lactic Acid Titer (g/L) | L-Lactic Acid Purity (%) | L-Lactic Acid Yield (g/g DM) b |
|---|---|---|---|---|
| YE + peptone, reagent grade | 15 for YE; 10 for peptone | 105.0 ± 0.5 | 99.5 ± 0.1 | 0.33 ± 0.01 |
| YE + peptone, industrial grade | 15 for YE; 10 for peptone | 102.0 ± 0.8 | 99.6 ± 0.1 | 0.32 ± 0.02 |
| DCSLP #1 | 20 | 87.2 ± 1.4 | 94.9 ± 0.1 | 0.26 ± 0.01 |
| DCSLP #2 | 20 | 85.4 ± 1.0 | 95.3 ± 0.1 | 0.25 ± 0.01 |
| Nitrogen a | Price b (USD/kg) | Protein (mg/g DM) | Lactic Acid (mg/g DM) | L-Lactic Acid (mg/g DM) | D-Lactic Acid (mg/g DM) |
|---|---|---|---|---|---|
| YE, reagent grade | 34.92 | 658.6 ± 1.6 | 3.3 ± 0.2 | 1.8 ± 0.1 | 1.5 ± 0.1 |
| YE, industrial grade | 17.46 | 769.3 ± 9.5 | 2.1 ± 0.1 | 0.9 ± 0.1 | 1.2 ± 0.2 |
| Peptone, reagent grade | 80.00 | 760.2 ± 14.3 | 2.0 ± 0.1 | 1.1 ± 0.1 | 0.9 ± 0.1 |
| Peptone, industrial grade | 11.11 | 728.4 ± 12.5 | ND c | ND c | ND c |
| DCSLP #1 | 1.43 | 451.2 ± 5.3 | 215.8 ± 3.5 | 85.6 ± 1.4 | 130.2 ± 2.1 |
| DCSLP #2 | 0.32 | 467.5 ± 6.1 | 196.3 ± 2.7 | 85.1 ± 0.4 | 111.2 ± 2.3 |
| Nitrogen | Price (USD/kg) | Protein (mg/g DM) | Lactic Acid (mg/g DM) | L-Lactic Acid (mg/g DM) | D-Lactic Acid (mg/g DM) |
|---|---|---|---|---|---|
| Soybean meal | 0.32 | 456.3 ± 9.5 | ND * | ND * | ND * |
| Cottonseed meal | 1.59 | 545.3 ± 11.3 | ND * | ND * | ND * |
| Case 1 [23] | Case 2 [This Study] | Case 3 [This Study] | |
|---|---|---|---|
| Strain | P. acidilactici TY112 | P. acidilactici ZY271 | P. acidilactici ZY271 |
| Xylose utilization | No | Yes | Yes |
| Raw feedstock | Corn stover | Wheat straw | Wheat straw |
| Pretreatment acid dosage | 5.0%, w/w (dry matter) | 4.1%, w/w (dry matter) | 4.1%, w/w (dry matter) |
| Fermentation solids loading | 30% (w/w) | 25% (w/w) | 25% (w/w) |
| Nitrogen sources | |||
| Complex nitrogen source a | 20 g/L DCSLP | 15 g/L YE + 10 g/L Peptone | 20 g/L cottonseed hydrolysate |
| Available nitrogen source | 2 g/L diammonium phosphate | 2 g/L diammonium hydrogen citrate | 10 g/L ammonium sulfate |
| SSCF period (h) | 72 | 72 | 72 |
| Titer (g/L) | 104.5 | 102.0 | 96.5 |
| Yield (g/g dry raw feedstock) | 0.27 | 0.33 | 0.31 |
| Productivity (g/L/h) | 1.45 | 1.46 | 1.32 |
| Chiral purity (%) | 95.3 | 99.5 | 99.7 |
| Material | USD Price (2022) |
|---|---|
| Feedstock (wheat straw) | 71.24/ton |
| Sulfuric acid, 98% | 125.06/ton |
| Lime | 99.69/ton |
| Diammonium hydrogen citrate | 3166.11/ton |
| Ammonium sulfate | 87.07/ton |
| Manganese sulfate | 443.26/ton |
| Yeast extract (YE) | 17,413.60/ton |
| Peptone | 11,081.38/ton |
| Cottonseed meal | 1266.44/ton |
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Feedstock handling rate | 300,000 metric tons/year | 300,000 metric tons/year | 300,000 metric tons/year |
| Total capital investment a | USD 186 million | USD 193 million | USD 195 million |
| Lactic acid yield | 269 kg/ton corn stover | 330 kg/ton wheat straw | 310 kg/ton wheat straw |
| (95.3% L-purity) | (99.6% L-purity) | (99.7% L-purity) | |
| Plant water usage | 6. 10 kg/kg lactic acid product | 7.29 kg/kg lactic acid product | 8.10 kg/kg lactic acid product |
| Minimum lactic acid selling price (USD/kg lactic acid product) | 0.584 | 3.241 | 0.813 |
| Feedstock | 0.237 | 0.276 | 0.286 |
| Enzyme b | 0.130 | 0.101 | 0.104 |
| Complex nitrogen | 0.009 | 2.624 | 0.193 |
| Available nitrogen | 0.016 | 0.045 | 0.007 |
| Sulfuric acid c | / | / | 0.005 |
| CaCO3 d | / | / | 0.004 |
| None-enzyme conversion | 0.192 | 0.195 | 0.214 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Wu, L.; Liu, X.; Bao, J. Plant Proteins as an Alternative Nitrogen Source for Chiral Purity L-Lactic Acid Fermentation from Lignocellulose Feedstock. Fermentation 2022, 8, 546. https://doi.org/10.3390/fermentation8100546
Zhang B, Wu L, Liu X, Bao J. Plant Proteins as an Alternative Nitrogen Source for Chiral Purity L-Lactic Acid Fermentation from Lignocellulose Feedstock. Fermentation. 2022; 8(10):546. https://doi.org/10.3390/fermentation8100546
Chicago/Turabian StyleZhang, Bin, Lei Wu, Xiucai Liu, and Jie Bao. 2022. "Plant Proteins as an Alternative Nitrogen Source for Chiral Purity L-Lactic Acid Fermentation from Lignocellulose Feedstock" Fermentation 8, no. 10: 546. https://doi.org/10.3390/fermentation8100546
APA StyleZhang, B., Wu, L., Liu, X., & Bao, J. (2022). Plant Proteins as an Alternative Nitrogen Source for Chiral Purity L-Lactic Acid Fermentation from Lignocellulose Feedstock. Fermentation, 8(10), 546. https://doi.org/10.3390/fermentation8100546







