Abstract
Research on biosurfactants (BS) is consistently increasing in the past years, driven from their beneficial attributes over chemical surfactants. Lactobacilli (LAB) and yeast BS producers could prevail over the pathogenic strains, owing to their GRAS status, to broaden end-applications toward the food and pharmaceutical industries. However, the increased cost of production confers a bottleneck for the industrial manufacture. Hence, the exploitation of agro-industrial waste and by-products as fermentation supplements is currently on the spotlight. This study elaborates on the efficient production of lactobacilli and Moesziomyces BS via cheese whey (CW) bioprocessing. Subsequently, the obtained BS were individually assessed in fermented milk production using as starter culture the initial LAB strain used for BS synthesis. Physicochemical and microbiological assessment was performed during storage. Results indicated that LAB-BS addition increased the lactobacilli concentration, whereby mannosylerythritol lipids (MEL) inclusion showed a positive effect on syneresis. Aiming to develop a closed-loop process, for the first time, LAB cells after BS extraction were sequentially applied for sour milk production, demonstrating the ability for cell re-utilization. This study introduces a holistic and circular configuration that consolidates CW valorization for BS production, that are re-introduced in the food supply, to complement the resilience of the dairy industry.
1. Introduction
Bio-based surfactants or green surfactants can be obtained from the bioprocessing of renewable resources (via chemical or enzymatic routes) [1,2] or can be microbially produced through the fermentative bioconversion of yeast, bacteria, and fungi. The latter are commonly referred to as biosurfactants and are reported to be either produced as primary metabolites, in the early exponential phase, [3] or at the stationary phase as secondary products [4,5]. Biosurfactants are an heterogenous group of amphipathic compounds, eliciting diversified chemical configurations, depending on the microbial strain and the nutrient supplements used during fermentation [6]. Biosynthesis of microbial surfactants can occur on the cell membrane, e.g., for most lactobacilli strains or they can occur extracellularly (i.e., in the culture medium) by most yeast strains.
Unequivocally, several studies have evidenced the advantages of biosurfactants over the chemical surfactants with respect to biodegradability, lower toxicity, and the environmentally benign character. Biosurfactants have the ability to reduce the surface and interfacial tension, along with emulsifying and foaming ability, antimicrobial, and antioxidant properties. Still, biosurfactants are not yet economically competitive for large-scale production owing to the high cost of manufacture, associated primarily with the cost of raw materials and downstream processing. Thus, scientific research elaborates on manifold proposals for cost-efficient and sustainable biosurfactant production, taking into account that worldwide biosurfactant production is expected to reach USD 2.8 billion by 2026, and approximately 1.5 million metric tons of biosurfactants are generated in the EU [1]. Rhamnolipids, sophorolipids, and mannosylerythritol lipids (MEL) occupy the largest share of global biosurfactants market [7], whereas an increase of 5.6–8% for rhamnolipids and sophorolipids has been also predicted [8].
Apparently, the utilization of agro-industrial waste and food by-product streams as renewable resources for onset fermentation feedstock, exhibits the leading approach to reduce the cost of biosurfactants [9,10]. Equally, the end use of biosurfactants has attained emerging significance, since the initially suggested applications targeted enhanced oil recovery and bioremediation, whereas novel formulations are sought to endeavor cosmetics, pharmaceutical, and food industries [11,12]. Worth noting, the final application is associated with downstream and purification of the crude biosurfactant, that consequently will affect the production cost. Likewise, the majority of studies in the open literature reporting high production yields employed pathogenic strains, e.g., Pseudomonas sp., Bacillus sp. etc. Thereby, it is crucial to also implement non-pathogenic strains such as Lactobacillus, with specific focus on novel isolates from food sources [13]. In the context of designing cascade bioprocessing strategies to foster the pillars of bio-economy, the obtained biosurfactant will be produced from novel strains, using low-cost substrates and it will be further incorporated in the food supply chain.
Several investigations have undertaken the isolation of lactic acid bacteria (LAB) from fermented dairy or non-dairy sources in an attempt to elucidate the indigenous microflora, but also to identify potential probiotics or even novel strains to be used as starter cultures. On top of that, these newly isolated Lactobacillus strains could be evaluated for biosurfactants production [13,14,15].
Fermented (or sour or acidophilus) milk confers a quite versatile dairy product, enabling straightforward modifications (e.g., during formulation) and has been evaluated in several biotechnological applications (e.g., inclusion of probiotics in diversified forms) [16,17,18,19]. The development of functional food products is currently on the spotlight, induced by the consumers’ demand for diet modification via healthier food choices and green-labeled products. For instance, in accordance with the recommendations of health authorities to reduce saturated fat consumption, consumers are seeking for reduced fat (or low fat) dairy products, including beverages. To comply with these requirements, new product development targets the manufacture of reduced fat food products, i.e., yogurt and fermented milk. Fat reduction in yogurts and fermented milk is often accompanied with the addition of thickeners in the form of hydrocolloids (e.g., pectin, inulin or carboxymethyl cellulose) to modify and sustain an accepted texture and physicochemical properties of the end-product [20,21]. On the other hand, biosurfactants in food formulations are usually employed as thickeners and/or stabilizers because of their ability to form emulsions and enhance immiscible compounds to disperse in a mixture [22]. Glycolipids, rhamnolipids, and lipopeptides have been previously applied in baked food products with respect to organoleptic properties and rheological characteristics [23,24,25]. In another study, lipopetides deriving from B. licheniformis MS48 were used to assess the impact on the survival of Streptomyces thermophilus and L. delbrueckii subsp. bulgaricus [24]. Noteworthy, the authors observed that the yogurt produced with the biosurfactant and the starter cultures, evidenced lower syneresis, hence sustaining the potential emulsification claims. Moreover, a natural biosurfactant extracted from the corn-milling industry was applied in the formulation of drinkable yogurt, demonstrating a positive effect on the survival of the probiotic L. casei [26].
Thereby, the aim of the present study is to elaborate on the production of different types of biosurfactants, derived from LAB and yeasts, using cheese whey as fermentation substrate, in view of developing a sustainable bioprocess. More specifically, different LAB strains were selected based on our preceding study [27], while MEL biosurfactants were generated from cheese whey using Moesziomyces antarcticus [28]. Subsequently, LAB and yeast biosurfactants were applied in fermented dairy products using as starter culture the parental LAB strain used in the fermentative bioconversion process. After downstream processing, crude biosurfactants (LAB-BS and MEL) were employed for sour milk production, using different types of whole and semi-skimmed milk. The physicochemical properties as well as the effect of both biosurfactants was compared and meticulously discussed. Actually, our study constitutes one of the few in the literature including both lactobacilli and yeast biosurfactant production from food industry by-products alongside with their implementation in dairy products. The ultimate target is to conceptualize a closed-loop bioprocessing configuration for the dairy industry to mitigate dairy industry by-products (and specifically CW) via the production of high-added value compounds and their successive use in food products, aiming to compliment the pillars of circular economy.
2. Materials and Methods
2.1. Microorganisms and Culture Conditions
Lactobacilli used in this study were selected from our previous study [13,27]. More specifically, the selected strains used in this study were: Limosilactobacillus fermentum ACA-DC 0182, Limosilactobacillus fermentum ACA-DC 0183 from the Culture Collection of the Laboratory of Dairy Research, Agricultural University of Athens, Greece (ACA-DC), Lactiplantibacillus plantarum FMCC B-324 (E10) and Lactiplantibacillus pentosus FMCC B-329 (E108) from the Culture Collection of the Laboratory of Food Microbiology and Biotechnology, Agricultural University of Athens, Greece (FMCC). Strains were stored at −80 °C in previously autoclaved (121 °C, 20 min) De Man, Rogosa and Sharpe (MRS) broth (DifcoTM, Sparks, USA) supplemented with autoclaved glycerol (1:1, v/v). Before the designated use, all strains were streaked on MRS agar plates and incubated overnight (37 °C). For the experiments, bacterial inocula were prepared in Erlenmeyer flasks with sterile MRS broth, whereas the pre-culture volume was set at 10% of the final working volume.
Moesziomyces antarcticus was obtained from the Portuguese Yeast Culture Collection (PYCC), CREM, FCT/UNL, Caparica, Portugal. Stock cultures were prepared by culturing yeast cells in liquid medium, described below for the inoculum, and stored in 20% (v/v) glycerol aliquots, at −70 °C. For the fermentation experiments, yeast inoculum was prepared in Erlenmeyer flasks by the incubation of yeast stocks at 27 °C, 250 rpm, for 48 h, in liquid medium containing 0.3 g L−1 MgSO4, 3 g L−1 of NaNO3, 0.3 g L−1 KH2PO4, 1 g L−1 yeast extract (YE), 40 g L−1 D-glucose. The pre-culture volume was set at 10% of the final working volume of the fermentation volume.
Cheese whey was kindly provided by local dairy companies, and utilized as a low-cost fermentation feedstock. Before the fermentation process, de-proteinization of cheese whey was performed to obtain cheese whey permeate (CWP), following previous methods [28,29]. After the removal of protein fraction, CWP was autoclaved (121 °C, 20 min) and the pH value was adjusted (6.8 ± 0.2 for LAB and 6.0 for yeast) prior to inoculation. All chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Cheese Whey Fermentations for Biosurfactants Production
2.2.1. Lactobacilli Biosurfactant Production
Lactobacilli bioconversions were conducted using CWP as substrate at 37 °C and a pH value of 6.8 ± 0.2. Temperature was controlled using a thermocouple, whereas pH value was adjusted in the designated value using a peristaltic pump attached to a controller (SC200, HACH, Ames, IA, USA). The working volume was 1.5 L and the inoculum was 10% v/v of the final volume. Agitation was set at 600–800 rpm using magnetic stirrers to achieve optimum mixing of fermentation broth. Initial lactose was approximately 40 g L−1 and initial free amino nitrogen (FAN) was ~200 mg L−1. Samples (50 mL) were withdrawn at several time intervals to monitor fermentation parameters. Samples were centrifuged at 4200× g for 20 min at 4 °C (Rotina 420R, Hettich Zentrifugen) Andreas Hettich GmbH & Co. KG, Tuttlingen, Germany); supernatants were stored at −20°C for analysis. Cell mass was washed twice with deionized water and subsequently treated to extract cell-bound biosurfactants. LAB fermentation experiments were performed in duplicates and the results presented hereof correspond to the average values.
2.2.2. MEL Production
The production of MEL was performed in a shaken flask system, using CWP and vegetable oil (specifically sunflower oil) as substrates, at 27 °C with no pH control. The inoculum was 10% v/v of the final working volume (50 mL), in a medium containing: (i) 35% (v/v) of CWP supplemented with 20 g L−1 of vegetable oil (VO) at days 0, 4, and 8; (ii) the same substrate regime as (i) further supplemented with 0.3 g L−1 MgSO4, 3 g L−1 of NaNO3, 0.3 g L−1 L KH2PO4, 1 g L−1 yeast extract (YE). The initial lactose was approximately 40 g L−1. Samples (1 mL) were collected, centrifuged, and the pellet was dried at 60 °C to measure the total dry weight (TDW) whereas the supernatant was used for further analysis as previously described [28].
Experiments were performed in duplicates and the results presented hereof correspond to the average values.
2.3. Extraction of Biosurfactants
Extraction of cell bound LAB-biosurfactants was performed using a method previously reported [27]. Briefly, samples were centrifuged to obtain the cell pellet, that was washed twice with distilled water and resuspended in phosphate buffer saline (PBS, pH 7.0) (6:1 fermentation broth:PBS). The solutions were placed under gentle stirring in demineralized water at 4 °C at least for 6 h to release biosurfactants. The suspension was centrifuged to remove cell mass, the supernatant was collected and filtered (0.22 µm pore-size filter, Millipore) and further dialyzed with membranes (Cellu-Sep® molecular weight cutoff 6–8 kDa) before measuring surface tension.
MEL was obtained by a liquid–liquid extraction with ethyl acetate [30]. An equal volume of ethyl acetate and fermentation broth were added together in separator funnels, the mixture was shaken vigorously under manual agitation for 1 min and then it was allowed to settle in two distinct phases. The organic phase was separated; the procedure was performed twice and 0.5 g magnesium sulfate per 100 mL of ethyl acetate were added (to remove water residues) before filtration. Ethyl acetate was removed from the filtrate using a rotary evaporator under vacuum. MELs formed a brown gum extract after evaporation and were stored at 4 °C.
2.4. β-Galactosidase Assay
Selected lactobacilli strains used for the fermented milk experiments were screened for β-galactosidase assay following preculture for 12 h and 14 h. Samples were harvested at the designated timepoints and centrifuged at 13,000× g for 10 min. The cell pellet was washed twice with PBS solution and resuspended in sodium phosphate buffer (pH 6.8). The cell pellet was sonicated and the obtained solution was centrifuged and used in enzymatic activity [31]. β-galactosidase was measured using the method described by Osman et al. [32]. Briefly, the reaction mixture contained 250 μL 2-nitrophenyl β-D-galactopyranoside solution (20 mM) in sodium phosphate buffer (o-NPG), 200 μL of sodium phosphate buffer (pH 6.8), 10 μL of MgCl2 (50 mM), and 40 μL of crude enzyme. The reaction was performed at 40 °C for 10 min in a water bath and the reaction was terminated by the addition of 500 μL of sodium carbonate (Na2CO3, 1 M), followed by measuring the absorbance at 420 nm. One unit of activity was defined as the amount of enzyme required to release 1 μmol of o-nitrophenol (o-NP) in one minute assayed under the described conditions.
2.5. Surface Tension Measurement
Crude biosurfactant extracts deriving from lactobacilli bioconversions on CWP were used for surface tension measurements to assess biosurfactant production. Surface tension was estimated via the Wilhelmy plate method, at 25 °C, using a tensiometer (K20 Krüss tensiometer, KRÜSS GmbH, Hamburg, Germany) equipped with a 1.9 cm platinum plate (wetted length: 40.20 mm). The tensiometer was calibrated with distilled water and the production of biosurfactants was determined based on the decrease compared to the control sample (distilled water and phosphate saline buffer, PBS). All measurements were conducted in triplicate (n = 3) and the results represent the mean values. The critical micelle concentration (CMC) was measured as previously described [27].
2.6. Fermented Milk Production
Different types (whole and semi-skimmed) of commercial homogenized and pasteurized cow’s and goat’s milk were obtained from a local market and further used for fermented milk production. The protein content was 3.2–3.6% for all types of milk, initial pH was 6.7 ± 0.1, and fat content was 3.7% and 1.5% for cow’s milk (whole and semi-skimmed respectively) and 2.2% and 1.5% for goat’s milk (whole and semi-skimmed, respectively). The fat, protein, lactose, and total solids’ concentration was also confirmed by MilkoScanTM Mars, Foss (Hillerød, Denmark).
Lactobacilli cells were collected, centrifuged, and washed with milk to remove MRS broth residues prior to inoculation. Fermented milk was produced in sterile glass containers at a final volume of 200 mL using a 10% preculture (initial LAB cells concentration in the range of 108–109 CFU mL−1) at 37 °C until the pH reached <4.5 (after 14–16 h, depending on the strain). LAB biosurfactants (LAB-BS) and MEL were aseptically added prior to the acidification process, before inoculating with the starter culture at the designated CMC which was different for each biosurfactant compound. The fermented milk was subsequently rapidly cooled in a water-bath and stored at 4 °C up to 3 weeks.
2.7. Microbiological Analysis
The enumeration of each lactobacilli strain was evaluated at specific time intervals during storage (1, 5, 10, 15, and 20 days) by the standard enumeration methods using ten-fold serial dilutions. Ten grams of sample was homogenized with 90 mL Ringer’s solution followed by serial dilutions. The obtained suspensions were incorporated into MRS agar plates to determine viable cell counts after incubation at 37 °C for 48 h. The number of populations was expressed as log CFU mL−1.
2.8. Physicochemical Analysis
Titratable acidity was assessed using 0.1 mol L−1 NaOH and phenolphthalein as an indicator and expressed as lactic acid per 100 g of fermented milk. Syneresis was evaluated using the centrifugal method described previously [33]. Briefly, 5 g of each sample was weighed and centrifuged at 5000 rpm for 10 min at 4 °C. Syneresis % was estimated using the following formula (Equation (1)):
where Ws is the supernatant weight after centrifugation and Wg the total weight of the sample.
2.9. Analytical Methods
Lactose, glucose, galactose, and lactic acid were determined using high performance liquid chromatography analysis (HPLC, Agilent, Santa Clara, CA, USA), equipped with a ROA-organic acid H+ (300 mm × 7.8 mm, Phenomenex, Torrance, CA, USA) column coupled to a differential refractometer (RID). Operating conditions were as follows: sample volume 10 μL; mobile phase 10 mM H2SO4; flow rate 0.6 mL min−1; column temperature 65 °C. Samples were diluted and filtered (Whatman®, 0.2 μm) prior to analysis. Free amino nitrogen (FAN) was determined following the ninhydrin colorimetric method previously described [34].
Total dry weight (TDW, g L−1) was determined gravimetrically by drying the cell pellet after centrifugation at 65 °C until a constant weight was obtained. MEL was quantified as previously described [30].
2.10. Statistical Analysis
Statistical analysis was performed using Microsoft Excel 2018, and values are presented as average ± standard deviation.
3. Results and Discussion
3.1. Production of Lactobacilli Biosurfactants (LAB-BS) Using Different Strains
In our first screening study, we assessed 50 Lactobacillus strains for their potential to generate biosurfactants when cultivated in synthetic media containing glucose and subsequently on CWP as a renewable substrate [13]. The most promising candidates were subsequently studied under different bioprocessing strategies in shake flasks and bench-top bioreactors, whereby the potential of L. fermentum ACA-DC 0183 to produce biosurfactants on CWP was well evidenced [27]. In accordance with the literature, both studies indicated the prominence of the fermentation supplement, specifically carbon and nitrogen source on the production of biosurfactants. Herein, we evaluated three more strains to assess their ability to produce biosurfactants, namely L. fermentum ACA-DC 0182, isolated from cheese, L. plantarum E10 (FMCC B-324) and L. pentosus E108 (FMCC B-329), isolated from fermented olives [35]. In fact, the last two strains have previously demonstrated potential probiotic properties in vitro [35], hence were selected to be further evaluated, prospering the development of novel functional food. The experimental procedure was initiated with the strain L. plantarum E10 using CWP to assess the effect of lactose during controlled conditions of pH, temperature, and agitation (Figure 1A). Surface tension was reduced to 54.7 mN/m, compared to PBS (72 mN/m), after 24 h of fermentation; however, biomass production was rather low (1.5 g L−1), indicating a nutrient deficiency in CWP for this specific strain. Subsequently, the potential probiotic strain L. pentosus E108 was employed (Figure 1Β). Similarly, in this case, surface tension reduction was observed within the first hours of fermentation, from 69.3 to 42.3 mN/m reaching approximately 41 mN/m at the end of fermentation (24 h). Compared to the strain E10, L. pentosus E108 consumed lactose more efficiently, which was also evidenced by the higher biomass (2.1 g L−1) and lactic acid production (12 g L−1). It should be mentioned that surface tension values were compared to PBS to assess biosurfactant synthesis, a method widely employed in the open literature.
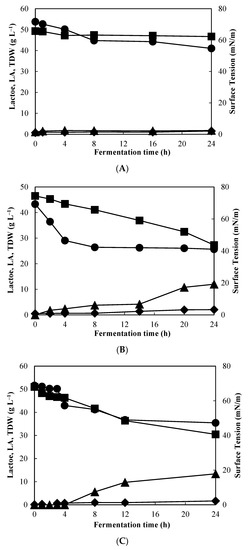
Figure 1.
Profile change of (●) ST, (■) lactose, (▲) lactic acid (LA), and (◆) total dry weight (TDW) during fermentations using CWP with the strains L. plantarum E10 (A) and L. pentosus E108 (B) and L. fermentum ACA-DC 0182 (C).
Likewise, Figure 1C shows the fermentation profile of L. fermentum ACA-DC 0182 on CWP. In this series of experiments and after 8 h of fermentation, surface tension was 55 mN/m, but followed a steadily decreasing trend up to 24 h (reaching 47.3 mN/m), that is equal to a reduction of ~24 mN/m. Overall, the strains L. pentosus and L. fermentum, demonstrated the ability to consume lactose and synthesize cell-bound biosurfactants as evidenced by the reduction in surface tension, within the first hours of fermentation, postulating that biosurfactant production occurs in the early exponential phase. Interestingly, a close observation in these results in the Figures indicates an adverse relation of biosurfactant synthesis with lactic acid production, considering that surface tension reduction remained relatively stable at the timepoint where lactic acid synthesis was boosted. As a matter of fact, there are scarce references in the open literature that correlate lactic acid production with cell-bound biosurfactants, thus indicating that further investigation is suggested. Thereof, these strains were selected to be subsequently evaluated for fermented milk production.
3.2. Production of MEL Biosurfactants
Cheese whey was previously assessed as a successful medium component for Moesziomyces spp. growth and MEL production [28], as opposed to the sole use of vegetable oils as carbon source, widely used in Moesziomyces spp. fermentations to achieve high MEL production [36]. The combination of CWP with vegetable oils may positively impact the overall MEL process economics, first by reducing the amount of vegetable oil used in fermentation with positive effect on the downstream processing, and consequently by reducing substrate costs. The efficacy of CWP on reducing the substrate-derived costs can be enhanced in the case that CWP provides all the essential compounds to serve as a sole carbon and nutrients source, allowing to exclude mineral medium and yeast extract (YE). In this work, CWP was assessed in the fermentation of Moesziomyces antarcticus, combined with a vegetable oil feeding strategy (at 0, 4, 8 days), with and without mineral medium, YE, and NaNO3. The latter constitute common medium components for MEL production. The biomass expressed as total dry weight (TDW) and MELs production, as well as sugar and residual lipids profiles, are presented in Figure 2, where it can be seen that the maximum production of MEL (25.4 g L−1) was obtained after 14 days of fermentation in the supplemented CWP substrate.
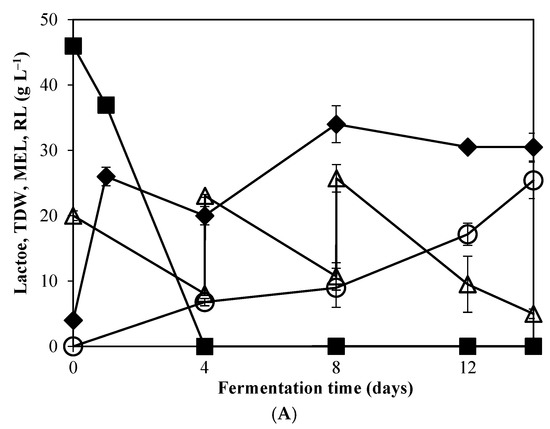
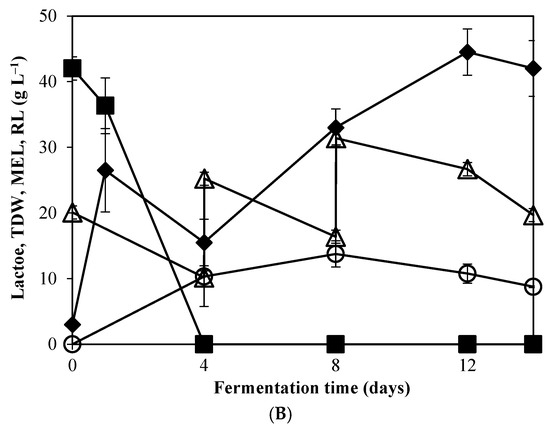
Figure 2.
Profile change of (○) MEL, (■) lactose, (△) residual lipids (RL), and (◆)total dry weight (TDW) during fermentation of M. antarcticus on supplemented CWP with yeast extract and NaNO3 (A) and CWP without any supplementation (B).
Nonetheless, a comparison of the two experiments reveals that different maximum MEL production and fermentation profiles were observed for the two conditions assessed. For instance, the highest MELs concentration was obtained after 14 days in fermentations with CW and VO, supplemented with mineral medium and YE (Figure 2A), representing a productivity of 1.81 g L−1 d−1 and MEL yield of 0.25 (g g−1) (Table 1). However, it is worth noting that on the condition that only CW and VO were used for M. antarcticus growth, the highest production of MEL (13.75 g L−1) was obtained on the 8th day, with a productivity of 1.72 g L−1 d−1. Compared to the supplemented CW (Figure 2B), at the same timepoint (day 8), a lower MEL concentration was observed (7 g L−1). On the other hand, MEL production in supplemented cultures increased after the 8th day, followed by further addition of VO.

Table 1.
Maximum concentrations of TDW and MEL production (g L−1); yield of produced MEL (gMEL/gSubstrate), maximum productivity (g L−1 d−1), and purity (g g−1) of M. antarcticus PYCC 5048T using CW and VO (20 g L−1) in the presence or absence of mineral medium with YE and NaNO3.
On the later stages of the fermentation, TDW continued to increase only in the experiments performed using solely CW and VO, while the consumption rate of residual lipids decreased. This is postulated to relate with the observation that MEL production was terminated. This phenomenon can be attributed to an earlier depletion of essential elements important for the secondary metabolism associated with MEL synthesis. It could be speculated that, the lower nitrogen availability in the non-supplemented CW medium might trigger MEL synthesis before day 8, but equally entails the early depletion of essential elements, thus impairing on the prolonged MEL production in the following days, regardless the addition of VO.
Overall, the results regarding MEL production indicate the importance of productivity, conversion yield and the use of conventional nutrients during the development of a bioprocess. All the above merit significant factors to be considered to sustain the economic feasibility. Equally, the end-application of the product can determine economics, specifically when food applications constitute the target market outlet.
3.3. Fermented Milk Production
In the context of proposing a comprehensive approach that includes both production and application of lactobacilli and Moesziomyces biosurfactants, this study undertook the inclusion of biosurfactants in fermented dairy beverage formulation. The target was to assess the effect on microbial population along with physicochemical properties.
The first step of the experimental set up to evaluate the best performing lactobacilli strains for fermented milk production, employed β-galactosidase assay on the precultures. For this reason, samples were collected after 12 and 14 h of incubation on MRS substrate and the enzymatic activity was assayed, and the results are presented in Figure 3. It can be easily observed that ACA-DC 0183 presented the highest β-galactosidase activity, followed by ACA-DC 0182 and E108, after 14 h of fermentation. Actually, both ACA-DC 0182 and 0183 are L. fermentum strains, and the ability to produce biosurfactants was recently demonstrated [27]. In fact, L. fermentum strains have been widely evaluated as starter cultures in dairy products. On the other hand, FMCC E108 corresponds to a non-dairy L. pentosus strain with potential probiotic properties [35]. Thereof, these three strains were further assessed for sour milk production, considering as well the ability to secrete β-galactosidase and also biosurfactant production.
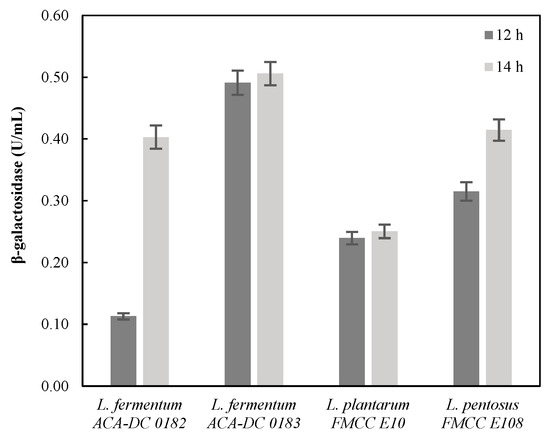
Figure 3.
β-galactosidase of lactobacilli strains (L. fermentum ACA-DC 0182 and 0183; L. plantarum FMCC E10 and L. pentosus FMCC E108) on MRS medium after 12 h and 14 h of preculture.
Sour milk production experiments were initiated with the strain ACA-DC 0183, evidencing BS production, using whole and semi-skimmed cow milk. LAB and MEL biosurfactants were added in their respective CMC value, whereas one experiment was conducted without exogenous addition of BS (control). Table 2 presents the microbiological and physicochemical properties during cold storage of the sour milk made with two types of cow milk. The addition of LAB-BS entailed a positive effect on microbial counts throughout the cold storage, corresponding to an increase (up to 8.6 log10CFU/mL) compared to MELs (8.37 log10CFU/mL), indicating a positive effect of LAB-BS addition. This actually resulted in higher titratable acidity that was accompanied with an analogous increase in syneresis.

Table 2.
Microbiological and physicochemical changes during cold storage of fermented milk prepared using the strain L. fermentum ACA-DC 0183 on whole and semi skimmed milk with and without the addition of lactobacilli and Moesziomyces biosurfactants.
Actually, syneresis increased during storage time from 34.8% to 50% for the control sample after 20 days. On the other hand, LAB-BS entailed the highest syneresis at the end of storage (51%), whereas MELs addition resulted in lower syneresis (47%) percentage on the same timepoint (Day 20). Based on the results of Table 2, one can note the positive effect of MEL biosurfactants on syneresis, as this case showed the lowest increase.
This could be related to the improvement of the colloidal stability of the oil-soluble surfactants, since their orientation is directed to the lipid-core micelle of the protein (where oil droplets are entrapped). On the other hand, LAB-BS (as hydrophilic compounds) cover the outside of the protein, and render it more hydrophobic, inhibiting at the same time the interactions with Ca+2 at low pH values that will strengthen their structure. In the semi-skimmed cow milk, the results indicated a same pattern for growth and acidity regardless the examined case. Still syneresis was even higher in all experiments, which can be attributed to the lower content of fat globules that would fill the protein–oil matrix, hence formulating softer gels.
The strain L. pentosus FMCC E108 was also evaluated for sour milk production, following its ability to produce biosurfactants (along with its potential probiotic characteristics). Similarly, all experiments were performed with LAB-BS and MELs addition and the results are presented in Table 3. Microbial populations were higher after the acidification compared to the strain ACA-DC 0183 that demonstrated the highest β-galactosidase production, hence would be expected to perform better. Microbial counts followed the same decreasing order (LAB-BS > Control > MELs) as in the ACA-DC 0183 case, and similarly MELs resulted in the lowest syneresis. LAB-BS addition along with the high increase in populations is speculated to elicit a protective role on the cells, considering also that BS synthesis in LAB relates to their physiological cell function. Likewise, probiotic bacteria elicit an increased tolerance in low pH values, hence a synergistic effect on microbial increase in storage could be hypothesized. However, the advantage of increased cell viability might not benefit food applications, for technological reasons, as the increase in acidity would hinder the syneresis (e.g., yoghurt, sour milk). MELs on the other hand, seems to better sustain syneresis levels. Still, cell viability is lower, which might be a disadvantage in probiotic product development, where sustaining the probiotic concentrations is a pre-requisite.

Table 3.
Microbiological and physicochemical changes during cold storage of fermented milk prepared using the strain L. pentosus FMCC-E108 on whole and semi skimmed milk with and without the addition of lactobacilli and Moesziomyces biosurfactants.
To better illustrate the positive effect of LAB-BS, Figure 4 presents the correlation of microbial populations with titratable acidity during cold storage. It can be observed that the increase in titratable acidity is associated with the increase in cells concentration, and actually the experiment performed with LAB-BS resulted in higher concentrations, thereby postulating a protective effect on the probiotic bacteria. The increase in titratable acidity also relates with lactic acid production that impacts casein micelle dissociation [17]. It has been previously suggested that lactic acid production acts on the negative charge on the casein micelle surface, leading to the modification of aggregates and subsequently the destabilization [37]. Equally, several recent studies have reported on the interaction mechanisms between surfactants, including biosurfactants and casein [38]. Biosurfactants elicit great diversity with respect to their structure, hence numerous interactions can be developed with casein. These interactions can affect surface tension and the formation of aggregates, that could be associated with syneresis. On the other hand, several biosurfactants possess antimicrobial activity and could negatively affect probiotic bacteria [39]. The results of Figure 4, however, speculate that specifically LAB-BS, produced by the same strain used as starter culture, enhanced the survival of the lactobacilli, compared to MELs that were synthesized from M. antarcticus yeast.
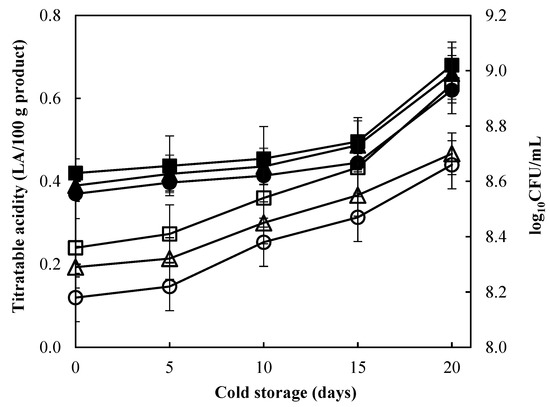
Figure 4.
Microbiological population of lactobacilli (△:control experiment, □: LAB-BS, ○: MEL) correlated with titratable acidity (▲: control experiment, ■: LAB-BS, ●: MEL) during cold storage of sour milk produced with the strain L. pentosus FMCC E108 and full fat cow milk.
An additional experiment was performed using goat milk for sour milk formulation, considering that the casein content (e.g., casein fraction αs-1) differs from cow milk, and it could affect syneresis and acid gel formation [40]. Goat milk also presents improved digestibility in the lower gastrointestinal tract compared to cow milk [41], providing the potential for functional product development for lactose intolerant consumers. Goat milk has been also reported to have smaller fat globule size leading to softer gel formation hence affecting syneresis, and previous studies presented higher syneresis in beverages or yoghurts with goat milk compared to cow milk [41,42]. In our experiments, fermented goat milk indicated similar results to the semi-skimmed milk (data not shown). More specifically, MEL addition resulted in the lowest microbial counts, lower acidity and improved syneresis, whereas LAB-BS and the control experiments demonstrated high cell viability.
The physiological role of biosurfactant synthesis in microbial entities is postulated to relate with this observation, as previously mentioned. For instance, probiotic bacteria produce primarily cell-bound biosurfactants as a physiological response to adhere on interfaces and survive [43], compared to yeast strains that produce biosurfactants extracellularly, in an attempt to emulsify and metabolize water insoluble compounds.
The last experiments included the evaluation of L. fermentum ACA-DC 0182 strain using whole and semi-skimmed cow milk and the results are presented in Table 4. In this case, the initial microbial populations, were higher after the acidification process specifically with the addition of LAB-BS, compared to the previous strains used (e.g., ACA-DC 0183 and E108). Microbial counts were maintained lower via MELs inclusion. Still, the initial high acidity obtained at the end of acidification, which could be related with the starting higher microbial concentrations, presented high syneresis that further increased during storage.

Table 4.
Microbiological and physicochemical changes during cold storage of fermented milk prepared using the strain L. fermentum ACA-DC 0182 on whole and semi skimmed cow milk, and cell recycling fermented milk, with and without the addition of lactobacilli and Moesziomyces biosurfactants.
Additionally, we performed one experiment that employed a cell recycling process in an attempt to valorize the cell extract remaining after the extraction of cell-bound biosurfactants, toward developing a closed-loop process. In this case, lactobacilli cells were freeze dried and used as the starter culture in sour milk production and the results are shown also in Table 4. The starting populations at storage were lower to the previous experiment, still an increase is observed in the case of LAB-BS during 20 days of storage, along with a rise in syneresis and acidity, that is proportionally lower compared with the previous experiments. MELs addition resulted in the lowest LAB counts (after 20 days of storage), postulating a disadvantage for probiotic bacteria, considering that prolonged storage could employ further decrease and exceed the critical limit set for the viability of probiotic bacteria to confer health benefits.
Worth noting, this observation is contingent with the cell exhaustion before milk acidification, obtained after CWP bioconversion for BS production, PBS extraction and freeze-drying. Nonetheless, the significant outcome of this experiment relates to the re-utilization of LAB cells (even with a decrease in fermentative ability). Apparently, the addition of LAB-BS is speculated to elicit a synergistic effect and a protective role toward the parental LAB cells, suggesting a beneficial attribute for probiotic bacteria, and the subsequent application in sour milk.
Evidently, this hypothesis will be confirmed in a forthcoming study, via the application of probiotic bacteria along with BS, considering that sustained probiotic cell concentration and viability through storage is of utmost importance.
To the best of our knowledge, no previous reports in the open literature have evaluated the LAB cell fraction that remains after the extraction of cell-bound biosurfactants. This configuration is expected to expand the potential food applications of probiotic LAB-BS, envisaging the transition to circular economy. Likewise, following the observation that LAB-BS addition enhances the parental cell proliferation and the well-established effect of solids addition in improving syneresis, LAB-BS immobilization on functional and food grade supports will result in sustained probiotic concentration, reduced syneresis, and the inclusion of bioactive components to formulate functional food products.
Regarding technological consideration, it is well established that the dominant disadvantage of biosurfactants is the high cost of production, reciprocal to the majority of bio-based products. On top of that, the formation of microbial surfactants, particularly from lactobacilli, often demonstrates low productivities. On the other hand, chemical surfactants exhibit a low production cost that entails low market prices, affected primarily by the prices of ethylene oxide, as raw material used for synthesis. Still, the enforcement of stringent directives for the environment induced a boost on biosurfactant market demand. In addition, consumers’ rising awareness on environmentally benign products should not be disregarded. Therefore, envisaging to enhance the economic feasibility of biosurfactant production, research should target the development of circular configuration bioprocesses, generating minimal waste streams. Likewise, the development of such processes should encompass food, cosmetic, and pharmaceutical applications in an attempt to increase the sustainability of the proposed scheme.
4. Conclusions
The outcomes of this study demonstrated the effective valorization of cheese whey for the fermentative production of lactobacilli and Moesziomyces biosurfactants. The obtained food-grade biosurfactants were subsequently applied for fermented milk production. Results indicated a protective effect on LAB population during storage of the parental strains when LAB-BS were applied. MELs on the other hand demonstrated a better effect on sustaining the syneresis, that is beneficial for specific food formulations (e.g., sour milk). Ultimately, for the first time, LAB cells after downstream of BS, were sequentially applied for sour milk production, demonstrating the ability for cell re-utilization and the development of a closed-loop process to enhance the sustainability of the dairy industry.
Author Contributions
Conceptualization, V.K., N.K.; methodology, V.K., M.A., N.T.F., N.K.; investigation, V.K., M.A., D.A., M.F.N., N.T.F.; resources, A.P., F.C.F., and N.K.; writing—original draft preparation, V.K., M.A., M.F.N., and N.T.F.; writing—review and editing, V.K., M.A., A.P., F.C.F., and N.K.; supervision, A.P., F.C.F., and N.K.; project administration, N.K. All authors have read and agreed to the published version of the manuscript.
Funding
We acknowledge the support of this work by the project “Research Infrastructure on Food Bioprocessing Development and Innovation Exploitation—Food Innovation RI” (MIS 5027222), which is implemented under the Action “Reinforcement of the Research and Innovation Infrastructure”, funded by the Operational Programme “Competitiveness, Entrepreneurship and Innovation” (NSRF 2014–2020) and co-financed by Greece and the European Union (European Regional Development Fund). This work was also supported by FCT—Fundação para a Ciência e a Tecnologia, I.P., through iBB (UIDB/04565/2020 and UIDP/04565/2020), i4HB (LA/P/0140/2020) and the PhD grant (SFRH/BD/137007/2018).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are contained within the article.
Acknowledgments
The authors also acknowledge the Portuguese Yeast Culture Collection (PYCC), UCIBIO/Requimte, FCT/UNL and Lacticínios Paiva SA for providing the yeast strains and the CW, respectively, for MEL production.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ismail, N.L. Chapter 5. In Surfactants and Detergents; Shahruddin, S., Ed.; IntechOpen: London, UK, 2022; ISBN 978-1-83962-897-9. [Google Scholar]
- Hayes, D.G.; Smith, G.A. Chapter 1—Biobased surfactants: Overview and industrial state of the art. In Biobased Surfactants, 2nd ed.; Hayes, D.G., Solaiman, D.K.Y., Ashby, R.D.B.T.-B.S., Eds.; AOCS Press: Urbana, IL, USA, 2019; pp. 3–38. ISBN 978-0-12-812705-6. [Google Scholar]
- Montoya Vallejo, C.; Flórez Restrepo, M.A.; Guzmán Duque, F.L.; Quintero Díaz, J.C. Production, Characterization and Kinetic Model of Biosurfactant Produced by Lactic Acid Bacteria. Electron. J. Biotechnol. 2021, 53, 14–22. [Google Scholar] [CrossRef]
- Banat, I.M.; Carboué, Q.; Saucedo-Castañeda, G.; de Jesús Cázares-Marinero, J. Biosurfactants: The Green Generation of Speciality Chemicals and Potential Production Using Solid-State Fermentation (SSF) Technology. Bioresour. Technol. 2021, 320, 124222. [Google Scholar] [CrossRef] [PubMed]
- Markande, A.R.; Patel, D.; Varjani, S. A Review on Biosurfactants: Properties, Applications and Current Developments. Bioresour. Technol. 2021, 330, 124963. [Google Scholar] [CrossRef] [PubMed]
- Jimoh, A.A.; Lin, J. Biotechnological Applications of Paenibacillus Sp. D9 Lipopeptide Biosurfactant Produced in Low-Cost Substrates. Appl. Biochem. Biotechnol. 2020, 191, 921–941. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Vaccari, M.; Prasad, S.; Rtimi, S. Preparation, Characterization and Application of Biosurfactant in Various Industries: A Critical Review on Progress, Challenges and Perspectives. Environ. Technol. Innov. 2021, 24, 102090. [Google Scholar] [CrossRef]
- Farias, C.B.B.; Almeida, F.C.G.; Silva, I.A.; Souza, T.C.; Meira, H.M.; Soares da Silva, R.d.C.F.; Luna, J.M.; Santos, V.A.; Converti, A.; Banat, I.M.; et al. Production of Green Surfactants: Market Prospects. Electron. J. Biotechnol. 2021, 51, 28–39. [Google Scholar] [CrossRef]
- Gaur, V.K.; Sharma, P.; Sirohi, R.; Varjani, S.; Taherzadeh, M.J.; Chang, J.-S.; Yong Ng, H.; Wong, J.W.C.; Kim, S.-H. Production of Biosurfactants from Agro-Industrial Waste and Waste Cooking Oil in a Circular Bioeconomy: An Overview. Bioresour. Technol. 2022, 343, 126059. [Google Scholar] [CrossRef]
- Domínguez Rivera, Á.; Martínez Urbina, M.Á.; López y López, V.E. Advances on Research in the Use of Agro-Industrial Waste in Biosurfactant Production. World J. Microbiol. Biotechnol. 2019, 35, 155. [Google Scholar] [CrossRef]
- Ferreira, A.; Vecino, X.; Ferreira, D.; Cruz, J.M.; Moldes, A.B.; Rodrigues, L.R. Novel Cosmetic Formulations Containing a Biosurfactant from Lactobacillus Paracasei. Colloids Surf. B Biointerfaces 2017, 155, 522–529. [Google Scholar] [CrossRef]
- Adu, S.A.; Naughton, P.J.; Marchant, R.; Banat, I.M. Microbial Biosurfactants in Cosmetic and Personal Skincare Pharmaceutical Formulations. Pharmaceutics 2020, 12, 1099. [Google Scholar] [CrossRef]
- Kachrimanidou, V.; Papadaki, A.; Lappa, I.; Papastergiou, S.; Kleisiari, D.; Kopsahelis, N. Biosurfactant Production from Lactobacilli: An Insight on the Interpretation of Prevailing Assessment Methods. Appl. Biochem. Biotechnol. 2022, 194, 882–900. [Google Scholar] [CrossRef] [PubMed]
- Gudiña, E.J.; Teixeira, J.A.; Rodrigues, L.R. Biosurfactant-Producing Lactobacilli: Screening, Production Profiles, and Effect of Medium Composition. Appl. Environ. Soil Sci. 2011, 201254. [Google Scholar] [CrossRef]
- Cornea, C.P.; Roming, F.I.; Sicuia, O.A.; Voaideş, C.; Zamfir, M.; Grosu-Tudor, S.S. Biosurfactant Production by Lactobacillus Spp. Strains Isolated from Romanian Traditional Fermented Food Products. Rom. Biotechnol. Lett. 2016, 21, 11312–11320. [Google Scholar]
- Schoina, V.; Terpou, A.; Papadaki, A.; Bosnea, L.; Kopsahelis, N.; Kanellaki, M. Enhanced Aromatic Profile and Functionality of Cheese Whey Beverages by Incorporation of Probiotic Cells Immobilized on Pistacia Terebinthus Resin. Foods 2020, 9, 13. [Google Scholar] [CrossRef]
- Dimitrellou, D.; Kandylis, P.; Kourkoutas, Y. Assessment of Freeze-Dried Immobilized Lactobacillus Casei as Probiotic Adjunct Culture in Yogurts. Foods 2019, 8, 374. [Google Scholar] [CrossRef]
- Dimitrellou, D.; Salamoura, C.; Kontogianni, A.; Katsipi, D.; Kandylis, P.; Zakynthinos, G.; Varzakas, T. Effect of Milk Type on the Microbiological, Physicochemical and Sensory Characteristics of Probiotic Fermented Milk. Microorganisms 2019, 7, 274. [Google Scholar] [CrossRef]
- Sakandar, H.A.; Zhang, H. Trends in Probiotic(s)-Fermented Milks and Their in Vivo Functionality: A Review. Trends Food Sci. Technol. 2021, 110, 55–65. [Google Scholar] [CrossRef]
- Gallardo-Escamilla, F.J.; Kelly, A.L.; Delahunty, C.M. Mouthfeel and Flavour of Fermented Whey with Added Hydrocolloids. Int. Dairy J. 2007, 17, 308–315. [Google Scholar] [CrossRef]
- Teimouri, S.; Abbasi, S.; Scanlon, M.G. Stabilisation Mechanism of Various Inulins and Hydrocolloids: Milk–Sour Cherry Juice Mixture. Int. J. Dairy Technol. 2018, 71, 208–215. [Google Scholar] [CrossRef]
- Mouafo, H.T.; Sokamte, A.T.; Mbawala, A.; Ndjouenkeu, R.; Devappa, S. Biosurfactants from Lactic Acid Bacteria: A Critical Review on Production, Extraction, Structural Characterization and Food Application. Food Biosci. 2022, 46, 101598. [Google Scholar] [CrossRef]
- Zouari, R.; Besbes, S.; Ellouze-Chaabouni, S.; Ghribi-Aydi, D. Cookies from Composite Wheat–Sesame Peels Flours: Dough Quality and Effect of Bacillus Subtilis SPB1 Biosurfactant Addition. Food Chem. 2016, 194, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, A.; Kiran, G.S.; Selvin, J. Revealing the Effect of Lipopeptide on Improving the Probiotics Characteristics: Flavor and Texture Enhancer in the Formulated Yogurt. Food Chem. 2022, 375, 131718. [Google Scholar] [CrossRef]
- Ribeiro, B.G.; Guerra, J.M.C.; Sarubbo, L.A. Potential Food Application of a Biosurfactant Produced by Saccharomyces Cerevisiae URM 6670. Front. Bioeng. Biotechnol. 2020, 8, 434. [Google Scholar] [CrossRef]
- López-Prieto, A.; Rodríguez-López, L.; Rincón-Fontán, M.; Moldes, A.B.; Cruz, J.M. Effect of Biosurfactant Extract Obtained from the Corn-Milling Industry on Probiotic Bacteria in Drinkable Yogurt. J. Sci. Food Agric. 2019, 99, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Kachrimanidou, V.; Alimpoumpa, D.; Papadaki, A.; Lappa, I.; Alexopoulos, K.; Kopsahelis, N. Cheese Whey Utilization for Biosurfactant Production: Evaluation of Bioprocessing Strategies Using Novel Lactobacillus Strains. Biomass Convers. Biorefinery 2022, 12, 4621–4635. [Google Scholar] [CrossRef]
- Nascimento, M.F.; Barreiros, R.; Oliveira, A.C.; Ferreira, F.C.; Faria, N.T. Moesziomyces Spp. Cultivation Using Cheese Whey: New Yeast Extract-Free Media, β-Galactosidase Biosynthesis and Mannosylerythritol Lipids Production. Biomass Convers. Biorefinery 2022, 1–14. [Google Scholar] [CrossRef]
- Sharma, D.; Saharan, B.S.; Chauhan, N.; Bansal, A.; Procha, S. Production and Structural Characterization of Lactobacillus Helveticus Derived Biosurfactant. Sci. World J. 2014, 493548. [Google Scholar] [CrossRef]
- Faria, N.T.; Santos, M.; Ferreira, C.; Marques, S.; Ferreira, F.C.; Fonseca, C. Conversion of Cellulosic Materials into Glycolipid Biosurfactants, Mannosylerythritol Lipids, by Pseudozyma Spp. under SHF and SSF Processes. Microb. Cell Fact. 2014, 13, 155. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Splechtna, B.; Steinböck, M.; Kneifel, W.; Lettner, H.P.; Kulbe, K.D.; Haltrich, D. Purification and Characterization of Two Novel β-Galactosidases from Lactobacillus Reuteri. J. Agric. Food Chem. 2006, 54, 4989–4998. [Google Scholar] [CrossRef]
- Osman, A.; Tzortzis, G.; Rastall, R.A.; Charalampopoulos, D. BbgIV Is an Important Bifidobacterium β-Galactosidase for the Synthesis of Prebiotic Galactooligosaccharides at High Temperatures. J. Agric. Food Chem. 2012, 60, 740–748. [Google Scholar] [CrossRef]
- Terpou, A.; Gialleli, A.I.; Bekatorou, A.; Dimitrellou, D.; Ganatsios, V.; Barouni, E.; Koutinas, A.A.; Kanellaki, M. Sour Milk Production by Wheat Bran Supported Probiotic Biocatalyst as Starter Culture. Food Bioprod. Process. 2017, 101, 184–192. [Google Scholar] [CrossRef]
- Kachrimanidou, V.; Kopsahelis, N.; Vlysidis, A.; Papanikolaou, S.; Kookos, I.K.; Monje Martínez, B.; Escrig Rondán, M.C.; Koutinas, A.A. Downstream Separation of Poly(Hydroxyalkanoates) Using Crude Enzyme Consortia Produced via Solid State Fermentation Integrated in a Biorefinery Concept. Food Bioprod. Process. 2016, 100, 323–334. [Google Scholar] [CrossRef]
- Argyri, A.A.; Zoumpopoulou, G.; Karatzas, K.-A.G.; Tsakalidou, E.; Nychas, G.-J.E.; Panagou, E.Z.; Tassou, C.C. Selection of Potential Probiotic Lactic Acid Bacteria from Fermented Olives by in Vitro Tests. Food Microbiol. 2013, 33, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Morita, T.; Fukuoka, T.; Imura, T.; Kitamoto, D. Production of Mannosylerythritol Lipids and Their Application in Cosmetics. Appl. Microbiol. Biotechnol. 2013, 97, 4691–4700. [Google Scholar] [CrossRef]
- El Bouchikhi, S.; Pagès, P.; El Alaoui, Y.; Ibrahimi, A.; Bensouda, Y. Syneresis Investigations of Lacto-Fermented Sodium Caseinate in a Mixed Model System. BMC Biotechnol. 2019, 19, 57. [Google Scholar] [CrossRef]
- Tian, Q.; Lai, L.; Zhou, Z.; Mei, P.; Lu, Q.; Wang, Y.; Xiang, D.; Liu, Y. Interaction Mechanism of Different Surfactants with Casein: A Perspective on Bulk and Interfacial Phase Behavior. J. Agric. Food Chem. 2019, 67, 6336–6349. [Google Scholar] [CrossRef] [PubMed]
- Albadran, H.A.; Chatzifragkou, A.; Khutoryanskiy, V.V.; Charalampopoulos, D. Development of Surfactant-Coated Alginate Capsules Containing Lactobacillus Plantarum. Food Hydrocoll. 2018, 82, 490–499. [Google Scholar] [CrossRef]
- Domagała, J. Instrumental Texture, Syneresis and Microstructure of Yoghurts Prepared from Goat, Cow and Sheep Milk. Int. J. Food Prop. 2009, 12, 605–615. [Google Scholar] [CrossRef]
- Joon, R.; Mishra, S.K.; Singh Brar, G.; Kumar Singh, P.; Kumar Mishra, S.; Panwar, H. Instrumental Texture and Syneresis Analysis of Yoghurt Prepared from Goat and Cow Milk. Pharma Innov. J. 2017, 6, 971–974. [Google Scholar]
- Gomes, J.J.L.; Duarte, A.M.; Batista, A.S.M.; de Figueiredo, R.M.F.; de Sousa, E.P.; de Souza, E.L.; Queiroga, R.d.C.R.d.E. Physicochemical and Sensory Properties of Fermented Dairy Beverages Made with Goat’s Milk, Cow’s Milk and a Mixture of the Two Milks. LWT Food Sci. Technol. 2013, 54, 18–24. [Google Scholar] [CrossRef]
- Sharma, D.; Saharan, B.S.; Kapil, S. Biosurfactants of Probiotic Lactic Acid Bacteria; Springer: Cham, Switzerland, 2016; ISBN 9783319262130. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).