Methods for Oxygenation of Continuous Cultures of Brewer’s Yeast, Saccharomyces cerevisiae
Abstract
1. Introduction
2. Materials and Methods
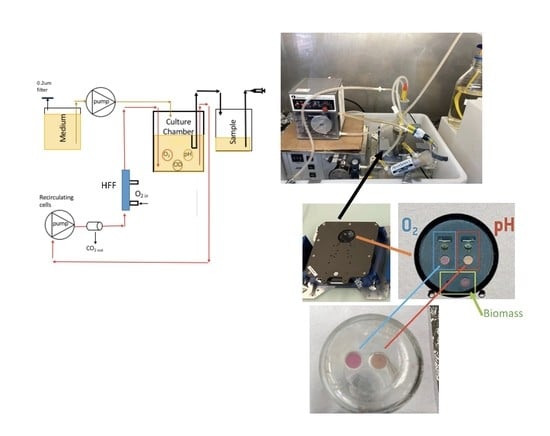
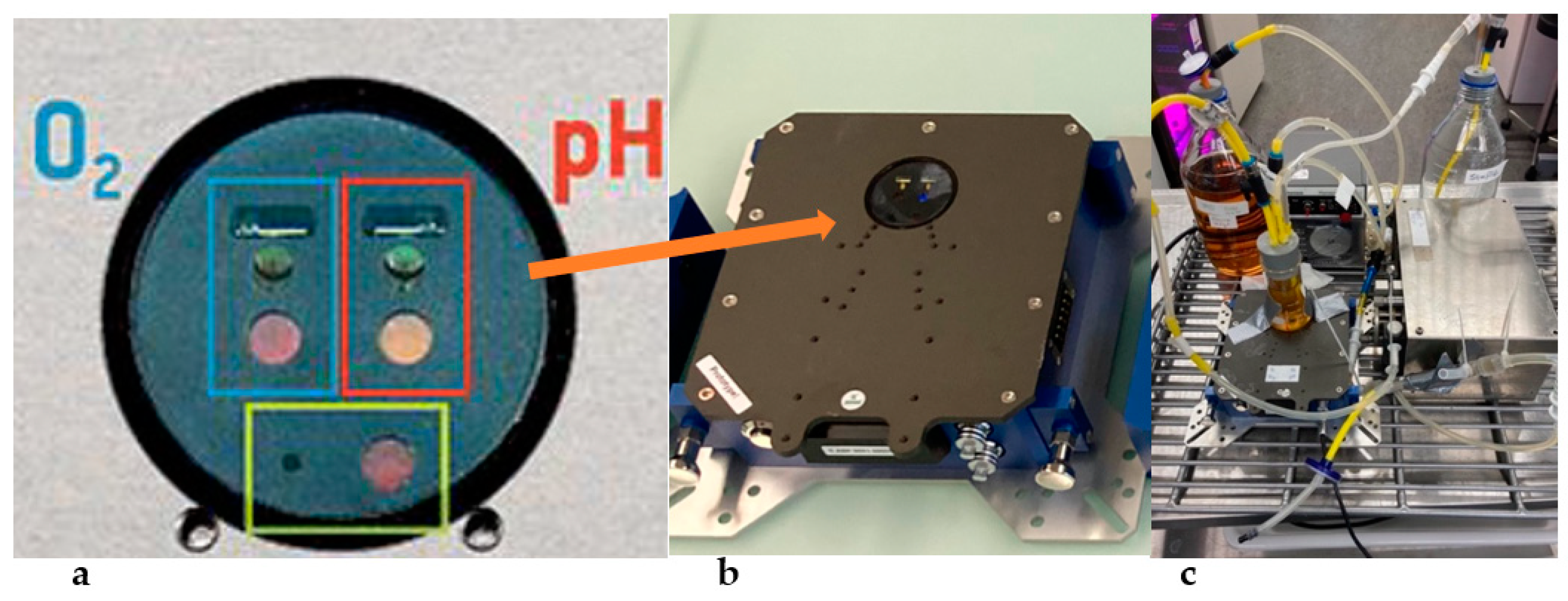
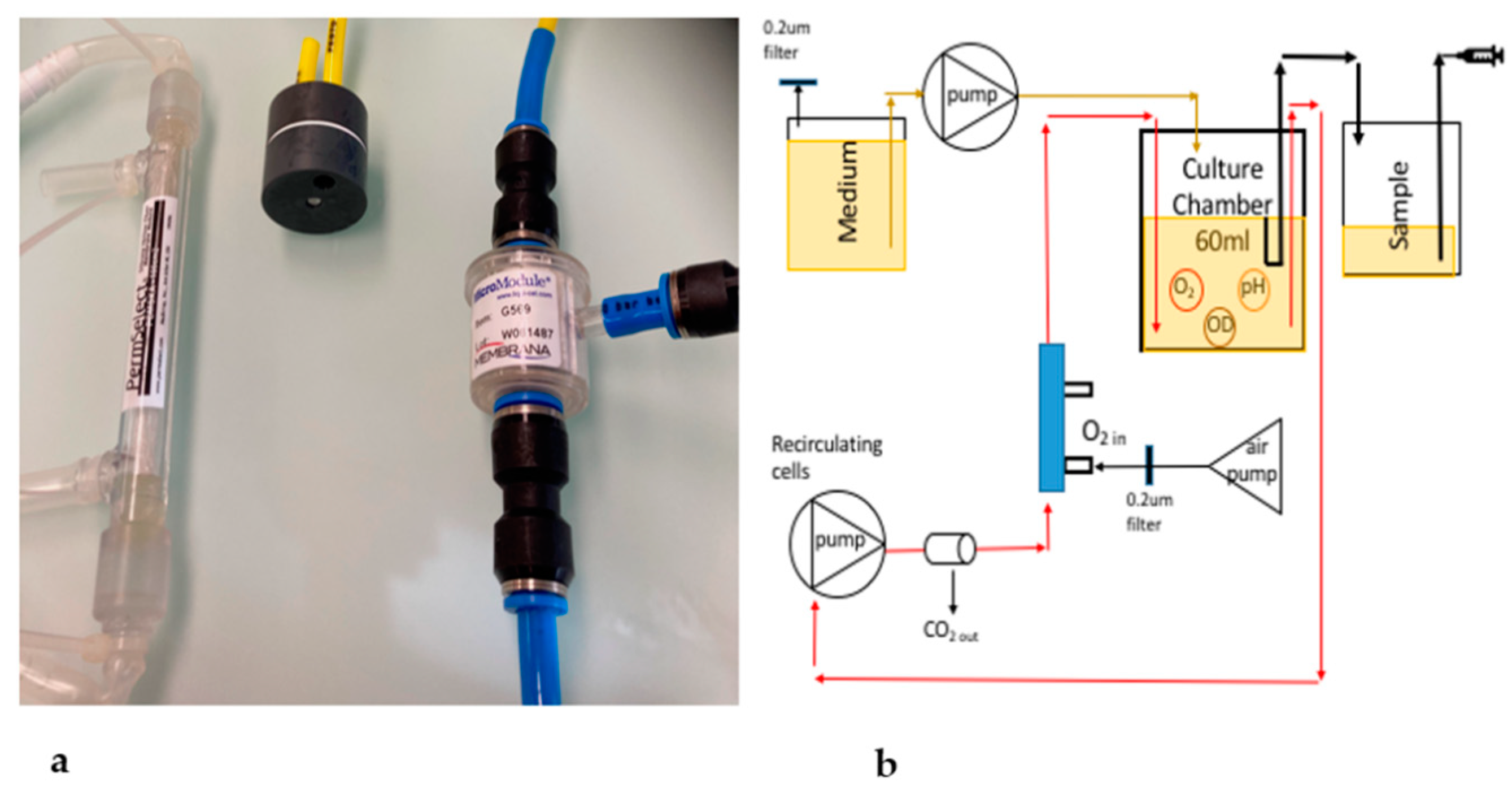
2.1. The Bioreactor and Data Analysis
2.2. Culture Conditions
2.3. Oxygenation Methods
3. Results
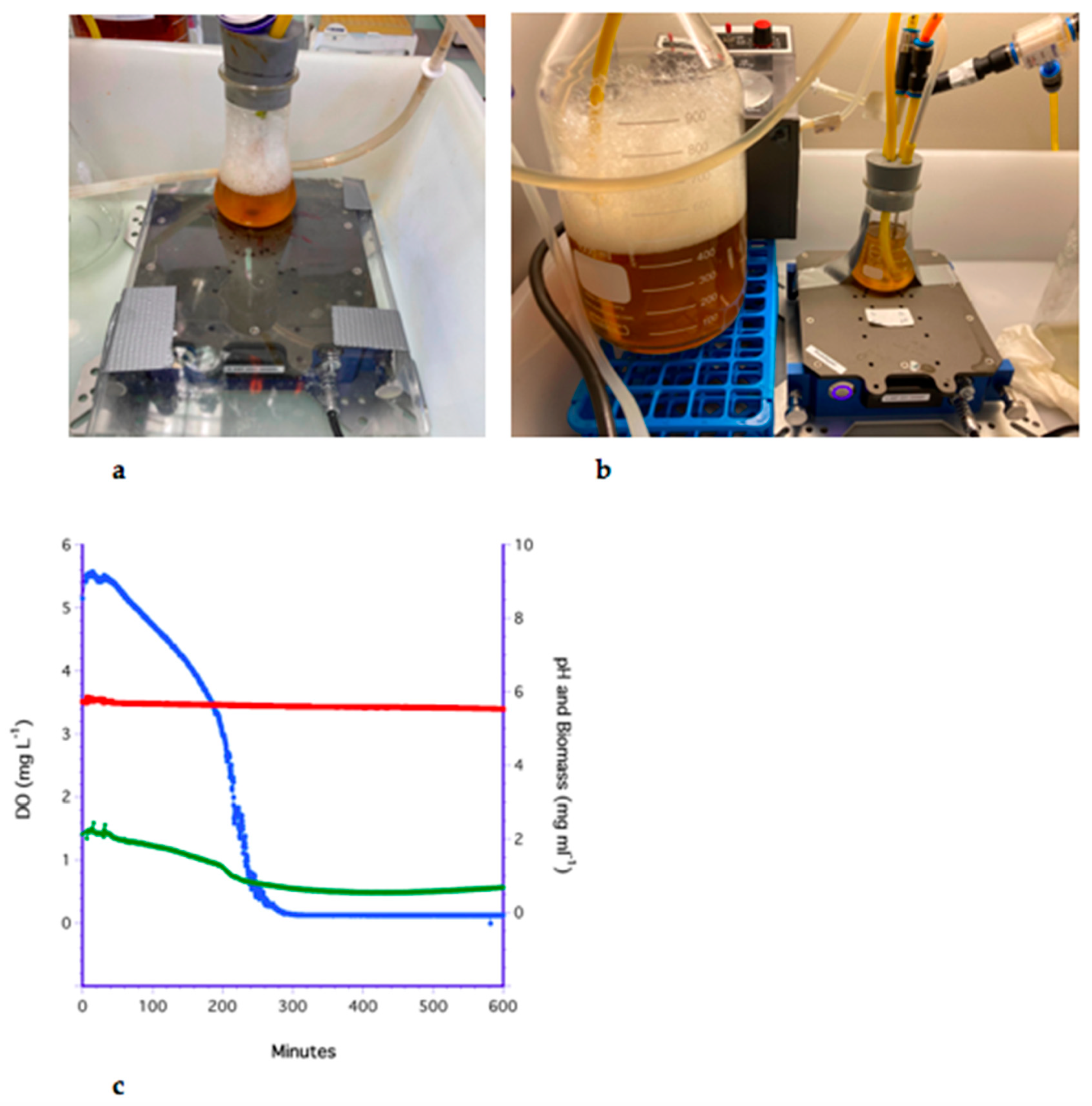
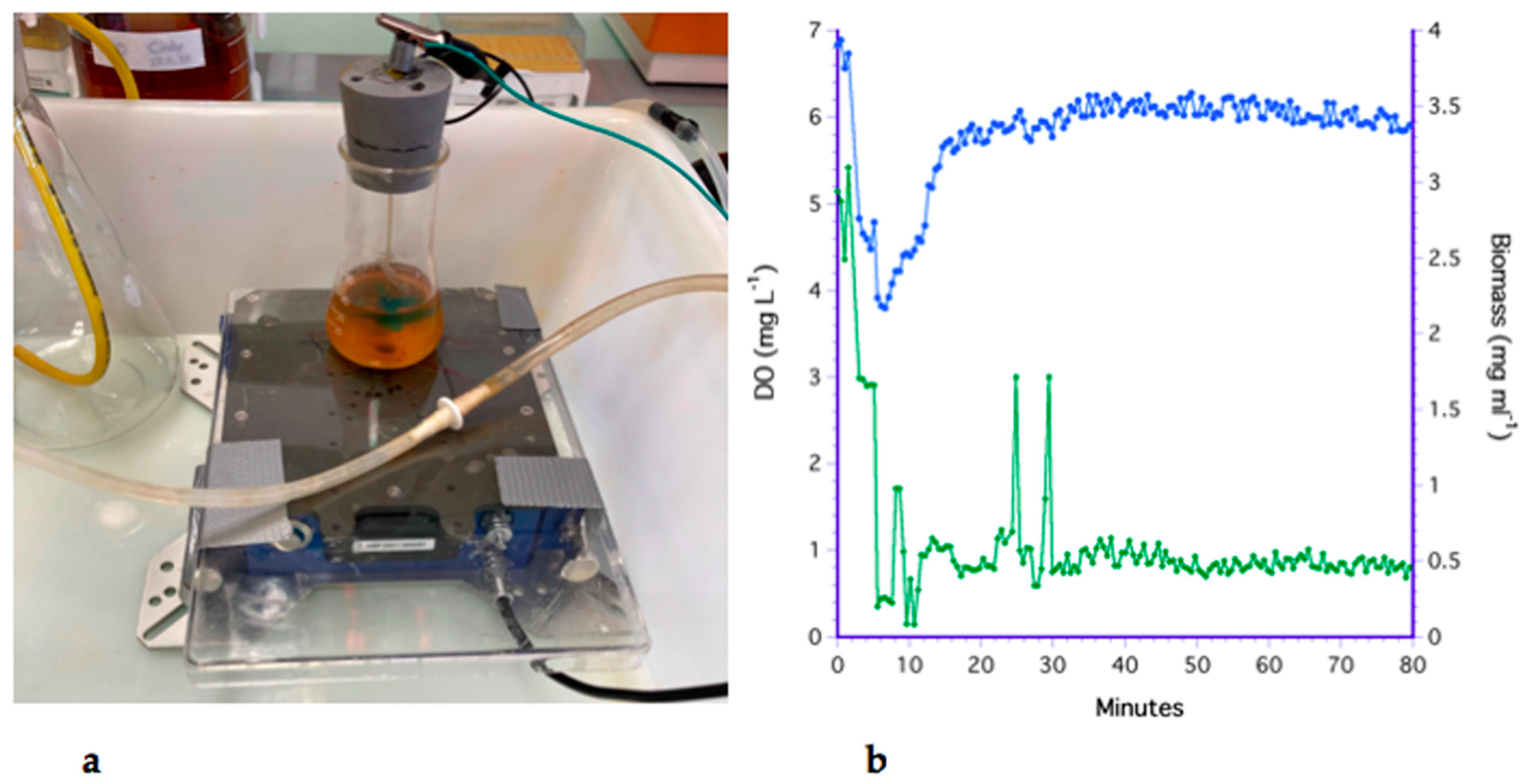
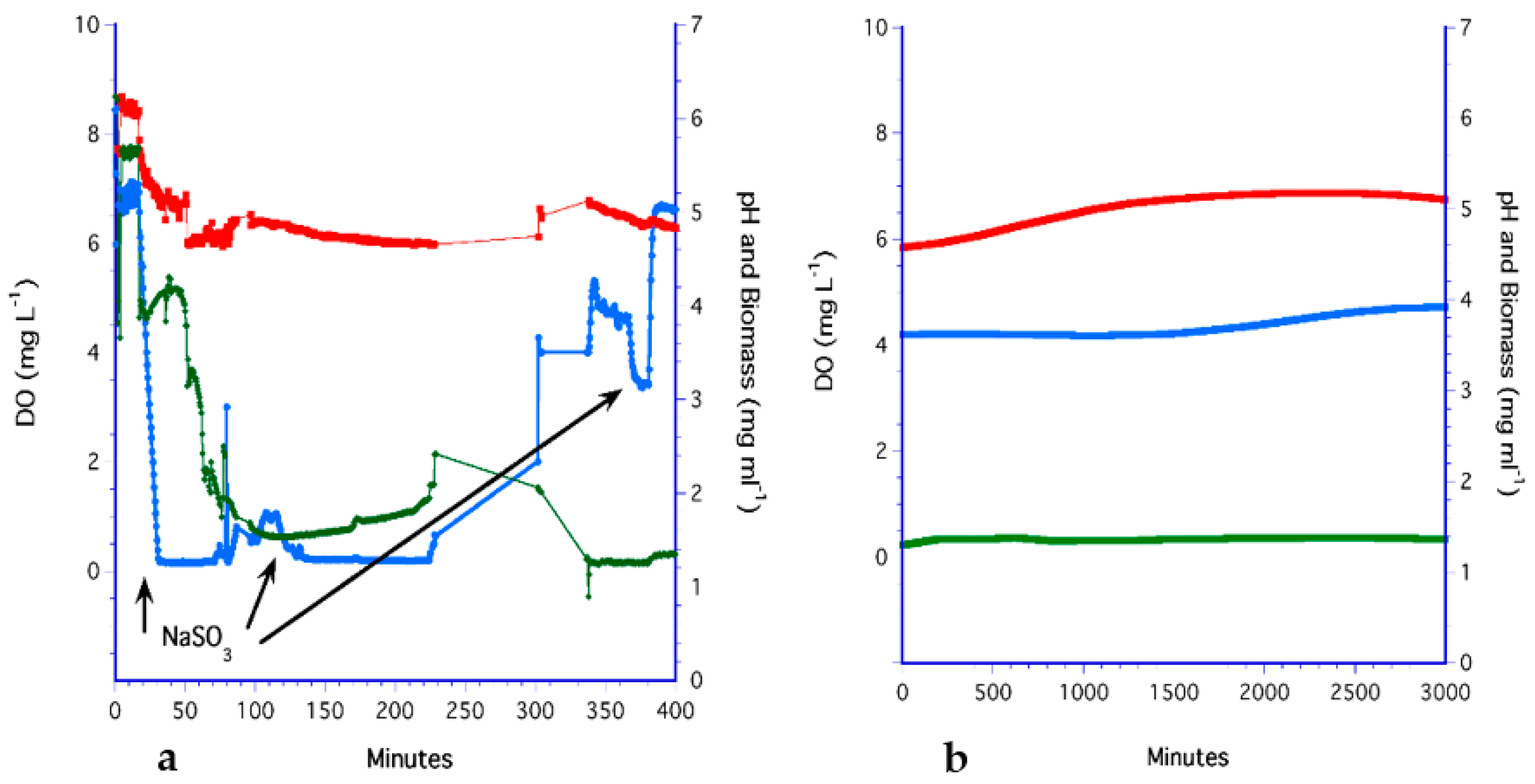
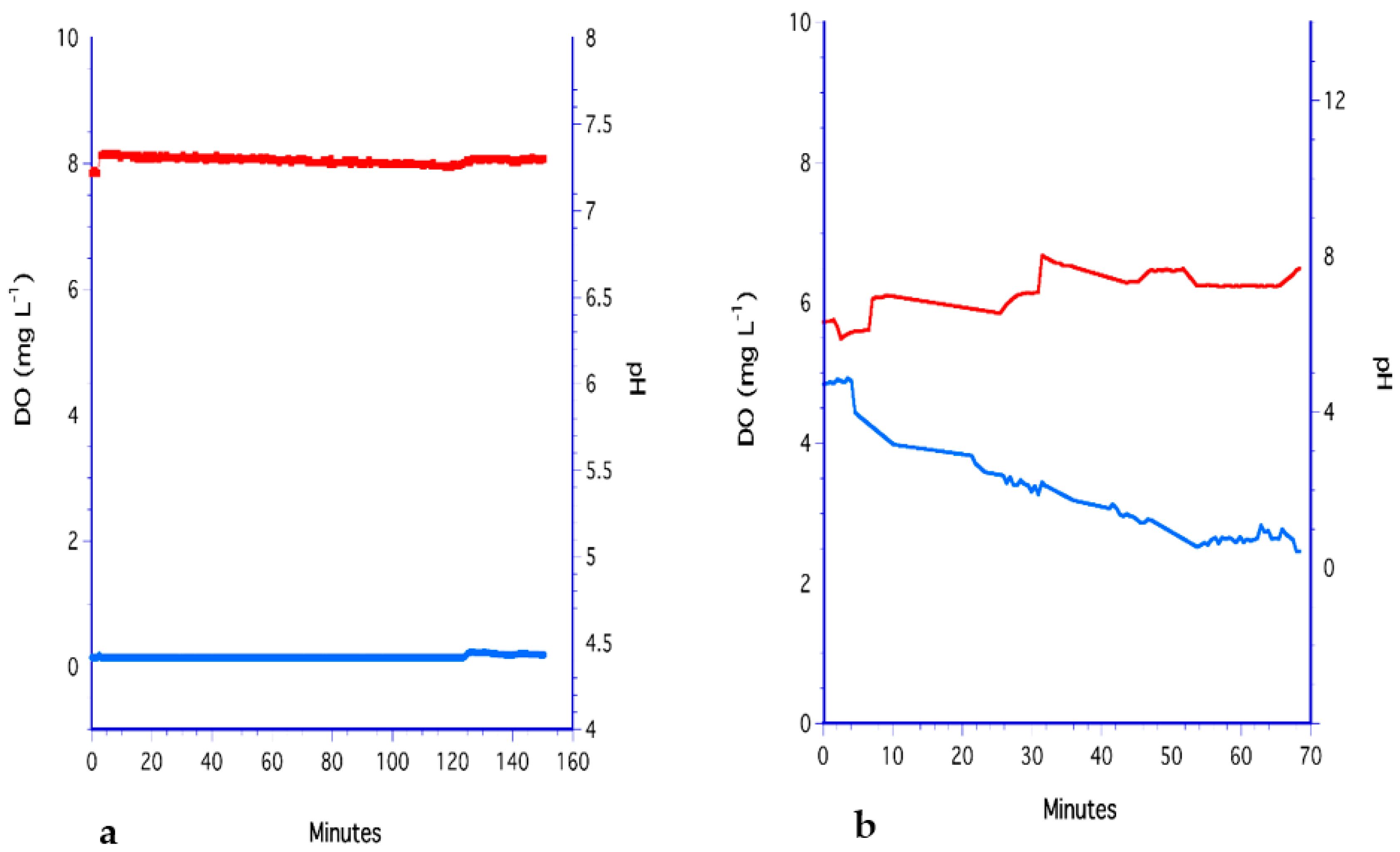
3.1. The Bubbling Method
3.2. The Impeller Method
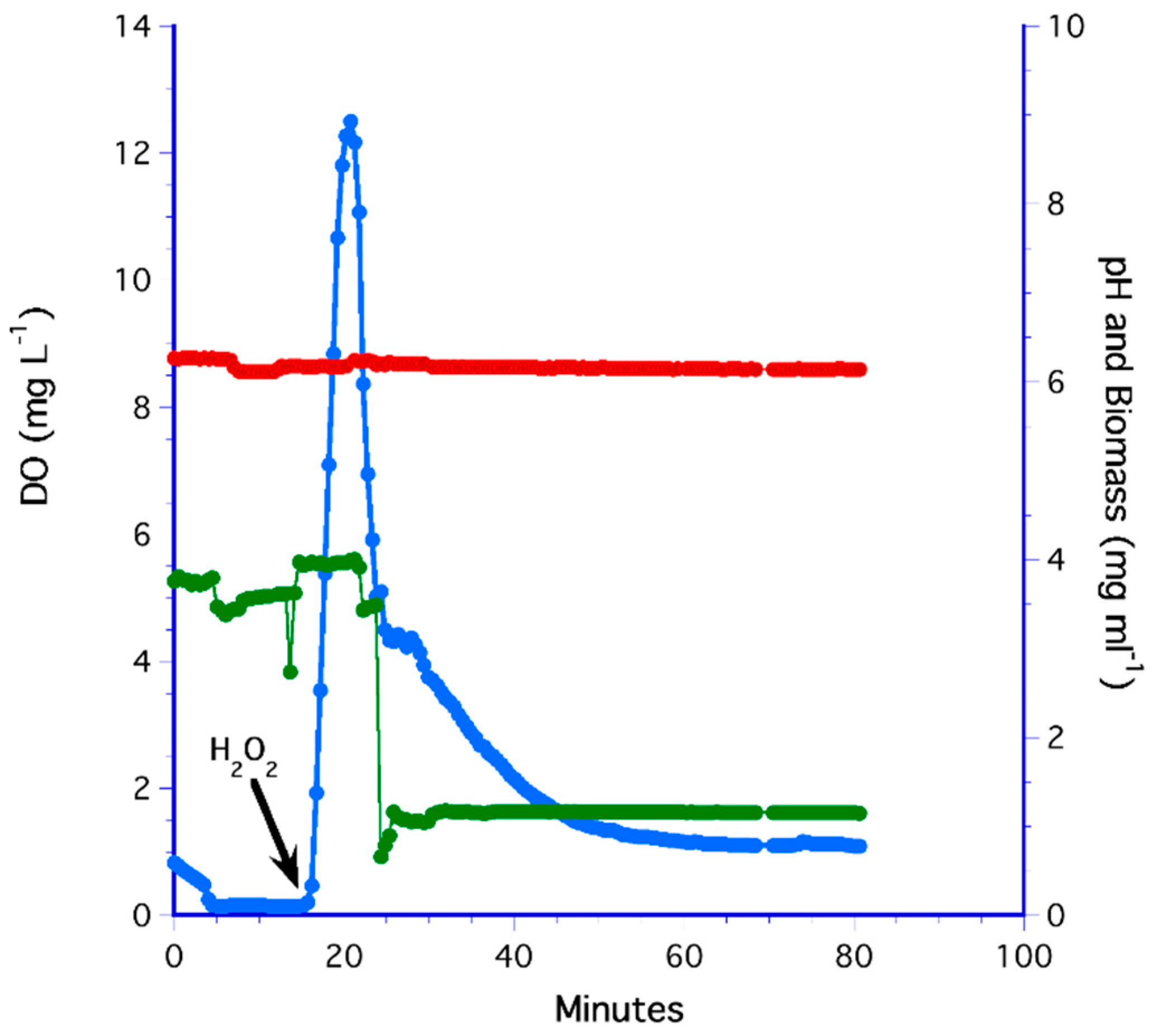
3.3. The H2O2 Method
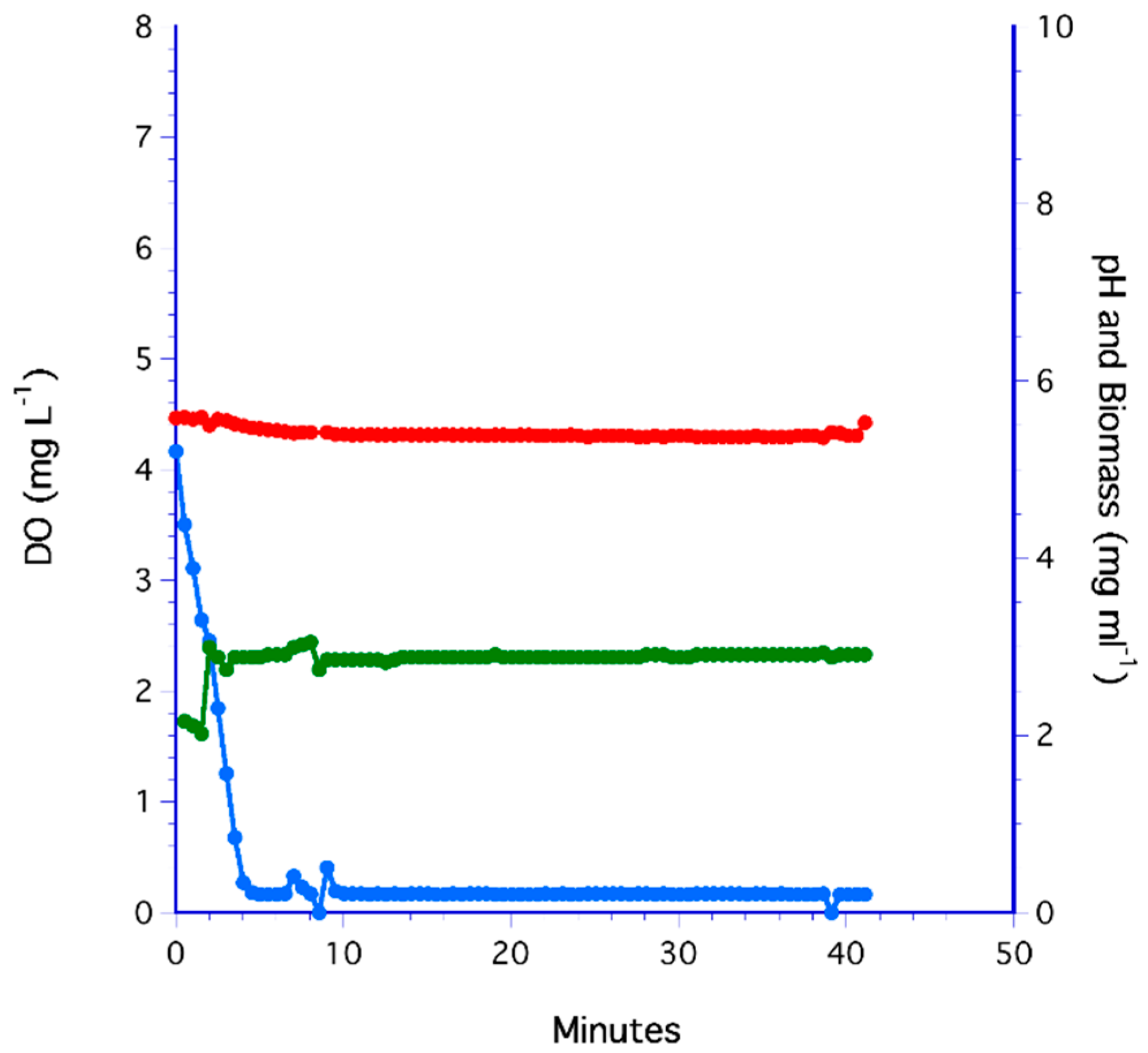
3.4. Flat Membrane Filter Method
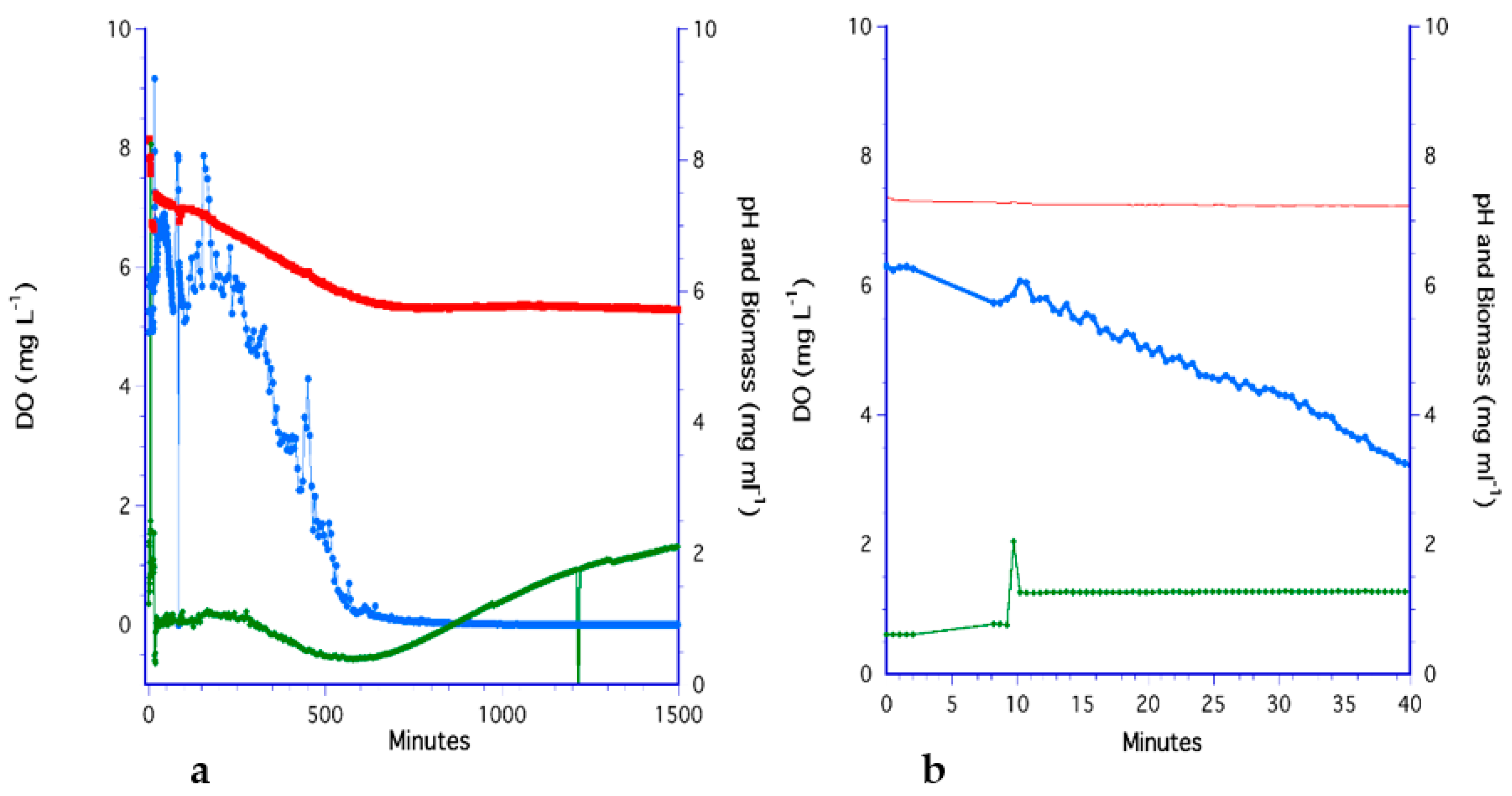
3.5. The LiquiCel Hollow-Fiber Filter (HFF) Method
3.6. The PDMS Hollow-Fiber Filter (HFF) Method
3.7. Analysis of Methods
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Montes, F.J.; Catalán, J.; Galán, M.A. Prediction of kLa in yeast broths. Process. Biochem. 1999, 34, 549–555. [Google Scholar] [CrossRef]
- Emery, A.N.; Jan, D.C.-H.; Al-Rubeai, M. Oxygenation of intensive cell-culture. Appl. Microbiol. Biotechnol. 1995, 43, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- Walther, I. Space bioreactors and their applications. Adv. Space Biol. Med. 2002, 8, 197–213. [Google Scholar] [CrossRef]
- Garcia-Ochoa, F.; Gomez, E. Bioreactor scale-up and oxygen transfer rate in microbial processes: An overview. Biotechnol. Adv. 2009, 27, 153–176. [Google Scholar] [CrossRef]
- Aroniada, M.; Maina, S.; Koutinas, A.; Kookos, I.K. Estimation of volumetric mass transfer coefficient (kLa)—Review of classical approaches and contribution of a novel methodology. Biochem. Eng. J. 2020, 155, 107458. [Google Scholar] [CrossRef]
- I Betts, J.; Baganz, F. Miniature bioreactors: Current practices and future opportunities. Microb. Cell Factories 2006, 5, 21. [Google Scholar] [CrossRef][Green Version]
- Cruz, A.J.G.; Silva, A.S.; Araujo, M.L.G.C.; Giordano, R.C.; Hokka, C.O. Estimation of the volumetric oxygen transfer coefficient (KLa) from the gas balance and using a neural network technique. Braz. J. Chem. Eng. 1999, 16, 179–183. [Google Scholar] [CrossRef]
- Badino, A.; Facciotti, M.; Schmidell, W. Volumetric oxygen transfer coefficients (kLa) in batch cultivations involving non-Newtonian broths. Biochem. Eng. J. 2001, 8, 111–119. [Google Scholar] [CrossRef]
- Marrot, B.; Barrios-Martinez, A.; Moulin, P.; Roche, N. Experimental Study of Mass Transfer Phenomena in a Cross Flow Membrane Bioreactor: Aeration and Membrane Separation. Eng. Life Sci. 2005, 5, 409–414. [Google Scholar] [CrossRef]
- Orgill, J.J.; Atiyeh, H.; Devarapalli, M.; Phillips, J.R.; Lewis, R.S.; Huhnke, R. A comparison of mass transfer coefficients between trickle-bed, hollow fiber membrane and stirred tank reactors. Bioresour. Technol. 2013, 133, 340–346. [Google Scholar] [CrossRef]
- Martínez-Force, E.; Benítez, T. Effects of varying media, temperature, and growth rates on the intracellular concentrations of yeast amino acids. Biotechnol. Prog. 2005, 11, 386–392. [Google Scholar] [CrossRef]
- García-Ochoa, F.; Santos, V.E.; Casas, J.A.; Gomez, E. Xanthan gum: Production, recovery, and properties. Biotechnol. Adv. 2000, 18, 549–579. [Google Scholar] [CrossRef]
- Russell, A.B.; Thomas, C.R.; Lilly, M.D. Oxygen transfer measurements during yeast fermentations in a pilot scale airlift fermenter. Bioprocess Eng. 1995, 12, 71–79. [Google Scholar] [CrossRef]
- Zedníková, M.; Orvalho, S.; Fialová, M.; Ruzicka, M.C. Measurement of Volumetric Mass Transfer Coefficient in Bubble Columns. ChemEngineering 2018, 2, 19. [Google Scholar] [CrossRef]
- Kim, H.J.; Jong, H.K.; Hyuck, J.O.; Chul, S.S. Morphological control of Monascus cells and scale-up of pigment fermentation. Process Biochem. 2002, 38, 649–655. [Google Scholar] [CrossRef]
- Schwippel, J.; Votruba, J. Oxygen and/or glucose limitation in a chemostat culture of Candida utilis mathematical model identification. Bioprocess Eng. 1995, 13, 133–140. [Google Scholar] [CrossRef]
- Rieger, M.; Käppeli, O.; Fiechter, A. The Role of Limited Respiration In The Incomplete Oxidation Of Glucose By Saccharomyces Cerevisiae. J. Gen. Microbiol. 1983, 129, 653–661. [Google Scholar] [CrossRef]
- Banti, D.C.; Tsali, A.; Mitrakas, M.; Samaras, P. The Dissolved Oxygen Effect on the Controlled Growth of Filamentous Microorganisms in Membrane Bioreactors. Environ. Sci. Proc. 2020, 2, 39. [Google Scholar] [CrossRef]
- Al-Mayah, A.M.R.; Muallah, S.K.; Al-Jabbar, A.A. Prediction of Oxygen Mass Transfer Coefficients in Stirred Bioreactor with Rushton Turbine Impeller for Simulated (Non-Microbial) Medias. Al-Khwarizmi Eng. J. 2014, 10, 1–14. [Google Scholar]
- Raghavendran, V.; Webb, J.P.; Cartron, M.L.; Springthorpe, V.; Larson, T.R.; Hines, M.; Mohammed, H.; Zimmerman, W.; Poole, R.K.; Green, J. A microbubble-sparged yeast propagation–fermentation process for bioethanol production. Biotechnol. Biofuels 2020, 13, 104. [Google Scholar] [CrossRef]
- Terasaka, K.; Shibata, H. Oxygen transfer in viscous non-Newtonian liquids having yield stress in bubble columns. Chem. Eng. Sci. 2003, 58, 5331–5337. [Google Scholar] [CrossRef]
- Caşcaval, D.; Galaction, A.-I.; Turnea, M. Comparative analysis of oxygen transfer rate distribution in stirred bioreactor for simulated and real fermentation broths. J. Ind. Microbiol. Biotechnol. 2011, 38, 1449–1466. [Google Scholar] [CrossRef] [PubMed]
- Chisti, Y.; Jauregui-Haza, U.J. Oxygen transfer and mixing in mechanically agitated airlift bioreactors. Biochem. Eng. J. 2002, 10, 143–153. [Google Scholar] [CrossRef]
- Galaction, A.-I.; Cascaval, D.; Oniscu, C.; Turnea, M. Prediction of oxygen mass transfer coefficients in stirred bioreactors for bacteria, yeasts and fungus broths. Biochem. Eng. J. 2004, 20, 85–94. [Google Scholar] [CrossRef]
- Klein, T.; Schneider, K.; Heinzle, E. A system of miniaturized stirred bioreactors for parallel continuous cultivation of yeast with online measurement of dissolved oxygen and off-gas. Biotechnol. Bioeng. 2013, 110, 535–542. [Google Scholar] [CrossRef]
- Jouhten, P.; Rintala, E.; Huuskonen, A.; Tamminen, A.; Toivari, M.; Wiebe, M.; Ruohonen, L.; Penttilä, M.; Maaheimo, H. Oxygen dependence of metabolic fluxes and energy generation of Saccharomyces cerevisiae CEN.PK113-1A. BMC Syst. Biol. 2008, 2, 60. [Google Scholar] [CrossRef]
- Lee, J.H.; Lim, Y.B.; Park, K.M.; Lee, S.W.; Baig, S.Y.; Shin, H.T. Factors Affecting Oxygen Uptake by Yeast Issatchenkia orientalis as Microbial Feed Additive for Ruminants. Asian Australas. J. Anim. Sci. 2003, 16, 1011–1014. [Google Scholar] [CrossRef]
- Özbek, B.; Gayik, S. The studies on the oxygen mass transfer coefficient in a bioreactor. Process. Biochem. 2001, 36, 729–741. [Google Scholar] [CrossRef]
- Peddie, F.L.; Simpson, W.J.; Kara, B.V.; Robertson, S.C.; Hammond, J.R.M. Measurement of Endogenous Oxygen Uptake Rates of Brewers’ Yeasts. J. Inst. Brew. 1991, 97, 21–25. [Google Scholar] [CrossRef]
- Ju, L.-K.; Sundararajan, A. The effects of cells on oxygen transfer in bioreactors. Bioprocess Eng. 1995, 13, 271–278. [Google Scholar] [CrossRef]
- Yildiz, E.; Keskinler, B.; Pekdemir, T.; Akay, G.; Nuhoglu, A. High strength wastewater treatment in a jet loop membrane bioreactor: Kinetics and performance evaluation. Chem. Eng. Sci. 2005, 60, 1103–1116. [Google Scholar] [CrossRef]
- Millward, H.; Bellhouse, B.; Sobey, I. The vortex wave membrane bioreactor: Hydrodynamics and mass transfer. Chem. Eng. J. 1996, 62, 175–181. [Google Scholar] [CrossRef]
- Ahmed, T.; Semmens, M.J.; Voss, M.A. Oxygen transfer characteristics of hollow-fiber, composite membranes. Adv. Environ. Res. 2004, 8, 637–646. [Google Scholar] [CrossRef]
- Kuriyama, H.; Kobayashi, H. Effects of oxygen supply on yeast growth and metabolism in continuous fermentation. J. Ferment. Bioeng. 1993, 75, 364–367. [Google Scholar] [CrossRef]
- Levy, E.D.; De, S.; Teichmann, S. Cellular crowding imposes global constraints on the chemistry and evolution of proteomes. Proc. Natl. Acad. Sci. USA 2012, 109, 20461–20466. [Google Scholar] [CrossRef]
- Stafford, R.A. Yeast Physical (Shear) Stress: The Engineering Perspective. In Brewing Yeast Fermentation Performance, 2nd ed.; Smart, K., Ed.; Wiley: New York, NY, USA, 2003. [Google Scholar]


| Composition 1 | YPD 2 (mg L−1) | SC (mg L−1) | Composition 1 | YPD 2 (mg L−1) | SC (mg L−1) |
|---|---|---|---|---|---|
| (NH4)2SO4 | 0 | 5000 | Lysine | 183 | 76 |
| YNB | 17,000 | 6700 | Methionine | 61 | 76 |
| Amino acids | 2611 | 1216 | Phenylalanine | 192 | 76 |
| Alanine | 257 | 76 | Proline | ||
| Arginine | 292 | 76 | Pyrrolysine 2 | ||
| Aspartic acid | Serine | 122 | 76 | ||
| Asparagine | 102 | 76 | Selenocysteine | ||
| Cysteine | 76 | Threonine | 95.1 | 76 | |
| Glutamine | 397 | 76 | Tryptophan | ||
| Glutamic acid | Tyrosine | 102 | 76 | ||
| Glycine | 120 | 76 | Valine | 170 | 76 |
| Histidine | 42.5 | 76 | Nucleic acids | ||
| Isoleucine | Adenine | ND 3 | 18 | ||
| Leucine | 212 | 176 | Uracil | ND 3 | 76 |
| Experiment | Repeated | Medium | Temperature (°C) | Pressure (mbars) | DOS1 (mg L−1) |
|---|---|---|---|---|---|
| 1 Bubbling | 4 | YPD | 26.4 ± 1.1 | 973.8 ± 0.31 | 7.74 |
| 2 Impeller | 3 | YPD | 26.4 ± 1.1 | 973.8 ± 0.31 | 7.74 |
| 3 H2O2 | 3 | YPD | 26.8 ± 0.18 | 973.8 ± 0.50 | 7.69 |
| 4 Flat Membrane | 2 2 | YPD | 26.6 ± 0.10 | 965.0 ± 2.4 | 7.65 |
| 5 Liqui-Cel HFF | 3 | YPD | 26.6 ± 0.10 | 965.0 ± 2.4 | 7.65 |
| 6 PDMS HFF | 8 | YPD | 26.3 ± 0.65 | 957.2 ± 0.11 | 7.62 |
| 7 PDMS HFF | 5 | SC | 26.8 ± 0.23 | 949.1 ± 0.35 | 7.54 |
| Aeration Process | Medium | KLa (min−1) | N 1 | References |
|---|---|---|---|---|
| Bubbled | Broth | 0.30−2.4 | 4 | [12,13] |
| Bubbled | DIW | 1.5−3.4 | 2 | [14] |
| Impeller (CSTR) | Broth | 0.09−1.7 | 4 | [2,15] |
| Impeller-Sparged | Broth | 0.30−2.4 | 15 | [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30] |
| Impeller-Sparged | DIW | 0.24−5.5 | 8 | [5,12,23,28] |
| Membranes | WW 2, Broth | 0.13−8.1 | 3 | [9,31,32] |
| Various HFF | DIW | 0.03−2.7 | 8 | [9,10,33] |
| PDMS 2500 | DIW | 0.33−7.8 | 2 | [10] |
| PDMS 100 1 | FDIW | 1.3−1.7 | 5 | This study |
| PDMS 100 1 | SC | 1.1−1.5 | 4 | This study |
| PDMS 100 1 | YPD | ≤−0.09 | 4 | This study |
| PDMS 100 1 | YPD (no glucose) | ≤−0.02 | 3 | This study |
| Method | Biomass (mg mL−1) | KLa (min−1) | OUR2 (mg O2 L−1 min−1) | qO2 (mg O2 gDwt−1 min−1) | N 3 |
|---|---|---|---|---|---|
| H2O2/YPD | 2.3−3.5 | 2.3 ± 1.2 | 2.3 ± 0.29 | 3.7 ± 5.7 | 3 |
| Impeller/YPD | 0.3−2.67 | 0.87 ± 0.02 | 6.0 ± 3.1 | 2.3 ± 1.2 | 3 |
| PDMS/YPD | 0.6–1.2 | negligible | −0.18 ± 0.32 | 0.06 ± 0.09 | 4 |
| PDMS/ SC | 1.4–38 | 1.3 ± 0.17 | 5.6 ± 4.3 | 3.0 ± 4.1 | 4 |
| Sparged-Impeller [20,23,24,25,26,27,29,30,34] | 1.4−50 | 1.3 ± 0.17 | 0.88 ± 1.0 | 4.8 ± 5.7 | 13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Granata, T.; Follonier, C.; Burkhardt, C.; Rattenbacher, B. Methods for Oxygenation of Continuous Cultures of Brewer’s Yeast, Saccharomyces cerevisiae. Fermentation 2021, 7, 282. https://doi.org/10.3390/fermentation7040282
Granata T, Follonier C, Burkhardt C, Rattenbacher B. Methods for Oxygenation of Continuous Cultures of Brewer’s Yeast, Saccharomyces cerevisiae. Fermentation. 2021; 7(4):282. https://doi.org/10.3390/fermentation7040282
Chicago/Turabian StyleGranata, Timothy, Cindy Follonier, Chiara Burkhardt, and Bernd Rattenbacher. 2021. "Methods for Oxygenation of Continuous Cultures of Brewer’s Yeast, Saccharomyces cerevisiae" Fermentation 7, no. 4: 282. https://doi.org/10.3390/fermentation7040282
APA StyleGranata, T., Follonier, C., Burkhardt, C., & Rattenbacher, B. (2021). Methods for Oxygenation of Continuous Cultures of Brewer’s Yeast, Saccharomyces cerevisiae. Fermentation, 7(4), 282. https://doi.org/10.3390/fermentation7040282