Abstract
The looming climate and energy crises, exacerbated by increased waste generation, are driving research and development of sustainable resource management systems. Research suggests that organic materials, such as food waste, grass, and manure, have potential for biotransformation into a range of products, including: high-value volatile fatty acids (VFAs); various carboxylic acids; bioenergy; and bioplastics. Valorizing these organic residues would additionally reduce the increasing burden on waste management systems. Here, we review the valorization potential of various sustainably sourced feedstocks, particularly food wastes and agricultural and animal residues. Such feedstocks are often micro-organism-rich and well-suited to mixed culture fermentations. Additionally, we touch on the technologies, mainly biological systems including anaerobic digestion, that are being developed for this purpose. In particular, we provide a synthesis of VFA recovery techniques, which remain a significant technological barrier. Furthermore, we highlight a range of challenges and opportunities which will continue to drive research and discovery within the field. Analysis of the literature reveals growing interest in the development of a circular bioeconomy, built upon a biorefinery framework, which utilizes biogenic VFAs for chemical, material, and energy applications.
1. Introduction
As both climate change and the global energy crisis escalate, it becomes ever more critical to implement sustainable resource management strategies such as biorefineries and resource recovery systems. These systems typically utilize innovative resource recovery technologies and novel renewable materials. The valorization of biomass can play a foundational role within these systems, supporting the generation of energy (biofuel) as well as a wide range of bio-based products through the biorefinery concept [1,2,3,4]. Biomass can be broadly classified as either an energy crop or residue. Energy crops are specifically cultivated for energy generation. These crops are typically cultivated using intensive farming practices, and since they are often edible, using them for energy generation results in less food and less food-crop land. In contrast, biomass residues are non-edible and are generally composed of waste products or agro-industrial side streams.
Among biomass residues, food waste and agricultural waste have demonstrated their promising potential for biorefinery applications [2,3,5,6,7]. In Europe, these biomasses are valorized using biological, chemical, and thermochemical methods. However, variability in the quantity and composition of biomass limits the technological and economic viability of these valorization methods. Therefore, these highly variable biomass resources are better suited for processes such as anaerobic digestion (AD), which is able to convert a wide range of organics into products such as volatile fatty acids (VFAs), biohydrogen, polyhydroxyalkanoates (PHAs), and bioenergy. AD can serve as a sustainable and economically attractive biological pretreatment for lignocellulosic biomass, facilitating its conversion into bio-based products by exposing lignin and undigested fibers for further valorization.
The ultimate purpose of an AD-based biorefinery system is to optimize resource-use efficiency while minimizing waste; this is typically accomplished by maximizing energy/biogas production. The generation of alternative valuable by-products, in addition to biogas, represents a new opportunity to enhance resource recovery (Figure 1). An important value-added by-product, VFAs, are produced during the initial phases of AD in a process known as acidogenic fermentation. VFAs have a wide range of potential applications in the biorefinery industry where they can be used as feedstock for various bio-based products. For instance, VFAs are considered a potential platform for the production of biodegradable PHA polymers [8]. Currently, synthetically produced VFAs are used in the food and beverage industries, as well as in pharmaceutical and synthetic chemistry. The ratios of the specific volatile fatty acids that are produced via acidogenic fermentation are dependent on the feedstock biomass’ composition, the extent of hydrolysis, operational conditions, reactor design, and the structure of the microbial community. Research investigating these parameters is being carried out and promises to greatly improve the efficiency and stability of the acid-forming stage.
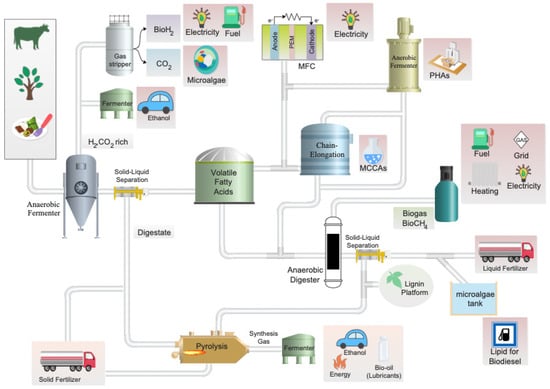
Figure 1.
Potential biorefinery process focusing on maximizing VFA production. The process begins with the valorization of residual feedstocks and culminates in the potential production of various high-value end-products (highlighted in pink).
This review elucidates the potential for low-value biomass to be used as feedstock for VFA generation within a biorefinery model. Specifically, we discuss various techniques and system designs which optimize energy use and product yield. Finally, we discuss current trends and challenges for a biorefinery concept, and the outstanding research necessary to support a functioning bioeconomy.
2. Methods of Valorizing Low-Value Feedstocks
Biomass valorization has gained popularity and traction in recent years given its potential to sustainably meet regulatory requirements in terms of energy, chemicals, and materials. Research suggests that within the biorefinery concept, biomass valorization—where all fractions are processed selectively towards a variety of products—is generally achieved using two strategies: thermochemical and/or biological conversion [9]. While this review focuses on biological processes, we will also briefly mention trends in thermochemical techniques.
2.1. Thermochemical Approach
Among the thermochemical conversion processes, gasification and pyrolysis are commonly used to produce heat, biochar, and syngas from lignocellulosic biomass. Conventional gasification technologies include fixed beds, fluidized beds, and entrained flow reactors [10]. However, these technologies still struggle with process inefficiencies related to biomass moisture content and tar production. Recently, efforts have been made to mitigate these factors—using technologies such as pyrolysis and supercritical water gasification [11,12,13,14,15,16]. Unlike gasification, pyrolysis is a technology that converts biomass into bio-oil, syngas, and biochar in the absence of oxygen [17]. Pyrolysis can be used to valorize different types of recalcitrant biomass, such as agricultural residues and wood wastes. The resulting syngas can then be converted by anaerobic bacteria into biochemicals and biofuels independent of the original biomass composition; a process known as hybrid thermochemical-biochemical [18]. However, the high cost and safety risk of the pyrolysis process make it unviable for large scale applications [17].
Recently, supercritical water gasification has been considered as a potential technique to valorize lignocellulosic biomass and wastes with high moisture content—up to 80% wet weight [16]. Supercritical water gasification is being studied especially for hydrogen production, as the composition of the resulting synthesis gas is higher in hydrogen and lower in carbon monoxide [19]; in addition, the low production of tar and char is an advantage compared to other technologies [20]. However, supercritical water gasification is a technology still unfeasible for large scale applications in biorefineries—its implementation requires improvements in terms of pump energy efficiency [16], and reactor designs which can withstand corrosion [16] and high pressure [21].
2.2. Biological Approach
Biological conversion processes encompass both AD and fermentation and are commonly used to valorize biomass such as food waste, agricultural residues, organic fraction of the municipal solid waste (OFMSW), and energy crops. Unlike the thermochemical conversion method where the primary product is biofuel, the biological conversion of biomass can produce biofuel and chemicals. Due to the high moisture content of most biomass, direct valorization using thermochemical technologies is challenging. Therefore, biological conversion technologies are reported to be more eco-friendly and appropriate for waste biomass with high moisture content [22].
AD is a well-established process for the sustainable management of solid organic feedstock [22]. AD can be used to convert various organic substrates into methane-rich gas destined for energy generation. In this context, organic residues are conveniently used to meet global energy demand while reducing the burden of fuel consumption and waste disposal. In Europe, the success of AD is witnessed by its dynamic ascent with a total of 18,202 biogas installations, producing 11,082 MW, and 63,511 GWh worth of biogas as recorded in 2018 [23,24]. Despite this continued growth, AD technology is still not cost-competitive with natural gas without fiscal incentives. This is due to high costs associated with biogas production, whereas natural gas is available at lower cost worldwide. Therefore, increasing the efficiency of AD processes is critical to improving its economic attractiveness. To this end, feedstock pre-treatment, reactor configuration, and feedstock co-digestion have been studied as potential means of improving resource recovery [25,26].
2.3. Valorization—Selecting a Method
The selection of a particular valorization method is highly dependent on the biomass characteristics and composition. For instance, biological approaches are suitable for readily degradable, high-moisture-content biomass such as food waste. Thermochemical methods are more commonly used for recalcitrant feedstocks such as lignocellulosic biomass. While both treatment options entail installation and operation costs, research and application suggest that the biological approach may be more flexible in terms of feedstock and products. Moreover, AD and fermentative processes result in fewer undesirable effects such as tar production.
3. Sustainable Feedstock Types
3.1. Food Waste
Food waste makes up a significant portion of anthropogenically derived organic waste and constitutes an environmental burden where landfill disposal is employed. One third of all food produced in the world for human consumption goes to waste [27], with 14% of food waste occurring during production processes alone [28]. While post-consumer waste can be minimized through prevention campaigns, production wastage (peelings, damaged or diseased matter, inedible plant parts) is likely to remain at similar or increasing values. Generally, food waste is composed of fruits, vegetables, and tubers [28]. These materials all have relatively high moisture and energy contents and, therefore, qualify as high value feedstock for AD [29]. Through AD, this waste stream can be converted into a renewable resource while simultaneously reducing waste-related challenges in the long term [30].
Food waste composition varies greatly but is fundamentally a mix of carbohydrates, proteins, and lipids. The ratio of these three biomolecules largely determines the material’s energy generation potential. Lipids have higher energy content than carbohydrates and proteins; however, they have been reported to be difficult to breakdown in AD bioreactors, even destabilizing digesters at high concentrations [31,32,33]. Most food waste is primarily composed of complex carbohydrates, including lignocellulosic and/or hemicellulosic compounds (25–30% of total solids (TS)) [34,35]. These carbohydrates originate from plant matter and are challenging to hydrolyze. Indeed, hydrolysis is frequently reported as the rate limiting step in AD [36]. Efforts to facilitate hydrolysis have been made, primarily the investigation of various pre-treatment methods including alkaline [25,37], thermal [38], acid [25] and enzymatic pre-treatments [39]. However, these treatments all increase operational costs. Whereas biological strategies, such as tailoring operational conditions to promote the growth and persistence of key microbial hydrolysers within AD bioreactors, represent a promising alternative [35].
3.2. Agricultural Residues
Agricultural residual biomasses comprise crop and plant residues, vegetable waste, forest residues, grass, and livestock manure [40]. These are largely composed of lignocellulose which can be converted via AD and fermentation to bioenergy and biochemicals. The efficiency of these conversions is determined by specific lignocellulosic characteristics such as lignin content, degree of polymerization, hemicellulose structure, cellulose crystallinity, porosity, and specific area [41,42].
Many studies in the literature review the use of pre-treatments that would decrease the recalcitrance of this biomass by improving the accessibility of cellulose to cellulases. This is achieved either by decreasing the hemicellulose content (e.g., dilute acids and bases) or by applying physical treatment (e.g., high temperature and pressure) to disrupt the lignin matrix [40,42]. Of course, all pre-treatment processes entail a trade-off between the cost of pre-treatment and the desired end-product yield increase [43]. While ionic liquids and deep-eutectic solvents have been recently investigated, full-scale biorefineries generally employ steam explosion, organosolv, or dilute acids [43,44]. The use of these pretreatment technologies may negatively impact the indigenous microbiome of the feedstock, which can be critical to the fermentative process.
The use of lignocellulosic waste as feedstock for biogas production through AD is well established. However, the potential of lignocellulosic waste for VFA production has been garnering increased attention [45]. In a biorefinery context, carboxylic acids are a desirable product with high market value [46,47,48,49]. Among lignocellulosic wastes, grass is an abundant, renewable, and cheap feedstock that has been largely employed to produce biogas in AD [50]. Relatedly, silage is grass which has been fermented to facilitate preservation during storage. During fermentation, lactic acid bacteria use soluble carbohydrates present on the surface of grass in the production of lactic acid, causing a decrease in pH, which allows the feedstock to be preserved for animal feed without risk of spoilage [51].
While grass is considered a sustainable feedstock due to its carbon-sequestering capacity, co-digestion with other agricultural residues, such as cattle slurry, may further enhance the sustainability of the process [50,52]. Cattle slurry is an abundant agricultural waste and is cheaper and richer in nutrients than grassland feedstocks. Furthermore, by co-digesting this waste stream with grass, greenhouse gas (GHG) emissions from slurry are reduced [50]. The co-digestion of different grassland forages with grass could also improve AD yields due to an improvement in nutrient availability for the microbial community [53]. Moreover, the combined growth of multi-species grassland mixtures (herbs, legumes, and grass) in intensively managed grassland may enhance yield while mitigating disturbances, such as drought and environmental impact, when compared to monocultures [52].
3.3. Animal Residues
Animal manure is a primary contributor to environmental pollution in rural areas. This is usually due to emissions from land-spreading and manure storage facilities, which release harmful substances to the soil, water, and atmosphere. Animal manure/slurry has high concentrations of nutrients (such as nitrogen and phosphorus) and metals (such as copper, zinc, arsenic, and cadmium). Leaching of these metals into the surrounding environment increases phytotoxicity, reduces soil fertility and productivity, and increases toxicity of crops and food products grown on the contaminated soil [54]. Meanwhile, leaching of nutrients contributes to water quality degradation and eutrophication. Moreover, the storage and land-spreading of animal manure/slurry can release GHG, such as methane, nitrous oxide, and ammonia, into the air contributing to climate change [55,56].
To mitigate the environmental burden of the manure/slurry, researchers have engaged in developing techniques for its sustainable treatment. Although composting, incineration, pyrolysis, and gasification have been evaluated, AD is outstanding in its capacity to reduce pollution while generating valuable by-products such as fertilizers and renewable energy [54]. However, there are some factors at play which limit the use of slurry/manure fed AD: (i) slurry bio-methane potential is low due to high moisture and low organic content [55]; (ii) a large volume of feedstock, usually collected from multiple sites, is required for efficiencies of scale [54]; and (iii) slurry has a low C:N ratio, which tends to inhibit methane production.
These issues can be mitigated or avoided entirely by employing co-digestion, specifically, by making a mixed feedstock composed of slurry with other organic wastes/residues/energy crops that have a high C:N ratio. Several researchers have reported that co-digestions improved biogas production [57,58,59] or VFA production [60,61]. Although the co-digestion of manure/slurry with other feedstock provides a means to increase economic feasibility, the nutrient and metal-rich liquid digestate remains an issue in an AD-based facility. Therefore, complete valorization of the manure/slurry within an AD-based biorefinery concept could result in a more desirable digestate product.
4. State-of-the-Art System Designs
4.1. Single-Stage System Design and Application
Anaerobic bioreactors may be designed to optimize the processing of a selected biomass and for the production of a specific desired product. Many bioreactor types have the capacity to produce VFAs, hydrogen associated with VFA as a by-product, or biogas. Several reactors, including the continuous stirred tank reactor (CSTR), the packed bed biofilm column reactor, leach bed reactor (LBR), two-stage anaerobic bioreactor, and continuous flow fermentation reactors, have been used to produce VFAs (Table 1). Studies using solid feedstocks generally use CSTRs or LBRs and have generated promising results. The CSTR is perhaps the most widely used single-stage wet AD design [62]. CSTRs are suitable for materials with solids content up to 10% [63] and work by thoroughly mixing feedstock and microbes in the presence of suspended solids [64]. Previous studies have reported successful production of VFAs from food waste and OFMSW using the CSTR configuration (Table 1). However, this reactor design has significant inherent inefficiencies, including (i) a tendency for biomass washout, (ii) the need for size reduction of the substrates, (iii) energy input required for continuous stirring, and (iv) the low solids content (<10%) requirement [65,66]. In an attempt to overcome these limitations, a novel CSTR design consisting of a solid–liquid separator was proposed to retain undigested biomass with the active community in the system [67]. This approach addresses the issue of biomass washout, but not the limitations for feedstock processing (size reduction, low solids) or energy consumption.
LBRs are a promising alternative to the CSTR for VFA production from high-solid waste such as food waste, OFMSW and vegetable waste, and grass (Table 1). Compared to CSTRs, these reactors have been reported to permit higher loads and high VFA production [35,68]. In LBRs, solid material is loaded into the reactor and irrigated with water, which is recycled through the system continuously. Hydrolysis occurs in the solid bed, while fermentation occurs in the liquid phase, thus, decoupling the hydrolysis and fermentation processes. The recirculation mechanism allows for the dilution of inhibitory compounds and increases the moisture in the solid bed which facilitates micro-organism growth and activity, all with a relatively low water requirement [69,70].
Compared to CSTRs, LBRs have several financial advantages—less instrumentation, maintenance, and investment are required, making it an attractively low-cost, high-solids AD reactor [71]. However, since LBRs process solid feedstock which is not stirred, VFA product accumulation can occur. Furthermore, high levels of VFA can inhibit micro-organisms involved in the hydrolysis and fermentation stages [72,73,74]. In-line VFA separation, which could remove VFA product from the LBR leachate, is currently being investigated [75,76]. However, there is currently no consensus or single outstanding technology being used to recover VFA from fermentation liquor. Therefore, researchers have focused on developing two-stage systems in which VFAs generated in LBRs are removed and valorized through processes such as chain elongation (CE), PHA production, or even biogas production.

Table 1.
Chemical characteristics of feedstocks and inoculum sources used for VFA production.
Table 1.
Chemical characteristics of feedstocks and inoculum sources used for VFA production.
| Raw Material | TS | VS | COD/TOC | Lignocellulosic | Remarks | Ref |
|---|---|---|---|---|---|---|
| (%ww) | (%ww) | (gO2.kg−1ww) | (%TS) | |||
| Feedstock: | ||||||
| Cattle manure | 5.0–9.5 | 7.0–7.3 | 44–54 | n/r | TKN: 1.9–3.6 gN.kg−1ww | [3] |
| Ryegrass silage | 35–40 | 31.3–36.0 | 312–360 | n/r | TKN: 4.7–5.9 gN.kg−1ww | [3] |
| Napier grass | 15.12 g.L−1 | 12.65 g.L−1 | 0.92 g.g−1 | Cel: 36.81%, Hem: 26.16%, Lig: 8.27% | [77] | |
| Ryegrass silage | 25.5 | 24.1 | n/a | Cel: 34.3%, Hem: 29.6%, Lig: 8.6% | Ddata based on fresh ryegrass before ensiling | [78] |
| Food waste | 42.46 ± 0.78 | 38.45 ± 1.87 | 53.02 ± 2.29% a | n/a | TKN: 2.10 ± 0.17% | [79] |
| Dried farmland grass | 83.6 ± 0.6 | 72.8 ± 1.1 | n/a | n/a | [80] | |
| OFMSW | 12 ± 1.4 | 10.7 ± 0.7 | 102.8 ± 13.0 | n/a | Mix of feed, inoculum, and tap water to a TS of 7–8 %ww. TKN: 3.12 ± 0.51 gN.kg−1ww | [81] |
| OFMSW | 28.14 ± 4.01 | 25.98 ± 2.29 | 312.6 ± 120.8 | n/a | A mix of OFMSW and water was used as inoculum after it was acclimatized to 55 °C. TKN: 8.16 ± 1.83 gN.kg−1ww | [82] |
| Food waste | 28.19 ± 2.32 | 25.96 ± 2.08 | 376.4 ± 51.3 b, 28.6 ± 2.3 c | Cel: 2.82 ± 0.95%, Hem: 32.58 ± 4.48% | Lipids: 27.50 ± 1.45 %ww; Protein: 20.69 ± 1.17 %ww | [35] |
| Food waste | 17.8 | 17.1 | 320 gO2.L−1 b, 95 gO2.L−1 c | n/a | Lipids: 0.59 %ww; Protein: 3.79 %ww | [83] |
| Food waste | 16.5 ± 0.2 | 15.5 ± 0.7 | 264 ± 27 gO2.L−1 b | n/a | Sludge inoculum acclimatized for 5 days at 37 °C. Inoculum was treated with BES to inhibit methanogenesis | [84] |
| Kitchen waste | 128.92 ± 2.33 g.L−1 | 115.91 ± 2.84 g.L−1 | n/a | n/a | [85] | |
| Inoculum: | ||||||
| Cow manure | 16.81 g.L−1 | 11.78 g.L−1 | 0.19 g.g−1 | Cel: 18.29%, Hem: 9.07%, Lig: 11.77% | [77] | |
| Liquid digestate from the co-digestion of pig manure and grass silage | 2.30% | 1.60% | 0.58 gO2.L−1 c | n/a | Stored at 35 °C until CH4 production was minimal. | [78] |
| Cow manure | 18.22 ± 0.77 | 16.33 ± 0.76 | 50.76 ± 2.96% a | n/a | TKN: 1.40 ± 0.02% | [79] |
| Anaerobic digested food waste | 2.98 ± 0.00 | 2.67 ± 0.00 | 34.80 ± 0.98% a | n/a | TKN: 1.99 ± 0.03% | [79] |
| Anaerobic granular sludge | 9.01 ± 0.09 | 7.85 ± 0.04 | n/a | n/a | [35] | |
| Anaerobic digestion sludge | 0.4 ± 0.1 | 0.3 ± 0.1 | 10.5 ± 1.2 gO2.L−1 b, 5.1 ± 0.8 gO2.L−1,c | n/a | [84] | |
| Anaerobic digestion sludge | 31.31 ± 0.49 g.L−1 | 19.67 ± 0.35 g.L−1 | n/a | n/a | Pre-treated with heat-shock at 70 °C for 30 min | [85] |
Acronyms: TS—total solids, vs.—volatile solids, COD—chemical oxygen demand, TOC—total organic carbon, TKN—total kjendahl nitrogen, Cel—cellulose, Hem—hemicellulose, Lig—lignin, BES—2-bromoethane sulfonic, and n/r—not reported. Notes: a—TOC, b—Total COD, and c—Soluble COD.
4.2. Multi-Stage System Design and Application
A multi-stage bioreactor is, broadly, any system with two or more bioreactors. This design facilitates the segregation of different microbial processes into separate reactors, allowing the environment of each reactor to be optimized for a specific functional microbiome. Such systems are capable of efficiently treating organic waste in terms of degradation yield and biogas production [86], and of producing valuable products such as VFA, lactic acid, alcohols, and medium-chain carboxylic acids (MCCAs) [87,88,89]. In a multi-stage system, hydrolysis and acidification stages occur in one reactor, while CE, PHA production, and methanogenesis occur in a separate reactor. In this way, the inhibition of the methanogens is avoided in the first reactor and different operating conditions can be used in each stage to maximize yields. This approach has been found to be more stable than single-stage systems in treating organic waste with high solid content [90,91]. The observed enhanced performance is reportedly due to the flexibility in process control offered by two-stage systems [8,92].
The number of multi-stage systems throughout Europe was expected to rise due to their ability to handle higher loading rates and improved process stability and flexibility. However, less than 10% of AD capacity in Europe are multi-stage systems [93,94]. This discrepancy is likely due to the complexity and cost of building and operating such systems. Nevertheless, the versatility and potential of multi-stage systems to improve process performance has encouraged ongoing research, especially within the biorefinery context. The viability of the multi-stage bioreactor systems was evaluated in a previous study in which one- and two-stage systems for the enzymatic hydrolysis of a municipal solid waste were compared using a techno-economic assessment (TEA) approach. The authors reported, on average, a 15–22% return on investment (ROI) and a 4–6 year payback period (PP) for two-stage systems, compared to 4–7% and 13–25 years for one-stage systems [95]. Regalado et al. (2022) pointed out that a multi-stage processing system, in which biogas is simultaneously recovered with other value-added products, offers a possible solution for achieving a more robust circular economy [96]. In addition, multi-stage systems allow for the treatment of large quantities of recalcitrant biomass which otherwise could not be treated with one-stage systems. This enhances the carbon-neutral energy output.
5. Process Optimization for Carboxylic Acid Production
5.1. Producing Carboxylic Acids
Carboxylic acids could serve as the foundation for a circular bioeconomy; here we focus especially on lactic acid and VFAs. Lactic acid can be used by the cosmetic, dairy, and pharmaceutical industries. It is also a precursor for the synthesis of bioplastics (polylactic acid) and MCCAs [80,97]. VFAs are aliphatic organic acids with less than six carbons that, likewise, have applications in the pharmaceutical, dairy, food, animal food, textile, and cosmetic industries [97]. However, the commercialization of biogenic, mixed VFAs is still challenging, as a commercially feasible technology to recover and purify individual VFAs is still needed [98]. Alternatively, many studies have proposed the use of mixed VFAs as building blocks in a biorefinery to produce MCCAs, polyesters, PHAs, bioenergy, and electricity [98,99].
The fermentative process for VFA production requires further development. As compared to established chemical routes, the process has lower productivity and yield with higher production costs [100]. Of course, the chemical synthesis and petrochemical route produces more GHG emissions and consumes unrenewable energy sources [98]. Our review of the literature indicates that VFA production costs could be minimized by valorizing low-cost residual biomass, such as food waste, grass, and manure. Moreover, productivity yields and concentration can be improved by optimizing operational parameters such as inoculum, feedstock, temperature, pH, organic loading rate, and leachate dilution (Table 1 and Table 2). Therefore, it seems that from a sustainability standpoint, the biological production of VFA is the most promising option.

Table 2.
Summary of the operational conditions for VFA production using the raw materials described in Table 1.
5.2. Inoculum—Providing an Appropriate Microbial Community
Fermentative processes are carried out by microbial communities, and their composition and activities are directly related to reactor operational conditions. VFAs can be produced through fermentation employing either pure cultures or mixed cultures as inoculum. The use of pure culture fermentation, however, requires synthetic media, pure substrates, and media sterilization, increasing production costs [101]. Alternatively, the use of residual biomass feedstocks during mixed-culture AD does not require energy for sterilization or pure substrate supplementation, thus, providing greater cost-efficiency [99].
AD is catalyzed by a microbial consortium composed of different hydrolytic and fermentative bacteria, and methanogenic archaea [102]. VFAs are produced in the second step (acidogenesis) of the AD process, alongside lactic acid, CO2, and H2. However, complete mineralization involves the consumption of VFAs by methanogenic archaea producing acetic acid, CO2, and H2 and, ultimately biogas [50]. Therefore, to optimize VFA production, methanogenic archaea must be inhibited using chemical (e.g., BES) [83] or physical (e.g., heat-shock) [85] pre-treatments, or by manipulating operational conditions (e.g., pH) [103]. Balancing the trade-off between VFA productivity and the cost of these pre-treatments is necessary to ensure process feasibility.
The use and choice of inoculum is another crucial consideration in VFA production. The absence of inoculum in grass fermentation led to very low biomass degradation and VFA production (Table 2) [77,78]. The use of cow manure as inoculum increased VFA production as well as the degradation of grass. The quantity of inoculum was also shown to be an important consideration; adding more than 20% of cow manure to the solid fraction proved to negatively impact the process due to the high solid content inside the reactor [77]. The digestate from an anaerobic co-digestion of pig manure and silage was responsible for increasing VFA production from grass silage, enhancing biomass degradation [78]. Additionally, rumen bacteria from cow manure caused a shift in the profile of VFAs obtained from food waste, leading to increased propionic, butyric acid, and ethanol concentrations [79].
5.3. Two-Stage Design for Optimized Carboxylic Acid Production
As discussed previously, two-stage systems are preferable for the accumulation of VFAs in AD since the optimal operational conditions of acidogenesis and methanogenesis are drastically different. Moreover, a second stage reactor can also be used to further convert VFAs into MCCAs, PHAs, and other valuable products. A two-stage strategy for the AD of grass was found to increase hydrolysis of grass silage and biogas productivity [50]. Additionally, a two-stage AD of OFMSW using a mesophilic CSTR produced 24.4 g COD.L−1 of VFA while maximizing acidification [81]. While this study was designed to optimize biogas production in the second stage, the observed accumulation of propionic and valeric acids in the first stage highlight the potential for PHA production. Another two-stage study recorded grass fermentation and subsequent microbial CE of the lactic acid produced [80]. The native micro-organisms on the surface of the grass were responsible for the lactic acid production (9.36 g L−1) at low pH, while caproic acid, acetic acid, and butyric were obtained in a CE reactor. Caproic acid has very low solubility in water and is immiscible at concentrations above 11 g.L−1 (20 °C). Therefore, optimizing the operational conditions to obtain caproic acid above this concentration would be beneficial for the process, not only due to its high market value but to decrease costs associated with product recovery.
5.4. Leachate Dilution in LBRs Affects VFA Production
A recent study demonstrated that diluting LBR leachate resulted in increased VFA production and grass solubilization [77]. Reactors with undiluted leachate had lower VFA production; the degradation of grass was also limited, represented by the low soluble COD produced (51.5 g). Meanwhile, leachate dilution led to a higher production of VFAs—observed as an accumulation of acetic acid (54.17%). The accumulation of higher chain VFAs (e.g., butyric) can have inhibitory effects on micro-organisms, negatively impacting overall acid production and feedstock degradation [77,78]. As an alternative to leachate dilution, in-line selective VFA extraction may remove higher-chain VFAs with a higher market value to produce PHAs and MCCAs, while separating and concentrating the acetic acid for biomethane production in a high-rate reactor [3,97,98,104,105].
5.5. pH Directly Affects Biomass Degradability and VFA Profile
In fermentative processes, it has been found that the pH directly affects the microbial community, as well as biomass degradability [79]. Further, pH is directly correlated with VFA and H2 production in the fermentation of food waste [82]. Low pH (below 6.5) effectively inhibits methanogenesis but may also inhibit hydrolysis when lower than 4 [78,79,103]. Recently, high VFA production during fermentation of OFMSW was attributed to an operational pH of 6.6, which may have been high enough to avoid the inhibitory effects of acidic environments [81]. Moreover, studies showed that pH values ranging from 6 to 7 improved food waste hydrolysis and maize silage solubilization while increasing VFA accumulation [106,107]. In the fermentation of grass silage, slightly lower pH levels were responsible for higher VFA yield with stable operational conditions and suppressed methane generation [108]. In the fermentation of grass pellets, controlling the pH levels at 5.50 using 6 M sodium hydroxide led to an efficient degradation of grass to produce 4.5 g/L−1 of VFAs [105]. In two-stage systems, the recycling of anaerobic leachate from a second reactor can eliminate the need for external buffering agents to control the pH in a first stage LBR—resulting in overall reductions in operational costs, downstream processing, and environmental impacts [50].
5.6. Temperature Implications for VFA Accumulation
Temperature is also an important factor when considering the digestion of high-solid biomass for the production of VFAs. Although it does not affect the VFA profile as significantly as pH, temperature can affect the microbial community dynamics [101]. Lower temperatures, in particular, are reported to reduce the hydrolysis of grass but may have a positive effect on VFA accumulation [47]. Mesophilic temperatures (37 °C) during the fermentation of OFMSW prevented sudden drops in pH, which consequently prevented inhibition of VFA production [81]. Conversely, thermophilic temperatures (55 °C) during the fermentation of food waste in a similar CSTR reactor with no inoculum addition led to a 43% reduction in VFA production [82,107]. A final consideration when deciding upon an operational temperature is the cost of heating the system—additional heating can significantly add to the operational costs of the treatment. In light of these considerations, low-temperature operational conditions may be ideal for VFA generation.
5.7. Organic Loading Rate and Hydraulic Retention Time
Organic loading rate (OLR) and hydraulic retention time (HRT) are also important parameters in the production of VFAs. At higher HRTs, micro-organisms are retained in the bioreactor for longer periods, leading to more thorough conversion of the biomass. Although, a high HRT will also increase operational costs [101]. OLR is an important parameter in VFA production [109,110] — A higher OLR translates into increased overall substrate availability and may also cause a decrease in pH, inhibiting methanogenesis [103]. Indeed, increasing the OLR has been shown to successfully inhibit the methane production without the aid of additional methanogen inhibitors [83]. This inhibition and nutrient availability leads to a increased accumulation of VFAs, e.g., during fermentation of OFMSW in plug-flows, doubling the OLR resulted in a 150% increase in VFA production and 30% decrease in specific biogas production [110]. On the other hand, the pH decrease that comes with a high OLR may also function to hinder hydrolysis [78]. It was also observed that increasing the OLR and pH levels lower than 5 led to a predominance of ethanol production instead of VFAs in the fermentation of food waste in leach-bed reactors [109]. Therefore, an optimal pH range associated with both a balanced and appropriate OLR is necessary to support optimal production.
5.8. Feedstock Choice Influences VFA Profile
As discussed in Section 3, feedstock has a direct impact on VFA production (Table 1) similar to OLR. Characteristics, such as ammonia, COD, pH, and micronutrient availability, could affect the microbial conversion of the biomass to the desired acids [101]. Notably, the hydrolysis of biomasses that have higher total COD than soluble COD is directly impaired due to the degradation of particulate compounds. Feedstock type is also known to affect the profile of VFAs produced due to its characteristics and/or indigenous microbial community [80,101,107]. For example, propionic and valeric acids were the main VFAs obtained in the fermentation of food waste at 5-day HRT, 14–15 kgVS.m−3.day−1 [81], while butyric and acetic acids were the main VFAs produced from fermentation of grass and grass silage (Table 2) [77].
5.9. The Challenge: VFA Recovery and Concentration
The choice of which operational conditions to employ in VFA generation must be informed by the intended purpose of those VFAs. For example, when designing a second stage to produce biomethane or electricity using microbial fuel cells, conditions should maximize the accumulation of acetic acid [101]. However, if the primary function is to produce PHAs in a second reactor, conditions should support the accumulation of butyric acid or propionic and valeric acids. In this way, optimizing the operational conditions to produce VFAs is extremely important. Although focus has been placed on VFA production and optimization, the separation of individual VFAs remains a substantial challenge [97].
The separation of individual VFAs is inherently challenging due to (i) the similar physical properties of VFAs, (ii) their potential to form azeotropes with water, and (iii) their oftentimes low concentrations in fermentation media [99]. In one recent study attempting to isolate caproic acid, a VFA-rich stream was treated via electrochemical extraction. It successfully concentrated caproic acid above its solubility concentration in water, which resulted in the formation of a hydrophobic layer making the separation feasible with 70%wt purity [80]. However, scaled application would require external electricity supplementation. In a recent study, two techniques were investigated: a combination of ultrafiltration and reverse osmosis membrane and liquid-liquid extraction with diethyl ether and methyl-isobutyl-ketone [97]. Another combination of techniques using solid screening, microfiltration, pervaporation, and electrodialysis was successful in recovering 4.5 g.L−1 of VFAs from a 80 L reactor fermenting grass while separating the solids [105]. However, to date, the selective separation of individual acids is still unavailable, which directly impacts the commercial feasibility of isolating specific VFAs to sell as individual products [98,99].
Moreover, VFA separation from complex media, such as fermentation liquor, is primarily limited to lab-scale investigations which implement a wide variety of technologies. Analyzing trends in this work may facilitate consensus building and eventual pilot-scale and real-world applications. Current trends suggest that separation methods often consist of multiple steps and technologies, making up process cascades (Figure 2). Most require one or more media preparation steps, such as pH correction or solids removal. These steps each require equipment and operational input and greatly influence the overall efficiency of the pipeline. For instance, approximately 75% of these processes used a solid removal method (Figure 2). Most used centrifugation or filtration as opposed to more efficient, passive solids removal technologies such as tank separation. Additionally, while pH correction was implemented in most fermentation systems, it was only considered a step in the VFA separation process if (i) it was corrected to a very high or very low pH [111,112], (ii) the pH was specifically referenced as facilitating separation [113], or (iii) if the fermentation broth was pH-corrected after collection from reactor [114].
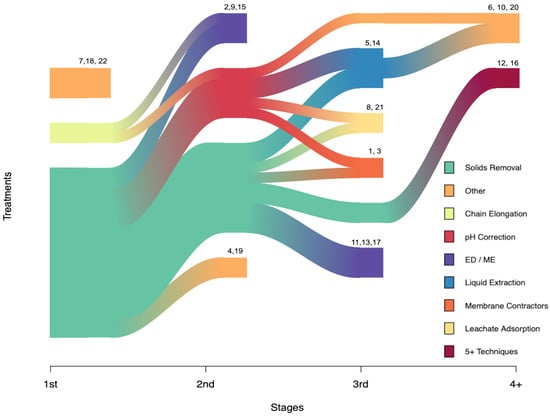
Figure 2.
Processes used to separate VFAs from fermentation liquors. Sankey plot showing the variety of VFA harvesting processes as a flow of stages (1st, 2nd, 3rd, and 4+). Technologies using similar principles and materials were grouped together; for example, electrodialysis and membrane electrolysis were grouped together since they both use membranes and current driven separation of solutes. Technologies were classified as “other” if they were used by only a single study at any given stage. References: 1—[115]; 2—[75]; 3—[114]; 4—[116]; 5—[117]; 6—[46]; 7—[113]; 8—[118]; 9—[119]; 10—[97]; 11—[120]; 12—[105]; 13—[121]; 14—[88]; 15—[80]; 16—[122]; 17—[123]; 18—[124]; 19—[125]; 20—[112]; 21—[111]; and 22—[126].
CE was used in only 10% of these treatments. Generally, CE alone is not sufficient to accomplish VFA separation. Also referred to as a secondary fermentation, it is a microbially mediated process that lengthens the carbon chains of fatty acids, making them more hydrophobic and easier to separate from an aqueous solution. Often CE requires electron donor supplementation (e.g., ethanol). However, one study instead relied on donors produced during primary fermentation [80]. Because fermentation liquors have significant levels of electron donors (e.g., lactic acid) they may be well-suited for use as feedstock for CE processes.
In VFA separation processes, research suggests that the last step is the most intensive and effective. Roughly 30% of treatments terminated with the use of electrodialysis or membrane electrolysis, 20% with membrane contractors/membrane based solvent extraction/membrane-based reactant extraction, 14% used leachate absorption, and 14% used liquid extraction (Figure 2). Only 14% of the terminal treatments were classified as “other” technologies which were used two or fewer times (e.g., distillation, pervaporation). Each separation technology generally entails a trade-off between cost and productivity [97]. The cost of using membranes with electricity is dependent upon the price of the membrane material. Meanwhile, the use of organic solvents is controversial in terms of sustainability since most of these solvents are fossil-fuel derived chemicals. The use of distillation to recover these solvents and VFAs could be feasible provided a low-cost energy source is available. As mentioned previously, some studies have avoided separation altogether by converting mixed VFAs into the desired products directly from the fermentation liquor [99,101,127,128,129]. However, more work needs to be done in this area to maximize productivity and improve commercial feasibility.
6. Innovative VFA Applications
6.1. VFAs for Bioplastic Production
PHAs are biodegradable thermoplastic polymers synthesized by micro-organisms from VFA (Figure 1) and, therefore, are considered an environmentally friendly substitution to fossil fuel-derived plastics [101,128,130]. The characteristics of the final bioplastic are directly related to the polymer chain-length and the monomers and co-monomers used in its formation [128,130,131]. Although PHAs are already commercially produced, high operational costs still hinder large-scale production of these bioplastics [130]. Most commercial productions are performed by pure or genetically modified cultures, consequently resulting in high operational costs due to downstream processing (separation, filtration, and centrifugation), energy input (media and reactor sterilization), substrate formulation (pure VFAs), and equipment cost [101,130]. However, many studies have shifted focus to the development of processes using mixed cultures and low-cost biomass, thereby improving economic feasibility [101,128,129,131,132]. The profitability of PHA production seems to be associated with selecting (i) low-cost feedstocks and (ii) specialized mixed cultures (as opposed to pure cultures) to decrease operational costs. Optimizing the conditions for PHA production would also decrease the costs associated with product recovery [101].
6.2. VFAs for Chain Elongation Chemicals
MCCAs are aliphatic and straight carboxylic acids composed of 6–12 atoms of carbon [127]. MCCAs are produced from the biological CE of ethanol and/or short-chain carboxylic acids (SCCAs) through the reverse β-oxidation pathway by anaerobic bacteria [133]. The MCCAs have longer carbon chains and are hydrophobic as compared to SCCAs. Thus, the recovery of MCCAs from liquid media is easier, translating into lower downstream separation costs for higher-market-value compounds [127]. As with PHA production, the use of mixed-species cultures rather than pure cultures would increase commercial feasibility. Therefore, many studies on optimizing the production of MCCAs from low-cost feedstock and VFA-rich streams have been conducted [35,80,115,127,134,135,136,137,138]. Challenges remain in terms of optimizing operational conditions to selectively produce the MCCAs of interest and concentrate them to a solubility level that would facilitate separation from the liquid media. Moreover, the development of effective and cheap separation processes would also increase the competitiveness of MCCAs.
6.3. VFAs for Bioenergy and Biofuel
Biogenic VFAs can also be converted into bioenergy and biofuels such as biogas, biomethane, biohydrogen, and electricity (Figure 1). Although not as profitable an application as the synthesis of PHAs and MCCAs, bioenergy is essential to the function of a biorefinery. In order to support chemical platforms of the biorefinery, VFAs can be converted to energy and used to sustainably maintain the biorefinery processes. Surplus energy could be sold to the grid, while biogas can be used as a source of heat, for combined heat and power (CHP) plants [139], or upgraded to biomethane [140]. Biohydrogen has also attracted attention as a fossil fuel substitute for transport due to its clean combustion (generating water) and high energetic value [141]. VFAs can be used to generate biohydrogen through photo fermentation and microbial electrolysis cell [101,142,143]. Alternatively, VFAs can also be used to produce electricity from microbial fuel cells [101]. These processes, however, have not yet achieved a commercial state and are highly dependent upon an acetic acid rich VFA stream to maximize efficiency.
7. Fermentative Microbial Communities
7.1. Microbial Communities—Why Bother?
Microbiology provides a validation that communities develop and adapt to conditions inside engineered systems [35,144,145,146,147]. Understanding their responses and ongoing development can give us confidence around applications of new technologies. Understanding that there are degradative processes that can be linked to biological processes and further linked to design [148,149] is critical for the future of the field. Furthermore, understanding how microbial communities develop under these conditions and underpin efficient conversions, supports the application of biotechnologies under conditions which were previously considered unsuitable. Gaining a deeper understanding of the interactions, ongoing development, and functions of built-ecosystem microbiomes will move us one step further toward harnessing their transformative metabolisms at full capacity—resulting in more efficient systems and a wider range of bioproducts at higher yields.
7.2. Key Fermentative Groups
The structure and function of a bioreactor’s microbial consortium directly depend upon the applied operating conditions; thus, community profiles vary from study to study. For example, operational choices, such as pre-treatment [150], temperature [151], inoculum [152], and even digester design [148] all induce shifts in microbial community structure and function. However, while fermentative systems support a diverse range of community profiles, several common trends and notable findings reoccur. Namely, Clostridia are consistently cited for efficient production of VFA across a wide range of studies with differing operational conditions [35,48,150,153]. Other important players in terms of efficient VFA production are Sporanaerobacter, Tissierella, Bacillus, and Firmicutes [152,153]. Interestingly, however, one study noted that Chloroflexi were negatively associated with increased VFA yields [152].
8. Future Perspectives: Biorefinery Concept, Application, and Challenges
Most existing AD plants generate biofuel and/or biochemicals in single production chains, generating low-value products or residues which are treated as waste or land spread. In contrast, AD plants can function within a biorefinery as part of a zero-waste strategy, resulting in the complete conversion of wastes into valuable products. The biorefinery concept is parallel to the refinery process in the oil industry, where crude oil is taken in and separated into a myriad of petrochemical products including fuels, lubricants, waxes, asphalt, and polymer production chemicals. Similarly, a biorefinery is a facility that takes in biogenic feedstock to produce biofuels, power, and biochemicals [154].
However, unlike an oil refinery, biorefineries must cope with an extensive variability of feedstock in terms of carbohydrate composition, recalcitrance, ash content, etc. In order to optimally produce zero-waste end-products the biorefinery must integrate physical, chemical, biological, and thermochemical processes to convert each fraction into product [2,7]. Via process integration, these systems convert heterogenous biogenic and waste streams into a multitude of value-added products. AD has great potential as a valuable core technology within the biorefinery concept (Figure 1) due to its diverse functionality. It can carry out waste remediation, bioenergy production, bio-based product synthesis, and biological pretreatment of lignocellulosic biomass [2,3,7].
AD is a proven process that generally produces energy-rich biogas as the main attractive product. However, biogas production is usually not stable in digester systems dealing with heterogeneous feedstock (e.g., food waste, grass, and slurry). Therefore, the yield of biogas is reduced, further reducing its already low economical added value. An alternative approach is to bioengineer the AD process for the production of carboxylic acids alongside biogas and other products, thereby converting biogas plants into biorefineries.
Carboxylic acids, such as lactic, succinic, and VFA, have been successfully generated from the initial anaerobic fermentation of food waste, agricultural waste (silage and cattle liquid manure), and OFMSW (Table 1). These carboxylic acids are valuable products when separated from the fermentation broth. However, due to their high solubility in water, the recovery has proven difficult and economically unattractive [125,155]. These carboxylic acids may be further processed into biogas or converted through biological and chemical process into alcohol-based fuels (e.g., ethanol and butanol) or other value-added products (e.g., PHAs and MCCAs), or they can be used directly to generate electricity in microbial fuel cells [3,7,156]. In addition to organic acids, gaseous molecular hydrogen and carbon dioxide are normally produced during the anaerobic fermentation of organic substrates. These can be biologically converted to methane [157] or chemically processed into methanol [158].
The solid residue obtained in the anaerobic treatment of biomass, known as digestate, has been viewed as a low-value product, conventionally managed as a fertilizer or animal bedding. However, recent studies have proposed innovative concepts and techniques for its valorization to biogas and bio-based products [2,3,159,160]. The digestate, together with a low VFA-liquor and biogas, can be processed to a methane-rich biogas which, in turn, can be used to supply heat and electricity for facility operation. Furthermore, studies have demonstrated that AD can act as a biological pre-treatment for lignocellulosic feedstock as it degrades hemicellulose faster than cellulose, thereby facilitating the subsequent enzymatic hydrolysis of cellulose in downstream processes [161,162]. Following the cellulose extraction, the lignin-rich residue can be thermochemically processed to biofuel or other valuable products.
Interestingly, a significant opportunity exists to valorize the nutrient-rich liquor from the digester for macro- and micro-algae production. This approach not only allows for the production of algal biomass, which can be further processed into biofuels and bio-based products, but also accomplishes nutrient-removal from AD effluents which can then be recycled back as process-water into the AD plant [7,163]. Despite the potential value offered by biomass biorefineries technological, spatial, and logistical barriers impact its economic viability, thus hindering widespread application [164]. Notably, the technological barrier seems to be the most pressing challenge as it directly affects production yield. To maximize product yield from biomass, various pre-treatment methods and enzymatic hydrolysis techniques have been used within the AD-based biorefinery. However, these techniques present many limitations (e.g., low efficiency and high cost). Apart from the technological barriers, challenges such as the recovery of products from effluent, the transportation of the fuel and feedstock also impact the success of AD-based biorefinery (Figure 3).
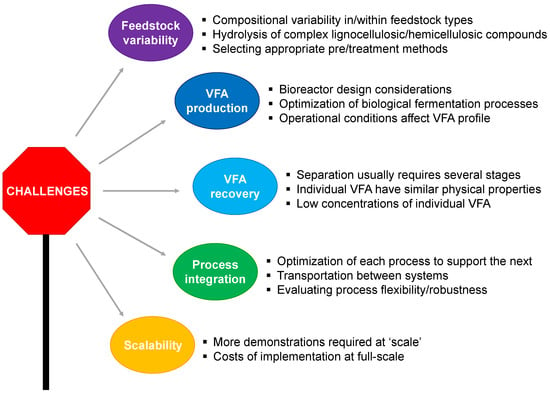
Figure 3.
Challenges and opportunities for future research. The promise of a biorefinery-based bioeconomy will rely on innovative and interdisciplinary solutions. The literature suggests that key challenges with respect to feedstock variability, VFA production and recovery, process integration, and scalability all need to be tackled.
Addressing these challenges by developing novel, sustainable, and economically viable technologies will contribute towards the development of an economically attractive biomass biorefinery. For instance, some studies have pointed out potential nanotechnology applications in pre-treatment methods [165]. The enzymatic hydrolysis pre-treatment method could be improved by using magnetic nanoparticles to immobilize hydrolytic enzymes, thus allowing them to be re-used in multiple cycles of hydrolysis [166]. Novel nanotechnological solutions should be further investigated, with an aim to improve product yield and process efficiency.
9. Conclusions
Although there is clearly room for technological advancements and for closing research gaps, literature suggests that the use of residual biomass within the biorefinery framework is paving the way for a closed-loop bioeconomy. Within such a framework, VFA production could serve as the core platform to produce both energy and a range of bio-based products. This would propel us towards a functioning circular economy that minimizes waste and maximizes production.
Author Contributions
All authors designed the review. C.N., F.C., E.D. and A.T. reviewed the literature and drafted the manuscript and V.O. revised the document. All authors approve the paper and agree for accountability of the work therein. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by grants from the Higher Education Authority (HEA) of Ireland through: the Programme for Research at Third Level Institutions, Cycle 5 (PRTLI-5), co-funded by the European Regional Development Fund (ERDF); the Enterprise Ireland Technology Centres Programme (TC/2014/0016) and Science Foundation Ireland (14/IA/2371 and 16/RC/3889).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank the funding bodies which supported this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Badgujar, K.C.; Bhanage, B.M. Dedicated and Waste Feedstocks for Biorefinery: An Approach to Develop a Sustainable Society. In Waste Biorefinery; Elsevier: Amsterdam, The Netherlands, 2018; pp. 3–38. [Google Scholar]
- Coma, M.; Martinez-Hernandez, E.; Abeln, F.; Raikova, S.; Donnelly, J.; Arnot, T.C.; Allen, M.J.; Hong, D.D.; Chuck, C.J. Organic Waste as a Sustainable Feedstock for Platform Chemicals. Faraday Discuss. 2017, 202, 175–195. [Google Scholar] [CrossRef] [PubMed]
- Righetti, E.; Nortilli, S.; Fatone, F.; Frison, N.; Bolzonella, D. A Multiproduct Biorefinery Approach for the Production of Hydrogen, Methane and Volatile Fatty Acids from Agricultural Waste. Waste Biomass Valorization 2020, 11, 5239–5246. [Google Scholar] [CrossRef]
- Wainaina, S.; Lukitawesa; Kumar Awasthi, M.; Taherzadeh, M.J. Bioengineering of Anaerobic Digestion for Volatile Fatty Acids, Hydrogen or Methane Production: A Critical Review. Bioengineered 2019, 10, 437–458. [Google Scholar] [CrossRef] [PubMed]
- Lytras, G.; Lytras, C.; Mathioudakis, D.; Papadopoulou, K.; Lyberatos, G. Food Waste Valorization Based on Anaerobic Digestion. Waste Biomass Valorization 2021, 12, 1677–1697. [Google Scholar] [CrossRef]
- Michalopoulos, I.; Lytras, G.M.; Mathioudakis, D.; Lytras, C.; Goumenos, A.; Zacharopoulos, I.; Papadopoulou, K.; Lyberatos, G. Hydrogen and Methane Production from Food Residue Biomass Product (FORBI). Waste Biomass Valorization 2020, 11, 1647–1655. [Google Scholar] [CrossRef]
- Surendra, K.C.; Sawatdeenarunat, C.; Shrestha, S.; Sung, S.; Khanal, S.K. Anaerobic Digestion-Based Biorefinery for Bioenergy and Biobased Products. Ind. Biotechnol. 2015, 11, 103–112. [Google Scholar] [CrossRef]
- Demirer, G.N.; Chen, S. Two-Phase Anaerobic Digestion of Unscreened Dairy Manure. Process Biochem. 2005, 40, 3542–3549. [Google Scholar] [CrossRef]
- Ning, P.; Yang, G.; Hu, L.; Sun, J.; Shi, L.; Zhou, Y.; Wang, Z.; Yang, J. Recent Advances in the Valorization of Plant Biomass. Biotechnol. Biofuels 2021, 14, 102. [Google Scholar] [CrossRef]
- Farzad, S.; Mandegari, M.A.; Görgens, J.F. A Critical Review on Biomass Gasification, Co-Gasification, and Their Environmental Assessments. Biofuel Res. J. 2016, 3, 483–495. [Google Scholar] [CrossRef]
- Bonilla, J.; Gordillo, G.; Cantor, C. Experimental Gasification of Coffee Husk Using Pure Oxygen-Steam Blends. Front. Energy Res. 2019, 7, 127. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, S.; Huelsman, C.M.; Savage, P.E. Products, Pathways, and Kinetics for Reactions of Indole under Supercritical Water Gasification Conditions. J. Supercrit. Fluids 2013, 73, 161–170. [Google Scholar] [CrossRef]
- Heidenreich, S.; Foscolo, P.U. New Concepts in Biomass Gasification. Prog. Energy Combust. Sci. 2015, 46, 72–95. [Google Scholar] [CrossRef]
- Kalinci, Y.; Hepbasli, A.; Dincer, I. Exergoeconomic Analysis and Performance Assessment of Hydrogen and Power Production Using Different Gasification Systems. Fuel 2012, 102, 187–198. [Google Scholar] [CrossRef]
- Sikarwar, V.S.; Zhao, M.; Clough, P.; Yao, J.; Zhong, X.; Memon, M.Z.; Shah, N.; Anthony, E.J.; Fennell, P.S. An Overview of Advances in Biomass Gasification. Energy Environ. Sci. 2016, 9, 2939–2977. [Google Scholar] [CrossRef]
- Lee, C.S.; Conradie, A.V.; Lester, E. Review of Supercritical Water Gasification with Lignocellulosic Real Biomass as the Feedstocks: Process Parameters, Biomass Composition, Catalyst Development, Reactor Design and Its Challenges. Chem. Eng. J. 2021, 415, 128837. [Google Scholar] [CrossRef]
- Hu, X.; Gholizadeh, M. Biomass Pyrolysis: A Review of the Process Development and Challenges from Initial Researches up to the Commercialisation Stage. J. Energy Chem. 2019, 39, 109–143. [Google Scholar] [CrossRef]
- Latif, H.; Zeidan, A.A.; Nielsen, A.T.; Zengler, K. Trash to Treasure: Production of Biofuels and Commodity Chemicals via Syngas Fermenting Microorganisms. Curr. Opin. Biotechnol. 2014, 27, 79–87. [Google Scholar] [CrossRef]
- Gemechu, E.D.; Kumar, A. Chapter 12—The Environmental Performance of Hydrogen Production Pathways Based on Renewable Sources; Ren, J.B.T.-R.-E.-D.F., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 375–406. ISBN 978-0-12-820539-6. [Google Scholar]
- Casademont, P.; García-Jarana, M.B.; Sánchez-Oneto, J.; Portela, J.R.; Martínez de la Ossa, E.J. Supercritical Water Gasification: A Patents Review. Rev. Chem. Eng. 2017, 33, 237–261. [Google Scholar] [CrossRef]
- De Blasio, C.; Järvinen, M. Supercritical Water Gasification of Biomass; Elsevier: Oxford, UK, 2017; pp. 171–195. ISBN 978-0-12-804792-7. [Google Scholar]
- Sevillano, C.A.; Pesantes, A.A.; Peña Carpio, E.; Martínez, E.J.; Gómez, X. Anaerobic Digestion for Producing Renewable Energy—The Evolution of This Technology in a New Uncertain Scenario. Entropy 2021, 23, 145. [Google Scholar] [CrossRef]
- Cesaro, A. The Valorization of the Anaerobic Digestate from the Organic Fractions of Municipal Solid Waste: Challenges and Perspectives. J. Environ. Manag. 2021, 280, 111742. [Google Scholar] [CrossRef]
- EBA European Biogas Association. EBA European Biogas Association Annual Report; EBA European Biogas Association: Brussels, Belgium, 2020; p. 38. [Google Scholar]
- Cheah, Y.-K.; Vidal-Antich, C.; Dosta, J.; Mata-Álvarez, J. Volatile Fatty Acid Production from Mesophilic Acidogenic Fermentation of Organic Fraction of Municipal Solid Waste and Food Waste under Acidic and Alkaline PH. Environ. Sci. Pollut. Res. 2019, 26, 35509–35522. [Google Scholar] [CrossRef]
- Menzel, T.; Neubauer, P.; Junne, S. Role of Microbial Hydrolysis in Anaerobic Digestion. Energies 2020, 13, 5555. [Google Scholar] [CrossRef]
- FAO. Food Loss and Food Waste: Causes and Solutions; Food and Agriculture Organization: Rome, Italy, 2011; ISBN 9781788975391. [Google Scholar]
- FAO. The State of Food and Agriculture 2019: Moving forward on Food Loss and Waste Reduction; Food and Agriculture Organization: Rome, Italy, 2019; ISBN 9781315764788. [Google Scholar]
- Matharu, A.S.; de Melo, E.M.; Houghton, J.A. Opportunity for High Value-Added Chemicals from Food Supply Chain Wastes. Bioresour. Technol. 2016, 215, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Morales-Polo, C.; Cledera-Castro, M.D.M.; Moratilla Soria, B.Y. Reviewing the Anaerobic Digestion of Food Waste: From Waste Generation and Anaerobic Process to Its Perspectives. Appl. Sci. 2018, 8, 1804. [Google Scholar] [CrossRef]
- Fernández, A.; Sanchez, A.; Font, X. Anaerobic Co-Digestion of a Simulated Organic Fraction of Municipal Solid Wastes and Fats of Animal and Vegetable Origin. Biochem. Eng. J. 2005, 26, 22–28. [Google Scholar] [CrossRef]
- Girault, R.; Bridoux, G.; Nauleau, F.; Poullain, C.; Buffet, J.; Peu, P.; Sadowski, A.G.; Béline, F. Anaerobic Co-Digestion of Waste Activated Sludge and Greasy Sludge from Flotation Process: Batch versus CSTR Experiments to Investigate Optimal Design. Bioresour. Technol. 2012, 105, 1–8. [Google Scholar] [CrossRef]
- Neves, L.; Goncalo, E.; Oliveira, R.; Alves, M.M. Influence of Composition on the Biomethanation Potential of Restaurant Waste at Mesophilic Temperatures. Waste Manag. 2008, 28, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Fisgativa, H.; Tremier, A.; Dabert, P. Characterizing the Variability of Food Waste Quality: A Need for Efficient Valorisation through Anaerobic Digestion. Waste Manag. 2016, 50, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Nzeteu, C.O.; Trego, A.C.; Abram, F.; O’Flaherty, V. Reproducible, High-Yielding, Biological Caproate Production from Food Waste Using a Single-Phase Anaerobic Reactor System. Biotechnol. Biofuels 2018, 11, 108. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Frear, C.; Wang, Z.; Yu, L.; Zhao, Q.; Li, X.; Chen, S. A Simple Methodology for Rate-Limiting Step Determination for Anaerobic Digestion of Complex Substrates and Effect of Microbial Community Ratio. Bioresour. Technol. 2013, 134, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Ji, S.; Huang, D.; Huang, Z.; Huang, Z.; Zeng, Y.; Liu, Y. Impact of Alkaline Pretreatment to Enhance Volatile Fatty Acids (VFAs) Production from Rice Husk. Biochem. Res. Int. 2019, 2019, 8489747. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Jiang, H.; Chang, J.; Sun, J.; Tu, W.; Wang, H. Effect of Thermal Hydrolysis Pretreatment on Volatile Fatty Acids Production in Sludge Acidification and Subsequent Polyhydroxyalkanoates Production. Bioresour. Technol. 2019, 279, 92–100. [Google Scholar] [CrossRef]
- Kim, J.K.; Oh, B.R.; Chun, Y.N.; Kim, S.W. Effects of Temperature and Hydraulic Retention Time on Anaerobic Digestion of Food Waste. J. Biosci. Bioeng. 2006, 102, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Paudel, S.R.; Banjara, S.P.; Choi, O.K.; Park, K.Y.; Kim, Y.M.; Lee, J.W. Pretreatment of Agricultural Biomass for Anaerobic Digestion: Current State and Challenges. Bioresour. Technol. 2017, 245, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.K.; Jagtap, S.S.; Bedekar, A.A.; Bhatia, R.K.; Rajendran, K.; Pugazhendhi, A.; Rao, C.V.; Atabani, A.E.; Kumar, G.; Yang, Y.-H. Renewable Biohydrogen Production from Lignocellulosic Biomass Using Fermentation and Integration of Systems with Other Energy Generation Technologies. Sci. Total Environ. 2020, 765, 144429. [Google Scholar] [CrossRef] [PubMed]
- Zoghlami, A.; Paës, G. Lignocellulosic Biomass: Understanding Recalcitrance and Predicting Hydrolysis. Front. Chem. 2019, 7, 874. [Google Scholar] [CrossRef] [PubMed]
- Galbe, M.; Wallberg, O. Pretreatment for Biorefineries: A Review of Common Methods for Efficient Utilisation of Lignocellulosic Materials. Biotechnol. Biofuels 2019, 12, 294. [Google Scholar] [CrossRef] [PubMed]
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent Trends in the Pretreatment of Lignocellulosic Biomass for Value-Added Products. Front. Energy Res. 2018, 6, 141. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, L.; Loh, K.-C. Review and Perspectives of Enhanced Volatile Fatty Acids Production from Acidogenic Fermentation of Lignocellulosic Biomass Wastes. Bioresour. Bioprocess. 2021, 8, 68. [Google Scholar] [CrossRef]
- Cerrone, F.; Choudhari, S.K.; Davis, R.; Cysneiros, D.; O’Flaherty, V.; Duane, G.; Casey, E.; Guzik, M.W.; Kenny, S.T.; Babu, R.P.; et al. Medium Chain Length Polyhydroxyalkanoate (Mcl-PHA) Production from Volatile Fatty Acids Derived from the Anaerobic Digestion of Grass. Appl. Microbiol. Biotechnol. 2014, 98, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Cysneiros, D.; Thuillier, A.; Villemont, R.; Littlestone, A.; Mahony, T.; O’Flaherty, V. Temperature Effects on the Trophic Stages of Perennial Rye Grass Anaerobic Digestion. Water Sci. Technol. 2011, 64, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Joyce, A.; Ijaz, U.Z.; Nzeteu, C.; Vaughan, A.; Shirran, S.L.; Botting, C.H.; Quince, C.; O’Flaherty, V.; Abram, F. Linking Microbial Community Structure and Function during the Acidified Anaerobic Digestion of Grass. Front. Microbiol. 2018, 9, 540. [Google Scholar] [CrossRef]
- Tilman, D.; Hill, J.; Lehman, C. Carbon-Negative Biofuels from Low-Input High-Diversity Grassland Biomass. Science 2006, 314, 1598–1600. [Google Scholar] [CrossRef]
- Murphy, J.; Korres, N.; Singh, A.; Smyth, B.; Nizami, A.S.; Thamsiriroj, T. The Potential for Grass Biomethane as a Biofuel Compressed Biomethane Generated from Grass, Utilised as A Transport Biofuel CCRP Report; Environmental Protection Agency: Wexford, Ireland, 2013; ISBN 9781840954272.
- Kung, L.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage Review: Interpretation of Chemical, Microbial, and Organoleptic Components of Silages; Elsevier: Amsterdam, The Netherlands, 2018; Volume 101. [Google Scholar]
- Grange, G.; Finn, J.A.; Brophy, C. Plant Diversity Enhanced Yield and Mitigated Drought Impacts in Intensively Managed Grassland Communities. J. Appl. Ecol. 2021, 58, 1864–1875. [Google Scholar] [CrossRef]
- Cong, W.-F.; Moset, V.; Feng, L.; Møller, H.B.; Eriksen, J. Anaerobic Co-Digestion of Grass and Forbs—Influence of Cattle Manure or Grass Based Inoculum. Biomass Bioenergy 2018, 119, 90–96. [Google Scholar] [CrossRef]
- Díaz-Vázquez, D.; Alvarado-Cummings, S.C.; Meza-Rodríguez, D.; Senés-Guerrero, C.; de Anda, J.; Gradilla-Hernández, M.S. Evaluation of Biogas Potential from Livestock Manures and Multicriteria Site Selection for Centralized Anaerobic Digester Systems: The Case of Jalisco, México. Sustainability 2020, 12, 3527. [Google Scholar] [CrossRef]
- Nolan, S.; Thorn, C.E.; Ashekuzzaman, S.M.; Kavanagh, I.; Nag, R.; Bolton, D.; Cummins, E.; O’Flaherty, V.; Abram, F.; Richards, K.; et al. Landspreading with Co-Digested Cattle Slurry, with or without Pasteurisation, as a Mitigation Strategy against Pathogen, Nutrient and Metal Contamination Associated with Untreated Slurry. Sci. Total Environ. 2020, 744, 140841. [Google Scholar] [CrossRef]
- Wolter, M.; Prayitno, S.; Schuchardt, F. Greenhouse Gas Emission during Storage of Pig Manure on a Pilot Scale. Bioresour. Technol. 2004, 95, 235–244. [Google Scholar] [CrossRef]
- Clemens, J.; Trimborn, M.; Weiland, P.; Amon, B. Mitigation of Greenhouse Gas Emissions by Anaerobic Digestion of Cattle Slurry. Agric. Ecosyst. Environ. 2006, 112, 171–177. [Google Scholar] [CrossRef]
- Kougias, P.G.; Kotsopoulos, T.A.; Martzopoulos, G.G. Effect of Feedstock Composition and Organic Loading Rate during the Mesophilic Co-Digestion of Olive Mill Wastewater and Swine Manure. Renew. Energy 2014, 69, 202–207. [Google Scholar] [CrossRef]
- Orive, M.; Cebrián, M.; Zufía, J. Techno-Economic Anaerobic Co-Digestion Feasibility Study for Two-Phase Olive Oil Mill Pomace and Pig Slurry. Renew. Energy 2016, 97, 532–540. [Google Scholar] [CrossRef]
- Tampio, E.A.; Blasco, L.; Vainio, M.M.; Kahala, M.M.; Rasi, S.E. Volatile Fatty Acids (VFAs) and Methane from Food Waste and Cow Slurry: Comparison of Biogas and VFA Fermentation Processes. GCB Bioenergy 2019, 11, 72–84. [Google Scholar] [CrossRef]
- Yin, D.; Mahboubi, A.; Wainaina, S.; Qiao, W.; Taherzadeh, M.J. The Effect of Mono-and Multiple Fermentation Parameters on Volatile Fatty Acids (VFAs) Production from Chicken Manure via Anaerobic Digestion. Bioresour. Technol. 2021, 330, 124992. [Google Scholar] [CrossRef]
- Moran, S. An Applied Guide to Process and Plant Design; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 0128148616. [Google Scholar]
- Van, D.P.; Fujiwara, T.; Leu Tho, B.; Song Toan, P.P.; Hoang Minh, G. A Review of Anaerobic Digestion Systems for Biodegradable Waste: Configurations, Operating Parameters, and Current Trends. Environ. Eng. Res. 2020, 25, 1–17. [Google Scholar] [CrossRef]
- Pastor-Poquet, V.; Papirio, S.; Steyer, J.-P.; Trably, E.; Escudié, R.; Esposito, G. High-Solids Anaerobic Digestion Model for Homogenized Reactors. Water Res. 2018, 142, 501–511. [Google Scholar] [CrossRef]
- Kariyama, I.D.; Zhai, X.; Wu, B. Influence of Mixing on Anaerobic Digestion Efficiency in Stirred Tank Digesters: A Review. Water Res. 2018, 143, 503–517. [Google Scholar] [CrossRef] [PubMed]
- Lindmark, J.; Thorin, E.; Fdhila, R.B.; Dahlquist, E. Effects of Mixing on the Result of Anaerobic Digestion. Renew. Sustain. Energy Rev. 2014, 40, 1030–1047. [Google Scholar] [CrossRef]
- Karthikeyan, O.P.; Selvam, A.; Wong, J.W.C. Hydrolysis–Acidogenesis of Food Waste in Solid–Liquid-Separating Continuous Stirred Tank Reactor (SLS-CSTR) for Volatile Organic Acid Production. Bioresour. Technol. 2016, 200, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Doğan, E.; Demirer, G.N. Volatile Fatty Acid Production from Organic Fraction of Municipal Solid Waste through Anaerobic Acidogenic Digestion. Environ. Eng. Sci. 2009, 26, 1443–1450. [Google Scholar] [CrossRef]
- Pommier, S.; Chenu, D.; Quintard, M.; Lefebvre, X. A Logistic Model for the Prediction of the Influence of Water on the Solid Waste Methanization in Landfills. Biotechnol. Bioeng. 2007, 97, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Sanphoti, N.; Towprayoon, S.; Chaiprasert, P.; Nopharatana, A. The Effects of Leachate Recirculation with Supplemental Water Addition on Methane Production and Waste Decomposition in a Simulated Tropical Landfill. J. Environ. Manag. 2006, 81, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Degueurce, A.; Trémier, A.; Peu, P. Dynamic Effect of Leachate Recirculation on Batch Mode Solid State Anaerobic Digestion: Influence of Recirculated Volume, Leachate to Substrate Ratio and Recirculation Periodicity. Bioresour. Technol. 2016, 216, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Cadavid-Rodríguez, L.S.; Horan, N.J. Production of Volatile Fatty Acids from Wastewater Screenings Using a Leach-Bed Reactor. Water Res. 2014, 60, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, S.; Sarkar, O.; Swamy, Y.V.; Mohan, S.V. Acidogenic Fermentation of Food Waste for Volatile Fatty Acid Production with Co-Generation of Biohydrogen. Bioresour. Technol. 2015, 182, 103–113. [Google Scholar] [CrossRef]
- Hussain, A.; Filiatrault, M.; Guiot, S.R. Acidogenic Digestion of Food Waste in a Thermophilic Leach Bed Reactor: Effect of PH and Leachate Recirculation Rate on Hydrolysis and Volatile Fatty Acid Production. Bioresour. Technol. 2017, 245, 1–9. [Google Scholar] [CrossRef]
- Arslan, D.; Zhang, Y.; Steinbusch, K.J.J.; Diels, L.; Hamelers, H.V.M.; Buisman, C.J.N.; De Wever, H. In-Situ Carboxylate Recovery and Simultaneous PH Control with Tailor-Configured Bipolar Membrane Electrodialysis during Continuous Mixed Culture Fermentation. Sep. Purif. Technol. 2017, 175, 27–35. [Google Scholar] [CrossRef]
- Roume, H.; Arends, J.; Ameril, C.P.; Patil, S.A.; Rabaey, K. Enhanced Product Recovery from Glycerol Fermentation into 3-Carbon Compounds in a Bioelectrochemical System Combined with in Situ Extraction. Front. Bioeng. Biotechnol. 2016, 4, 73. [Google Scholar] [CrossRef] [PubMed]
- Kullavanijaya, P.; Chavalparit, O. The Production of Volatile Fatty Acids from Napier Grass via an Anaerobic Leach Bed Process: The Influence of Leachate Dilution, Inoculum, Recirculation, and Buffering Agent Addition. J. Environ. Chem. Eng. 2019, 7, 103458. [Google Scholar] [CrossRef]
- Xie, S.; Lawlor, P.G.; Frost, J.P.; Wu, G.; Zhan, X. Hydrolysis and Acidification of Grass Silage in Leaching Bed Reactors. Bioresour. Technol. 2012, 114, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.H.; Selvam, A.; Wong, J.W.C. Application of Rumen Microbes to Enhance Food Waste Hydrolysis in Acidogenic Leach-Bed Reactors. Bioresour. Technol. 2014, 168, 64–71. [Google Scholar] [CrossRef]
- Khor, W.C.; Andersen, S.; Vervaeren, H.; Rabaey, K. Electricity-Assisted Production of Caproic Acid from Grass. Biotechnol. Biofuels 2017, 10, 180. [Google Scholar] [CrossRef]
- Valentino, F.; Munarin, G.; Biasiolo, M.; Cavinato, C.; Bolzonella, D.; Pavan, P. Enhancing Volatile Fatty Acids (VFA) Production from Food Waste in a Two-Phases Pilot-Scale Anaerobic Digestion Process. J. Environ. Chem. Eng. 2021, 9, 106062. [Google Scholar] [CrossRef]
- Gottardo, M.; Micolucci, F.; Bolzonella, D.; Uellendahl, H.; Pavan, P. Pilot Scale Fermentation Coupled with Anaerobic Digestion of Food Waste—Effect of Dynamic Digestate Recirculation. Renew. Energy 2017, 114, 455–463. [Google Scholar] [CrossRef]
- Lukitawesa; Patinvoh, R.J.; Millati, R.; Sárvári-Horváth, I.; Taherzadeh, M.J. Factors Influencing Volatile Fatty Acids Production from Food Wastes via Anaerobic Digestion. Bioengineered 2020, 11, 39–52. [Google Scholar] [CrossRef]
- Xiong, Z.; Hussain, A.; Lee, J.; Lee, H.-S. Food Waste Fermentation in a Leach Bed Reactor: Reactor Performance, and Microbial Ecology and Dynamics. Bioresour. Technol. 2019, 274, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Swiatkiewicz, J.; Slezak, R.; Krzystek, L.; Ledakowicz, S. Production of Volatile Fatty Acids in a Semi-Continuous Dark Fermentation of Kitchen Waste: Impact of Organic Loading Rate and Hydraulic Retention Time. Energies 2021, 14, 2993. [Google Scholar] [CrossRef]
- Pohland, F.G.; Ghosh, S. Developments in Anaerobic Stabilization of Organic Wastes-the Two-Phase Concept. Environ. Lett. 1971, 1, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Grootscholten, T.I.M.; Strik, D.; Steinbusch, K.J.J.; Buisman, C.J.N.; Hamelers, H.V.M. Two-Stage Medium Chain Fatty Acid (MCFA) Production from Municipal Solid Waste and Ethanol. Appl. Energy 2014, 116, 223–229. [Google Scholar] [CrossRef]
- Kannengiesser, J.; Sakaguchi-Söder, K.; Mrukwia, T.; Jager, J.; Schebek, L. Extraction of Medium Chain Fatty Acids from Organic Municipal Waste and Subsequent Production of Bio-Based Fuels. Waste Manag. 2016, 47, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Hao, J.; Guzman, J.J.L.; Spirito, C.M.; Harroff, L.A.; Angenent, L.T. Temperature-Phased Conversion of Acid Whey Waste into Medium-Chain Carboxylic Acids Via Lactic Acid: No External e-Donor. Joule 2019, 3, 885–888. [Google Scholar] [CrossRef]
- Fezzani, B.; Cheikh, R. Ben Two-Phase Anaerobic Co-Digestion of Olive Mill Wastes in Semi-Continuous Digesters at Mesophilic Temperature. Bioresour. Technol. 2010, 101, 1628–1634. [Google Scholar] [CrossRef]
- Khalid, A.; Arshad, M.; Anjum, M.; Mahmood, T.; Dawson, L. The Anaerobic Digestion of Solid Organic Waste. Waste Manag. 2011, 31, 1737–1744. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.K.; Park, S.C.; Chang, H.N. Biochemical Methane Potential and Solid State Anaerobic Digestion of Korean Food Wastes. Bioresour. Technol. 1995, 52, 245–253. [Google Scholar] [CrossRef]
- De Baere, L.; Mattheeuws, B. Anaerobic Digestion of the Organic Fraction of Municipal Solid Waste in Europe-Status, Experience and Prospects. In Proceedings of the ISTANBUL3WCONGRESS 2013, Istanbul, Turkey, 22–24 May 2013; Volume 38. [Google Scholar]
- Rapport, J.; Zhang, R.; Jenkins, B.M.; Williams, R.B. Current Anaerobic Digestion Technologies Used for Treatment of Municipal Organic Solid Waste; California Integrated Waste Management Board: Sacramento, CA, USA, 2008; Volume 236.
- Climent Barba, F.; Grasham, O.; Puri, D.J.; Blacker, A.J. A Simple Techno-Economic Assessment for Scaling-Up the Enzymatic Hydrolysis of MSW Pulp. Front. Energy Res. 2022, 10. [Google Scholar] [CrossRef]
- Hernández Regalado, R.E.; Häner, J.; Brügging, E.; Tränckner, J. Techno-Economic Assessment of Solid–Liquid Biogas Treatment Plants for the Agro-Industrial Sector. Energies 2022, 15, 4413. [Google Scholar] [CrossRef]
- Jänisch, T.; Reinhardt, S.; Pohsner, U.; Böringer, S.; Bolduan, R.; Steinbrenner, J.; Oechsner, H. Separation of Volatile Fatty Acids from Biogas Plant Hydrolysates. Sep. Purif. Technol. 2019, 223, 264–273. [Google Scholar] [CrossRef]
- Atasoy, M.; Owusu-Agyeman, I.; Plaza, E.; Cetecioglu, Z. Bio-Based Volatile Fatty Acid Production and Recovery from Waste Streams: Current Status and Future Challenges. Bioresour. Technol. 2018, 268, 773–786. [Google Scholar] [CrossRef]
- Ramos-Suarez, M.; Zhang, Y.; Outram, V. Current Perspectives on Acidogenic Fermentation to Produce Volatile Fatty Acids from Waste. Rev. Environ. Sci. Bio/Technol. 2021, 20, 439–478. [Google Scholar] [CrossRef]
- Luo, H.; Yang, R.; Zhao, Y.; Wang, Z.; Liu, Z.; Huang, M.; Zeng, Q. Recent Advances and Strategies in Process and Strain Engineering for the Production of Butyric Acid by Microbial Fermentation. Bioresour. Technol. 2018, 253, 343–354. [Google Scholar] [CrossRef]
- Lee, W.S.; Chua, A.S.M.; Yeoh, H.K.; Ngoh, G.C. A Review of the Production and Applications of Waste-Derived Volatile Fatty Acids. Chem. Eng. J. 2014, 235, 83–99. [Google Scholar] [CrossRef]
- Biosantech, T.A.S.; Rutz, D.; Janssen, R.; Drosg, B. 2—Biomass Resources for Biogas Production. In Woodhead Publishing Series in Energy; Wellinger, A., Murphy, J., Baxter, D.B.T.-T.B.H., Eds.; Woodhead Publishing: Cambridge, UK, 2013; pp. 19–51. ISBN 978-0-85709-498-8. [Google Scholar]
- Magdalena, J.A.; Greses, S.; González-Fernández, C. Impact of Organic Loading Rate in Volatile Fatty Acids Production and Population Dynamics Using Microalgae Biomass as Substrate. Sci. Rep. 2019, 9, 18374. [Google Scholar] [CrossRef] [PubMed]
- Vartoukian, S.R.; Palmer, R.M.; Wade, W.G. The Division Synergistes. Anaerobe 2007, 13, 99–106. [Google Scholar] [CrossRef]
- Jones, R.J.; Massanet-Nicolau, J.; Fernandez–Feito, R.; Dinsdale, R.M.; Guwy, A.J. Fermentative Volatile Fatty Acid Production and Recovery from Grass Using a Novel Combination of Solids Separation, Pervaporation, and Electrodialysis Technologies. Bioresour. Technol. 2021, 342, 125926. [Google Scholar] [CrossRef] [PubMed]
- Cysneiros, D.; Banks, C.J.; Heaven, S.; Karatzas, K.-A.G. The Effect of PH Control and ‘Hydraulic Flush’ on Hydrolysis and Volatile Fatty Acids (VFA) Production and Profile in Anaerobic Leach Bed Reactors Digesting a High Solids Content Substrate. Bioresour. Technol. 2012, 123, 263–271. [Google Scholar] [CrossRef]
- Moza, A.; Ram, N.R.; Srivastava, N.K.; Nikhil, G.N. Bioprocessing of Low-Value Food Waste to High Value Volatile Fatty Acids for Applications in Energy and Materials: A Review on Process-Flow. Bioresour. Technol. Rep. 2022, 19, 101123. [Google Scholar] [CrossRef]
- Steinbrenner, J.; Oskina, A.; Müller, J.; Oechsner, H. PH-Depended Flushing in an Automatized Batch Leach Bed Reactor System for Volatile Fatty Acid Production. Bioresour. Technol. 2022, 360, 127611. [Google Scholar] [CrossRef]
- Campuzano, R.; Trejo-Aguilar, G.M.; Cuetero-Martínez, Y.; Ramírez-Vives, F.; Monroy, O. Acidogenesis of Food Wastes at Variable Inlet and Operational Conditions. Environ. Technol. Innov. 2022, 25, 102162. [Google Scholar] [CrossRef]
- Rossi, E.; Pecorini, I.; Paoli, P.; Iannelli, R. Plug-Flow Reactor for Volatile Fatty Acid Production from the Organic Fraction of Municipal Solid Waste: Influence of Organic Loading Rate. J. Environ. Chem. Eng. 2022, 10, 106963. [Google Scholar] [CrossRef]
- Yousuf, A.; Bonk, F.; Bastidas-Oyanedel, J.-R.; Schmidt, J.E. Recovery of Carboxylic Acids Produced during Dark Fermentation of Food Waste by Adsorption on Amberlite IRA-67 and Activated Carbon. Bioresour. Technol. 2016, 217, 137–140. [Google Scholar] [CrossRef]
- Yin, J.; Wang, K.; Yang, Y.; Shen, D.; Wang, M.; Mo, H. Improving Production of Volatile Fatty Acids from Food Waste Fermentation by Hydrothermal Pretreatment. Bioresour. Technol. 2014, 171, 323–329. [Google Scholar] [CrossRef]
- Dessì, P.; Asunis, F.; Ravishankar, H.; Cocco, F.G.; De Gioannis, G.; Muntoni, A.; Lens, P.N.L. Fermentative Hydrogen Production from Cheese Whey with In-Line, Concentration Gradient-Driven Butyric Acid Extraction. Int. J. Hydrogen Energy 2020, 45, 24453–24466. [Google Scholar] [CrossRef]
- Aydin, S.; Yesil, H.; Tugtas, A.E. Recovery of Mixed Volatile Fatty Acids from Anaerobically Fermented Organic Wastes by Vapor Permeation Membrane Contactors. Bioresour. Technol. 2018, 250, 548–555. [Google Scholar] [CrossRef]
- Agler, M.T.; Spirito, C.M.; Usack, J.G.; Werner, J.J.; Angenent, L.T. Chain Elongation with Reactor Microbiomes: Upgrading Dilute Ethanol to Medium-Chain Carboxylates. Energy Environ. Sci. 2012, 5, 8189–8192. [Google Scholar] [CrossRef]
- Bak, C.; Yun, Y.-M.; Kim, J.-H.; Kang, S. Electrodialytic Separation of Volatile Fatty Acids from Hydrogen Fermented Food Wastes. Int. J. Hydrogen Energy 2019, 44, 3356–3362. [Google Scholar] [CrossRef]
- Braune, M.; Yuan, B.; Sträuber, H.; McDowall, S.C.; Nitzsche, R.; Gröngröft, A. A Downstream Processing Cascade for Separation of Caproic and Caprylic Acid from Maize Silage-Based Fermentation Broth. Front. Bioeng. Biotechnol. 2021, 9, 725578. [Google Scholar] [CrossRef] [PubMed]
- Fernando-Foncillas, C.; Cabrera-Rodríguez, C.I.; Caparrós-Salvador, F.; Varrone, C.; Straathof, A.J.J. Highly Selective Recovery of Medium Chain Carboxylates from Co-Fermented Organic Wastes Using Anion Exchange with Carbon Dioxide Expanded Methanol Desorption. Bioresour. Technol. 2021, 319, 124178. [Google Scholar] [CrossRef]
- Hassan, G.K.; Jones, R.J.; Massanet-Nicolau, J.; Dinsdale, R.; Abo-Aly, M.M.; El-Gohary, F.A.; Guwy, A. Increasing 2-Bio-(H2 and CH4) Production from Food Waste by Combining Two-Stage Anaerobic Digestion and Electrodialysis for Continuous Volatile Fatty Acids Removal. Waste Manag. 2021, 129, 20–25. [Google Scholar] [CrossRef]
- Jones, R.J.; Fernández-Feito, R.; Massanet-Nicolau, J.; Dinsdale, R.; Guwy, A. Continuous Recovery and Enhanced Yields of Volatile Fatty Acids from a Continually-Fed 100 L Food Waste Bioreactor by Filtration and Electrodialysis. Waste Manag. 2021, 122, 81–88. [Google Scholar] [CrossRef]
- Jones, R.J.; Massanet-Nicolau, J.; Fernandez-Feito, R.; Dinsdale, R.M.; Guwy, A.J. Recovery and Enhanced Yields of Volatile Fatty Acids from a Grass Fermentation via In-Situ Solids Separation and Electrodialysis. J. Clean. Prod. 2021, 296, 126430. [Google Scholar] [CrossRef]
- Kucek, L.A.; Xu, J.; Nguyen, M.; Angenent, L.T. Waste Conversion into N-Caprylate and n-Caproate: Resource Recovery from Wine Lees Using Anaerobic Reactor Microbiomes and in-Line Extraction. Front. Microbiol. 2016, 7, 1892. [Google Scholar] [CrossRef]
- Tao, B.; Passanha, P.; Kumi, P.; Wilson, V.; Jones, D.; Esteves, S. Recovery and Concentration of Thermally Hydrolysed Waste Activated Sludge Derived Volatile Fatty Acids and Nutrients by Microfiltration, Electrodialysis and Struvite Precipitation for Polyhydroxyalkanoates Production. Chem. Eng. J. 2016, 295, 11–19. [Google Scholar] [CrossRef]
- Yesil, H.; Calli, B.; Tugtas, A.E. A Hybrid Dry-Fermentation and Membrane Contactor System: Enhanced Volatile Fatty Acid (VFA) Production and Recovery from Organic Solid Wastes. Water Res. 2021, 192, 116831. [Google Scholar] [CrossRef]
- Yesil, H.; Tugtas, A.E.; Bayrakdar, A.; Calli, B. Anaerobic Fermentation of Organic Solid Wastes: Volatile Fatty Acid Production and Separation. Water Sci. Technol. 2014, 69, 2132–2138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Angelidaki, I. Bioelectrochemical Recovery of Waste-Derived Volatile Fatty Acids and Production of Hydrogen and Alkali. Water Res. 2015, 81, 188–195. [Google Scholar] [CrossRef] [PubMed]
- De Groof, V.; Coma, M.; Arnot, T.; Leak, D.J.; Lanham, A.B. Medium Chain Carboxylic Acids from Complex Organic Feedstocks by Mixed Culture Fermentation. Molecules 2019, 24, 398. [Google Scholar] [CrossRef] [PubMed]
- Mannina, G.; Presti, D.; Montiel-Jarillo, G.; Carrera, J.; Suárez-Ojeda, M.E. Recovery of Polyhydroxyalkanoates (PHAs) from Wastewater: A Review. Bioresour. Technol. 2020, 297, 122478. [Google Scholar] [CrossRef]
- Pagliano, G.; Galletti, P.; Samorì, C.; Zaghini, A.; Torri, C. Recovery of Polyhydroxyalkanoates From Single and Mixed Microbial Cultures: A Review. Front. Bioeng. Biotechnol. 2021, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Sabapathy, P.C.; Devaraj, S.; Meixner, K.; Anburajan, P.; Kathirvel, P.; Ravikumar, Y.; Zabed, H.M.; Qi, X. Recent Developments in Polyhydroxyalkanoates (PHAs) Production—A Review. Bioresour. Technol. 2020, 306, 123132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Alvarez-Gaitan, J.P.; Dastyar, W.; Saint, C.P.; Zhao, M.; Short, M.D. Value-Added Products Derived from Waste Activated Sludge: A Biorefinery Perspective. Water 2018, 10, 545. [Google Scholar] [CrossRef]
- Costa, S.S.; Miranda, A.L.; de Morais, M.G.; Costa, J.A.V.; Druzian, J.I. Microalgae as Source of Polyhydroxyalkanoates (PHAs)—A Review. Int. J. Biol. Macromol. 2019, 131, 536–547. [Google Scholar] [CrossRef]
- Angenent, L.T.; Richter, H.; Buckel, W.; Spirito, C.M.; Steinbusch, K.J.J.; Plugge, C.M.; Strik, D.P.B.T.B.; Grootscholten, T.I.M.; Buisman, C.J.N.; Hamelers, H.V.M. Chain Elongation with Reactor Microbiomes: Open-Culture Biotechnology To Produce Biochemicals. Environ. Sci. Technol. 2016, 50, 2796–2810. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.V.; Mohan, S.V.; Chang, Y.-C. Medium-Chain Fatty Acids (MCFA) Production Through Anaerobic Fermentation Using Clostridium Kluyveri: Effect of Ethanol and Acetate. Appl. Biochem. Biotechnol. 2018, 185, 594–605. [Google Scholar] [CrossRef]
- Roghair, M.; Hoogstad, T.; Strik, D.P.B.T.B.; Plugge, C.M.; Timmers, P.H.A.; Weusthuis, R.A.; Bruins, M.E.; Buisman, C.J.N. Controlling Ethanol Use in Chain Elongation by CO2 Loading Rate. Environ. Sci. Technol. 2018, 52, 1496–1505. [Google Scholar] [CrossRef] [PubMed]
- Roghair, M.; Liu, Y.; Adiatma, J.C.; Weusthuis, R.A.; Bruins, M.E.; Buisman, C.J.N.; Strik, D.P.B.T.B. Effect of N-Caproate Concentration on Chain Elongation and Competing Processes. ACS Sustain. Chem. Eng. 2018, 6, 7499–7506. [Google Scholar] [CrossRef] [PubMed]
- Steinbusch, K.J.J.; Hamelers, H.V.M.; Plugge, C.M.; Buisman, C.J.N. Biological Formation of Caproate and Caprylate from Acetate: Fuel and Chemical Production from Low Grade Biomass. Energy Environ. Sci. 2011, 4, 216–224. [Google Scholar] [CrossRef]
- Nzeteu, C.O.; Coelho, F.; Trego, A.C.; Abram, F.; Ramiro-Garcia, J.; Paulo, L.; O’Flaherty, V. Development of an Enhanced Chain Elongation Process for Caproic Acid Production from Waste-Derived Lactic Acid and Butyric Acid. J. Clean. Prod. 2022, 338, 130655. [Google Scholar] [CrossRef]
- O’Flaherty, V.; Collins, G.; Mahony, T. Anaerobic Digestion of Agricultural Residues. Environ. Microbiol. 2010, 11, 259–279. [Google Scholar]
- Angelidaki, I.; Treu, L.; Tsapekos, P.; Luo, G.; Campanaro, S.; Wenzel, H.; Kougias, P.G. Biogas Upgrading and Utilization: Current Status and Perspectives. Biotechnol. Adv. 2018, 36, 452–466. [Google Scholar] [CrossRef]
- Toledo-Alarcón, J.; Capson-Tojo, G.; Marone, A.; Paillet, F.; Júnior, A.D.N.F.; Chatellard, L.; Bernet, N.; Trably, E. Basics of Bio-Hydrogen Production by Dark Fermentation. In Bioreactors for Microbial Biomass and Energy Conversion; Springer: Berlin/Heidelberg, Germany, 2018; pp. 199–220. [Google Scholar]
- Kadier, A.; Kalil, M.S.; Abdeshahian, P.; Chandrasekhar, K.; Mohamed, A.; Azman, N.F.; Logroño, W.; Simayi, Y.; Hamid, A.A. Recent Advances and Emerging Challenges in Microbial Electrolysis Cells (MECs) for Microbial Production of Hydrogen and Value-Added Chemicals. Renew. Sustain. Energy Rev. 2016, 61, 501–525. [Google Scholar] [CrossRef]
- Reungsang, A.; Zhong, N.; Yang, Y.; Sittijunda, S.; Xia, A.; Liao, Q. Hydrogen from Photo Fermentation. In Bioreactors for Microbial Biomass and Energy Conversion; Springer: Berlin/Heidelberg, Germany, 2018; pp. 221–317. [Google Scholar]
- Keating, C.; Hughes, D.; Mahony, T.; Cysneiros, D.; Ijaz, U.Z.; Smith, C.J.; O’Flaherty, V. Cold Adaptation and Replicable Microbial Community Development during Long-Term Low-Temperature Anaerobic Digestion Treatment of Synthetic Sewage. FEMS Microbiol. Ecol. 2018, 94, fiy095. [Google Scholar] [CrossRef]
- Treu, L.; Kougias, P.G.; Campanaro, S.; Bassani, I.; Angelidaki, I. Deeper Insight into the Structure of the Anaerobic Digestion Microbial Community; the Biogas Microbiome Database Is Expanded with 157 New Genomes. Bioresour. Technol. 2016, 216, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Trego, A.C.; McAteer, P.G.; Nzeteu, C.; Mahony, T.; Abram, F.; Ijaz, U.Z.; O’Flaherty, V. Combined Stochastic and Deterministic Processes Drive Community Assembly of Anaerobic Microbiomes During Granule Flotation. Front. Microbiol. 2021, 12, 1165. [Google Scholar] [CrossRef] [PubMed]
- Trego, A.C.; Galvin, E.; Sweeney, C.; Dunning, S.; Murphy, C.; Mills, S.; Nzeteu, C.; Quince, C.; Connelly, S.; Ijaz, U.Z.; et al. Growth and Break-Up of Methanogenic Granules Suggests Mechanisms for Biofilm and Community Development. Front. Microbiol. 2020, 11, 1126. [Google Scholar] [CrossRef] [PubMed]
- McAteer, P.G.; Trego, A.C.; Thorn, C.; Mahony, T.; Abram, F.; O’Flaherty, V. Reactor Configuration Influences Microbial Community Structure during High-Rate, Low-Temperature Anaerobic Treatment of Dairy Wastewater. Bioresour. Technol. 2020, 307, 123221. [Google Scholar] [CrossRef] [PubMed]
- Trego, A.C.; Holohan, B.C.; Keating, C.; Graham, A.; O’Connor, S.; Gerardo, M.; Hughes, D.; Zeeshan Ijaz, U.; O’Flaherty, V. First Proof of Concept for Full-Scale, Direct, Low-Temperature Anaerobic Treatment of Municipal Wastewater. Bioresour. Technol. 2021, 341, 125786. [Google Scholar] [CrossRef]
- Yang, G.; Wang, J. Kinetics and Microbial Community Analysis for Hydrogen Production Using Raw Grass Inoculated with Different Pretreated Mixed Culture. Bioresour. Technol. 2018, 247, 954–962. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Liu, Y.; Li, B.; Wang, B.; Wang, S.; Peng, Y. Short-Chain Fatty Acids Production and Microbial Community in Sludge Alkaline Fermentation: Long-Term Effect of Temperature. Bioresour. Technol. 2016, 211, 685–690. [Google Scholar] [CrossRef]
- Atasoy, M.; Eyice, O.; Schnürer, A.; Cetecioglu, Z. Volatile Fatty Acids Production via Mixed Culture Fermentation: Revealing the Link between PH, Inoculum Type and Bacterial Composition. Bioresour. Technol. 2019, 292, 121889. [Google Scholar] [CrossRef]
- Wu, Q.-L.; Guo, W.-Q.; Zheng, H.-S.; Luo, H.-C.; Feng, X.-C.; Yin, R.-L.; Ren, N.-Q. Enhancement of Volatile Fatty Acid Production by Co-Fermentation of Food Waste and Excess Sludge without PH Control: The Mechanism and Microbial Community Analyses. Bioresour. Technol. 2016, 216, 653–660. [Google Scholar] [CrossRef]
- Cherubini, F. The Biorefinery Concept: Using Biomass Instead of Oil for Producing Energy and Chemicals. Energy Convers. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- Li, Q.; Wang, D.; Wu, Y.; Li, W.; Zhang, Y.; Xing, J.; Su, Z. One Step Recovery of Succinic Acid from Fermentation Broths by Crystallization. Sep. Purif. Technol. 2010, 72, 294–300. [Google Scholar] [CrossRef]
- Chen, H.; Meng, H.; Nie, Z.; Zhang, M. Polyhydroxyalkanoate Production from Fermented Volatile Fatty Acids: Effect of PH and Feeding Regimes. Bioresour. Technol. 2013, 128, 533–538. [Google Scholar] [CrossRef]
- Leonzio, G. Upgrading of Biogas to Bio-Methane with Chemical Absorption Process: Simulation and Environmental Impact. J. Clean. Prod. 2016, 131, 364–375. [Google Scholar] [CrossRef]
- Yao, Y.; Sempuga, B.C.; Liu, X.; Hildebrandt, D. Production of Fuels and Chemicals from a CO2/H2 Mixture. Reactions 2020, 1, 130–146. [Google Scholar] [CrossRef]
- Budzianowski, W.M. High-Value Low-Volume Bioproducts Coupled to Bioenergies with Potential to Enhance Business Development of Sustainable Biorefineries. Renew. Sustain. Energy Rev. 2017, 70, 793–804. [Google Scholar] [CrossRef]
- Khoshnevisan, B.; Tsapekos, P.; Zhang, Y.; Valverde-Pérez, B.; Angelidaki, I. Urban Biowaste Valorization by Coupling Anaerobic Digestion and Single Cell Protein Production. Bioresour. Technol. 2019, 290, 121743. [Google Scholar] [CrossRef] [PubMed]
- MacLellan, J.; Chen, R.; Kraemer, R.; Zhong, Y.; Liu, Y.; Liao, W. Anaerobic Treatment of Lignocellulosic Material to Co-Produce Methane and Digested Fiber for Ethanol Biorefining. Bioresour. Technol. 2013, 130, 418–423. [Google Scholar] [CrossRef]
- Yue, Z.; Teater, C.; MacLellan, J.; Liu, Y.; Liao, W. Development of a New Bioethanol Feedstock—Anaerobically Digested Fiber from Confined Dairy Operations Using Different Digestion Configurations. Biomass Bioenergy 2011, 35, 1946–1953. [Google Scholar] [CrossRef]
- Rajagopal, R.; Mousavi, S.E.; Goyette, B.; Adhikary, S. Coupling of Microalgae Cultivation with Anaerobic Digestion of Poultry Wastes: Toward Sustainable Value Added Bioproducts. Bioengineering 2021, 8, 57. [Google Scholar] [CrossRef]
- Philippini, R.R.; Martiniano, S.E.; Ingle, A.P.; Franco Marcelino, P.R.; Silva, G.M.; Barbosa, F.G.; dos Santos, J.C.; da Silva, S.S. Agroindustrial Byproducts for the Generation of Biobased Products: Alternatives for Sustainable Biorefineries. Front. Energy Res. 2020, 8, 152. [Google Scholar] [CrossRef]
- Ingle, A.P.; Philippini, R.R.; da Silva, S.S. Pretreatment of Sugarcane Bagasse Using Two Different Acid-Functionalized Magnetic Nanoparticles: A Novel Approach for High Sugar Recovery. Renew. Energy 2020, 150, 957–964. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Pandit, R.; Paralikar, P.; Biswas, J.K.; da Silva, S.S. Emerging Role of Nanobiocatalysts in Hydrolysis of Lignocellulosic Biomass Leading to Sustainable Bioethanol Production. Catal. Rev. 2019, 61, 1–26. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).