Abstract
The growing interest in the consumption and study of traditionally fermented food worldwide has led to the development of numerous scientific investigations that have focused on analyzing the microbial and nutritional composition and the health effects derived from the consumption of these foods. Traditionally fermented foods and beverages are a significant source of nutrients, including proteins, essential fatty acids, soluble fiber, minerals, vitamins, and some essential amino acids. Additionally, fermented foods have been considered functional due to their prebiotic content, and the presence of specific lactic acid bacterial strains (LAB), which have shown positive effects on the balance of the intestinal microbiota, providing a beneficial impact in the treatment of diseases. This review presents a bibliographic compilation of scientific studies assessing the effect of the nutritional content and LAB profile of traditional fermented foods on different conditions such as obesity, diabetes, and gastrointestinal disorders.
1. Introduction
Fermentation is the oldest and most affordable preservation process used by humans around the world [1]. It results from the biochemical transformation of some of the food compounds by the action of specific microorganisms, conferring nutritional properties and modified attributes of taste, aroma, and texture to the transformed product [2]. According to Wang et al. [3], the earliest reports on the use of fermentation are registered in the province of Henan, China, around 7000 B.C., where the local people consumed a fermented beverage prepared by mixing rice, honey, and fruit.
Similarly, historical data describe the manufacture of other fermented beverages such as kombucha in 220 B.C. and kefir, which through a recent proteomic analysis, revealed to have been made for 3500 years in Asia [4]. In Mesoamerica, the most distinctive fermented products were those derived from corn, such as tesgüino and pozol, from honey, and other fruits, mainly mead extracted from different species of Agave that was used for the production of alcoholic beverages, which had a significant cultural and social impact [5,6,7].
Recently, there has been an increasing interest in consuming traditional fermented foods prepared using different raw materials, microorganisms, and techniques for their preparation [8]. Most traditional fermented foods are made in Asia, Africa, and Middle Eastern countries, in homes, villages, or small-scale industries [9,10]. Similarly, over the past 15 years, there has been a growing number of studies involving traditional fermented foods and beverages, which have primarily focused on analyzing their microbial and nutritional composition and their health and probiotic effects [11]. However, information on the relationship between specific fermented products such as non-dairy beverages and their impact on human health is limited. Hypothetically, any such beverage may provide a health benefit, yet this affirmation requires scientific evidence in the form of randomized, controlled, and repeatable human interventions [12].
Alternatively, the health benefits provided by fermented foods have been demonstrated in several in vitro and in vivo studies, displaying antihypertensive, hypoglycemic, antidiarrheal, and antithrombotic effects, among others [13,14,15,16,17,18]. In India, for instance, 60% of the milk production is transformed into traditional fermented dairy products such as dahi, mishti doi, shrikhand, lassi, chach, or mohi, among others. Features including the reduction of gastrointestinal infections and serum cholesterol levels and their antimutagenic activity have made these products largely consumed by the Indian population [19]. In this context, it has been demonstrated that food products made from fermented milk confer modulatory effects on the human brain and anticarcinogenic activities [20]. Among these products, research on the consumption of kefir, a worldwide known fermented beverage, has reported a positive impact on the gastrointestinal tract, stimulations of the immune system, anti-inflammatory, and anticancer effects [1,20].
Meanwhile, in Japan, the most traditional fermented foods are miso, natto, and tempeh, which use traditional methods to mix cultures of beneficial microorganisms (Lactobacillus species, A. oryzae, S. cerevisiae, B. subtilis, etc.); these products have attracted worldwide attention for promoting longevity and be considered as functional foods [21].
As a result, fermented foods could be categorized as functional foods, since besides providing nutrients, they also confer health benefits to the host, as they typically contain probiotics, prebiotics, proteins, and peptides that might display specific biological activities [22,23]. Microorganisms have played an essential role in the production of food for human consumption [24]. Generally, LAB of different genera, mainly, Lactobacillus, Streptococcus, and Leuconostoc, predominate in fermented foods, although other bacteria, fungi, and yeasts also contribute to the fermentation process [25]. Thus, some bacteria, yeasts, and fungi can also be considered probiotics, which are living microorganisms that, when administered in adequate amounts, confer health benefits to the host; these probiotics serve as a supplement to the host’s microbiota [26,27]. The amount varies from country to country depending on its legislation; however, generally, a probiotic product should contain more than 106–108 CFU/g or more than 108–1010 CFU/day [28,29].
Moreover, during meat fermentation, an ancient preservation technique [30], one of the most important processes undergone is proteolytic degradation, leading to the release of ACE-I inhibitors and antioxidant peptides [31]. In this regard, a few studies have described some peptides and free amino acids from fermented meats that have antioxidant and antihypertensive effects [32]. However, to the best of our knowledge, no studies analyzed the effect of the administration of traditional fermented meat products on obesity, type 2 Diabetes Mellitus (T2DM), and irritable bowel syndrome; as a result, this review does not include information concerning this. Therefore, the objective of this review is to carry out a bibliographic compilation of different traditional fermented foods and beverages in the world, emphasizing those in which their effect on specific diseases affecting human health has been evaluated.
2. Traditional Fermented Foods and Beverages in the World, and Their Association with Health
Recently, interest in consuming traditional fermented foods made from a wide variety of raw materials, microorganisms, and manufacturing techniques has increased worldwide. It is estimated that around 3500 different fermented foods and drinks based on milk, vegetables, or fruits are produced globally. Traditional fermented foods and beverages provide a significant source of nutrients depending on the substrate used for their production. Fermented foods based on soybean and cabbage (chungkookjang, doenjang, meju, gochujang, natto, sauerkraut, tempeh) are a source of protein, essential fatty acids (linoleic and linolenic), soluble fiber, minerals such as iron and zinc, as well as vitamin K, vitamin B9, B1, and B6. Similarly, certain fermented foods made from milk (yogurt, kefir, dahi) contain mainly high-biological value proteins, calcium, and vitamins B2, B12, and B9. In contrast, fermented foods made from cereals (pozol, red yeast rice) possess essential amino acids (threonine, valine, isoleucine, leucine) and insoluble fiber (Table 1 and Figure 1).

Table 1.
Traditional fermented foods and beverages around the world.
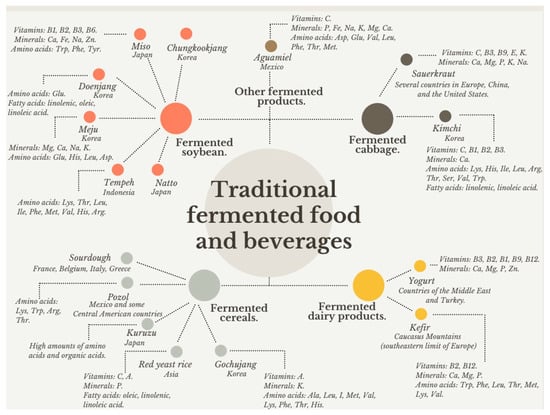
Figure 1.
Classification of traditional fermented foods and beverages based on their substrate and their nutritional content.
Among the nutrients mentioned above, several studies have also confirmed the participation of fiber in carbohydrate metabolism by delaying glucose absorption in the gut, increasing insulin sensitivity in people with obesity, and decreasing preprandial and postprandial glucose levels in patients with T2DM [105,106]. Meanwhile, essential fatty acids have been demonstrated to decrease symptoms of irritable bowel syndrome by reducing inflammation in the intestine. Likewise, polyunsaturated fatty acids have been shown to reduce cardiovascular risk by lowering triglyceride levels and possessing atheroprotective properties (reduction of inflammation markers, cholesterol crystal formation, and endothelial dysfunction) [107,108,109]. Accordingly, Vitamin B9 promotes the decrease of homocysteine, a protein involved in increasing the risk of cardiovascular disease, obesity, hypertension, and T2DM [110,111]. Homocysteine has also been related to the inhibition of the phosphorylation of substrate 2 of the insulin receptor (IRS2) and serine-threonine protein kinase (Akt), leading to insulin resistance and an increase in glucose levels [112,113]. Furthermore, it has been reported that the fermentation process developed by probiotic bacteria increases the number of nutrients and bioactive compounds in the food [114,115]. For instance, according to Jung et al. [116], the probiotic bacteria contained in kimchi (L. mesenteroides and Latilactobacillus sakei) contribute to the increase of vitamin B9 and B2; in tempeh, the presence of R. oligosporus aids in the increase of vitamins B9, B3, B2, and B6 [117].
It is widely recognized that traditional fermented dairy foods are the primary source of probiotic strains of the Lactobacillus genus [118,119]; however, these bacteria have also been isolated from other fermented foods. In this regard, Lee et al. [120] reported the presence of Lactiplantibacillus plantarum in kimchi, reporting that these probiotic bacteria prevented the growth of H. pylori, a bacterium causing stomach infection. Besides, some LAB produce antimicrobial compounds such as bacteriocins, which have exhibited antagonistic activity against L. monocytogenes, S. aureus, E. coli, and S. typhimurium [121]. Similarly, Ibarra-Sánchez et al. [122] informed that L. lactis, a probiotic strain isolated from dahi, produced nisin Z, an effective bacteriocin that inhibited the growth of L. monocytogenes and S. aureus. Moreover, it has been reported that Japanese foods such as natto, chungkookjang, and tempeh, possess antioxidant activity due to the hydrolytic activity of LAB [123,124]. A brief description of traditional fermented foods worldwide, the microorganisms that have been identified, their physical-chemical features, and their nutritional content are presented in Table 1 and Figure 1.
Several studies have demonstrated that fermented foods provide health benefits to the host, showing positive effects in treating diseases such as obesity, diabetes, and gastrointestinal disorders. The following section will address the effects of administering traditional fermented food and beverages for the treatment of the conditions mentioned above.
3. Use of Fermented Foods and Beverages as an Adjuvant in the Treatment of Obesity
According to the World Health Organization, over the last 40 years, overweight rates and obesity have tripled in both developed and undeveloped countries for adult and child populations, with approximately 1.9 billion overweight adults, 650 million obese, and 340 million overweight and obese children in the world [125]. Obesity is a pathological condition determined by an accumulation of visceral and abdominal fat accompanied by chronic low-grade inflammation, which contributes to the appearance of the metabolic syndrome characterized by insulin resistance, dyslipidemia, hepatic steatosis, and cardiovascular disease according to Jung and Choi [126]. The etiology of this disease is multifactorial, with dietary (imbalance between energy intake and expenditure), environmental (sedentary lifestyle), genetic, and metabolic factors being involved. However, other influencing factors have been described, such as psychological and microbiota dysbiosis [127,128].
Moreover, the development of this disease is followed by the production of inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and IL-β [129,130]. These cytokines lead to systemic inflammation contributing to the development of obesity-related complications [13]. Given their elevated hospitalization costs, these complications are of great concern in terms of global public health, thus the increased interest in looking for treatment alternatives focused on the prevention of obesity. Among these, the use of traditional fermented foods and beverages arises as a practical and low-cost alternative [131,132,133]. Different studies in vitro and in vivo have shown that traditional fermented foods such as kimchi, doenjang, meju, cheonggukjang, gochujang, chungkookjang, kefir, and kurozu can aid in reducing body weight, fat tissue, serum, liver triglycerides, cholesterol, inflammatory cytokines, and oxidative stress markers [13,14,15,64,73,134].
In a murine model study, Nam et al. [13] found that mice fed with a high-fat diet and doenjang (14.4%) for 11 weeks showed a decrease in body weight, fat tissue weight, levels of oxidative stress markers (p40phox involved in superoxide production), and a reduction in specific inflammatory adipokines (TNF-α and MCP-1) compared to mice fed with unfermented soybean (11%). These results are in agreement with those observed by Kwak et al. [135], who fed rats (Sprague-Dawley) with a high-fat/doenjang diet for eight weeks, reporting a decrease in visceral fat content, adipocyte size, and fat deposits compared with rats fed with a high-fat and cooked soybean diet. Meanwhile, in an intervention study of 51 overweight adults (men and women) supplemented with doenjang (9.9 g/day) for 12 weeks, a significant decrease in body weight, fat mass, and visceral fat was observed as compared to the placebo group; however, no changes in subcutaneous fat and lipid profile were detected [136]. In contrast, in another study, 60 overweight patients were treated with doenjang (9.8 g/day) for 12 weeks, and their amounts of visceral and subcutaneous fat were assessed; only the former exhibited a significant decrease in those patients with PPAR-γ on the T allele compared to the placebo group [137].
Additional studies using meju, gochujang, cheonggukjang, chungkookjang have reported similar effects to the ones described above. According to Shin et al. [49], rats fed with a high-fat diet and 10% of gochujang for five weeks showed reduced weight gain, decreased adipose tissue weight, serum LDL cholesterol, serum leptin levels, triglyceride levels, and total cholesterol in the liver compared to rats fed with a high-fat diet. Similar results were obtained by Ahn et al. [14], who performed an in vitro test treating adipocyte cells (3T3-L1) with 1 mg/mL of gochujang for three days, obtaining a decrease in leptin, glycerol secretion, and adipocyte size compared to the untreated group. Likewise, Bae et al. [73] detected a reduction in weight gain and serum triglyceride levels, an increase in HDL cholesterol levels, and a decrease in the expression of lipid metabolism genes (SREBP-1c, PPAR-γ, and ACC) when mice (C7BL/6J) were fed with a high-fat diet with 30% traditionally fermented meju (60–90 days of fermentation) for 16 weeks, compared with mice fed with a high-fat diet and standardized fermented meju (6 days of fermentation).
However, in the study conducted by Cha et al. [138] in which 53 overweight patients were treated with 30 g/day of gochujang for 12 weeks, no decrease in triglyceride, LDL, HDL, nor a weight reduction was observed, only reporting a reduction in visceral fat compared to the untreated group. Interesting results were obtained by Byun et al. [134] after treating 83 overweight and obese patients with chungkookjang for 12 weeks, reporting that only female patients had a decreased body fat percentage, waist circumference, waist-hip index, and C-reactive protein levels compared to the placebo group. Conversely, Back et al. [139] reported significant decreases in blood triglyceride, LDL levels, and Apo B levels in 55 patients, men and women included, after being treated with chungkookjang (26 g three times a day) for 12 weeks compared to the placebo group.
On the other hand, several investigations have compared the effect of spontaneous- and inoculated-fermented foods on specific obesity markers. Some of these studies reveal that inoculated fermented products might yield better results than spontaneously fermented ones. For instance, Park et al. [63] reported better effects on the reduction of body weight, leptin, total cholesterol levels, hepatic triglycerides concentration, serum insulin levels, and increased HDL concentration when mice (C57BL/6J) were fed with a high-fat diet complemented with kimchi at 3% inoculated with W. koreensis OK1–6 for 12 weeks. In the case of Lee et al. [64], a reduction in triglyceride levels and a decreased expression of anabolic lipid genes (PPAR-γ, C/EBP-α and FAS) were observed when fat tissue cells (3T3-L1) were supplemented with 100 µg/mL of kimchi inoculated with L. mesenteroides and L. plantarum for 24 h. Conversely, Kim et al. [65] demonstrated that not only had the fermented nature of the food products a significant role in obesity markers, but also the fermentation time. In this study, 22 overweight and obese patients were treated with 1-day-old or 10-day-old kimchi and found that those treated with 10-day-old kimchi (300 g/day) for four weeks had significant decreases in their waist-hip index, body fat, blood pressure, insulin levels, total cholesterol, leptin, and fasting glucose compared to those patients treated with the same dose of fresh kimchi.
Studies on diverse traditional fermented foods such as kefir that use milk as the substrate have shown an improved liver lipid profile in mice. Regarding this, Bourrie et al. [140] reported that mice (C57BL/6) fed with a high-fat diet and 100 μL of traditionally fermented kefir showed a reduction in plasma total cholesterol levels, PPAR-γ gene expression, and in IL-1β compared to those mice treated with commercial kefir. The authors associated these effects with the presence of LAB, which effectively inhibits weight in obese animals by preventing the accumulation of intracellular lipids in the adipocyte [141,142].
On the other hand, the traditional fermented food kurozu, which uses vinegar as a substrate, has improved human liver lipid profile. For example, Hamadate et al. [15] supplemented 48 obese adults with 879 mg/day of this fermented product for 12 weeks. Although no significant reduction in waist circumference was detected, the treated patients showed a significant decrease in body weight compared to the placebo group. Complementary parameters that included total, subcutaneous, and visceral fat were detected to increase in both groups; however, a significantly lower increase was detected in the females treated with kurozu. There is also evidence that L. plantarum reduces adipogenesis and lipogenesis by inhibiting the activation of PPAR-γ, C/EBP-α, and FAS [143]. In addition, the researchers hypothesized that the fermentation process of soybean favors the increase of isoflavone-aglycones, mainly genistein and daidzein. Genistein has been found to play an essential role in regulating lipid metabolism and preventing the production of cytokines and reactive oxygen species (ROS) [144,145]. Furthermore, genistein may increase the activity of the liver enzyme carnitine palmitoyltransferase I (CPT-1), which regulates another enzyme involved in the oxidation of fatty acids. Increased fatty acid oxidation results in increased energy expenditure, decreasing both body fat and weight [146].
According to the results reported in this section, it is observed that traditionally fermented foods might be an alternative for both the treatment and prevention of obesity, since the fermentation process favors the presence of LAB, which can participate in the regulation of lipid metabolism and the expression of specific genes involved in adipogenesis. Moreover, these foods have been shown to increase the bioavailability of isoflavone-aglycones such as genistein, which participate in reducing ROS and inflammatory cytokines. Nonetheless, further interventions are needed to ensure these effects, since although most of the reviewed studies showed promising results in key obesity parameters (weight, percentage of body and visceral fat, waist circumference, and waist-hip index), not all of them offered an evident decrease in the lipid profile of patients.
4. Use of Fermented Foods and Beverages as an Adjuvant in the Treatment of Type 2 Diabetes Mellitus (T2DM)
According to the World Health Organization, in the last 34 years, the prevalence of diabetes has doubled from 4.7% to 8.5% in the world adult population, mainly affecting undeveloped countries where more than 80% of deaths occur due to the disease alone [147]. Diabetes is a severe chronic disease that ensues when β cells in the pancreas do not secrete enough insulin or the tissues cannot use it [148,149]. Three types of diabetes exist, type 1 diabetes mellitus (insulin-dependent), type 2 diabetes, and gestational diabetes. According to the American Diabetes Association [150], there are different tests for the diagnosis of diabetes; one of the most important is the glycosylated hemoglobin (HbA1C) test that measures the blood glucose average for the past two to three months and if it has a value of 6.5% or more indicates that the person has diabetes.
To prevent this disease, it is essential to modify lifestyle choices, like reducing energy intake, choosing better fats, trying to avoid refined carbohydrates, and choosing a diet rich in fiber and low glycemic foods [151]. There is evidence that low glycemic foods can reduce glucose and insulin responses, protect against weight gain, and reduce the risk of developing T2DM [152,153].
In this respect, fermented foods have shown hypoglycemic effects in model animals and humans. The mechanism implicated is that the process of fermentation can reduce the concentration of soluble carbohydrates, increase dietary fiber levels, and increase levels of resistant starch [154]. For instance, during sourdough fermentation, acetic and lactic acids are produced; both participate in the decrease of the glycemic index by delaying gastric emptying and reducing the availability of starch, respectively [155,156].
In search of a potential alternative treatment to overcome this global health issue, numerous researchers have performed in vivo tests to determine the effect of traditionally fermented foods (kefir, kimchi, meju, chungkookjang, natto, sourdough, and hon-chi) on diabetes markers. To date, results obtained comprise decreased serum glucose and insulin levels, increased liver insulin sensitivity, improved oxidative stress levels, reduced cholesterol and triglyceride levels, and regulated glucose homeostasis, among others [157,158,159,160].
In the first instance, tests employing traditionally fermented foods using soybean as the substrate have shown hypoglycemic effects in rats with T2DM by favoring liver insulin signaling cascades. Yang et al. [16] observed that rats fed with a high-fat diet supplemented with 10% traditionally or standardized fermented meju for 60 and 6 days, respectively, during eight weeks, exhibited lower glucose levels than the control group (no treatment). The results were attributed to an increase in liver insulin sensitivity and insulin secretion due to the phosphorylation of Akt and a decrease in the expression of phosphoenolpyruvate carboxykinase (PEPCK). Moreover, after performing a glucose tolerance test (GTT), the researchers found that blood glucose decreased in rats treated with both types of meju due to an increase in insulin levels in the first phase; furthermore, the glucose binding technique also displayed an increase in insulin levels in the first and second phases. These effects agree with those reported by Kwon et al. [17], who treated diabetic rats with 5% of standardized or traditionally fermented gochujang for 6 and 60 days, respectively, for eight weeks; in both treatments, rats showed a decrease in blood glucose levels, an increase in liver insulin sensitivity, and a reduction of glucose levels during a GTT, compared to the untreated rats; however, an increase in insulin secretion levels was not observed in rats. As a complement, the glucose binding technique revealed an increase in insulin levels only during the first phase due to phosphorylation of signal transducer and transcription activator 3 (STAT 3), AMP-activated protein kinase (AMPK), and the decrease in the expression of PEPCK.
Similarly, Kwon et al. [161] reported an increased insulin production during a GTT that improved glucose homeostasis on diabetic rats fed with a high-fat diet and chungkookjang for eight weeks. Results on the glucose binding technique displayed a decrease in glucose levels attributed to an enhanced insulin sensitivity related to phosphorylation of IRS2 and Akt and decreased expression of PEPCK compared to those fed with casein. The effect of the administration of natto in 11 overweight patients with hyperinsulinemia and glucose intolerance was studied by Taniguchi-Fukatsu et al. [79]; the treatment was complemented with viscous vegetables and white rice, while the control group was treated with white rice and cooked soybeans. After eight weeks of treatment, improved insulin and postprandial glucose profiles were observed. Interestingly, when the treatment was administered during breakfast, insulin sensitivity was enhanced, and cholesterol level and oxidative stress were reduced, thus decreasing malondialdehyde-modified low-density lipoprotein and N-carboxyl methyl kinase. The authors concluded that the high fiber content of viscous vegetables modified the carbohydrate metabolism, and the consumption of natto and rice improved insulin sensitivity in patients.
Results obtained in assessing the relationship between the administration of soybean-based fermented food and diabetes markers have been associated with several potential factors. Particularly, Hyun Jung Yang et al. [48] pointed out that the effects are related to the fermentation process of soybeans and the involved LAB, which increase the presence of small peptides, esters, organic acids, and aglycone isoflavones (genistein, daidzein, and glycitein). Moreover, Sanjukta and Rai [162] dismiss that these effects are due to the presence of phytochemicals as capsaicin, present in gochujang, which decreases significantly in the fermentation process.
Regarding the effect of milk-based fermented food products on the treatment of diabetes, Ostadrahimi et al. [52] treated 60 adult patients with T2DM with 600 mL of kefir containing S. thermophilus, L. casei, L. acidophilus, and B. lactis for eight weeks. Significant reductions in serum glucose levels and HbA1C compared to the control group (treated with conventional fermented milk containing S. thermophilus and L. bulgaricus) were observed at the end of the treatment. However, no significant reduction in cholesterol and triglyceride levels was detected, attributed to the differences in probiotic strains and patients’ genetics. The authors associated these effects with the presence of LAB, which have a hypoglycemic effect by producing insulinotropic polypeptides and glucagon-like peptides that induce glucose absorption into the muscle and stimulate the liver to store glucose. Additionally, these LAB are related to antioxidant activity by their interaction with various metabolic pathways, which triggers the reduction of blood glucose and fasting Hb1AC [163].
In contrast, Alihosseini et al. [51] reported no decrease in insulin levels when 60 patients with type 2 diabetes were treated with 600 mL of kefir or conventional fermented milk per day. Nevertheless, a reduction in the insulin resistance index in the group treated with kefir was recorded. Moreover, both treatments reduced homocysteine levels at the end of the study, with no significant difference between them. In this sense, Alihosseini [51] mentions that probiotic bacteria in food improve blood glucose levels by inhibiting the production of reactive oxygen species and cytokines that participate in the destruction of pancreatic cells β. Similarly, according to Zulfania et al. [164], the reduction of homocysteine is owed to the presence of probiotic bacteria, which contribute to the synthesis of folates, which in turn participate in the conversion of homocysteine into methionine by yielding a methyl group, thus reducing cardiovascular disease risk in patients with T2DM.
Meanwhile, in the study of Punaro et al. [165], where rats with type 1 diabetes were treated with 1.8 mL of kefir per day for eight weeks, a decrease in ROS production was observed compared to the control group (no treatment). According to the authors, oxidative stress is associated with an immunological modulation and the anti-inflammatory properties exerted by probiotics, which modify the intestinal microbiota and maintain the integrity of the intestine. Still, the precise mechanism of the diabetes–probiotic relationship remains unknown; Miraghajani et al. [166] investigated the mechanisms related to the diabetes–probiotic relationship and proposed four main mechanisms (1) the local effects of probiotics, (2) the effects of probiotics on inflammatory and immune response, (3) the effects on probiotics in oxidative stress and (4) the effects of probiotics on gene expression.
Additionally, studies using different substrates such as fermented cabbage (kimchi), sourdough, and fermented rice (hon-chi), have also exhibited antidiabetic properties. For instance, Islam and Choi [167] treated rats with a high-fat diet complemented with 0.5 or 2% of kimchi, finding that β cells function increased, and HbA1C decreased in rats compared to the control group (no treatment); they also detected a significant difference in the reduction of serum insulin in the 2% kimchi-treated group. The results were attributed to the fermentation of the ingredients in kimchi (Chinese cabbage, onion, ginger, garlic, and red chili), which have demonstrated hypoglycemic, insulinotropic, and antidiabetic effects. In the study of Maioli et al. [168], a comparison between two formulations of sourdough on diabetes markers of 16 glucose intolerance patients was performed. Results indicated that 100 g of sourdough fermented with S. cerevisiae, Levilactobacillus brevis, and L. plantarum exhibited a significant reduction in insulin and plasma glucose levels compared to sourdough fermented with S. cerevisiae. These effects were associated with the presence of LAB, which transformed simple sugars into lactic acid, favoring the delay of glucose intolerance in diabetes. While in the study carried out by Cheng et al. [169], who fed diabetic male Wistar rats with hon-chi at different doses (50, 100 y 350 mg/kg), it was observed that the treatment was able to reduce the plasma glucose, being concentration-dependent. It was also found that hon-chi exerted a beneficial effect on glucose metabolism in diabetic rats with a lack of insulin; this mechanism was attributed to a change produced in the hepatic protein PEPCK, which contributes to the suppression of gluconeogenesis.
In brief, the information cited in this section confirms that the consumption of specific fermented foods might provide several benefits in the treatment of diabetes, including reductions in serum glucose, insulin levels, postprandial glucose, and oxidative stress, as well as increasing insulin sensitivity in liver and muscle, both in rats and humans. In this regard, researchers attribute such effects mainly to the presence of LAB that produce metabolites involved in glucose homeostasis and regulate molecular mechanisms that lead to the decreased expression of PEPCK protein and Akt phosphorylation.
5. Use of Fermented Foods and Beverages as an Adjuvant in the Treatment of Irritable Bowel Syndrome (IBS)
IBS is among the most common gastrointestinal diseases treated by gastroenterologists, affecting approximately 7–21% of the world population, with a higher prevalence in women [170,171]. IBS’s etiology is still unknown; however, several factors have been associated with this disease, such as poor eating habits, stress, and alterations in intestinal microbiota [172,173]. IBS is characterized by abdominal pain and abnormal bowel habits, such as diarrhea, constipation, or both, in addition to having other symptoms such as abdominal distention, bloating, flatulence, and urge to defecate, which can significantly affect people’s life quality [174,175]. These symptoms have also been associated with the consumption of oligosaccharides, disaccharides, monosaccharides, and fermentable polyols (FODMAPs), which exist in fruits (melon, pear, apple), dairy products, rye, wheat, artichoke, garlic, onions, broccoli, mushrooms, cauliflower, and artificial sweeteners [176,177].
On the other hand, recent studies have shown that dysbiosis in the microbiota is linked with an increase in the severity of IBS symptoms as it can cause abdominal hypersensitivity, epithelial barrier dysfunction, and loss of intestinal motility [178,179]. Therefore, treatments are focused on improving gut health either by intervention with probiotics, prebiotics, or low FODMAP diets [180,181]. Thus, traditional fermented foods might aid in treating IBS due to the presence of LAB and their prebiotic content.
In this context, Costabile et al. [182] conducted an in vitro trial isolating bacteria from feces of patients with and without IBS; feces were cultured with predigested sourdough and fermented for eight hours. In both cases, increased Bifidobacterium counts were observed after the fermentation process, while this effect was not observed when the groups were treated with bread containing S. cerevisiae as fermenting microorganism; thus, the authors suggested that sourdough might be beneficial to IBS patients by promoting a change in the composition and metabolism of the intestinal microbiota. Equally, Muir et al. [183] associated the beneficial effects of sourdough in IBS patients with the reduction of FODMAPs in bread due to the presence of LAB [184,185]. During the fermentation process, LAB can degrade carbohydrates such as fructose and fructans; to do so, the control of processing factors such as fermentation time and the microorganisms involved is essential [186]. Such processing leads to a lower load of undigested carbohydrates in the large intestine, reducing gastrointestinal symptoms [187].
In an intervention with patients with IBS (n = 26), Laatikainen et al. [188] reported no improvement in gastrointestinal symptoms or inflammation markers after consuming six sourdough pieces (fermented for 12 h) per day for seven days, compared to patients who consumed traditional bread. Nevertheless, sourdough displayed a significant reduction of FODMAPs and amylase and trypsin inhibitors (ATIs) due to the fermentative activity of LAB. The authors associate these results with the intervention time, which was truly short, and the placebo effect, which had a determinant role in IBS patients; then, a crossover study might yield better results.
Further traditional fermented foods such as sauerkraut and yogurt have shown promising results in alleviating IBS symptoms. In the study of Nielsen et al. [90], fifteen patients were treated with pasteurized or unpasteurized sauerkraut (75 g/day in both cases) for six weeks, with both groups showing a decrease in the total IBS symptom severity score. Besides, patients treated with unpasteurized sauerkraut manifested reduced days of pain, improved bowel habits, and enhanced presence of L. plantarum and L. brevis in the stool, compared to those treated with pasteurized sauerkraut. These findings were attributed to the fermentation process of LAB, which as aforementioned, promote the metabolism of glucose, fructose, sorbitol, galactooligosaccharides, and fructooligosaccharides present in the cabbage by using them as substrate. Meanwhile, in the research performed by Noorbakhsh et al. [189], it was observed that patients treated with 100 g of yogurt added with lactic acid bacteria (L. bulgaricus, S. thermophilus, L. plantarum, and Limosilactobacillus fermentum) and a prebiotic (xylooligosaccharides) for four weeks exhibited a significant reduction in abdominal distention and improvement in their quality of life, compared with the untreated group. Furthermore, an increased presence of Lactobacillus was found in the treated group’s feces, along with a reduction in homocysteine levels. The authors remarked that the alleviation of IBS symptoms in the treated patients is due to the presence of high numbers of Lactobacillus. The presence of Lactobacillus suppresses the production of inflammatory cytokines and reduces the homocysteine levels, leading to a dysfunction of the intestinal barrier, producing inflammation [190,191].
In short, traditionally fermented foods can also assist in IBS treatments due to the presence of LAB, which given their metabolic process, allow for the reduction of FODMAPs and ATIs, which are entirely associated with the development of the symptoms of this disease; in addition, these bacteria can also improve the recovery of the intestinal microbiota in patients. Notwithstanding, it is imperative to consider the type of studies to perform, the interventions’ length, the treated patients, and uncontrolled factors such as the nocebo effect, which has significantly impacted the treatment of this disease.
6. Conclusions
Traditionally fermented food and beverages, mainly those made from soybeans (doenjang, meju, gochujang, chungkookjang), milk (kefir and yogurt), and cabbage (kimchi and sauerkraut), have effects on lipid and glucose metabolism, regulate genes involved in adipogenesis, decrease ROS production and improve insulin sensitivity. Such effects have been attributed to LAB, which might be involved in the regulation of genes related to lipid and glucose metabolism; moreover, the LAB fermentation process aids in the increase of nutrients (B vitamins and fiber) and bioactive compounds such as the aglycone isoflavones that are implicated in the reduction of inflammatory cytokines and ROS. Therefore, these food products can potentially be used as adjuvant treatment and prevent chronic non-communicable diseases, such as diabetes and obesity. Correspondingly, yogurt, sourdough, and sauerkraut can be used as adjuvants in the treatment of IBS since LAB allow the reduction of symptoms, aid to restore dysbiosis of the microbiota and promote the removal of compounds such as FODMAPs and ATIs involved in the development of IBS symptoms. Most of the interventions performed in humans are of short duration and with small samples, so these factors must be considered for future research.
Author Contributions
B.N.-R., C.V.-O. and G.A.B.-D. conceived the review topic, performed the research, and wrote the manuscript. B.P.-A. and G.A.C.-U. conceived the review topic, supervised the work, revised and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
Authors Negrete-Romero, Valencia-Olivares, and Baños-Dossetti thank the Consejo Nacional de Ciencia y Tecnología (CONACYT) and Universidad Popular Autónoma del Estado de Puebla (UPAEP) for their financial support for their graduate studies.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kabak, B.; Dobson, A.D.W. An Introduction to the Traditional Fermented Foods and Beverages of Turkey. Crit. Rev. Food Sci. Nutr. 2011, 51, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Escamilla-Hurtado, M.d.L.; Escamilla-Hurtado, M.G. Los Alimentos Fermentados Que Comían Nuestros Bisabuelos Prehisánicos. Ciencia 2007, 58, 75–84. [Google Scholar]
- Wang, J.; Jiang, L.; Sun, H. Early Evidence for Beer Drinking in a 9000-Year-Old Platform Mound in Southern China. PLoS ONE 2021, 16, e0255833. [Google Scholar] [CrossRef]
- Silva, K.A.; Uekane, T.M.; de Miranda, J.F.; Ruiz, L.F.; da Motta, J.C.B.; Silva, C.B.; Pitangui, N.d.S.; Gonzalez, A.G.M.; Fernandes, F.F.; Lima, A.R. Kombucha Beverage from Non-Conventional Edible Plant Infusion and Green Tea: Characterization, Toxicity, Antioxidant Activities and Antimicrobial Properties. Biocatal. Agric. Biotechnol. 2021, 34, 102032. [Google Scholar] [CrossRef]
- Sánchez-Muniz, F.J.; Macho-González, A.; Garcimartín, A.; Santos-López, J.A.; Benedí, J.; Bastida, S.; González-Muñoz, M.J. The Nutritional Components of Beer and Its Relationship with Neurodegeneration and Alzheimer’s Disease. Nutrients 2019, 11, 1558. [Google Scholar] [CrossRef]
- Correa-Ascencio, M.; Robertson, I.G.; Cabrera-Cortés, O.; Cabrera-Castro, R.; Evershed, R.P. Pulque Production from Fermented Agave Sap as a Dietary Supplement in Prehispanic Mesoamerica. Proc. Natl. Acad. Sci. USA 2014, 111, 14223–14228. [Google Scholar] [CrossRef]
- Mc Govern, P.E. Pre-Hispanic Distillation? A Biomolecular Archaeological Investigation. Open Access J. Archaeol. Anthropol. 2019, 1. [Google Scholar] [CrossRef]
- Şanlier, N.; Gökcen, B.B.; Sezgin, A.C. Health Benefits of Fermented Foods. Crit. Rev. Food Sci. Nutr. 2019, 59, 506–527. [Google Scholar] [CrossRef]
- Bell, V.; Ferrão, J.; Pimentel, L.; Pintado, M.; Fernandes, T. One Health, Fermented Foods, and Gut Microbiota. Foods 2018, 7, 195. [Google Scholar] [CrossRef]
- Jiang, S.; Ma, C.; Peng, Q.; Huo, D.; Li, W.; Zhang, J. Microbial Profile and Genetic Polymorphism of Predominant Species in Some Traditional Fermented Seafoods of the Hainan Area in China. Front. Microbiol. 2019, 10, 564. [Google Scholar] [CrossRef]
- Rizo, J.; Guillén, D.; Farrés, A.; Díaz-Ruiz, G.; Sánchez, S.; Wacher, C.; Rodríguez-Sanoja, R. Omics in Traditional Vegetable Fermented Foods and Beverages. Crit. Rev. Food Sci. Nutr. 2020, 60, 791–809. [Google Scholar] [CrossRef]
- Marsh, A.J.; Hill, C.; Ross, R.P.; Cotter, P.D. Fermented Beverages with Health-Promoting Potential: Past and Future Perspectives. Trends Food Sci. Technol. 2014, 38, 113–124. [Google Scholar] [CrossRef]
- Nam, Y.R.; Won, S.B.; Chung, Y.S.; Kwak, C.S.; Kwon, Y.H. Inhibitory Effects of Doenjang, Korean Traditional Fermented Soybean Paste, on Oxidative Stress and Inflammation in Adipose Tissue of Mice Fed a High-Fat Diet. Nutr. Res. Pract. 2015, 9, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Ahn, I.S.; Do, M.S.; Kim, S.O.; Jung, H.S.; Kim, Y.I.; Kim, H.J.; Park, K.Y. Antiobesity Effect of Kochujang (Korean Fermented Red Pepper Paste) Extract in 3T3-L1 Adipocytes. J. Med. Food 2006, 9, 15–21. [Google Scholar] [CrossRef]
- Hamadate, N.; Nakamura, K.; Hirai, M.; Yamamoto, T.; Yamaguchi, H.; Iizuka, M.; Yamamoto, E.; Iwama, Y.; Yazawa, K. Effect of a Dietary Supplement Containing Kurozu (a Japanese Traditional Health Drink) Concentrate on Several Obesity-Related Parameters in Obese Japanese Adults: A Randomized, Double-Blind, Placebo-Controlled Trial. Funct. Foods Health Dis. 2013, 3, 310. [Google Scholar] [CrossRef]
- Yang, H.J.; Kwon, D.Y.; Kim, M.J.; Kang, S.; Park, S. Meju, Unsalted Soybeans Fermented with Bacillus Subtilis and Aspergilus Oryzae, Potentiates Insulinotropic Actions and Improves Hepatic Insulin Sensitivity in Diabetic Rats. Nutr. Metab. 2012, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.Y.; Hong, S.M.; Ahn, I.S.; Kim, Y.S.; Shin, D.W.; Park, S. Kochujang, a Korean Fermented Red Pepper plus Soybean Paste, Improves Glucose Homeostasis in 90% Pancreatectomized Diabetic Rats. Nutrition 2009, 25, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Wilburn, J.R.; Ryan, E.P. Fermented Foods in Health Promotion and Disease Prevention: An Overview; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Rasane, P.; Kailey, R.; Singh, S.K. Fermented Indigenous Indian Dairy Products: Standards, Nutrition, Technological Significance and Opportunities for Its Processing. J. Pure Appl. Microbiol. 2017, 11, 1199–1213. [Google Scholar] [CrossRef]
- Azizi, N.F.; Kumar, M.R.; Yeap, S.K.; Abdullah, J.O.; Khalid, M.; Omar, A.R.; Osman, M.A.; Mortadza, S.A.S.; Alitheen, N.B. Kefir and Its Biological Activities. Foods 2021, 10, 1210. [Google Scholar] [CrossRef]
- Murooka, Y.; Yamshita, M. Traditional Healthful Fermented Products of Japan. J. Ind. Microbiol. Biotechnol. 2008, 35, 791–798. [Google Scholar] [CrossRef]
- Dinçoğlu, A.H.; Rugji, J. Use of Rose Oil in Probiotic Fermented Whey as a Functional Food. J. Food Sci. Technol. 2021, 58, 2705–2713. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J.M. Potential Health Benefits of Plant Food-Derived Bioactive Components: An Overview. Foods 2021, 10, 839. [Google Scholar] [CrossRef]
- Sáez, G.D.; Flomenbaum, L.; Zárate, G. Lactic Acid Bacteria from Argentinean Fermented Foods: Isolation and Characterization for Their Potential Use as Vegetable Starters. Food Technol. Biotechnol. 2018, 56, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Rezac, S.; Kok, C.R.; Heermann, M.; Hutkins, R. Fermented Foods as a Dietary Source of Live Organisms. Front. Microbiol. 2018, 9, 1785. [Google Scholar] [CrossRef]
- FAO, E.; WHO, E. Probióticos En Los Alimentos Propiedades Saludables y Nutricionales y Directrices Para La Evaluación. Estud. FAO Aliment. Y Nutr. 2006, 85, 52. [Google Scholar]
- George, R.; Patra, J.K.; Gouda, S.; Park, Y.; Shin, H.S.; Das, G. Benefaction of Probiotics for Human Health: A Review. J. Food Drug Anal. 2018, 26, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.T.; Ruiz, M.A.; Morales, M.E. Microorganismos Probióticos y Salud. Ars Pharm. 2015, 56, 45–59. [Google Scholar] [CrossRef]
- Champagne, C.P.; Ross, R.P.; Saarela, M.; Hansen, K.F.; Charalampopoulos, D. Recommendations for the Viability Assessment of Probiotics as Concentrated Cultures and in Food Matrices. Int. J. Food Microbiol. 2011, 149, 185–193. [Google Scholar] [CrossRef]
- Arihara, K. Strategies for Designing Novel Functional Meat Products. Meat Sci. 2006, 74, 219–229. [Google Scholar] [CrossRef]
- Ferranti, P.; Nitride, C.; Nicolai, M.A.; Mamone, G.; Picariello, G.; Bordoni, A.; Valli, V.; Di Nunzio, M.; Babini, E.; Marcolini, E.; et al. In Vitro Digestion of Bresaola Proteins and Release of Potential Bioactive Peptides. Food Res. Int. 2014, 63, 157–169. [Google Scholar] [CrossRef]
- Stadnik, J.; Kęska, P. Meat and Fermented Meat Products as a Source of Bioactive Peptides. Acta Sci. Pol. Technol. Aliment. 2015, 14, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Bautista, D.N.; Arias, A.G.C. Estudio Químico Bromatológico de Aguamiel de Agave americana L. (Maguey). Cienc. E Investig. 2008, 11, 46–51. [Google Scholar] [CrossRef]
- Muñiz-Márquez, D.B.; Rodríguez-Jasso, R.M.; Rodríguez-Herrera, R.; Contreras-Esquivel, J.C.; Aguilar-González, C.N. Artisanal Production of Aguamiel: A Traditional Mexican Beverage Artisanal Production of Aguamiel: A Traditional Mexican Beverage. Rev. Científica La Univ. Autónoma Coahuila Prod. 2013, 5, 12–19. [Google Scholar]
- Romero-López, M.R.; Osorio-Díaz, P.; Flores-Morales, A.; Robledo, N.; Mora-Escobedo, R. Composición Química, Capacidad Antioxidante y El Efecto Prebiótico Del Aguamiel (Agave atrovirens) Durante Su Fermentación In Vitro. Rev. Mex. Ing. Quim. 2015, 14, 281–292. [Google Scholar]
- Guzmán-Pedraza, R.; Contreras-Esquivel, J.C. Aguamiel y Su Fermentación: Ciencia Más Allá de La Tradición. Mex. J. Biotechnol. 2018, 3, 1–22. [Google Scholar] [CrossRef]
- Leal-Díaz, A.M.; Noriega, L.G.; Torre-Villalvazo, I.; Torres, N.; Alemán-Escondrillas, G.; López-Romero, P.; Sánchez-Tapia, M.; Aguilar-López, M.; Furuzawa-Carballeda, J.; Velázquez-Villegas, L.A.; et al. Aguamiel Concentrate from Agave Salmiana and Its Extracted Saponins Attenuated Obesity and Hepatic Steatosis and Increased Akkermansia Muciniphila in C57BL6 Mice. Sci. Rep. 2016, 6, 34242. [Google Scholar] [CrossRef]
- Kwak, C.S.; Lee, M.S.; Park, S.C. Higher Antioxidant Properties of Chungkookjang, a Fermented Soybean Paste, May Be Due to Increased Aglycone and Malonylglycoside Isoflavone during Fermentation. Nutr. Res. 2007, 27, 719–727. [Google Scholar] [CrossRef]
- Kwon, D.Y.; Hong, S.M.; Lee, J.E.; Sung, S.R.; Park, S. Long-Term Consumption of Fermented Soybean-Derived Chungkookjang Attenuates Hepatic Insulin Resistance in 90% Pancreatectomized Diabetic Rats. Horm. Metab. Res. 2007, 39, 752–757. [Google Scholar] [CrossRef]
- Jung, K.O.; Park, S.Y.; Park, K.Y. Longer Aging Time Increases the Anticancer and Antimetastatic Properties of Doenjang. Nutrition 2006, 22, 539–545. [Google Scholar] [CrossRef]
- Jeong, J.K.; Chang, H.K.; Park, K.Y. Doenjang Prepared with Mixed Starter Cultures Attenuates Azoxymethane and Dextran Sulfate Sodium-Induced Colitis-Associated Colon Carcinogenesis in Mice. J. Carcinog. 2014, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Tamang, J.P. Ethnic Fermented Foods and Alcoholic Beverages of Asia; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar] [CrossRef]
- Carlsen, H.; Haugen, F.; Zadelaar, S.; Kleemann, R.; Kooistra, T.; Drevon, C.A.; Blomhoff, R. Diet-Induced Obesity Increases NF-ΚB Signaling in Reporter Mice. Genes Nutr. 2009, 4, 215–222. [Google Scholar] [CrossRef]
- Kwon, D.Y.; Hong, S.M.; Ahn, I.S.; Kim, M.J.; Yang, H.J.; Park, S. Isoflavonoids and Peptides from Meju, Long-Term Fermented Soybeans, Increase Insulin Sensitivity and Exert Insulinotropic Effects in Vitro. Nutrition 2011, 27, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.J.; Kim, Y.J.; Park, J.M.; Park, Y.S. Analysis of Microflora in Gochujang, Korean Traditional Fermented Food. Food Sci. Biotechnol. 2011, 20, 1435–1440. [Google Scholar] [CrossRef]
- Shin, D.; Jeong, D. Korean Traditional Fermented Soybean Products: Jang. J. Ethn. Foods 2015, 2, 2–7. [Google Scholar] [CrossRef]
- Kim, S.H.; Chung, K.R.; Yang, H.J.; Kwon, D.Y. Sunchang Gochujang (Korean Red Chili Paste): The Unfolding of Authenticity. J. Ethn. Foods 2016, 3, 201–208. [Google Scholar] [CrossRef]
- Yang, H.J.; Lee, Y.S.; Choi, I.S. Comparison of Physicochemical Properties and Antioxidant Activities of Fermented Soybean-Based Red Pepper Paste, Gochujang, Prepared with Five Different Red Pepper (Capsicum annuum L.) Varieties. J. Food Sci. Technol. 2018, 55, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.W.; Jang, E.S.; Moon, B.S.; Lee, J.J.; Lee, D.E.; Lee, C.H.; Shin, C.S. Anti-Obesity Effects of Gochujang Products Prepared Using Rice Koji and Soybean Meju in Rats. J. Food Sci. Technol. 2016, 53, 1004–1013. [Google Scholar] [CrossRef]
- Son, H.; Shin, H.; Jang, E.; Moon, B.; Lee, C.H.; Lee, J. Gochujang Prepared Using Rice and Wheat Koji Partially Alleviates High-fat Diet-induced Obesity in Rats. Food Sci. Nutr. 2020, 8, 1562–1574. [Google Scholar] [CrossRef]
- Alihosseini, N.; Moahboob, S.A.; Farrin, N.; Mobasseri, M.; Taghizadeh, A.; Ostadrahimi, A.R. Effect of Probiotic Fermented Milk (KEFIR) on Serum Level of Insulin and Homocysteine in Type 2 Diabetes Patients. Acta Endocrinol. 2017, 13, 431–436. [Google Scholar] [CrossRef]
- Ostadrahimi, A.; Taghizadeh, A.; Mobasseri, M.; Farrin, N.; Payahoo, L.; Beyramalipoor Gheshlaghi, Z.; Vahedjabbari, M. Effect of Probiotic Fermented Milk (Kefir) on Glycemic Control and Lipid Profile in Type 2 Diabetic Patients: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Iran. J. Public Health 2015, 44, 228–237. [Google Scholar] [PubMed]
- Marshall, V.M.; Cole, W.M. Methods for Making Kefir and Fermented Milks Based on Kefir. J. Dairy Res. 1985, 52, 451–456. [Google Scholar] [CrossRef]
- Simova, E.; Beshkova, D.; Angelov, A.; Hristozova, T.; Frengova, G.; Spasov, Z. Lactic Acid Bacteria and Yeasts in Kefir Grains and Kefir Made from Them. J. Ind. Microbiol. Biotechnol. 2002, 28, 1–6. [Google Scholar] [CrossRef]
- Goncu, A.; Alpkent, Z. Sensory and Chemical Properties of White Pickled Cheese Produced Using Kefir, Yoghurt or a Commercial Cheese Culture as a Starter. Int. Dairy J. 2005, 15, 771–776. [Google Scholar] [CrossRef]
- Irigoyen, A.; Arana, I.; Castiella, M.; Torre, P.; Ibáñez, F.C. Microbiological, Physicochemical, and Sensory Characteristics of Kefir during Storage. Food Chem. 2005, 90, 613–620. [Google Scholar] [CrossRef]
- García Fontán, M.C.; Martínez, S.; Franco, I.; Carballo, J. Microbiological and Chemical Changes during the Manufacture of Kefir Made from Cows’ Milk, Using a Commercial Starter Culture. Int. Dairy J. 2006, 16, 762–767. [Google Scholar] [CrossRef]
- Chen, H.C.; Wang, S.Y.; Chen, M.J. Microbiological Study of Lactic Acid Bacteria in Kefir Grains by Culture-Dependent and Culture-Independent Methods. Food Microbiol. 2008, 25, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Monar, M.; Dávalos, I.; Zapata, S.; Caviedes, M.; Ramírez-Cárdenas, L. Chemical and Microbiological Characterization of Ecuadorian Homemade Water Kefir Caracterización Química y Microbiológica Del Kéfir de Agua Artesanal de Origen Ecuatoriano. ACI Av. En Cienc. E Ing. 2014, 6. [Google Scholar] [CrossRef]
- Hadisaputro, S.; Djokomoeljanto, R.R.; Soesatyo, M.H. The Effects of Oral Plain Kefir Supplementation on Proinflammatory Cytokine Properties of the Hyperglycemia Wistar Rats Induced by Streptozotocin. Acta Med. Indones. 2012, 44, 100–104. [Google Scholar] [PubMed]
- Cheigh, H.; Park, K.; Lee, C.Y.; Cheigh, H.-S.; Park, K.-Y. Nutrition Biochemical, Microbiological, and Nutritional Aspects of Kimchi (Korean Fermented Vegetable Products). Crit. Rev. Food Sci. Nutr. 1994, 34, 175–203. [Google Scholar] [CrossRef]
- Park, K.Y.; Jeong, J.K.; Lee, Y.E.; Daily, J.W. Health Benefits of Kimchi (Korean Fermented Vegetables) as a Probiotic Food. J. Med. Food 2014, 17, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Park, J.A.; Tirupathi Pichiah, P.B.; Yu, J.J.; Oh, S.H.; Daily, J.W.; Cha, Y.S. Anti-Obesity Effect of Kimchi Fermented with Weissella Koreensis OK1-6 as Starter in High-Fat Diet-Induced Obese C57BL/6J Mice. J. Appl. Microbiol. 2012, 113, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Song, J.L.; Park, E.S.; Ju, J.; Kim, H.Y.; Park, K.Y. Anti-Obesity Effects of Starter Fermented Kimchi on 3T3-L1 Adipocytes. Prev. Nutr. Food Sci. 2015, 20, 298–302. [Google Scholar] [CrossRef]
- Kim, E.K.; An, S.Y.; Lee, M.S.; Kim, T.H.; Lee, H.K.; Hwang, W.S.; Choe, S.J.; Kim, T.Y.; Han, S.J.; Kim, H.J.; et al. Fermented Kimchi Reduces Body Weight and Improves Metabolic Parameters in Overweight and Obese Patients. Nutr. Res. 2011, 31, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Haruta, S.; Ueno, S.; Egawa, I.; Hashiguchi, K.; Fujii, A.; Nagano, M.; Ishii, M.; Igarashi, Y. Succession of Bacterial and Fungal Communities during a Traditional Pot Fermentation of Rice Vinegar Assessed by PCR-Mediated Denaturing Gradient Gel Electrophoresis. Int. J. Food Microbiol. 2006, 109, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, H.; Tanaka, H.; Hashiguchi, K.; Nagano, M.; Arakawa, T.; Tokunaga, M. Rapid Detection of Acetic Acid Bacteria in the Traditional Pot-Fermented Rice Vinegar Kurozu. Food Sci. Technol. Res. 2009, 15, 587–590. [Google Scholar] [CrossRef]
- Shibayama, Y.; Kanouchi, H.; Fujii, A.; Nagano, M. A Review of Kurozu, Amber Rice Vinegar Made in Pottery Jars. Funct. Foods Health Dis. 2020, 10, 254. [Google Scholar] [CrossRef]
- Hong, S.B.; Kim, D.H.; Lee, M.; Baek, S.Y.; Kwon, S.W.; Houbraken, J.; Samson, R.A. Zygomycota Associated with Traditional Meju, a Fermented Soybean Starting Material for Soy Sauce and Soybean Paste. J. Microbiol. 2012, 50, 386–393. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, S.H.; Kwon, S.W.; Lee, J.K.; Hong, S.B. Mycoflora of Soybeans Used for Meju Fermentation. Mycobiology 2013, 41, 170. [Google Scholar] [CrossRef][Green Version]
- Park, D.H. Effect of Incubation Temperature on Variations in Bacterial Communities Grown in Fermenting Meju and the Nutritional Quality of Soy Sauce. Food Sci. Biotechnol. 2014, 23, 1921–1928. [Google Scholar] [CrossRef]
- Son, Y.J.; Kang, S.H.; Ko, J.M.; Lee, Y.K.; Hwang, I.K.; Kang, H.J. Changes in Physicochemical Characteristics and Nutritional Values of Soybean, Meju, and Doenjang by Varying Sowing Periods. Korean J. Food Sci. Technol. 2017, 49, 56–62. [Google Scholar] [CrossRef]
- Bae, C.R.; Kwon, D.Y.; Cha, Y.S. Anti-Obesity Effects of Traditional and Standardized Meju in High-Fat Diet-Induced Obese C57BL/6J Mice. J. Clin. Biochem. Nutr. 2014, 54, 45–50. [Google Scholar] [CrossRef]
- Shukla, S.; Park, H.K.; Kim, J.K.; Kim, M. Determination of Biogenic Amines in Japanese Miso Products. Food Sci. Biotechnol. 2011, 20, 851–854. [Google Scholar] [CrossRef]
- Nout, R. Quality, Safety, Biofunctionality and Fermentation Control in Soya. In Advances in Fermented Foods and Beverages: Improving Quality, Technologies and Health Benefits; Elsevier: Amsterdam, The Netherlands, 2015; pp. 409–434. [Google Scholar] [CrossRef]
- Wu, S.; Feng, S.; Ci, Z.; Jiang, C.; Cui, Y.; Sasaki, Y.; Ota, Y. Anti-Obesity Effect and Antioxidant Activity in High-Fat Diet Mice Fed Fermented Buckwheat Products (Miso). J. Food Nutr. Res. 2016, 4, 355–360. [Google Scholar] [CrossRef]
- Okouchi, R.; Sakanoi, Y.; Tsuduki, T. Miso (Fermented Soybean Paste) Suppresses Visceral Fat Accumulation in Mice, Especially in Combination with Exercise. Nutrients 2019, 11, 560. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Song, J.L.; Wang, Q.; Qian, Y.; Li, G.J.; Pang, L. Comparisons of Shuidouchi, Natto, and Cheonggukjang in Their Physicochemical Properties, and Antimutagenic and Anticancer Effects. Food Sci. Biotechnol. 2013, 22, 1077–1084. [Google Scholar] [CrossRef]
- Taniguchi-Fukatsu, A.; Yamanaka-Okumura, H.; Naniwa-Kuroki, Y.; Nishida, Y.; Yamamoto, H.; Taketani, Y.; Takeda, E. Natto and Viscous Vegetables in a Japanese-Style Breakfast Improved Insulin Sensitivity, Lipid Metabolism and Oxidative Stress in Overweight Subjects with Impaired Glucose Tolerance. Br. J. Nutr. 2012, 107, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Cravioto, R.O.; Massieu, G.; Guzman, J. Investigaciones Bromatológicas En Alimentos Mexicanos. Bol. Oficina Sanit. Panam. 1955, 38, 26–33. [Google Scholar] [PubMed]
- Jiménez Vera, R.; González Cortés, N.; Magaña Contreras, A.; Corona Cruz, A. Evaluación Microbiológica y Sensorial de Fermentados de Pozol Blanco, Con Cacao (Theobroma cacao) y Coco (Cocos nucifera). Rev. Venez. Cienc. Y Tecnol. Aliment. 2010, 1, 70–80. [Google Scholar]
- Sotelo, A.; Soleri, D.; Wacher, C.; Sánchez-Chinchillas, A.; Argote, R.M. Chemical and Nutritional Composition of Tejate, a Traditional Maize and Cacao Beverage from the Central Valleys of Oaxaca, Mexico. Plant Foods Hum. Nutr. 2012, 67, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Wachner, C. La Biotecnología Alimentaria Antigua: Los Alimentos Fermentados. Rev. Digit. Univ. 2014, 15, 4–5. [Google Scholar]
- Pérez-Armendáriz, B.; Cardoso-Ugarte, G.A. Traditional Fermented Beverages in Mexico: Biotechnological, Nutritional, and Functional Approaches. Food Res. Int. 2020, 136, 109307. [Google Scholar] [CrossRef]
- Li, C.; Zhu, Y.; Wang, Y.; Zhu, J.S.; Chang, J.; Kritchevsky, D. Monascus Purpureus-Fermented Rice (Red Yeast Rice): A Natural Food Product That Lowers Blood Cholesterol in Animal Models of Hypercholesterolemia. Nutr. Res. 1998, 18, 71–81. [Google Scholar] [CrossRef]
- Ma, J.; Li, Y.; Ye, Q.; Li, J.; Hua, Y.; Ju, D.; Zhang, D.; Cooper, R.; Chang, M. Constituents of Red Yeast Rice, a Traditional Chinese Food and Medicine. J. Agric. Food Chem. 2000, 48, 5220–5225. [Google Scholar] [CrossRef]
- Journoud, M.; Jones, P.J.H. Red Yeast Rice: A New Hypolipidemic Drug. Life Sci. 2004, 74, 2675–2683. [Google Scholar] [CrossRef]
- Xiong, T.; Li, X.; Guan, Q.; Peng, F.; Xie, M. Starter Culture Fermentation of Chinese Sauerkraut: Growth, Acidification and Metabolic Analyses. Food Control 2014, 41, 122–127. [Google Scholar] [CrossRef]
- Peñas, E.; Martinez-Villaluenga, C.; Frias, J. Chapter 24—Sauerkraut: Production, Composition, and Health Benefits; Academic Press: Boston, MA, USA, 2017; pp. 557–576. [Google Scholar] [CrossRef]
- Nielsen, E.S.; Garnås, E.; Jensen, K.J.; Hansen, L.H.; Olsen, P.S.; Ritz, C.; Krych, L.; Nielsen, D.S. Lacto-Fermented Sauerkraut Improves Symptoms in IBS Patients Independent of Product Pasteurisation-a Pilot Study. Food Funct. 2018, 9, 5323–5335. [Google Scholar] [CrossRef]
- Lopez, H.W.; Duclos, V.; Coudray, C.; Krespine, V.; Feillet-Coudray, C.; Messager, A.; Demigné, C.; Rémésy, C. Making Bread with Sourdough Improves Mineral Bioavailability from Reconstituted Whole Wheat Flour in Rats. Nutrition 2003, 19, 524–530. [Google Scholar] [CrossRef]
- Galle, S.; Schwab, C.; Arendt, E.; Gänzle, M. Exopolysaccharide-Forming Weissella Strains as Starter Cultures for Sorghum and Wheat Sourdoughs. J. Agric. Food Chem. 2010, 58, 5834–5841. [Google Scholar] [CrossRef] [PubMed]
- Ogunsakin, O.A.; Banwo, K.; Ogunremi, O.R.; Sanni, A.I. Microbiological and Physicochemical Properties of Sourdough Bread from Sorghum Flour. Int. Food Res. J. 2015, 22, 2610–2618. [Google Scholar]
- Hayta, M.; Hendek Ertop, M. Optimisation of Sourdough Bread Incorporation into Wheat Bread by Response Surface Methodology: Bioactive and Nutritional Properties. Int. J. Food Sci. Technol. 2017, 52, 1828–1835. [Google Scholar] [CrossRef]
- Stillings, B.R.; Hackler, L.R. Amino Acid Studies on the Effect of Fermentation Time and Heat-Processing of Tempeh. J. Food Sci. 1965, 30, 1043–1048. [Google Scholar] [CrossRef]
- Hachmeister, K.A.; Fung, D.Y.C. Tempeh: A Mold-Modified Indigenous Fermented Food Made from Soybeans and/or Cereal Grains. Crit. Rev. Microbiol. 1993, 19, 137–188. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.M.; Eriksson, A.R.B.; Schnürer, J. Growth of Lactic Acid Bacteria and Rhizopus Oligosporus during Barley Tempeh Fermentation. Int. J. Food Microbiol. 2005, 104, 249–256. [Google Scholar] [CrossRef]
- Shurtleff, W.; Aoyagi, A. History of Tempeh; Soyinfo Center: Lafayette, CA, USA, 2007. [Google Scholar]
- Tahir, A.; Anwar, M.; Mubeen, H.; Raza, S. Evaluation of Physicochemical and Nutritional Contents in Soybean Fermented Food Tempeh by Rhizopus Oligosporus. J. Adv. Biol. Biotechnol. 2018, 17. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Wu, B.-H.; Chu, Y.-L.; Chang, W.-C.; Wu, M.-C. Effects of Tempeh Fermentation with Lactobacillus Plantarum and Rhizopus Oligosporus on Streptozotocin-Induced Type II Diabetes Mellitus in Rats. Nutrients 2018, 10, 1143. [Google Scholar] [CrossRef]
- McKinley, M.C. The Nutrition and Health Benefits of Yoghurt. Int. J. Dairy Technol. 2005, 90, 613–620. [Google Scholar] [CrossRef]
- Surono, I.S.; Hosono, A. Fermented Milks: Types and Standards of Identity. In Encyclopedia of Dairy Sciences, 2nd ed.; Academic Press: Boston, MA, USA, 2011; pp. 470–476. [Google Scholar] [CrossRef]
- Serafeimidou, A.; Zlatanos, S.; Laskaridis, K.; Sagredos, A. Chemical Characteristics, Fatty Acid Composition and Conjugated Linoleic Acid (CLA) Content of Traditional Greek Yogurts. Food Chem. 2012, 134, 1839–1846. [Google Scholar] [CrossRef]
- Babio, N.; Mena-Sánchez, G.; Salas-Salvadó, J. Más Allá Del Valor Nutricional Del Yogur: ¿un Indicador de La Calidad de La Dieta? Nutr. Hosp. 2017, 34, 1567. [Google Scholar] [CrossRef]
- Dahl, W.J.; Stewart, M.L. Position of the Academy of Nutrition and Dietetics: Health Implications of Dietary Fiber. J. Acad. Nutr. Diet. 2015, 115, 1861–1870. [Google Scholar] [CrossRef]
- Da Porto, A.; Cavarape, A.; Colussi, G.; Casarsa, V.; Catena, C.; Sechi, L.A. Polyphenols Rich Diets and Risk of Type 2 Diabetes. Nutrients 2021, 13, 1445. [Google Scholar] [CrossRef]
- Lev-Tzion, R.; Griffiths, A.M.; Leder, O.; Turner, D. Omega 3 Fatty Acids (Fish Oil) for Maintenance of Remission in Crohn’s Disease. Cochrane Database Syst. Rev. 2014. [Google Scholar] [CrossRef] [PubMed]
- Lorente-Cebrián, S.; Costa, A.G.V.; Navas-Carretero, S.; Zabala, M.; Laiglesia, L.M.; Martínez, J.A.; Moreno-Aliaga, M.J. An Update on the Role of Omega-3 Fatty Acids on Inflammatory and Degenerative Diseases. J. Physiol. Biochem. 2015, 71, 341–349. [Google Scholar] [CrossRef]
- Preston Mason, R. New Insights into Mechanisms of Action for Omega-3 Fatty Acids in Atherothrombotic Cardiovascular Disease. Curr. Atheroscler. Rep. 2019, 21, 2. [Google Scholar] [CrossRef]
- Dehkordi, E.; Sedehi, M.; Shahraki, Z.; Najafi, R. Effect of Folic Acid on Homocysteine and Insulin Resistance of Overweight and Obese Children and Adolescents. Adv. Biomed. Res. 2016, 5, 88. [Google Scholar] [CrossRef] [PubMed]
- Fruchart, J.C.; Nierman, M.C.; Stroes, E.S.G.; Kastelein, J.J.P.; Duriez, P. New Risk Factors for Atherosclerosis and Patient Risk Assessment. Circulation 2004, 109. [Google Scholar] [CrossRef]
- Esse, R.; Barroso, M.; Tavares de Almeida, I.; Castro, R. The Contribution of Homocysteine Metabolism Disruption to Endothelial Dysfunction: State-of-the-Art. Int. J. Mol. Sci. 2019, 20, 867. [Google Scholar] [CrossRef]
- Gargari, B.P.; Aghamohammadi, V.; Aliasgharzadeh, A. Effect of Folic Acid Supplementation on Biochemical Indices in Overweight and Obese Men with Type 2 Diabetes. Diabetes Res. Clin. Pract. 2011, 94, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Steinkraus, K. Handbook of Indigenous Fermented Foods, Revised and Expanded; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar] [CrossRef]
- Thapa, N.; Tamang, J.P. Functionality and Therapeutic Values of Fermented Foods. In Health Benefits of Fermented Foods and Beverages; CRC Press: Boca Raton, FL, USA, 2015; pp. 111–168. [Google Scholar]
- Jung, S.J.; Park, S.H.; Choi, E.K.; Cha, Y.S.; Cho, B.H.; Kim, Y.G.; Kim, M.G.; Song, W.O.; Park, T.S.; Ko, J.K.; et al. Beneficial Effects of Korean Traditional Diets in Hypertensive and Type 2 Diabetic Patients. J. Med. Food 2014, 17, 161–171. [Google Scholar] [CrossRef]
- Astuti, M. Health Benefits of Tempe. In Health Benefits of Fermented Foods and Beverages; CRC Press: Boca Raton, FL, USA, 2015; pp. 371–394. [Google Scholar]
- Bourdichon, F.; Arias, E.; Babuchowski, A.; Bückle, A.; Bello, F.D.; Dubois, A.; Fontana, A.; Fritz, D.; Kemperman, R.; Laulund, S.; et al. The Forgotten Role of Food Cultures. FEMS Microbiol. Lett. 2021, 368. [Google Scholar] [CrossRef]
- Shah, N.P. Functional Properties of Fermented Milks. In Health Benefits of Fermented Foods; CRC Press: Boca Raton, FL, USA, 2015; pp. 261–274. [Google Scholar]
- Lee, M.; Song, J.H.; Park, J.M.; Chang, J.Y. Bacterial Diversity in Korean Temple Kimchi Fermentation. Food Res. Int. 2019, 126, 108592. [Google Scholar] [CrossRef]
- Tamang, J.P.; Shin, D.-H.; Jung, S.-J.; Chae, S.-W. Functional Properties of Microorganisms in Fermented Foods. Front. Microbiol. 2016, 7, 578. [Google Scholar] [CrossRef]
- Ibarra-Sánchez, L.A.; El-Haddad, N.; Mahmoud, D.; Miller, M.J.; Karam, L. Invited Review: Advances in Nisin Use for Preservation of Dairy Products. J. Dairy Sci. 2020, 103, 2041–2052. [Google Scholar] [CrossRef]
- Cai, J.-S.; Feng, J.-Y.; Ni, Z.-J.; Ma, R.-H.; Thakur, K.; Wang, S.; Hu, F.; Zhang, J.-G.; Wei, Z.-J. An Update on the Nutritional, Functional, Sensory Characteristics of Soy Products, and Applications of New Processing Strategies. Trends Food Sci. Technol. 2021, 112, 676–689. [Google Scholar] [CrossRef]
- Rahnama Vosough, P.; Habibi Najafi, M.B.; Edalatian Dovom, M.R.; Javadmanesh, A.; Mayo, B. Evaluation of Antioxidant, Antibacterial and Cytotoxicity Activities of Exopolysaccharide from Enterococcus Strains Isolated from Traditional Iranian Kishk. J. Food Meas. Charact. 2021, 15, 5221–5230. [Google Scholar] [CrossRef]
- WHO. Obesidad y Sobrepeso Datos y Cifras; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Jung, U.; Choi, M.-S. Obesity and Its Metabolic Complications: The Role of Adipokines and the Relationship between Obesity, Inflammation, Insulin Resistance, Dyslipidemia and Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2014, 15, 6184. [Google Scholar] [CrossRef]
- Perez Lizaur, A.; Garcia Campos, M. Dietas Normales y Terapeúticas, 6th ed.; Mc Graw Hill: Ciudad de México, Mexico, 2014. [Google Scholar]
- Escott Stump, S. Nutrición, Diagnóstico y Tratamiento, 8th ed.; Ippincott Williams and Wilkins: Philadelphia, PA, USA; Wolters Kluwer Health: Philadelphia, PA, USA, 2016. [Google Scholar]
- Kim, K.Y.; Kim, J.K.; Jeon, J.H.; Yoon, S.R.; Choi, I.; Yang, Y. C-Jun N-Terminal Kinase Is Involved in the Suppression of Adiponectin Expression by TNF-α in 3T3-L1 Adipocytes. Biochem. Biophys. Res. Commun. 2005, 327, 460–467. [Google Scholar] [CrossRef]
- Maury, E.; Brichard, S.M. Adipokine Dysregulation, Adipose Tissue Inflammation and Metabolic Syndrome. Mol. Cell. Endocrinol. 2010, 314, 1–16. [Google Scholar] [CrossRef]
- Cope, M.B.; Allison, D.B. Critical Review of the World Health Organization’s (WHO) 2007 Report on “evidence of the Long-Term Effects of Breastfeeding: Systematic Reviews and Meta-Analysis” with Respect to Obesity. Obes. Rev. 2008, 9, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.B. The Effect of Obesity on Health Outcomes. Mol. Cell. Endocrinol. 2010, 316, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Cawley, J.; Meyerhoefer, C. The Medical Care Costs of Obesity: An Instrumental Variables Approach. J. Health Econ. 2012, 31, 219–230. [Google Scholar] [CrossRef]
- Byun, M.S.; Yu, O.K.; Cha, Y.S.; Park, T.S. Korean Traditional Chungkookjang Improves Body Composition, Lipid Profiles and Atherogenic Indices in Overweight/Obese Subjects: A Double-Blind, Randomized, Crossover, Placebo-Controlled Clinical Trial. Eur. J. Clin. Nutr. 2016, 70, 1116–1122. [Google Scholar] [CrossRef]
- Kwak, C.S.; Park, S.C.; Song, K.Y. Doenjang, a Fermented Soybean Paste, Decreased Visceral Fat Accumulation and Adipocyte Size in Rats Fed with High Fat Diet More Effectively than Nonfermented Soybeans. J. Med. Food 2012, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cha, Y.S.; Yang, J.A.; Back, H.I.; Kim, S.R.; Kim, M.G.; Jung, S.J.; Song, W.O.; Chae, S.W. Visceral Fat and Body Weight Are Reduced in Overweight Adults by the Supplementation of Doenjang, a Fermented Soybean Paste. Nutr. Res. Pract. 2012, 6, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Cha, Y.S.; Park, Y.; Lee, M.; Chae, S.W.; Park, K.; Kim, Y.; Lee, H.S. Doenjang, a Korean Fermented Soy Food, Exerts Antiobesity and Antioxidative Activities in Overweight Subjects with the PPAR-Γ2 C1431T Polymorphism: 12-Week, Double-Blind Randomized Clinical Trial. J. Med. Food 2014, 17, 119–127. [Google Scholar] [CrossRef]
- Cha, Y.S.; Kim, S.R.; Yang, J.A.; Back, H.I.; Kim, M.G.; Jung, S.J.; Song, W.O.; Chae, S.W. Kochujang, Fermented Soybean-Based Red Pepper Paste, Decreases Visceral Fat and Improves Blood Lipid Profiles in Overweight Adults. Nutr. Metab. 2013, 10, 24. [Google Scholar] [CrossRef]
- Back, H.I.; Kim, S.R.; Yang, J.A.; Kim, M.G.; Chae, S.W.; Cha, Y.S. Effects of Chungkookjang Supplementation on Obesity and Atherosclerotic Indices in Overweight/Obese Subjects: A 12-Week, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J. Med. Food 2011, 14, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Bourrie, B.C.T.; Cotter, P.D.; Willing, B.P. Traditional Kefir Reduces Weight Gain and Improves Plasma and Liver Lipid Profiles More Successfully than a Commercial Equivalent in a Mouse Model of Obesity. J. Funct. Foods 2018, 46, 29–37. [Google Scholar] [CrossRef]
- Park, J.E.; Oh, S.H.; Cha, Y.S. Lactobacillus Brevis OPK-3 from Kimchi Prevents Obesity and Modulates the Expression of Adipogenic and pro-Inflammatory Genes in Adipose Tissue of Diet-Induced Obese Mice. Nutrients 2020, 12, 604. [Google Scholar] [CrossRef]
- Moszak, M.; Szulińska, M.; Bogdański, P. You Are What You Eat—the Relationship between Diet, Microbiota, and Metabolic Disorders—A Review. Nutrients 2020, 12, 1096. [Google Scholar] [CrossRef]
- Jung, S.; Lee, Y.J.; Kim, M.; Kim, M.; Kwak, J.H.; Lee, J.W.; Ahn, Y.T.; Sim, J.H.; Lee, J.H. Supplementation with Two Probiotic Strains, Lactobacillus Curvatus HY7601 and Lactobacillus Plantarum KY1032, Reduced Body Adiposity and Lp-PLA2 Activity in Overweight Subjects. J. Funct. Foods 2015, 19, 744–752. [Google Scholar] [CrossRef]
- Lu, C.; Wang, Y.; Xu, T.; Li, Q.; Wang, D.; Zhang, L.; Fan, B.; Wang, F.; Liu, X. Genistein Ameliorates Scopolamine-Induced Amnesia in Mice through the Regulation of the Cholinergic Neurotransmission, Antioxidant System and the ERK/CREB/BDNF Signaling. Front. Pharmacol. 2018, 9, 1153. [Google Scholar] [CrossRef] [PubMed]
- Ginwala, R.; Bhavsar, R.; Chigbu, D.G.I.; Jain, P.; Khan, Z.K. Potential Role of Flavonoids in Treating Chronic Inflammatory Diseases with a Special Focus on the Anti-Inflammatory Activity of Apigenin. Antioxidants 2019, 8, 35. [Google Scholar] [CrossRef]
- Xin, X.; Chen, C.; Hu, Y.Y.; Feng, Q. Protective Effect of Genistein on Nonalcoholic Fatty Liver Disease (NAFLD). Biomed. Pharmacother. 2019, 117, 109047. [Google Scholar] [CrossRef] [PubMed]
- OMS. Informe Mundial de La Diabetes; WHO: Geneva, Switzerland, 2016; p. 4. [Google Scholar]
- DeFronzo, R.A.; Ferrannini, E. Regulation of Intermediary Metabolism During Fasting and Feeding. In Endocrinology: Adult and Pediatric; Elsevier: Amsterdam, The Netherlands, 2015; Volume 1–2, pp. 598–626.e3. [Google Scholar] [CrossRef]
- OMS. ¿Qué Es La Diabetes? Available online: https://www.niddk.nih.gov/health-information/informacion-de-la-salud/diabetes/informacion-general/que-es (accessed on 25 October 2021).
- Association, A.D. Classification and Diagnosis of Diabetes. Diabetes Care 2017, 40, S11–S24. [Google Scholar] [CrossRef] [PubMed]
- Livesey, G.; Taylor, R.; Livesey, H.F.; Buyken, A.E.; Jenkins, D.J.A.; Augustin, L.S.A.; Sievenpiper, J.L.; Barclay, A.W.; Liu, S.; Wolever, T.M.S.; et al. Dietary Glycemic Index and Load and the Risk of Type 2 Diabetes: Assessment of Causal Relations. Nutrients 2019, 11, 1436. [Google Scholar] [CrossRef] [PubMed]
- Augustin, L.S.A.; Kendall, C.W.C.; Jenkins, D.J.A.; Willett, W.C.; Astrup, A.; Barclay, A.W.; Björck, I.; Brand-Miller, J.C.; Brighenti, F.; Buyken, A.E.; et al. Glycemic Index, Glycemic Load and Glycemic Response: An International Scientific Consensus Summit from the International Carbohydrate Quality Consortium (ICQC). Nutr. Metab. Cardiovasc. Dis. 2015, 25, 795–815. [Google Scholar] [CrossRef]
- Sacks, F.M.; Carey, V.J.; Anderson, C.A.M.; Miller, E.R.; Copeland, T.; Charleston, J.; Harshfield, B.J.; Laranjo, N.; McCarron, P.; Swain, J.; et al. Effects of High vs Low Glycemic Index of Dietary Carbohydrate on Cardiovascular Disease Risk Factors and Insulin Sensitivity. JAMA 2014, 312, 2531. [Google Scholar] [CrossRef]
- Scazzina, F.; Del Rio, D.; Pellegrini, N.; Brighenti, F. Sourdough Bread: Starch Digestibility and Postprandial Glycemic Response. J. Cereal Sci. 2009, 49, 419–421. [Google Scholar] [CrossRef]
- Demirkesen-Bicak, H.; Arici, M.; Yaman, M.; Karasu, S.; Sagdic, O. Effect of Different Fermentation Condition on Estimated Glycemic Index, In Vitro Starch Digestibility, and Textural and Sensory Properties of Sourdough Bread. Foods 2021, 10, 514. [Google Scholar] [CrossRef]
- Lukšič, L.; Bonafaccia, G.; Timoracka, M.; Vollmannova, A.; Trček, J.; Nyambe, T.K.; Melini, V.; Acquistucci, R.; Germ, M.; Kreft, I. Rutin and Quercetin Transformation during Preparation of Buckwheat Sourdough Bread. J. Cereal Sci. 2016, 69, 71–76. [Google Scholar] [CrossRef]
- Choi, J.H.; Pichiah, P.B.T.; Kim, M.J.; Cha, Y.S. Cheonggukjang, a Soybean Paste Fermented with B. Licheniformis-67 Prevents Weight Gain and Improves Glycemic Control in High Fat Diet Induced Obese Mice. J. Clin. Biochem. Nutr. 2016, 59, 31–38. [Google Scholar] [CrossRef]
- Razmpoosh, E.; Javadi, M.; Ejtahed, H.S.; Mirmiran, P. Probiotics as Beneficial Agents in the Management of Diabetes Mellitus: A Systematic Review. Diabetes Metab. Res. Rev. 2016, 32, 143–168. [Google Scholar] [CrossRef]
- Kumar, A.; Sahoo, S.; Sahu, S.; Nayak, L.; Ngangkham, U.; Parameswaran, C.; Bose, L.K.; Samantaray, S.; Kumar, G.; Sharma, S.G. Rice with Pulses or Cooking Oils Can Be Used to Elicit Lower Glycemic Response. J. Food Compos. Anal. 2018, 71, 1–7. [Google Scholar] [CrossRef]
- Rosa, D.D.; Dias, M.M.S.; Grześkowiak, Ł.M.; Reis, S.A.; Conceição, L.L.; Peluzio, M.D.C.G. Milk Kefir: Nutritional, Microbiological and Health Benefits. Nutr. Res. Rev. 2017, 30, 82–96. [Google Scholar] [CrossRef]
- Kwon, D.Y.; Jang, J.S.; Hong, S.M.; Lee, J.E.; Sung, S.R.; Park, H.R.; Park, S. Long-Term Consumption of Fermented Soybean-Derived Chungkookjang Enhances Insulinotropic Action Unlike Soybeans in 90% Pancreatectomized Diabetic Rats. Eur. J. Nutr. 2007, 46, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Sanjukta, S.; Rai, A.K. Production of Bioactive Peptides during Soybean Fermentation and Their Potential Health Benefits. Trends Food Sci. Technol. 2016, 50, 1–10. [Google Scholar] [CrossRef]
- Mirmiranpour, H.; Huseini, H.F.; Derakhshanian, H.; Khodaii, Z.; Tavakoli-Far, B. Effects of Probiotic, Cinnamon, and Synbiotic Supplementation on Glycemic Control and Antioxidant Status in People with Type 2 Diabetes; a Randomized, Double-Blind, Placebo-Controlled Study. J. Diabetes Metab. Disord. 2020, 19, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Zulfania, A.K.; Rehman, S.; Ghaffar, T. Association of Homocysteine with Body Mass Index, Blood Pressure, HbA1c and Duration of Diabetes in Type 2 Diabetics. Pak. J. Med. Sci. 2018, 34, 1483–1487. [Google Scholar] [CrossRef]
- Punaro, G.R.; Maciel, F.R.; Rodrigues, A.M.; Rogero, M.M.; Bogsan, C.S.B.; Oliveira, M.N.; Ihara, S.S.M.; Araujo, S.R.R.; Sanches, T.R.C.; Andrade, L.C.; et al. Kefir Administration Reduced Progression of Renal Injury in STZ-Diabetic Rats by Lowering Oxidative Stress. Nitric Oxide-Biol. Chem. 2014, 37, 53–60. [Google Scholar] [CrossRef]
- Miraghajani, M.; Dehsoukhteh, S.S.; Rafie, N.; Hamedani, S.G.; Sabihi, S.; Ghiasvand, R. Potential Mechanisms Linking Probiotics to Diabetes: A Narrative Review of the Literature. Sao Paulo Med. J. 2017, 135, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Choi, H. Antidiabetic Effect of Korean Traditional Baechu (Chinese Cabbage) Kimchi in a Type 2 Diabetes Model of Rats. J. Med. Food 2009, 12, 292–297. [Google Scholar] [CrossRef]
- Maioli, M.; Pes, G.M.; Sanna, M.; Cherchi, S.; Dettori, M.; Manca, E.; Farris, G.A. Sourdough-Leavened Bread Improves Postprandial Glucose and Insulin Plasma Levels in Subjects with Impaired Glucose Tolerance. Acta Diabetol. 2008, 45, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.T.; Huang, C.C.; Liu, I.M.; Tzeng, T.F.; Chih, J.C. Novel Mechanism for Plasma Glucose-Lowering Action of Metformin in Streptozotocin-Induced Diabetic Rats. Diabetes 2006, 55, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Gadour, E.; Hassan, Z.; Gadour, R. A Comprehensive Review of Transaminitis and Irritable Bowel Syndrome. Cureus 2021, 13, e16583. [Google Scholar] [CrossRef]
- Arishi, A.M.; Elmakki, E.E.; Hakami, O.M.; Alganmy, O.M.; Maashi, S.M.; Al-Khairat, H.K.; Sahal, Y.A.; Sharif, A.A.; Alfaifi, M.H. Irritable Bowel Syndrome: Prevalence and Risk Factors in Jazan Region, Saudi Arabia. Cureus 2021, 13, e15979. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.H.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human Gut Microbes Impact Host Serum Metabolome and Insulin Sensitivity. Nature 2016, 535, 376–381. [Google Scholar] [CrossRef]
- Oświęcimska, J.; Szymlak, A.; Roczniak, W.; Girczys-Połedniok, K.; Kwiecień, J. New Insights into the Pathogenesis and Treatment of Irritable Bowel Syndrome. Adv. Med Sci. 2017, 62, 17–30. [Google Scholar] [CrossRef]
- Marsh, A.; Eslick, E.M.; Eslick, G.D. Does a Diet Low in FODMAPs Reduce Symptoms Associated with Functional Gastrointestinal Disorders? A Comprehensive Systematic Review and Meta-Analysis. Eur. J. Nutr. 2016, 55, 897–906. [Google Scholar] [CrossRef]
- Kim, M.-Y.; Choi, S.-W. Dietary Modulation of Gut Microbiota for the Relief of Irritable Bowel Syndrome. Nutr. Res. Pract. 2021, 15, 411. [Google Scholar] [CrossRef]
- Stróżyk, A.; Horvath, A.; Muir, J.; Szajewska, H. Effect of a Low-FODMAP Diet for the Management of Functional Abdominal Pain Disorders in Children: A Study Protocol for a Randomized Controlled Trial. Nutr. J. 2021, 20, 1. [Google Scholar] [CrossRef]
- Mansueto, P.; Seidita, A.; D’Alcamo, A.; Carroccio, A. Role of FODMAPs in Patients With Irritable Bowel Syndrome. Nutr. Clin. Pract. 2015, 30, 665–682. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, Y.; Muniz Pedrogo, D.A.; Kashyap, P.C. Irritable Bowel Syndrome: A Gut Microbiota-Related Disorder? Am. J. Physiol.—Gastrointest. Liver Physiol. 2016, 312, G52–G62. [Google Scholar] [CrossRef] [PubMed]
- Tap, J.; Derrien, M.; Törnblom, H.; Brazeilles, R.; Cools-Portier, S.; Doré, J.; Störsrud, S.; Le Nevé, B.; Öhman, L.; Simrén, M. Identification of an Intestinal Microbiota Signature Associated With Severity of Irritable Bowel Syndrome. Gastroenterology 2017, 152, 111–123.e8. [Google Scholar] [CrossRef]
- Liu, H.N.; Wu, H.; Chen, Y.Z.; Chen, Y.J.; Shen, X.Z.; Liu, T.T. Altered Molecular Signature of Intestinal Microbiota in Irritable Bowel Syndrome Patients Compared with Healthy Controls: A Systematic Review and Meta-Analysis. Dig. Liver Dis. 2017, 49, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Fukudo, S.; Okumura, T.; Inamori, M.; Okuyama, Y.; Kanazawa, M.; Kamiya, T.; Sato, K.; Shiotani, A.; Naito, Y.; Fujikawa, Y.; et al. Evidence-Based Clinical Practice Guidelines for Irritable Bowel Syndrome 2020. J. Gastroenterol. 2021, 56, 193–217. [Google Scholar] [CrossRef]
- Costabile, A.; Santarelli, S.; Claus, S.P.; Sanderson, J.; Hudspith, B.N.; Brostoff, J.; Ward, J.L.; Lovegrove, A.; Shewry, P.R.; Jones, H.E.; et al. Effect of Breadmaking Process on in Vitrogut Microbiota Parameters in Irritable Bowel Syndrome. PLoS ONE 2014, 9, e111225. [Google Scholar] [CrossRef]
- Muir, J.G.; Varney, J.E.; Ajamian, M.; Gibson, P.R. Gluten-Free and Low-FODMAP Sourdoughs for Patients with Coeliac Disease and Irritable Bowel Syndrome: A Clinical Perspective. Int. J. Food Microbiol. 2019, 290, 237–246. [Google Scholar] [CrossRef]
- Bartkiene, E.; Bartkevics, V.; Pugajeva, I.; Krungleviciute, V.; Mayrhofer, S.; Domig, K. The Contribution of P. acidilactici, L. plantarum, and L. curvatus Starters and L-(+)-Lactic Acid to the Acrylamide Content and Quality Parameters of Mixed Rye—Wheat Bread. LWT Food Sci. Technol. 2017, 80, 43–50. [Google Scholar] [CrossRef]
- Struyf, N.; Laurent, J.; Verspreet, J.; Verstrepen, K.J.; Courtin, C.M. Saccharomyces Cerevisiae and Kluyveromyces Marxianus Cocultures Allow Reduction of Fermentable Oligo-, Di-, and Monosaccharides and Polyols Levels in Whole Wheat Bread. J. Agric. Food Chem. 2017, 65, 8704–8713. [Google Scholar] [CrossRef]
- Ziegler, J.U.; Steiner, D.; Longin, C.F.H.; Würschum, T.; Schweiggert, R.M.; Carle, R. Wheat and the Irritable Bowel Syndrome—FODMAP Levels of Modern and Ancient Species and Their Retention during Bread Making. J. Funct. Foods 2016, 25, 257–266. [Google Scholar] [CrossRef]
- Halmos, E.P.; Power, V.A.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. A Diet Low in FODMAPs Reduces Symptoms of Irritable Bowel Syndrome. Gastroenterology 2014, 146, 67–75.e5. [Google Scholar] [CrossRef] [PubMed]
- Laatikainen, R.; Koskenpato, J.; Hongisto, S.M.; Loponen, J.; Poussa, T.; Huang, X.; Sontag-Strohm, T.; Salmenkari, H.; Korpela, R. Pilot Study: Comparison of Sourdough Wheat Bread and Yeast-Fermented Wheat Bread in Individuals with Wheat Sensitivity and Irritable Bowel Syndrome. Nutrients 2017, 9, 1215. [Google Scholar] [CrossRef] [PubMed]
- Noorbakhsh, H.; Yavarmanesh, M.; Mortazavi, S.A.; Adibi, P.; Moazzami, A.A. Metabolomics Analysis Revealed Metabolic Changes in Patients with Diarrhea-Predominant Irritable Bowel Syndrome and Metabolic Responses to a Synbiotic Yogurt Intervention. Eur. J. Nutr. 2019, 58, 3109–3119. [Google Scholar] [CrossRef] [PubMed]
- Distrutti, E.; Monaldi, L.; Ricci, P.; Fiorucci, S. Gut Microbiota Role in Irritable Bowel Syndrome: New Therapeutic Strategies. World J. Gastroenterol. 2016, 22, 2219–2241. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Lehto, S.M.; Harty, S.; Dinan, T.G.; Cryan, J.F.; Burnet, P.W.J. Psychobiotics and the Manipulation of Bacteria–Gut–Brain Signals. Trends Neurosci. 2016, 39, 763–781. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).