Abstract
Ginseng is one of the most popular traditional Chinese medicines that have been widely used in China and other Asian countries for thousands of years. Ginsenosides are the unique bioactive saponins occurring in ginseng, and their biological activities have been extensively investigated. A large amount of ginseng residue is produced as waste product due to its applications in manufacturing functional food products, even though it may still contain bioactive components. Thus, the objective of this study was to investigate the hypoglycemic activities of American ginseng extraction residue (AmR) via fermentation with Ganoderma lucidum. Our results showed that the total phenolic contents and β-glucosidase activity of AmR profoundly increased after fermentation with G. lucidum. In 3T3-L1 adipocytes, stimulation of glucose uptake by treatment with AmR was not significant, while fermented AmR (FAmR) exhibited insulin-like glucose-uptake-stimulatory effects. Importantly, the hypoglycemic effects of FAmR were positively associated with the amount of the deglycosylated minor ginsenosides Rg1, Rg3, and compound K. Taken together, our current findings suggest that bioconversion of AmR by fermentation with G. lucidum may be a feasible and eco-friendly approach to developing a functional ingredient for the management of diabetes, while also resolving the problem of ginseng waste.
1. Introduction
Ginseng, the root of a plant in the genus Panax, is one of the most popular Chinese herbal medicines. The most recognized species of ginseng include Panax ginseng C.A. Meyer, known as Asian ginseng or Korean ginseng, and Panax quinquefolius L., commonly known as American ginseng. American ginseng roots are a popular dietary supplement in the United Sates, and statistical data have shown that American ginseng is 5–10 times more expensive than Asian ginseng on the market [1]. Ginsenosides, a series of tetracyclic triterpenoid saponins, are the major bioactive compounds in ginseng. These unique saponins can be categorized into two groups according to their structures: protopanaxatriols, and protopanaxadiols. With the R1, R2, and R3 groups bearing different glycosides, Rb1, Rc, compound K (CK), and Rg3 are protopanaxadiols, while protopanaxatriols include Re, Rg1, and Rg2 [2]. The structures of ginsenosides are given in Figure 1.
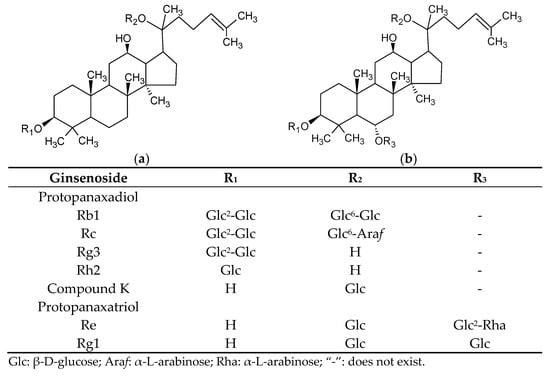
Figure 1.
Structures of ginsenosides. (a) Protopanaxadiol and (b) protopanaxatriol.
Accumulated data have shown that ginsenosides possess a broad spectrum of biological activities, such as antioxidant, anti-inflammatory, and neuroprotective effects, as well as the prevention of metabolic diseases, cardiovascular disease, and cancer [3,4,5,6,7]. Among these health-promoting effects, the antidiabetic effects of ginsenosides have been reported in both in vitro and in vivo studies [8]. In 3T3-L1 preadipocytes, the ginsenoside Re promoted glucose uptake through activation of translocation of GLUT4, upregulation of PPAR-γ2, IRS-1, and adiponectin, and inhibition of the TNF-α-mediated inflammatory response [9]. Similarly, the ginsenoside Rg1 significantly facilitated GLUT4 translocation to promote glucose uptake through activation of AMPK expression in the insulin-resistant differentiated C2C12 muscle cells. In addition, antidiabetic effects of ginsenosides have been demonstrated in different animal models. In an animal model of high-fat-diet-induced type 2 diabetes, administration of the ginsenoside Rb1 profoundly attenuated the level of fasting blood glucose, and enhanced glucose tolerance and insulin sensitivity through the suppression of 11β-hydroxysteroid hydrogenase type I [10]. In a type 2 diabetic mouse model, the ginsenoside Rg3 was able to stimulate glucagon-like peptide-1 (GLP-1) secretion through sweet taste receptor signaling, and reduced the blood glucose level via insulinotropic action [11]. Moreover, the therapeutic potential of American ginseng in the management of type 2 diabetes has also been reported in clinical trials [12,13].
The ginsenosides Rb1, Rb2, Rc, Re, and Rg1 account for more than 80% of the total ginsenosides. However, accumulated data have shown that the biological activities of the minor ginsenosides Rd, Rg3, and CK are superior to those of the major ginsenosides [14,15]. As a result, it is crucial to prepare the minor ginsenosides by transforming the major ginsenosides. Recent studies have developed several transformation methods to increase the biological activities of the ginsenosides, such as steaming, acid hydrolysis, and enzymatic and microbial bioconversion [16]. Microbial bioconversion methods are more convenient, efficient, and environmentally friendly for obtaining minor ginsenosides in comparison to physical and chemical approaches. For instance, the ginsenosides Rb1 and Rg1 were successfully converted to CK and F1 after fermentation with soil Cladosporium cladosporioides [17]. Edible fungi were also able to convert Rb1 to CK, Rh2, and F2 [16].
Along with ginseng, lingzhi (Ganoderma lucidum) is also a popular traditional Chinese medicine. Many studies have reported that G. lucidum possesses a broad spectrum of biological activities, such as immunomodulatory, antioxidant, hypoglycemic, antitumor, and anti-inflammatory activities [18]. In addition its beneficial effects on health, G. lucidum can produce an array of ligninolytic enzymes that synergistically degrade lignin [19].
In many Asian countries, large amounts of ginseng residue are produced as waste products due to its applications in manufacturing functional food products (e.g., ginseng drink), even though it still contains bioactive components. Thus, utilization of ginseng residues for potential applications is a subject of interest that provides possible solutions for waste management. Research has shown that the ethanolic extract of red ginseng marc exhibits anti-inflammatory effects by regulating IgA and IgE production after fermentation with Bacillus subtilis and Saccharomyces cerevisiae, suggesting that fermented red ginseng marc could be a natural resource used in the functional food industry [20]. Previously, we have investigated the biotransformation of ginsenosides in American ginseng extraction residue (AmR) by G. lucidum, as well as the determination of bioactive ginsenosides affected by different fermentation conditions of G. lucidum grown on AmR [21]. The results showed that the levels of Rg1, Rg3, and CK increased, while those of Re, Rb1, and Rc declined after fermentation with G. lucidum. In this study, the 3T3-L1 adipocytes were used to evaluate whether AmR exhibited hypoglycemic activity after fermentation with G. lucidum.
2. Materials and Methods
2.1. Chemicals and Reagents
Antibiotic–antimycotic solution, Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), bovine serum albumin (BSA), and trypsin-EDTA were purchased from Gibco (Grand Island, NY, USA). Dexamethasone, insulin, 3-isobutyl-1-methylxanthine, p-nitrophenyl-β-D-galactoside, and p-nitrophenol were sourced from Sigma (St. Louis, MO, USA). Folin–Ciocalteu phenol reagent was purchased from Merck (Darmstadt, Germany). 2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose (2-NBDG) was purchased from Invitrogen (Waltham, MA, USA). The ginsenosides Re, Rg1, Rb1, Rc, Rg3, and Rh2 were purchased from Advantage Chemical Co. (Taichung, Taiwan). CK was sourced from Tauto Biotech (Shanghai, China).
2.2. Materials
American ginseng extraction residue was obtained from a local food company. AmR fermentation products were prepared using G. lucidum (BCRC37066) from the Bioresource Collection and Research Center (Hsinchu City, Taiwan). The fermentation method was carried out as described in our previous study [21]. In brief, G. lucidum was first cultivated in malt extract agar supplemented with 2% ginseng residue at 25 °C for 7 days, and then subsequently seeded in AmR at 1% (low inoculation, L), 5% (medium inoculation, M), and 10% (high inoculation, H) inoculum quantities for incubation at 25 °C for 4 (L4, M4, and H4), 8 (L8, M8, and H8), or 13 (L13, M13, and H13) days. The extraction of ginsenosides from unfermented AmR and G. lucidum-fermented AmR (FAmR) were described previously [21]. Briefly, the sample (1 g) was extracted with 80% methanol by agitation at 50 °C for 1 h. The supernatant was collected, and the solvent and moisture were removed under vacuum. The dried extract was dissolved in water and partially purified using a Sep-Pak C18 cartridge (Phenomenex, Torrance, CA, USA). Quantification of ginsenosides was carried out as described in our previous study [21].
2.3. Total Phenol Content Determination
The content of total phenolic compounds was spectrophotometrically determined using a modified Folin–Ciocalteu colorimetric method [22]. In brief, 50 µL of AmR or FAmR was mixed with 1 mL of Folin–Ciocalteu reagent, and then 2.5 mL of 20% sodium carbonate was added. After 20 min, the wavelength at 735 nm was measured. The results were expressed as milligrams of gallic acid equivalents (GAE) per 1 g of sample.
2.4. Glucosidase Activity
The method for the determination of β-glucosidase was modified from the work of Matsuura and Obata [23]. The G. lucidum-fermented AmR was lyophilized with a freeze-drier and then homogenized into a fine powder. A total of 50 mg of the powder was mixed with 5 mL of 0.1 M phosphate-buffered saline (PBS, pH 6.0) and centrifuged at 5000× g for 20 min. The supernatant was mixed with 1 mM p-nitrophenyl-β-D-galactoside and incubated at 30 °C for 30 min. Subsequently, the reaction was interrupted by the addition of 2 mL of 0.5 M Na2CO3, and the absorbance was determined at a wavelength of 420 nm. Meanwhile, a calibration curve of p-nitrophenol was prepared in order to determine enzyme activity. A unit (U) was defined as the amount of β-glucosidase that would liberate 1 µM p-nitrophenol/min under the experimental conditions. The results are expressed as U/g of sample.
2.5. Cell Culture and Glucose Uptake
Murine 3T3-L1 preadipocytes were purchased from the Bioresource Collection and Research Center (Hsinchu City, Taiwan). The cells were grown in DMEM supplemented with 10% FBS, 4 mM L-glutamine, and 1% antibiotic–antimycotic solution (10,000 units of penicillin/mL and 10 mg of streptomycin/mL) in a 10 cm dish at 37 °C under a humidified atmosphere containing 5% CO2. For differentiation of 3T3-L1 preadipocytes, the cells were seeded into a 12-well plate at a density of 6000 cells/well and then incubated for 3 days. When the cells were confluent, they were incubated in the differentiation medium containing 0.5 mM 3-isobutyl-1-methylxanthine, 0.25 mM dexamethasone, 1 µM insulin, 10% fetal bovine serum, 4 mM glutamine, and 1% antibiotic–antimycotic solution. After 3 days, the cell medium was removed and replaced with the differentiation medium containing 1 µM insulin, 10% fetal bovine serum, 4 mM glutamins1e, and 1% antibiotic–antimycotic solution for an additional 2 days. Thereafter, the cells were incubated in the original propagation DMEM for 3 days. Each well was examined under the microscope to identify whether the cells had become adipocytes, which are round and full of easily distinguishable fat globules.
After differentiation, the cells were washed three times with the Krebs–Ringer HEPES (KRH) buffer composed of 0.5% BSA. Next, the cells were treated with AmR, FAmR, or ginsenosides in the presence and absence of 10 nM insulin dissolved in KRH buffer, which was allowed to proceed for 30 min at 37 °C. For measurement of glucose transport, 1 mM glucose and 0.1 mM 2-NBDG were added. After 20 min at 37 °C, glucose uptake was terminated by washing the cells with ice-cold PBS three times. Subsequently, the cells were digested with the lysis buffer containing Triton X-100, 10% glycerol, 100 µM glycerophosphate, 10 mM NaF, and 1 mM sodium pyrophosphate, and then the fluorescence intensity (excitation wavelength of 485 nm and emission wavelength of 525 nm) was measured using a microplate reader (Bio-Tek, Atlanta, GA, USA).
2.6. Statistical Analysis
Data are expressed as the mean ± S.D. from a minimum of three experimental replicates. Statistically significant differences were detected using Student’s t-test. A significant difference was considered when p < 0.05. Pearson’s correlation coefficients were estimated in order to assess the relationships between the capacity for glucose uptake and the total contents of minor ginsenosides.
3. Results
3.1. Total Phenolic Contents of AmR and FAmR
Although our previous study showed that the fermentation of AmR with G. lucidum significantly changed the levels of Rg1, Re, Rb1, Rc, Rg3, and Rh2 [21], information regarding other bioactive compounds in AmR and FAmR is still lacking. To characterize the AmR and FAmR used in glucose uptake in 3T3-L1 cells, the total phenolic contents of AmR and FAmR were quantified. AmR contained 6.3 mg of GAE/g of sample, while H4 contained the most phenolic compounds, with 8.9 mg of GAE/g of sample (Figure 2). It is noteworthy that the total phenolic contents were significantly affected by the inoculum quantity. The phenolic content of FAmR at 10% inoculation for 4 days was significantly higher than that at 1% and 5% inoculation for 4 days. However, fermentation periods did not significantly affect the phenolic contents of FAmR.
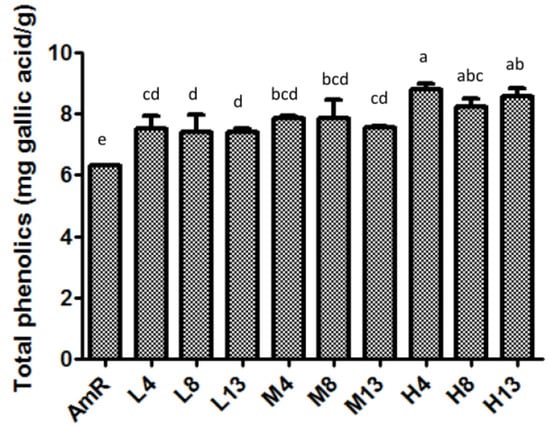
Figure 2.
Total phenolic contents of AmR and FAmR. Data are expressed as gallic acid equivalents/g of sample. AmR was incubated with low (L), medium (M), or high (H) inoculation quantities of G. lucidum, and then fermented for 4, 8, or 13 days. Each value represents a minimum of three experimental replicates. Data are expressed as the mean ± S.D., and were analyzed by one-way ANOVA and Duncan’s multiple range test. Within the same fermentation day, different letters of the alphabet represent significant differences (p < 0.05). AmR denotes American ginseng extraction residue. L4, L8, and L13 denote low inoculation quantities with fermentation for 4, 8, and 13 days, respectively. M4, M8, and M13 denote medium inoculation quantities with fermentation for 4, 8, and 13 days, respectively. H4, H8, and H13 denote high inoculation quantities with fermentation for 4, 8, and 13 days, respectively.
3.2. Glucosidase Activity
In this study, AmR and FAmR were screened for β-glucosidase activity. Before fermentation, the β-glucosidase activity of AmR was 0.12 U/g of sample (data not shown), while the activity of FAmR was 3.42 U/g of sample at 10% inoculation for 13 days. At 10% inoculation for 13 days, the enzyme activity of β-glucosidase was significantly higher than that at fermentation for 4 and 8 days. Similarly, at 1 and 5% inoculation for 13 days, the enzyme activity was higher than that at inoculation for 4 and 8 days. Altogether, β-glucosidase activity was positively associated with the fermentation time, regardless of inoculation quantity (Figure 3).
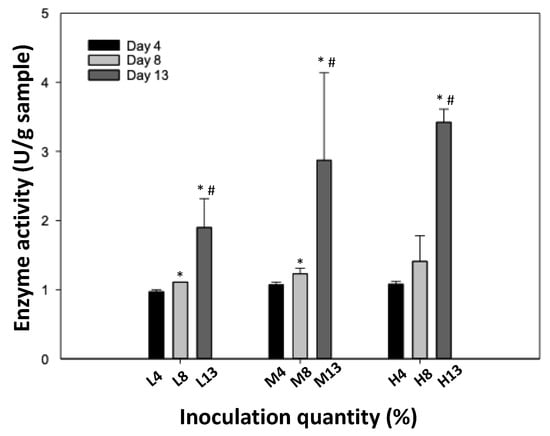
Figure 3.
The activity of β-glucosidase activity in FAmR. AmR was incubated with low, medium, or high inoculation quantities of G. lucidum, and then fermented for 4, 8, or 13 days. Data are expressed as the mean ± S.D. from three experimental replicates; * and # denote statistical significance in comparison to groups D4 and D8, respectively, as determined by Student’s t-test (p < 0.05). L4, L8, and L13 denote low inoculation quantities with fermentation for 4, 8, and 13 days, respectively. M4, M8, and M13 denote medium inoculation quantities with fermentation for 4, 8, and 13 days, respectively. H4, H8, and H13 denote high inoculation quantities with fermentation for 4, 8, and 13 days, respectively.
3.3. Glucose Uptake of 3T3-L1 Adipocytes
In the preliminary test, the cells were incubated with 1, 10, or 100 nM insulin for 30 min, followed by determination of 2-NBDG uptake. Figure 4 shows that the glucose uptake induced by 1 nM insulin was not significant, while significant increases were observed at 10 and 100 nM. The uptake of 2-NBDG induced by 10 and 100 nM insulin showed 1.35- and 1.95-fold increases relative to the vehicle group, respectively. Since exposure of adipocytes to 10 nM insulin was sufficient for stimulation of 2-NBDG uptake, this dose was used for evaluating the insulin-mediated 2-NBDG uptake activity of ginsenosides, AmR, and FAmR.
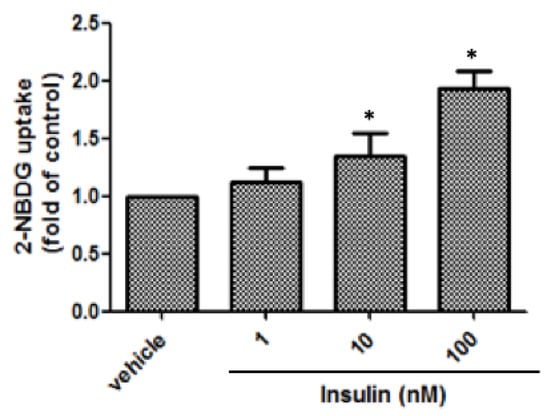
Figure 4.
The effects of insulin on the stimulation of glucose uptake in 3T3-L1 adipocytes. Cells were treated with different concentrations of insulin for 30 min. The 2-NBDG uptake by untreated cells was taken as 1.0. Data are expressed as the mean ± S.D. from three experimental replicates; * denotes statistical significance in comparison to the vehicle group, as determined by Student’s t-test (p < 0.05).
Before evaluating the hypoglycemic effects of AmR and FAmR, their cytotoxicity in 3T3-L1 adipocytes was tested. The results showed that AmR and FAmR did not cause significant cytotoxicity when their treatment concentrations were below 200 ppm (data not shown). The effects of 200 ppm AmR and FAmR on 2-NBDG uptake in the presence or absence of insulin are shown in Figure 5. The unfermented AmR did not significantly stimulate glucose uptake in the absence of 10 nM insulin, while the 2-NBDG uptake significantly dropped in the presence of 10 nM insulin. Upon fermentation for 4, 8, and 13 days, the 2-NBDG uptake of FAmR at 1% inoculation was 128%, 135%, and 132% higher than that of the vehicle group without the presence of 10 nM insulin, respectively. When the adipocytes were treated with FAmR at 5% inoculation for incubation for 8 and 13 days, the 2-NBDG uptake significantly increased as compared to the vehicle group in the absence of insulin (p < 0.05). Similarly, elevation of the 2-NBDG uptake of FAmR at 10% inoculation was positively associated with increased fermentation time in the absence of 10 nM insulin (Figure 5c). At 10% inoculation, the 2-NBDG uptake of FAmR at 8 days and 13 days was 132% and 145% higher than that of the vehicle group, respectively (p < 0.05). Finally, FAmR did not stimulate glucose uptake in the presence of 10 nM insulin, regardless of inoculation quantity and incubation days. Instead, AmR and FAmR L8 and H13 significantly reduced glucose uptake activities with the presence of insulin.
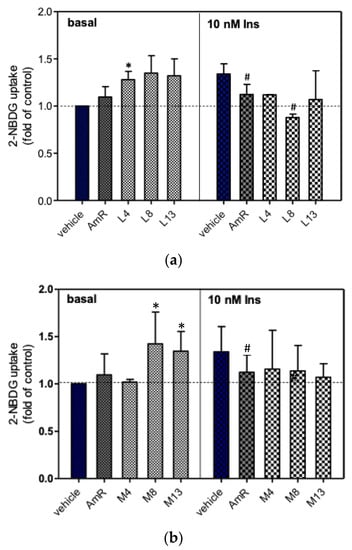
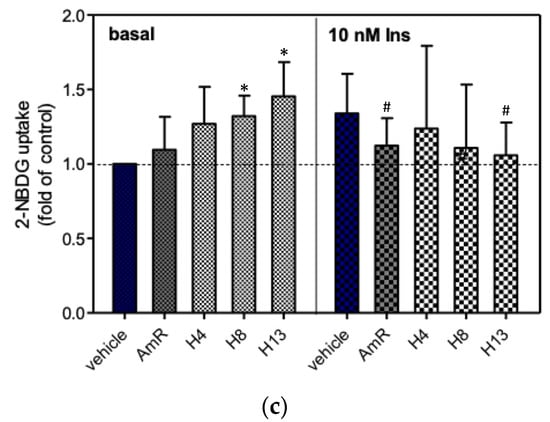
Figure 5.
The effects of AmR and FAmR on the stimulation of glucose uptake in 3T3-L1 adipocytes, with or without the presence of 10 nM insulin. The 2-NBDG uptake by untreated cells was taken as 1.0. Cells were treated with FAmR at (a) low (L), (b) medium (M), or (c) high (H) inoculation quantities for 4, 8, or 13 days. Data are expressed as the mean ± S.D. from a minimum of three experimental replicates; * denotes statistical significance in comparison to the basal vehicle group, as determined by Student’s t-test (p < 0.05); # denotes statistical significance in comparison to the insulin-treated vehicle group, as determined by Student’s t-test (p < 0.05). AmR denotes American ginseng extraction residue. L4, L8, and L13 denote low inoculation quantities with fermentation for 4, 8, and 13 days, respectively. M4, M8, and M13 denote medium inoculation quantities with fermentation for 4, 8, and 13 days, respectively. H4, H8, and H13 denote high inoculation quantities with fermentation for 4, 8, and 13 days, respectively.
Since ginsenosides are among the most important bioactive compounds in ginseng, and our previous study also demonstrated that fermentation with G. lucidum significantly affects the levels of Rg1, Re, Rb1, Rc, Rg3, CK, and Rh2 in AmR [21], their abilities in terms of 2-NBDG uptake were also compared in this study. In this study, the adipocytes were treated with different concentrations of ginsenosides in the presence or absence of 10 nM insulin, and their abilities in terms of 2-NBDG uptake were compared (Figure 6). In the absence of insulin, 0.1 and 1 µM Rg1 and Rb1 caused significant stimulation of 2-NBDG uptake in 3T3-L1 cells (Figure 6). It is noteworthy that Re, Rc, Rg3, and CK significantly stimulated 2-NBDG uptake only at the concentration of 1 µM without the presence of insulin treatment. The elevation of 2-NBDG uptake by Rh2 was not significant in the absence of insulin treatment. With the presence of 10 nM insulin, Rg1, Rc, Rg3, and Rb1 did not cause significant increases in 2-NBDG uptake, while only 1 µM Re and Rh2 significantly stimulated 2-NBDG uptake. Taken together, most of the tested ginsenosides significantly stimulated 2-NBDG uptake in the absence of insulin, whereas only Re and Rh2 elevated 2-NBDG uptake in the presence of 10 nM insulin.
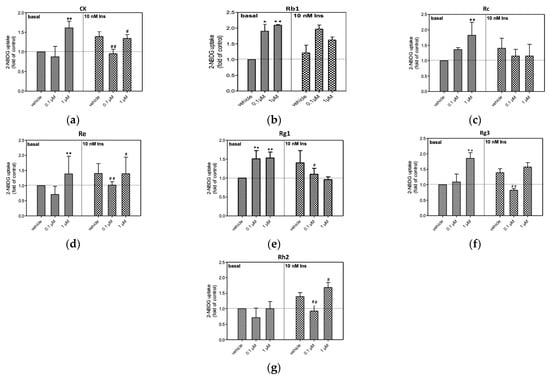
Figure 6.
The effects of the ginsenosides (a) CK, (b) Rb1, (c) Rc, (d) Re, (e) Rg1, (f) Rg3, and (g) Rh2 on the stimulation of glucose uptake in 3T3-L1 adipocytes, with or without the presence of 10 nM insulin (Ins). The 2-NBDG uptake by untreated cells was taken as 1.0. Data are expressed as the mean ± S.D. from a minimum of three experimental replicates; * and ** denote statistical significance in comparison to the basal vehicle group, as determined by Student’s t-test (p < 0.05 and p < 0.01, respectively); # and ## denote statistical significance in comparison to the insulin-treated vehicle group, as determined by Student’s t-test (p < 0.05 and p < 0.01, respectively).
In our previous study, the quantities of the minor ginsenosides Rg1, Rg3, and CK profoundly increased after fermentation with G. lucidum, especially at high inoculation quantities [21]. In this study, the percentages of minor ginsenosides of AmR during fermentation are given in Table 1. Formation of Rg3 was significantly elevated after fermentation with G. lucidum, regardless of inoculation quantity. At medium and high inoculation quantities, Rg1 levels significantly increased after fermentation with G. lucidum.

Table 1.
Percentage of ginsenoside contents of AmR during fermentation with G. lucidum.
Due to the fact that only FAmR stimulated glucose uptake in the absence of insulin, and that levels of Rg1, Rg3, and CK—the minor ginsenosides found in AmR—increased after fermentation with G. lucidum, the correlation between the amount of minor ginsenosides in FAmR and their activity on the stimulation of glucose uptake in the absence of insulin were evaluated. The total amounts of Rg1, Rg3, and CK in FAmR were positively associated with their activities in the stimulation of glucose uptake in adipocytes (R2 = 0.5578, p = 0.0131) (Figure 7).
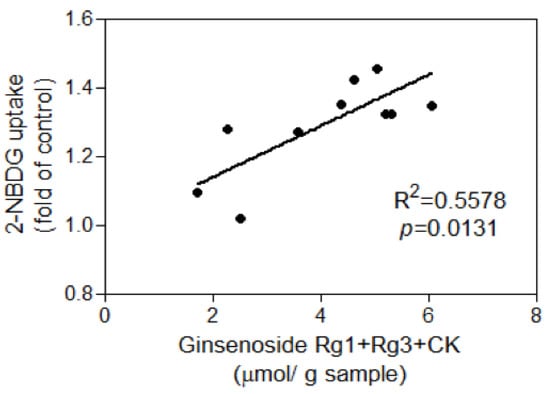
Figure 7.
Correlation between the amount of ginsenosides Rg1, Rg3, and CK and the 2-NBDG uptake of FAmR in the absence of insulin.
4. Discussion
Ginsenosides are regarded as the main components responsible for the bioactivity of ginseng. The main saponins include Rb1, Rb2, Re, and Rc, which comprise 80–90% of total ginsenosides. However, these naturally occurring glycosylated ginsenosides are poorly absorbed due to their large molecular size and poor permeability across the cell membrane [24,25]. In contrast, Rg3, CK, and Rd are the minor deglycosylated ginsenosides that exhibit higher pharmacological activity than the major ginsenosides. In our previous study, a microbial bioconversion approach was developed to convert ginsenosides in AmR via the fermentation of G. lucidum [21]. The results showed that the amounts of major ginsenosides in AmR—including Re and Rc—profoundly decreased, while the amounts of the minor ginsenosides Rg1, Rg3, and CK increased after incubation with G. lucidum. Likewise, microbial bioconversion of the major ginsenoside Rb1 to the minor ginsenoside Rd was also successfully developed using Indian fermented food bacteria [26]. In addition, the red ginseng extract was also converted to CK by fermentation with Saccharomyces cerevisiae HJ-014 [15]. The minor ginsenosides, which are present in low levels or absent in ginseng, can be produced by hydrolyzing the sugar moieties of major ginsenosides. Previous research has shown that glycoside-containing ginsenosides are the substrate to be hydrolyzed by β-glucosidase [27,28]. Importantly, hydrolysis of protopanaxadiol and protopanaxatriol by glycosidases has been comprehensively summarized previously [29]. For instance, the major ginsenosides Rc and Rb1 can be hydrolyzed by β-glucosidase to form Rg3 and CK, while enzymatic hydrolyzation of Re by β-glucosidase and α-rhamnosidase can produce Rg1. The present study also shows that the β-glucosidase activity of AmR profoundly increased after incubation with G. lucidum (Figure 3). Therefore, increases in the levels of minor ginsenosides—including Rg1, Rg3, and CK—of AmR after fermentation with G. lucidum are, at least in part, due to the cleavage of sugar moieties of the major ginsenosides Re, Rg1, and Rb1 by β-glucosidase. Altogether, microbial conversion using G. lucidum may serve as a feasible and eco-friendly bioconversion method for the production of minor ginsenosides.
In this study, the effects of the fermented products of ginseng residues on glucose uptake in 3T3-L1 adipocytes were examined in order to explore whether FAmR could serve as a viable new functional ingredient in the management of diabetes. The present study demonstrates that FAmR—especially at 5% and 10% inoculation—significantly stimulated glucose uptake in adipocytes without the presence of insulin, whereas unfermented AmR did not significantly enhance glucose uptake (Figure 5). Since ginsenosides are the main bioactive saponins in ginseng, their activities in glucose uptake were measured in this study. In the absence of insulin, Rb1, Rg1, Re, Rc, Rg3, and CK profoundly stimulated glucose uptake, whereas elevation of glucose uptake was not significantly affected by treatment with Rh2 (Figure 6). Similar results have been reported in 3T3-L1 adipocytes and C2C12 myotubes [30,31,32]. Both Rg1 and CK significantly enhanced glucose uptake in adipocytes, which is partly associated with GLUT4 translocation and activation of AMP-activated protein kinase (AMPK) and the phosphatidylinositol 3-kinase (PI3K) signaling pathway [30]. In addition, Rg3 and Re markedly improved glucose uptake via GLUT4 translocation involved with the expression of PI3K and insulin receptor substrate 1 (IRS-1) [31]. In 3T3-L1 adipocytes and C2C12 myotubes, Rb1 significantly improved basal and insulin-mediated glucose uptake in a dose-dependent manner, by promoting the translocation of GLUT1 and GLUT4 [32]. Recently, the therapeutic potential of ginsenosides in the management of diabetes has been comprehensively summarized, wherein the molecular targets mainly involved GLUTs, TNF-α, interleukin 6, caspase-3, bcl-2, the STAT5-PPAR γ pathway, the PI3K/Akt pathway, the AMPK-JNK pathway, and the NF-κB pathway [33,34]. Taken together, the hypoglycemic activity of FAmR found in the present study seems to have originated, at least in part, from its bioactive ginsenosides.
In this study, percentages of the minor ginsenosides in AmR and FAmR—including Rg1, Rg3, and CK—were calculated (Table 1). After fermentation with G. lucidum, Rg1 and Rg3 levels in FAmR significantly increased in comparison to those in AmR. The percentage of Rg1 + Rg3 + CK in AmR was only 1.85%, while their percentages were above 25% after fermentation with G. lucidum. Since stimulation of glucose uptake by treatment with FAmR significantly increased in comparison to AmR, and minor ginsenosides in AmR—especially Rg1 and Rg3—were elevated after fermentation, the correlation between the total amount of Rg1 + Rg3 + CK in FAmR and their glucose uptake activities was analyzed. A positive correlation (R2 = 0.5578) found between the total amounts of Rg1 + Rg3 + CK of FAmR and their glucose uptake activities implies that the minor deglycosylated ginsenosides might play an important role in the hypoglycemic effect of FAmR.
The present findings also show that the total phenolic contents of FAmR increased after fermentation with G. lucidum. A similar study has also reported that the total phenolic contents and antioxidative activities of red ginseng marc after fermentation with Saccharomyces cerevisiae and Bacillus subtilis were higher than those from unfermented red ginseng marc [20]. Meanwhile, the fermented red ginseng also exhibited antioxidant activity in streptozotocin-induced diabetic rats, elevating the activities of superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase, as well as reducing lipid peroxidation [35]. Recently, the polyphenol composition in different parts of ginseng has been comprehensively identified, wherein the major phenolic compounds occurring in ginseng roots included coumaric acid, chlorogenic acid, protocatechuic acid, gentisic acid, and hydroxybenzoic acid [36]. Moreover, rutin, resveratrol, naringin, naringenin, and hesperetin were also present in ginseng. Thus, total phenolic contents in AmR significantly increased after fermentation with G. lucidum, possibly due to increased production of these phenolic compounds. However, the formation of new phenolic compounds in AmR during fermentation with G. lucidum could not be ruled out in this study. Due to the fact that therapeutic potentials of these phenolic compounds occurring in ginseng against diabetes have been summarized previously [37,38,39], the hypoglycemic effect of FAmR found in this study might be from the result of increased production of polyphenol compounds.
In this study, the adipocytes were also treated with AmR and FAmR in the presence of 10 nM insulin to test whether they could improve insulin-mediated glucose uptake. Despite the positive outcome of glucose uptake stimulation with the treatment of FAmR in the absence of insulin, the present findings indicate that both AmR and FAmR were unable to stimulate glucose uptake in the presence of 10 nM insulin. These results suggest that AmR and FAmR have no additive or synergistic effects with insulin. On the other hand, AmR and FAmR L8 and H13 significantly reduced glucose uptake activity in the presence of insulin (Figure 5). Similar results were also found for ginsenosides (Figure 6); at a concentration of 0.1 µM, the ginsenosides Rg1, Re, Rg3, CK, and Rh2 significantly inhibited glucose uptake in the presence of insulin. A previous study showed similar results to the present findings observed in 3T3-L1 adipocytes, where the uptake of 2-deoxyglucose was significantly stimulated by treatment with a cinnamon water extract (CE). However, stimulation of glucose uptake by treatment with high concentrations of CE (0.3 and 0.4 mg/mL) was profoundly reduced in the presence of 50 nM insulin, indicating that CE predominated over insulin in the control of glucose uptake [40]; these results suggest that CE may compete with insulin at some points along the insulin-mediated signaling pathway. In addition, an extract of Lagerstroemia speciosa (banaba) also exhibited insulin-like glucose-uptake-stimulatory effects in the absence of insulin. However, in combination with insulin, stimulation of glucose uptake by banaba was profoundly diminished in a dose-dependent manner [41]. Banaba may interact with a protein factor that participates in the insulin-mediated glucose transport signaling pathway, or may structurally alter the conformation of a protein factor, subsequently inhibiting the glucose uptake signal initiated from insulin–insulin receptor binding.
Moreover, emerging data suggest that ginsenosides act as AMPK activators in the management of obesity and diabetes [31,34,42]. However, AMPK activators may inhibit insulin-mediated glucose uptake in adipocytes. 5-Aminoimidazole-4-carboxamide ribonucleoside (AICAR) is an AMPK activator that promotes glucose uptake in 3T3-L1 cells without the presence of insulin, whereas AICAR significantly reduces insulin-stimulated glucose uptake [43,44]. In adipocytes, insulin-stimulated glucose uptake is used anabolically to produce fatty acids and triacylglycerols for export to other tissues. Under the conditions of cellular stress by which AMPK is activated, insulin-mediated glucose uptake in adipocytes is inhibited to conserve ATP used in fatty acid and triacylglycerol synthesis. Unlike adipocytes, it is noteworthy that AICAR-mediated AMPK stimulation promotes glucose uptake to provide ATP as fuel in skeletal muscles. A similar study also showed the negative regulatory effect of quercetin on controlling insulin-mediated glucose homeostasis in 3T3-L1 adipocytes via AMPK-mediated mechanisms [45]. Taken together, further studies are required in order to demonstrate whether bioactive compounds occurring in FAmR—especially ginsenosides—can interfere with insulin-stimulated glucose uptake in adipocytes by competing with insulin, interacting with protein factors, or activating AMPK-mediated pathways.
5. Conclusions
Ginseng is a traditional Chinese medicine that has been widely used in China and other Asian countries for thousands of years. In many Asian countries, large amounts of ginseng residue are produced as waste products, due to its applications in manufacturing functional products, even though it still contains bioactive components. In this study, the β-glucosidase activity of AmR profoundly increased as a result of fermentation with G. lucidum. The production of the minor deglycosylated ginsenosides Rg1, Rg3, and CK in AmR after fermentation with G. lucidum was, at least in part, associated with the cleavage of sugar moieties of the major ginsenosides Re, Rg1, and Rb1 by β-glucosidase. In addition, the total phenolic contents of AmR significantly increased after fermentation with G. lucidum. In 3T3-L1 adipocytes, FAmR exerted insulin-like glucose-uptake-stimulatory effects, whereas stimulation of glucose uptake by treatment with AmR was not significant. The hypoglycemic effects of FAmR were positively associated with increased production of the minor ginsenosides Rg1, Rg3, and CK, as well as with total phenolic contents. Altogether, bioconversion of AmR by fermentation with G. lucidum may be a feasible and eco-friendly approach to not only resolve the problem of ginseng waste, but also develop a functional ingredient for the management of diabetes.
Author Contributions
Conceptualization, T.-J.L. and L.S.H.; methodology, B.-Y.H., C.-H.C. and W.-L.H.; resources, T.-J.L. and M.-H.P.; writing—original draft preparation, B.-Y.H., C.-H.C. and W.-L.H.; writing—review and editing, M.-H.P., C.-T.H. and W.-L.H.; supervision, C.-T.H. and L.S.H.; project administration, L.S.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shaw, P.C.; But, P.P.H. Authentication of panax species and their adulterants by random-primed polymerase chain-reaction. Planta Med. 1995, 61, 466–469. [Google Scholar] [CrossRef]
- Liu, Z.Q. Chemical insights into ginseng as a resource for natural antioxidants. Chem. Rev. 2012, 112, 3329–3355. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.M.; Xin, Y.Z.; Li, Y.J.; Xu, F.X.; Xi, X.Z.; Guo, H.; Cui, X.W.; Cao, H.; Zhang, X.; Han, C.C. Ginsenosides: A potential neuroprotective agent. Biomed. Res. Int. 2018, 2018, 8174345. [Google Scholar] [CrossRef]
- Yi, Y.S. Roles of ginsenosides in inflammasome activation. J. Ginseng Res. 2019, 43, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.X.; Huang, Y.L.; Zheng, H.; Li, S.Q.; Li, Z.H.; Yuan, L.; Cheng, X.; He, C.S.; Sun, J.F. Ginsenosides for the treatment of metabolic syndrome and cardiovascular diseases: Pharmacology and mechanisms. Biomed. Pharmacother. 2020, 132, 110915. [Google Scholar] [CrossRef]
- Park, S.K.; Hyun, S.H.; In, G.; Park, C.K.; Kwak, Y.S.; Jang, Y.J.; Kim, B.; Kim, J.H.; Han, C.K. The antioxidant activities of Korean Red Ginseng (Panax ginseng) and ginsenosides: A systemic review through in vivo and clinical trials. J. Ginseng Res. 2021, 45, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.S.T.; Che, C.M.; Leung, K.W. Recent advances in ginseng as cancer therapeutics: A functional and mechanistic overview. Nat. Prod. Rep. 2015, 32, 256–272. [Google Scholar] [CrossRef]
- Chen, W.; Balan, P.; Popovich, D.G. Review of ginseng anti-diabetic studies. Molecules 2019, 24, 4501. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yang, M.F.; Su, Y.P.; Jiang, H.M.; You, X.J.; Yang, Y.J.; Zhang, H.L. Ginsenoside Re reduces insulin resistance through activation of PPAR-gamma pathway and inhibition of TNF-alpha production. J. Ethnopharmacol. 2013, 147, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Ding, L.; Zhang, H.Q.; Chu, Y.F.; Chang, Z.H.; Yu, Y.L.; Guo, D.D.; Zhang, S.P.; Liu, X.Z. Ginsenoside Rb1 increases insulin sensitivity through suppressing 11 beta-hydroxysteroid dehydrogenase type I. Am. J. Transl. Res. 2017, 9, 1049–1057. [Google Scholar]
- Kim, K.S.; Yang, H.J.; Lee, I.S.; Kim, K.H.; Park, J.; Jeong, H.S.; Kim, Y.; Ahn, K.S.; Na, Y.C.; Jang, H.J. The aglycone of ginsenoside Rg3 enables glucagon-like peptide-1 secretion in enteroendocrine cells and alleviates hyperglycemia in type 2 diabetic mice. Sci. Rep. 2015, 5, 18325. [Google Scholar] [CrossRef]
- Jenkins, A.L.; Morgan, L.M.; Bishop, J.; Jovanovski, E.; Jenkins, D.J.A.; Vuksan, V. Co-administration of a konjac-based fibre blend and American ginseng (Panax quinquefolius L.) on glycaemic control and serum lipids in type 2 diabetes: A randomized controlled, cross-over clinical trial. Eur. J. Nutr. 2018, 57, 2217–2225. [Google Scholar] [CrossRef]
- Vuksan, V.; Xu, Z.Z.; Jovanovski, E.; Jenkins, A.L.; Beljan-Zdravkovic, U.; Sievenpiper, J.L.; Mark Stavro, P.; Zurbau, A.; Duvnjak, L.; Li, M.Z.C. Efficacy and safety of American ginseng (Panax quinquefolius L.) extract on glycemic control and cardiovascular risk factors in individuals with type 2 diabetes: A double-blind, randomized, cross-over clinical trial. Eur. J. Nutr. 2019, 58, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Lee, J.W.; Lee, K.Y.; Yang, D.C. Microbial conversion of major ginsenoside Rb-1 to pharmaceutically active minor ginsenoside Rd. J. Microbiol. 2005, 43, 456–462. [Google Scholar]
- Choi, H.J.; Kim, E.A.; Kim, D.H.; Shin, K.S. The bioconversion of red ginseng ethanol extract into compound K by saccharomyces cerevisiae HJ-014. Mycobiology 2014, 42, 256–261. [Google Scholar] [CrossRef]
- Zheng, M.M.; Xu, F.X.; Li, Y.J.; Xi, X.Z.; Cui, X.W.; Han, C.C.; Zhang, X.L. Study on transformation of ginsenosides in different methods. Biomed. Res. Int. 2017, 2017, 8601027. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.P.; Jin, Y.; Yin, C.R.; Bai, L.L. Co-transformation of Panax major ginsenosides Rb-1 and Rg(1) to minor ginsenosides C-K and F-1 by Cladosporium cladosporioides. J. Ind. Microbiol. Biotechnol. 2012, 39, 521–527. [Google Scholar] [CrossRef]
- Lu, J.H.; He, R.J.; Sun, P.L.; Zhang, F.M.; Linhardt, R.J.; Zhang, A.Q. Molecular mechanisms of bioactive polysaccharides from Ganoderma lucidum (Lingzhi), a review. Int. J. Biol. Macromol. 2020, 150, 765–774. [Google Scholar] [CrossRef]
- Songulashvili, G.; Elisashvili, V.; Wasser, S.P.; Nevo, E.; Hadar, Y. Basidiomycetes laccase and manganese peroxidase activity in submerged fermentation of food industry wastes. Enzym. Microb. Technol. 2007, 41, 57–61. [Google Scholar] [CrossRef]
- Lee, S.H.; Oh, M.; Park, J.; Jang, S.Y.; Cheong, S.H.; Lee, H.; Moon, S.H. Antioxidant and anti-inflammatory activities of the ethanolic extract of fermented red ginseng marc. Food Sci. Biotechnol. 2015, 24, 651–657. [Google Scholar] [CrossRef]
- Hsu, B.Y.; Chen, C.H.; Lu, T.J.; Hwang, L.S. Bioconversion of ginsenosides in the american ginseng (xi yang shen) extraction residue by fermentation with lingzhi (ling zhi, ganoderma lucidum). J. Tradit. Complement. Med. 2013, 3, 95–101. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.Z.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, M.; Obata, A. Beta-glucosidases from soybeans hydrolyze daidzin and genistin. J. Food Sci. 1993, 58, 144–147. [Google Scholar] [CrossRef]
- Xu, Q.F.; Fang, X.L.; Chen, D.F. Pharmacokinetics and bioavailability of ginsenoside Rb-1 and Rg(1) from Panax notoginseng in rats. J. Ethnopharmacol. 2003, 84, 187–192. [Google Scholar] [CrossRef]
- Yu, K.; Chen, F.; Li, C. Absorption, disposition, and pharmacokinetics of saponins from Chinese medicinal herbs: What do we know and what do we need to know more? Curr. Drug Metab. 2012, 13, 577–598. [Google Scholar] [CrossRef]
- Senthil, K.; Veena, V.; Mahalakshmi, M.; Pulla, R.; Yang, D.C.; Parvatham, R. Microbial conversion of major ginsenoside Rb1 to minor ginsenoside Rd by Indian fermented food bacteria. Afr. J. Biotechnol. 2009, 8, 6961–6966. [Google Scholar]
- Son, J.W.; Kim, H.J.; Oh, D.K. Ginsenoside Rd production from the major ginsenoside Rb-1 by beta-glucosidase from Thermus caldophilus. Biotechnol. Lett. 2008, 30, 713–716. [Google Scholar] [CrossRef]
- Ko, S.R.; Choi, K.J.; Suzuki, K.; Suzuki, Y. Enzymatic preparation of ginsenosides Rg(2), Rh-1, and F-1(1)). Chem. Pharm. Bull. 2003, 51, 404–408. [Google Scholar] [CrossRef]
- Park, C.S.; Yoo, M.H.; Noh, K.H.; Oh, D.K. Biotransformation of ginsenosides by hydrolyzing the sugar moieties of ginsenosides using microbial glycosidases. Appl. Microbiol. Biot. 2010, 87, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Lin, C.Y.; Huang, S.F.; Lin, H.C.; Chang, W.L.; Chang, T.C. Effect and mechanism of ginsenosides CK and Rg1 on stimulation of glucose uptake in 3T3-L1 adipocytes. J. Agric. Food Chem. 2010, 58, 6039–6047. [Google Scholar] [CrossRef]
- Lee, O.H.; Lee, H.H.; Kim, J.H.; Lee, B.Y. Effect of ginsenosides Rg3 and Re on glucose transport in mature 3T3-L1 adipocytes. Phytother. Res. 2011, 25, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Shang, W.B.; Yang, Y.; Zhou, L.B.; Jiang, B.R.; Jin, H.; Chen, M.D. Ginsenoside Rb-1 stimulates glucose uptake through insulin-like signaling pathway in 3T3-L1 adipocytes. J. Endocrinol. 2008, 198, 561–569. [Google Scholar] [CrossRef]
- Bai, L.T.; Gao, J.L.; Wei, F.; Zhao, J.; Wang, D.W.; Wei, J.P. Therapeutic potential of ginsenosides as an adjuvant treatment for diabetes. Front. Pharmacol. 2018, 9, 423. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.W.; Jiang, J.L.; Zou, J.J.; Yang, M.Y.; Chen, F.M.; Zhang, Y.J.; Jia, L. Therapeutic potential of ginsenosides on diabetes: From hypoglycemic mechanism to clinical trials. J. Funct. Foods 2020, 64, 103630. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, S.G.; Chae, I.G.; Kim, M.J.; Im, N.K.; Yu, M.H.; Lee, E.J.; Lee, I.S. Antioxidant Effects of Fermented Red Ginseng Extracts in Streptozotocin-Induced Diabetic Rats. J. Ginseng Res. 2011, 35, 129–137. [Google Scholar] [CrossRef]
- Malathy, R.; Prabakaran, M.; Kalaiselvi, K.; Chung, I.M.; Kim, S.H. Comparative polyphenol composition, antioxidant and anticorrosion properties in various parts of panax ginseng extracted in different solvents. Appl. Sci. 2021, 11, 93. [Google Scholar] [CrossRef]
- Vinayagam, R.; Jayachandran, M.; Xu, B.J. Antidiabetic effects of simple phenolic acids: A comprehensive review. Phytother. Res. 2016, 30, 184–199. [Google Scholar] [CrossRef]
- Ghorbani, A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed. Pharmacother. 2017, 96, 305–312. [Google Scholar] [CrossRef]
- Chen, R.; Qi, Q.L.; Wang, M.T.; Li, Q.Y. Therapeutic potential of naringin: An overview. Pharm. Biol. 2016, 54, 3203–3210. [Google Scholar] [CrossRef] [PubMed]
- Roffey, B.; Atwal, A.; Kubow, S. Cinnamon water extracts increase glucose uptake but inhibit adiponectin secretion in 3T3-L1 adipose cells. Mol. Nutr. Food Res. 2006, 50, 739–745. [Google Scholar] [CrossRef]
- Liu, F.; Kim, J.K.; Li, Y.H.; Liu, X.Q.; Li, J.; Chen, X.H. An extract of Lagerstroemia speciosa L. has insulin-like glucose uptake-stimulatory and adipocyte differentiation-inhibitory activities in 3T3-L1 cells. J. Nutr. 2001, 131, 2242–2247. [Google Scholar] [CrossRef]
- Liu, H.M.; Liu, M.H.; Jin, Z.B.; Yaqoob, S.; Zheng, M.Z.; Cai, D.; Liu, J.S.; Guo, S.D. Ginsenoside Rg2 inhibits adipogenesis in 3T3-L1 preadipocytes and suppresses obesity in high-fat-diet-induced obese mice through the AMPK pathway. Food Funct. 2019, 10, 3603–3614. [Google Scholar] [CrossRef] [PubMed]
- Salt, I.P.; Connell, J.M.C.; Gould, G.W. 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR) inhibits insulin-stimulated glucose transport in 3T3-L1 adipocytes. Diabetes 2000, 49, 1649–1656. [Google Scholar] [CrossRef] [PubMed]
- Sakoda, H.; Ogihara, T.; Anai, M.; Fujishiro, M.; Ono, H.; Onishi, Y.; Katagiri, H.; Abe, M.; Fukushima, Y.; Shojima, N.; et al. Activation of AMPK is essential for AICAR-induced glucose uptake by skeletal muscle but not adipocytes. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E1239–E1244. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, M.L.; Hu, J.J.; Zhao, W.W.; Gao, X.J.; Jiang, C.H.; Liu, K.; Liu, B.L.; Huang, F. Quercetin differently regulates insulin-mediated glucose transporter 4 translocation under basal and inflammatory conditions in adipocytes. Mol. Nutr. Food Res. 2014, 58, 931–941. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).