Quality Traits of Sourdough Bread Obtained by Novel Digital Technologies and Machine Learning Modelling
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Description
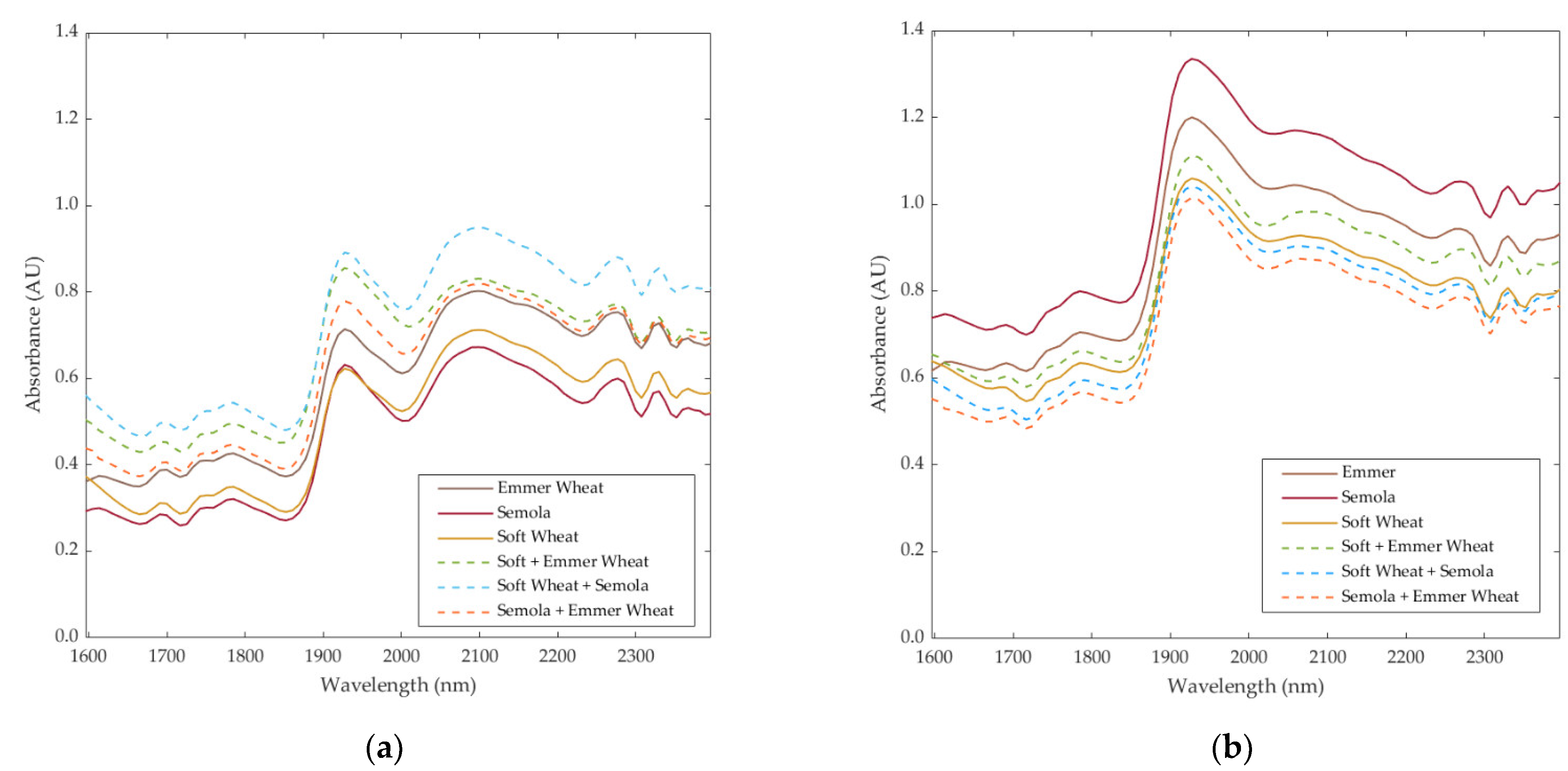
2.2. Near-Infrared Measurements
2.3. Gas Chromatography Mass-Spectroscopy
2.4. Electronic Nose Measurements
2.5. Physicochemical Measurements
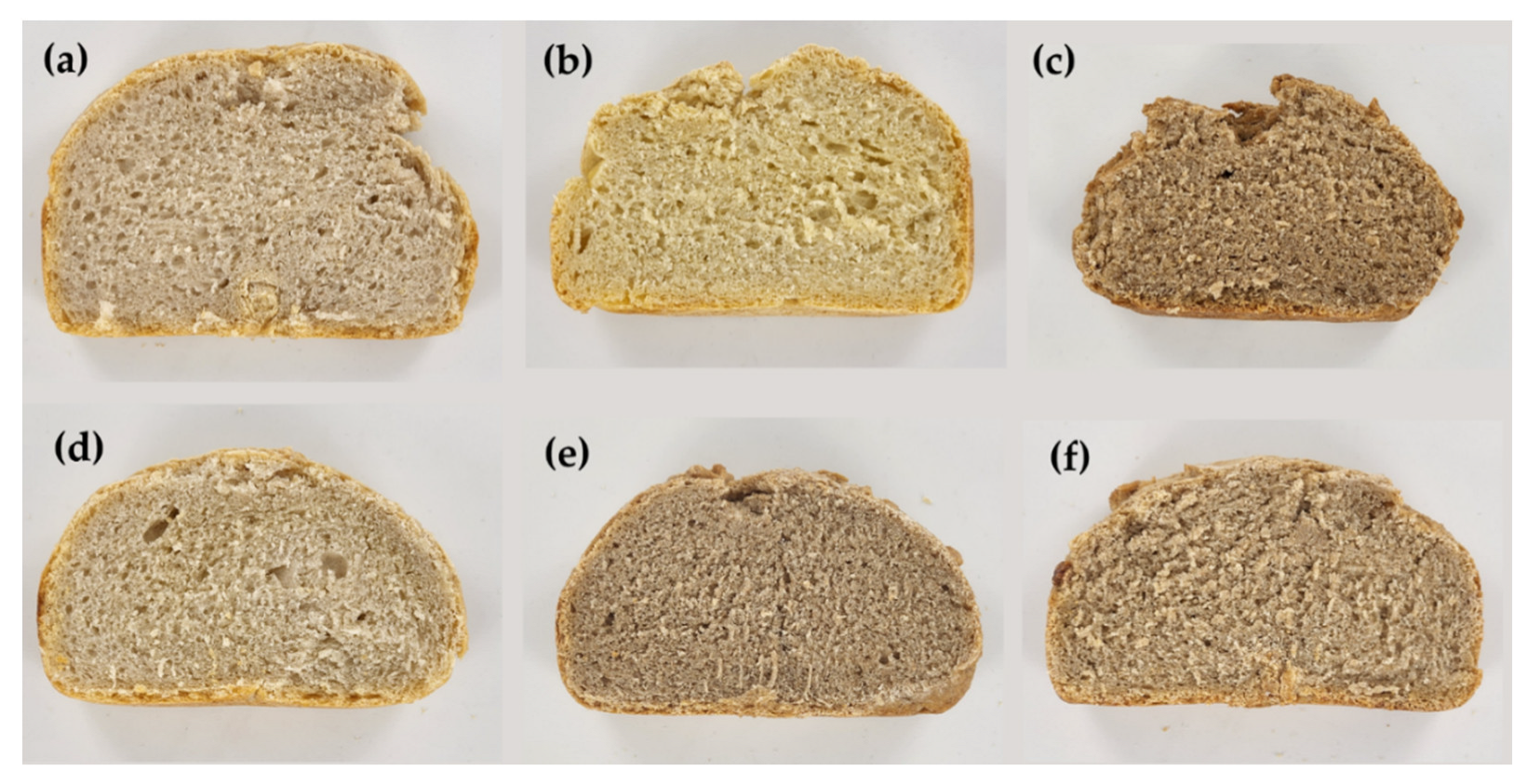
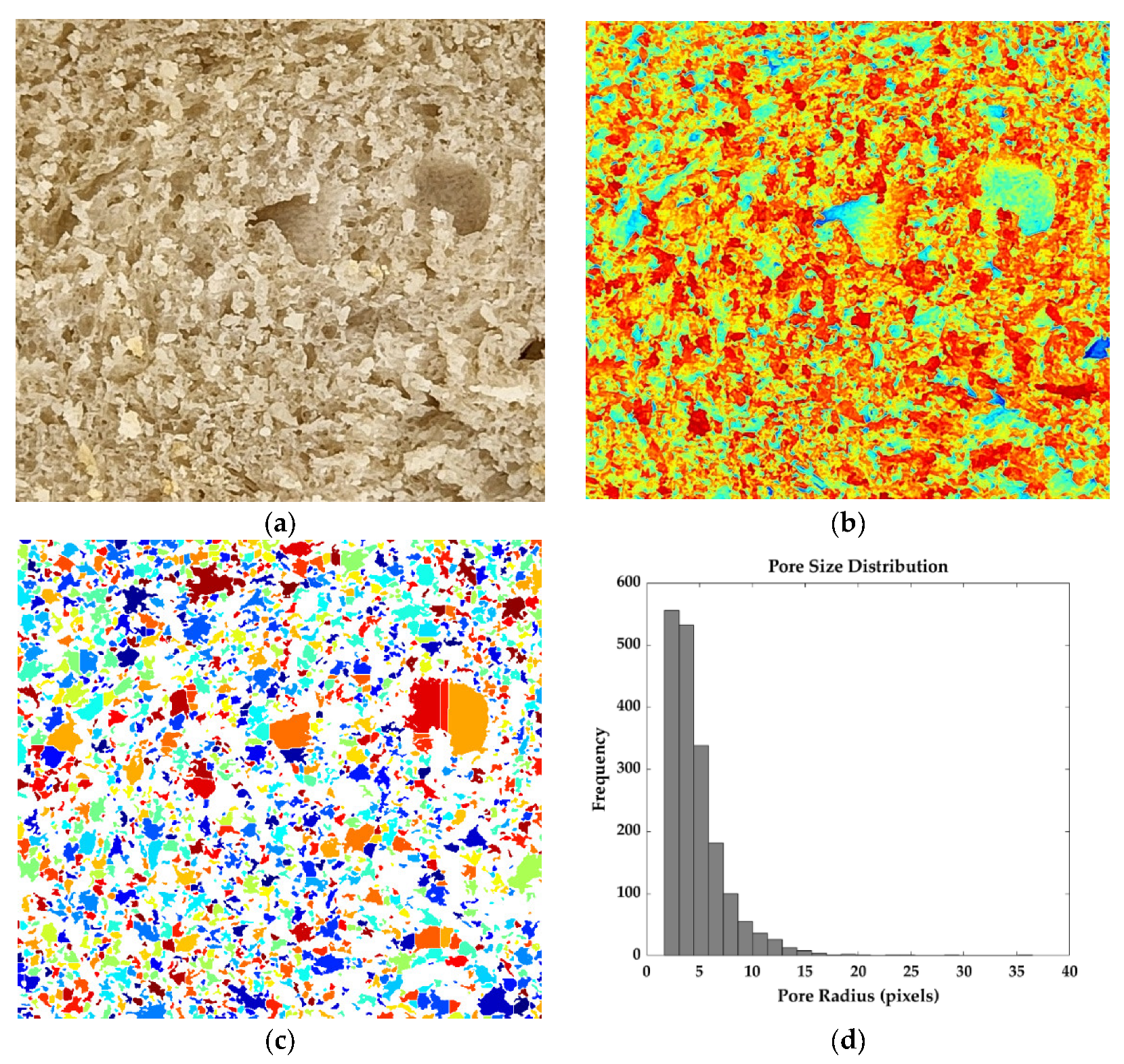
2.6. Computer Vision Analysis
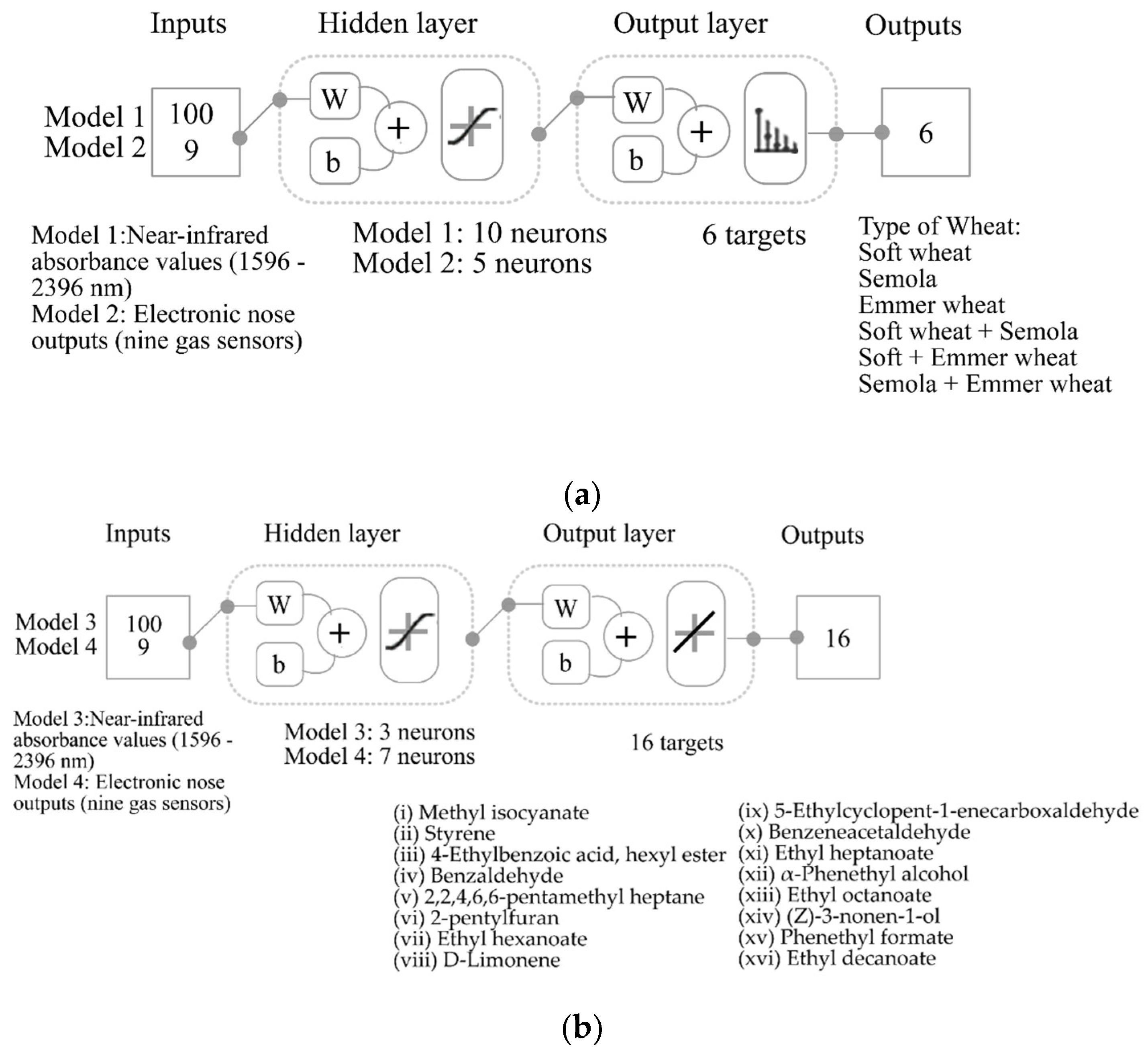
2.7. Statistical Analysis and Machine Learning Modelling
3. Results and Discussion
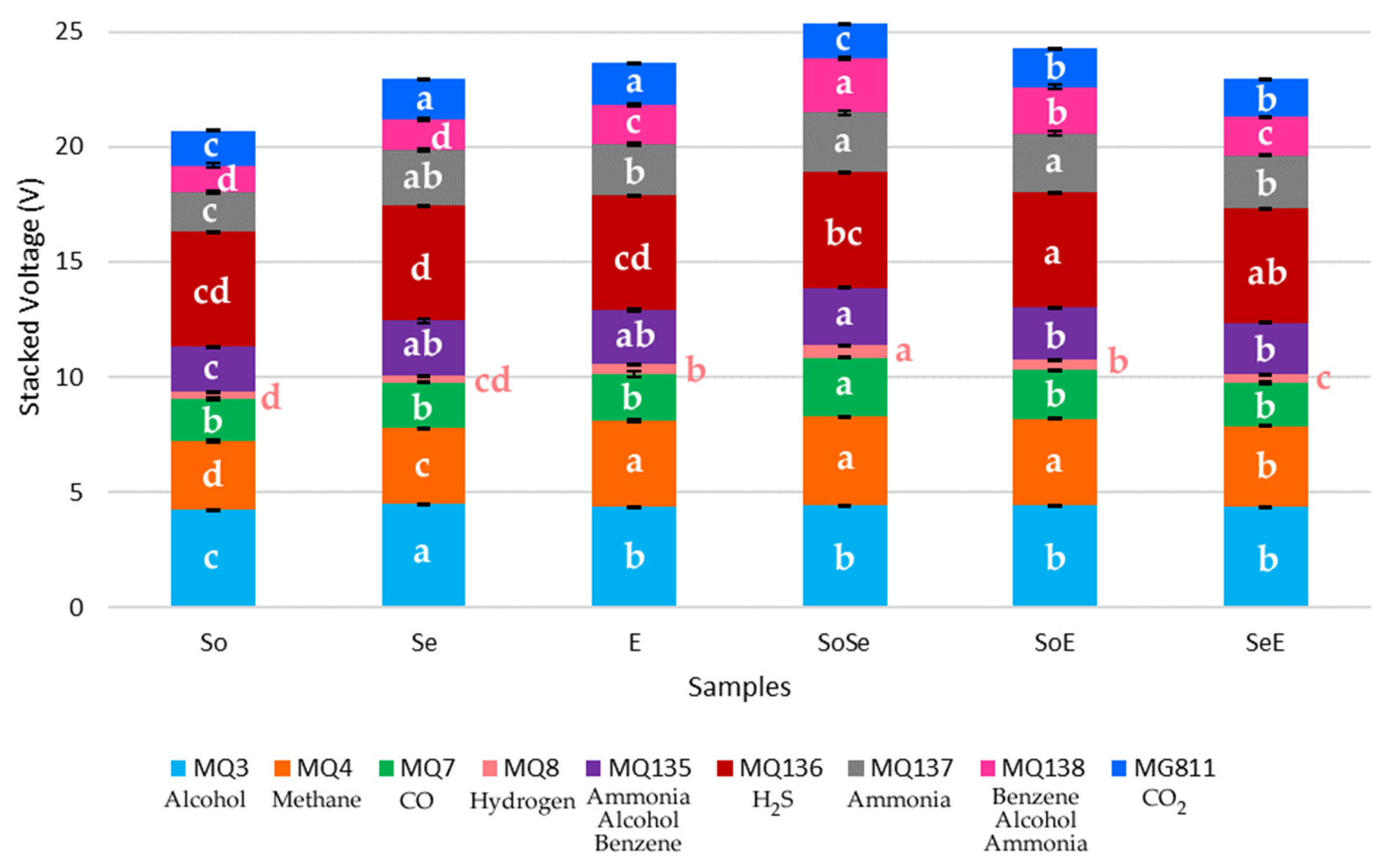
3.1. Analysis of Variance of Data from Digital Sensors Used (ANOVA)
3.2. Multivariate Data Analysis
3.3. Machine Learning Modelling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, S.; Wang, Z.; Guo, X.; Wang, F.; Huang, J.; Sun, B.; Wang, X. Sourdough improves the quality of whole-wheat flour products: Mechanisms and challenges—A review. Food Chem. 2021, 360, 130038. [Google Scholar] [CrossRef]
- Sofo, A.; Galluzzi, A.; Zito, F. A Modest Suggestion: Baking Using Sourdough-a Sustainable, Slow-Paced, Traditional and Beneficial Remedy against Stress during the Covid-19 Lockdown. Hum. Ecol. 2021, 49, 99–105. [Google Scholar] [CrossRef]
- Calvert, M.D.; Madden, A.A.; Nichols, L.M.; Haddad, N.M.; Lahne, J.; Dunn, R.R.; McKenney, E.A. A review of sourdough starters: Ecology, practices, and sensory quality with applications for baking and recommendations for future research. PeerJ 2021, 9, e11389. [Google Scholar] [CrossRef]
- Gänzle, M.G.; Zheng, J. Lifestyles of sourdough lactobacilli–Do they matter for microbial ecology and bread quality? Int. J. Food Microbiol. 2019, 302, 15–23. [Google Scholar] [CrossRef]
- Mezgebe, A.G.; Taylor, J.R.; de Kock, H.L. Influence of waxy (high amylopectin) and high protein digestibility traits in sorghum on injera sourdough-type flatbread sensory characteristics. Foods 2020, 9, 1749. [Google Scholar] [CrossRef]
- Pétel, C.; Onno, B.; Prost, C. Sourdough volatile compounds and their contribution to bread: A review. Trends Food Sci. Technol. 2017, 59, 105–123. [Google Scholar] [CrossRef]
- Hanis-Syazwani, M.; Bolarinwa, I.; Lasekan, O.; Muhammad, K. Influence of starter culture on the physicochemical properties of rice bran sourdough and physical quality of sourdough bread. Food Res. 2018, 2, 340–349. [Google Scholar] [CrossRef]
- Ogunsakin, O.; Banwo, K.; Ogunremi, O.; Sanni, A. Microbiological and physicochemical properties of sourdough bread from sorghum flour. Int. Food Res. J. 2015, 22, 2610–2618. [Google Scholar]
- Breads, M.S. Microbiological and Physicochemical Properties of Wholegrain. Int. J. 2019, 4, 074–082. [Google Scholar]
- Limbad, M.; Gutierrez Maddox, N.; Hamid, N.; Kantono, K. Sensory and physicochemical characterization of sourdough bread prepared with a coconut water kefir starter. Foods 2020, 9, 1165. [Google Scholar] [CrossRef]
- Lubis, Y.; Putri, C.; Rohaya, S. Physicochemical properties of sourdough bread made from local variety sweet potato and pineapple juice. IOP Conf. Ser. Earth Environ. Sci. 2020, 425, 012079. [Google Scholar]
- Olojede, A.; Sanni, A.; Banwo, K. Rheological, textural and nutritional properties of gluten-free sourdough made with functionally important lactic acid bacteria and yeast from Nigerian sorghum. LWT 2020, 120, 108875. [Google Scholar] [CrossRef]
- Torrieri, E.; Pepe, O.; Ventorino, V.; Masi, P.; Cavella, S. Effect of sourdough at different concentrations on quality and shelf life of bread. LWT-Food Sci. Technol. 2014, 56, 508–516. [Google Scholar] [CrossRef]
- Crowley, P.; Schober, T.J.; Clarke, C.I.; Arendt, E.K. The effect of storage time on textural and crumb grain characteristics of sourdough wheat bread. Eur. Food Res. Technol. 2002, 214, 489–496. [Google Scholar] [CrossRef]
- Fuentes, S.; Tongson, E.; Gonzalez Viejo, C. Novel digital technologies implemented in sensory science and consumer perception. Curr. Opin. Food Sci. 2021, 41, 99–106. [Google Scholar] [CrossRef]
- Fuentes, S.; Gonzalez Viejo, C.; Torrico, D.; Dunshea, F. Development of a biosensory computer application to assess physiological and emotional responses from sensory panelists. Sensors 2018, 18, 2958. [Google Scholar] [CrossRef]
- Plemmons, L.; Resurreccion, A. Note on computerized data collection in consumer sensory evaluation. J. Sens. Stud. 1999, 14, 19–34. [Google Scholar] [CrossRef]
- Gonzalez Viejo, C.; Fuentes, S.; Hernandez-Brenes, C. Smart Detection of Faults in Beers Using Near-Infrared Spectroscopy, a Low-Cost Electronic Nose and Artificial Intelligence. Fermentation 2021, 7, 117. [Google Scholar] [CrossRef]
- Gonzalez Viejo, C.; Fuentes, S. Beer Aroma and Quality Traits Assessment Using Artificial Intelligence. Fermentation 2020, 6, 56. [Google Scholar] [CrossRef]
- Gonzalez Viejo, C.; Caboche, C.H.; Kerr, E.D.; Pegg, C.L.; Schulz, B.L.; Howell, K.; Fuentes, S. Development of a Rapid Method to Assess Beer Foamability Based on Relative Protein Content Using RoboBEER and Machine Learning Modeling. Beverages 2020, 6, 28. [Google Scholar] [CrossRef]
- Gonzalez Viejo, C.; Fuentes, S. Low-Cost Methods to Assess Beer Quality Using Artificial Intelligence Involving Robotics, an Electronic Nose, and Machine Learning. Fermentation 2020, 6, 104. [Google Scholar] [CrossRef]
- Gonzalez Viejo, C.; Fuentes, S.; Torrico, D.; Howell, K.; Dunshea, F. Assessment of Beer Quality Based on a Robotic Pourer, Computer Vision, and Machine Learning Algorithms Using Commercial Beers. J. Food Sci. 2018, 83, 1381–1388. [Google Scholar] [CrossRef]
- Gonzalez Viejo, C.; Fuentes, S.; Li, G.; Collmann, R.; Condé, B.; Torrico, D. Development of a robotic pourer constructed with ubiquitous materials, open hardware and sensors to assess beer foam quality using computer vision and pattern recognition algorithms: RoboBEER. Food Res. Int. 2016, 89, 504–513. [Google Scholar] [CrossRef]
- Gonzalez Viejo, C.; Tongson, E.; Fuentes, S. Integrating a Low-Cost Electronic Nose and Machine Learning Modelling to Assess Coffee Aroma Profile and Intensity. Sensors 2021, 21, 2016. [Google Scholar] [CrossRef]
- Summerson, V.; Gonzalez Viejo, C.; Torrico, D.D.; Pang, A.; Fuentes, S. Digital Smoke Taint Detection in Pinot Grigio Wines Using an E-Nose and Machine Learning Algorithms Following Treatment with Activated Carbon and a Cleaving Enzyme. Fermentation 2021, 7, 119. [Google Scholar] [CrossRef]
- Summerson, V.; Gonzalez Viejo, C.; Pang, A.; Torrico, D.D.; Fuentes, S. Assessment of Volatile Aromatic Compounds in Smoke Tainted Cabernet Sauvignon Wines Using a Low-Cost E-Nose and Machine Learning Modelling. Molecules 2021, 26, 5108. [Google Scholar] [CrossRef]
- Winters, M.; Panayotides, D.; Bayrak, M.; Rémont, G.; Viejo, C.G.; Liu, D.; Le, B.; Liu, Y.; Luo, J.; Zhang, P. Defined co-cultures of yeast and bacteria modify the aroma, crumb and sensory properties of bread. J. Appl. Microbiol. 2019, 127, 778–793. [Google Scholar] [CrossRef]
- Gonzalez Viejo, C.; Torrico, D.; Dunshea, F.; Fuentes, S. Emerging Technologies Based on Artificial Intelligence to Assess the Quality and Consumer Preference of Beverages. Beverages 2019, 5, 62. [Google Scholar] [CrossRef]
- Gonzalez Viejo, C.; Fuentes, S.; Torrico, D.; Godbole, A.; Dunshea, F. Chemical characterization of aromas in beer and their effect on consumers liking. Food Chem. 2019, 293, 479–485. [Google Scholar] [CrossRef]
- Gonzalez Viejo, C.; Fuentes, S.; Godbole, A.; Widdicombe, B.; Unnithan, R.R. Development of a low-cost e-nose to assess aroma profiles: An artificial intelligence application to assess beer quality. Sens. Actuators B Chem. 2020, 308, 127688. [Google Scholar] [CrossRef]
- Park, Y.-H.; Jung, L.-H.; Jeon, E.-R. Quality characteristics of bread using sour dough. Prev. Nutr. Food Sci. 2006, 11, 323–327. [Google Scholar] [CrossRef]
- Rabbani, A.; Salehi, S. Dynamic modeling of the formation damage and mud cake deposition using filtration theories coupled with SEM image processing. J. Nat. Gas Sci. Eng. 2017, 42, 157–168. [Google Scholar] [CrossRef]
- Gonzalez Viejo, C.; Torrico, D.; Dunshea, F.; Fuentes, S. Development of Artificial Neural Network Models to Assess Beer Acceptability Based on Sensory Properties Using a Robotic Pourer: A Comparative Model Approach to Achieve an Artificial Intelligence System. Beverages 2019, 5, 33. [Google Scholar] [CrossRef]
- Ciurczak, E.W.; Igne, B.; Workman, J., Jr.; Burns, D.A. Handbook of Near-Infrared Analysis; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Burns, D.A.; Ciurczak, E.W. Handbook of Near-Infrared Analysis; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Ringsted, T.; Siesler, H.W.; Engelsen, S.B. Monitoring the staling of wheat bread using 2D MIR-NIR correlation spectroscopy. J. Cereal Sci. 2017, 75, 92–99. [Google Scholar] [CrossRef]
- Wilson, R.; Goodfellow, B.; Belton, P.; Osborne, B.; Oliver, G.; Russell, P. Comparison of Fourier transform mid infrared spectroscopy and near infrared reflectance spectroscopy with differential scanning calorimetry for the study of the staling of bread. J. Sci. Food Agric. 1991, 54, 471–483. [Google Scholar] [CrossRef]
- Cevoli, C.; Gianotti, A.; Troncoso, R.; Fabbri, A. Quality evaluation by physical tests of a traditional Italian flat bread Piadina during storage and shelf-life improvement with sourdough and enzymes. Eur. Food Res. Technol. 2015, 240, 1081–1089. [Google Scholar] [CrossRef]
- Gupta, M.K.; Viejo, C.G.; Fuentes, S.; Torrico, D.D.; Saturno, P.C.; Gras, S.L.; Dunshea, F.R.; Cottrell, J.J. Digital technologies to assess yoghurt quality traits and consumers acceptability. J. Sci. Food Agric. 2022, 102, 5642–5652. [Google Scholar] [CrossRef]
- Gonzalez Viejo, C.; Fuentes, S.; Torrico, D.; Howell, K.; Dunshea, F. Assessment of beer quality based on foamability and chemical composition using computer vision algorithms, near infrared spectroscopy and machine learning algorithms. J. Sci. Food Agric. 2018, 98, 618–627. [Google Scholar] [CrossRef]
- Gonzalez Viejo, C.; Fuentes, S. A Digital Approach to Model Quality and Sensory Traits of Beers Fermented under Sonication Based on Chemical Fingerprinting. Fermentation 2020, 6, 73. [Google Scholar] [CrossRef]
- Xie, F.; Dowell, F.E.; Sun, X.S. Comparison of near-infrared reflectance spectroscopy and texture analyzer for measuring wheat bread changes in storage. Cereal Chem. 2003, 80, 25–29. [Google Scholar] [CrossRef]
- Amann, L.S.; Frank, O.; Dawid, C.; Hofmann, T.F. The Sensory-Directed Elucidation of the Key Tastants and Odorants in Sourdough Bread Crumb. Foods 2022, 11, 2325. [Google Scholar] [CrossRef]
- Birch, A.N.; Petersen, M.A.; Arneborg, N.; Hansen, Å.S. Influence of commercial baker’s yeasts on bread aroma profiles. Food Res. Int. 2013, 52, 160–166. [Google Scholar] [CrossRef]
- The Good Scents Company. The Good Scents Company Information System. Available online: http://www.thegoodscentscompany.com/data/rw1038291.html (accessed on 3 September 2022).
- Li, Z.; Wang, J. Identification and similarity analysis of aroma substances in main types of Fenghuang Dancong tea. PLoS ONE 2020, 15, e0244224. [Google Scholar] [CrossRef] [PubMed]
- Ugliano, M.; Kolouchova, R.; Henschke, P.A. Occurrence of hydrogen sulfide in wine and in fermentation: Influence of yeast strain and supplementation of yeast available nitrogen. J. Ind. Microbiol. Biotechnol. 2011, 38, 423–429. [Google Scholar] [CrossRef]
- Farahmand, E.; Razavi, S.H.; Mohtasebi, S.S. Investigating effective variables to produce desirable aroma in sourdough using e-nose and sensory panel. J. Food Process. Preserv. 2021, 45, e15157. [Google Scholar] [CrossRef]
- Ghasemi-Varnamkhasti, M.; Lozano, J. Electronic nose as an innovative measurement system for the quality assurance and control of bakery products: A review. Eng. Agric. Environ. Food 2016, 9, 365–374. [Google Scholar] [CrossRef]
- Yan, B.; Sadiq, F.A.; Cai, Y.; Fan, D.; Zhang, H.; Zhao, J.; Chen, W. Identification of key aroma compounds in type I sourdough-based Chinese steamed bread: Application of untargeted metabolomics analysisp. Int. J. Mol. Sci. 2019, 20, 818. [Google Scholar] [CrossRef] [PubMed]
- Ventimiglia, G.; Alfonzo, A.; Galluzzo, P.; Corona, O.; Francesca, N.; Caracappa, S.; Moschetti, G.; Settanni, L. Codominance of Lactobacillus plantarum and obligate heterofermentative lactic acid bacteria during sourdough fermentation. Food Microbiol. 2015, 51, 57–68. [Google Scholar] [CrossRef]
- Pepe, O.; Ventorino, V.; Cavella, S.; Fagnano, M.; Brugno, R. Prebiotic content of bread prepared with flour from immature wheat grain and selected dextran-producing lactic acid bacteria. Appl. Environ. Microbiol. 2013, 79, 3779–3785. [Google Scholar] [CrossRef]
- Callejo, M.J. Present situation on the descriptive sensory analysis of bread. J. Sens. Stud. 2011, 26, 255–268. [Google Scholar] [CrossRef]
- Șerban, L.R.; Păucean, A.; Man, S.M.; Chiş, M.S.; Mureşan, V. Ancient Wheat Species: Biochemical Profile and Impact on Sourdough Bread Characteristics—A Review. Processes 2021, 9, 2008. [Google Scholar] [CrossRef]
- Deep, K.; Jain, M.; Salhi, S. Logistics, Supply Chain and Financial Predictive Analytics: Theory and Practices; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Hair, J.F.; Anderson, R.E.; Tatham, R.L.; Black, W.C. Multivariate Data Analysis New Jersey; Pearson Education: London, UK, 2010. [Google Scholar]
- Gonzalez Viejo, C.; Fuentes, S. Digital Assessment and Classification of Wine Faults Using a Low-Cost Electronic Nose, Near-Infrared Spectroscopy and Machine Learning Modelling. Sensors 2022, 22, 2303. [Google Scholar] [CrossRef]


| Ingredient | Amount |
|---|---|
| Starter | |
| Flour | 80 g |
| Salt | 2.5 g |
| Culture | 0.1 g |
| Water | 80 mL |
| Dough | |
| Sourdough Starter | 1 cup |
| Flour | 400 g |
| Salt | 5 g |
| Water | 150 mL |
| Label | Common Name | Aroma * |
|---|---|---|
| VAC1 | Methyl isocyanate | Pungent |
| VAC2 | Styrene | Balsamic/Floral/Sweet |
| VAC3 | 4-Ethylbenzoic acid, hexyl ester | NR |
| VAC4 | Benzaldehyde | Almond/Cherry |
| VAC5 | 2,2,4,6,6-pentamethyl heptane | Irritating odor (found in tea) ** |
| VAC6 | 2-pentylfuran | Fruity/Green/Earthy/Beany |
| VAC7 | Ethyl hexanoate | Fruity/Pineapple/Waxy/Green/Banana |
| VAC8 | D-Limonene | Citrus/Orange/Fresh |
| VAC9 | 5-Ethylcyclopent-1-enecarboxaldehyde | NR |
| VAC10 | Benzeneacetaldehyde | Green/Sweet/Floral/Clover/Honey/Cocoa |
| VAC11 | Ethyl heptanoate | Fruity/Pineapple/Cognac/Rum/Wine |
| VAC12 | α-Phenethyl alcohol | Sweet/Gardenia/Medicinal |
| VAC13 | Ethyl octanoate | Fruity/Winey/Mushroom/Banana |
| VAC14 | (Z)-3-nonen-1-ol | Fresh/Waxy/Green/Melon/Mushroom |
| VAC15 | Phenethyl formate | Rose/Green/Herbal |
| VAC16 | Ethyl decanoate | Waxy/Apple/Grape/Brandy |
| Sample | Weight | Height (mm) | Length (mm) | Width (mm) | Texture Crust NS | Texture of the Crumb NS | pH | Salt | L | a | b NS | R | G | B | Colour |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soft wheat | 604.05 | 90 | 175 | 129 | 44.38 | 49.83 | 4.30 d | 0.11 b | 66.13 a | 3.05 b | 18.04 | 175.67 ab | 158.50 a | 128.33 a | |
| ±0.00 | ±0.00 | ±0.00 | ±0.00 | ±7.78 | ±1.44 | ±0.01 | ±0.00 | ±2.91 | ±0.40 | ±1.77 | ±9.21 | ±7.42 | ±4.45 | ||
| Semola | 597.15 | 80 | 175 | 128 | 31.43 | 45.78 | 4.55 c | 0.13 a | 67.77 a | 2.89 b | 26.83 | 182.83 a | 162.50 a | 116.50 ab | |
| ±0.00 | ±0.00 | ±0.00 | ±0.00 | ±2.98 | ±6.01 | ±0.02 | ±<0.01 | ±2.17 | ±1.10 | ±2.02 | ±7.35 | ±5.61 | ±4.56 | ||
| Emmer wheat | 615.00 | 70 | 175 | 116 | 49.1 | 49.1 | 4.98 a | 0.13 a | 50.19 b | 9.17 a | 23.06 | 143.33 c | 113.33 b | 80.50 c | |
| ±0.00 | ±0.00 | ±0.00 | ±0.00 | ±9.08 | ±9.08 | ±0.00 | ±<0.01 | ±1.32 | ±1.61 | ±2.06 | ±1.69 | ±4.29 | ±6.35 | ||
| Soft wheat + Semola | 614.50 | 90 | 172 | 125 | 41.87 | 41.81 | 4.67 bc | 0.12 ab | 67.05 a | 3.67 b | 22.42 | 180.33 ab | 160.50 a | 122.67 a | |
| ±0.00 | ±0.00 | ±0.00 | ±0.00 | ±5.85 | ±5.88 | ±0.03 | ±<0.01 | ±1.86 | ±1.24 | ±3.59 | ±6.77 | ±4.85 | ±6.58 | ||
| Soft + Emmer wheat | 615.70 | 71 | 171 | 126 | 40.05 | 55.17 | 4.77 b | 0.13 a | 54.66 b | 7.53 ab | 22.14 | 153.00 bc | 125.50 b | 92.50 c | |
| ±0.00 | ±0.00 | ±0.00 | ±0.00 | ±4.11 | ±5 | ±0.03 | ±<0.01 | ±1.29 | ±1.39 | ±2.76 | ±5.37 | ±3.11 | ±4.45 | ||
| Semola + Emmer wheat | 557.55 | 71 | 173 | 130 | 35.68 | 47.05 | 4.63 bc | 0.11 b | 57.47 b | 7.14 ab | 23.4 | 160.33 abc | 133.00 b | 97.33 bc | |
| ±0.00 | ±0.00 | ±0.00 | ±0.00 | ±2.97 | ±3.01 | ±0.07 | ±0.01 | ±2.02 | ±1.29 | ±2.19 | ±6.30 | ±5.32 | ±5.40 |
| Stage | Samples | Accuracy | Error | Performance (MSE) |
|---|---|---|---|---|
| Model 1: Inputs: near-infrared; Targets: type of wheat | ||||
| Training | 76 | 100% | 0.0% | <0.01 |
| Validation | 16 | 87.5% | 12.5% | 0.04 |
| Testing | 16 | 87.5% | 12.5% | 0.03 |
| Overall | 108 | 96.3% | 3.7% | - |
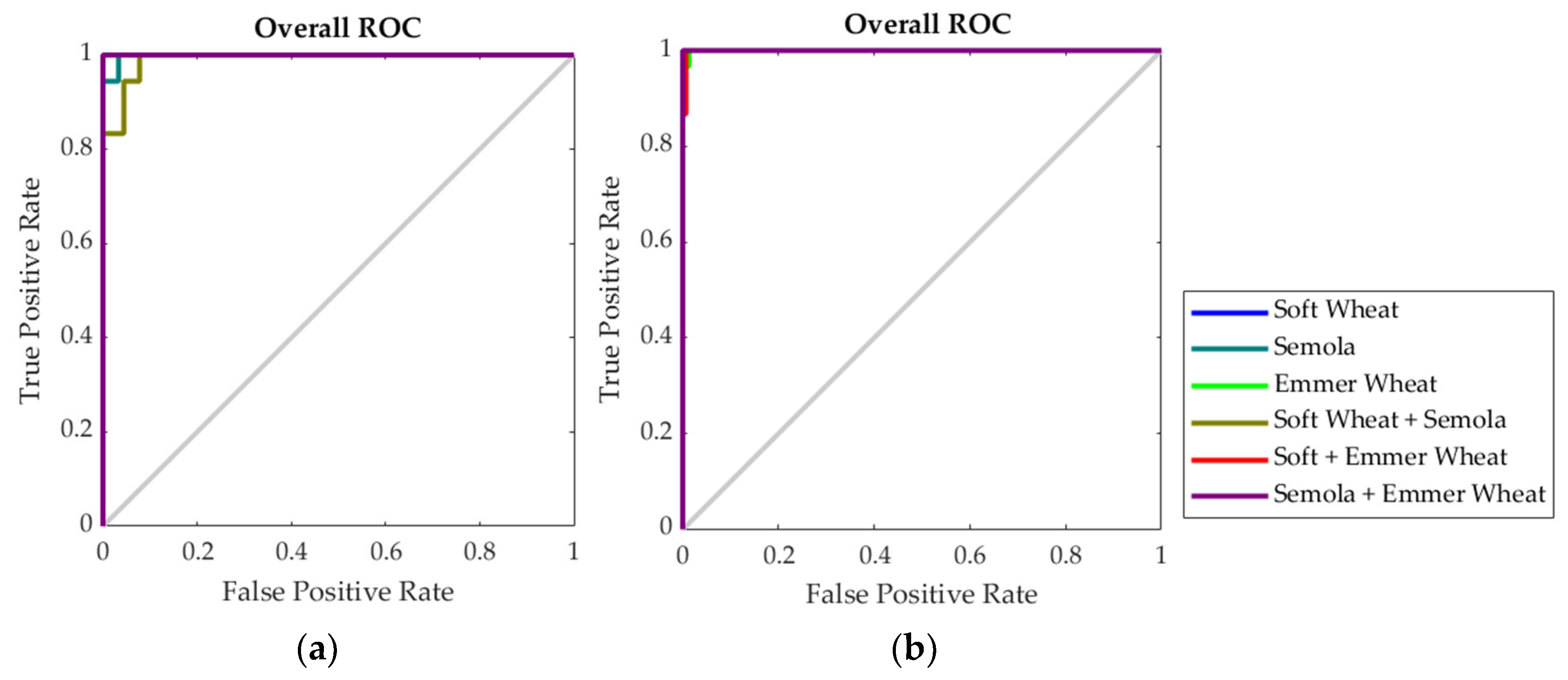
| Model 2: Inputs: electronic nose; Targets: type of wheat | ||||
| Training | 126 | 100% | 0.0% | <0.01 |
| Testing | 54 | 98.1% | 1.9% | 0.01 |
| Overall | 180 | 99.4% | 0.6% | - |
| Stage | Samples | Observations | R | R2 | Slope | Performance (MSE) |
|---|---|---|---|---|---|---|
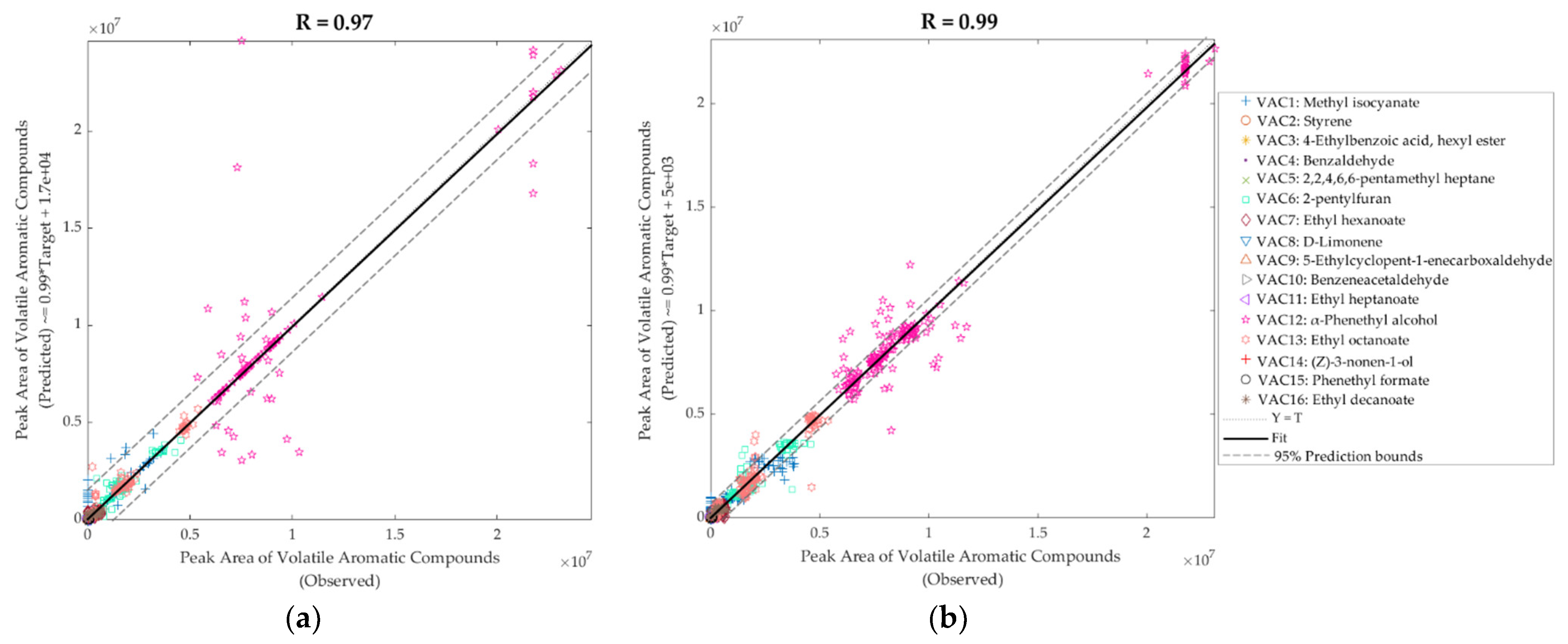
| Model 3: Inputs: near-infrared; Targets: volatile aromatic compounds | ||||||
| Training | 76 | 1216 | 1.00 | 1.00 | 1.00 | 0.66 × 1010 |
| Testing | 32 | 512 | 0.90 | 0.82 | 0.97 | 157.37 × 1010 |
| Overall | 108 | 1728 | 0.97 | 0.94 | 0.99 | - |
| Model 4: Inputs: electronic nose; Targets: volatile aromatic compounds | ||||||
| Training | 126 | 2016 | 1.00 | 1.00 | 1.00 | 3.21 × 1010 |
| Testing | 54 | 1024 | 0.99 | 0.97 | 0.98 | 18.64 × 1010 |
| Overall | 180 | 2880 | 0.99 | 0.99 | 0.99 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez Viejo, C.; Harris, N.M.; Fuentes, S. Quality Traits of Sourdough Bread Obtained by Novel Digital Technologies and Machine Learning Modelling. Fermentation 2022, 8, 516. https://doi.org/10.3390/fermentation8100516
Gonzalez Viejo C, Harris NM, Fuentes S. Quality Traits of Sourdough Bread Obtained by Novel Digital Technologies and Machine Learning Modelling. Fermentation. 2022; 8(10):516. https://doi.org/10.3390/fermentation8100516
Chicago/Turabian StyleGonzalez Viejo, Claudia, Natalie M. Harris, and Sigfredo Fuentes. 2022. "Quality Traits of Sourdough Bread Obtained by Novel Digital Technologies and Machine Learning Modelling" Fermentation 8, no. 10: 516. https://doi.org/10.3390/fermentation8100516
APA StyleGonzalez Viejo, C., Harris, N. M., & Fuentes, S. (2022). Quality Traits of Sourdough Bread Obtained by Novel Digital Technologies and Machine Learning Modelling. Fermentation, 8(10), 516. https://doi.org/10.3390/fermentation8100516








