Abstract
Isocitric acid (ICA) has found wide application in medicine as a promising compound with powerful antioxidant activity to combat oxidative stress. In the known microbiological processes of ICA production by non-conventional yeast Yarrowia lipolytica, the pure carbon sources are commonly used. ICA can be also synthetized by Y. lipolytica from ester-aldehyde fraction (EAF)-waste of the ethanol production process. A highly effective method of ICA production from EAF based on regulation of key enzymes (aconitate hydratase and isocitrate lyase) by metabolic regulators (iron and itaconic acid) and aeration was developed. It is recommended to cultivate Y. lipolytica VKM Y-2373 under nitrogen deficiency conditions, a high aeration (60% of air saturation), an addition of 15 mM itaconic acid, and 2.4 mg/L iron. Under optimal conditions, Y. lipolytica VKM Y-2373 produced 83 g/L ICA with isocitrate to citrate ratio of 4.1:1 and mass yield of 1.1 g/g. The putative mechanism of ICA overproduction from EAF by Y. lipolytica was suggested.
1. Introduction
In recent decades, interest in the study of non-conventional yeast Yarrowia lipolytica has increased due to their difference from the well-studied yeast Saccharomyces cerevisiae in terms of their phylogenetic evolution, physiology, genetics, and their use in biotechnology [1,2]. The yeast Y. lipolytica grows in the pH range from 2.5 to 8 on a minimal mineral salt medium with various carbon sources [1,2,3]. Y. lipolytica differs from facultative anaerobe of S. cerevisiae in that it is an obligate aerobe [3], that is, its metabolism depends entirely on the functioning of mitochondria. Y. lipolytica is easily separated from the cultivation medium, does not pollute the air with spores [1,2,3]. The synthesized metabolites of Y. lipolytica are admitted as safe [4]. Wild and recombinant strains of Y. lipolytica are considered as microbial factories to produce valuable metabolites in industrially significant quantities, such as lipids [5,6], polyols (erythritol and mannitol) [7,8,9,10], and citric acid (CA) [5,9,10,11,12].
Today, there is great interest in the production of (2R,3S)-isocitric acid (ICA) by Y. lipolytica [13,14,15]. In the above review articles, ICA is considered as an original, fundamentally new compound for the prevention and treatment of many socially significant illnesses. It has proven its effectiveness regarding iron deficiency anemia, the resorption of blood clots [13], and Parkinson’s disease [16]. In the form of lactone, ICA is considered as a forward-looking intermediate for synthesis of drugs with anti-cancer activity [17,18,19,20]. Monopotassium isocitrate is used to synthesize darunavir and brecanavir with positive activity against HIV/AIDS [21,22]. It was indicated that ICA favorably influences the infusorian cells with oxidative stress induced by various toxic compounds (Cu, Pb, Zn, Cd, H2O2) [23]. It also relieves the neurointoxication, restores the spatial memory, and accelerates learning in rats that have been reduced under the influence of heavy metals [24,25].
In the known processes of ICA production by Y. lipolytica, the pure carbon sources such as ethanol [23,24,26,27], rapeseed oil [28,29], sunflower oil [17,18,19,30], glucose [31,32,33], and glycerol [31,32,33] are commonly used. However, the high cost of ICA production from pure substrates determines the interest in finding cheap and renewable waste. It was reported that a reduced production cost of ICA can be realized by using low-cost glycerol-containing biodiesel waste [25,34,35].
Recently, we published the first paper on ether-aldehyde fraction (EAF) as a promising source of carbon for ICA synthesis by Y. lipolytica [36]. EAF (the head fraction of ethyl alcohol) is a by-product of ethanol industry. EAF is generated during the production of ethyl rectified alcohol from food raw materials at a distiller unit of spirit plants in a concentration of 8–15% from absolute ethanol. EAF contains 65–90 vol% of ethanol and impurities, that give it its characteristic color and odor. These impurities include saturated aldehydes, unsaturated aldehydes (acrolein, croton aldehyde, and furfurole), methanol, esters of lower alcohols (ethyl acetate, methyl acetate, ethyl propionate, methyl propionate, and ethyl formate), and carboxylic acids (acetic, propionic, and some others). The content of impurities is low, but their presence causes a strong irritating action on the mucous membranes of the eyes and upper respiratory tract [37]. Due to the presence of impurities of ethers and aldehydes, EAF is subject to further recycling or use for technical purposes in the chemical, paint, and varnish industries.
Since purification of EAF has considerable cost, the utilization of this by-product to produce ICA using yeast Y. lipolytica is of great environmental and economic importance. Using EAF as the only carbon source, we achieved 65 g/L ICA with a product yield of 0.65 g/g by optimizing culture conditions such as pH, ammonium sulfate, and EAF concentrations [36]. However, these indexes were lower than in the medium with pure ethanol [24,28].
According to the literature data, the aeration has the greatest influence on ICA production by Y. lipolytica, cultivated on purified ethanol [27], rapeseed oil [28], sunflower oil [30], and crude glycerol [35]. It is well known that the synthesis of ICA from the above substrates can be regulated by overexpression of the ACO1 gene encoding aconitase hydratase [32], the disruption of ICL1 gene encoding isocitrate lyase [31], or addition of iron, an activator aconitate hydrates [27], or itaconic and oxalic acids, inhibitors of isocitrate lyase in a nutrition medium [38,39]. However, at present no information is available on the effect of aeration and activators/inhibitors of key enzymes of ICA metabolism in Y. lipolytica yeast, cultivated on EAF and other ethanol-containing wastes.
The aim of this work was to study the effects of metabolic regulators (itaconic acid and iron) and aeration on the growth and acids synthesis by Y. lipolytica in the perspective to develop a highly effective method of ICA production in medium containing EAF as the carbon substrate.
2. Materials and Methods
The study was carried out with wild strain Y. lipolytica VKM Y-2373 previously selected as a producer of ICA from ether-aldehyde fraction (EAF) [36]. The culture was maintained at 4 °C on agar slants with n-alkanes.
All chemicals for cultivation medium were of analytical grade (Mosreactiv, Russia). EAF was purchased from NTM Pharm (Nizhny Novgorod, Russia); EAF consisted of 90 vol% ethanol, 1 vol% methanol, 0.5 g/L aldehydes (in terms of acetaldehyde), and 0.4 g/L esters (in terms of ethyl acetate). All chemicals and enzymes for enzyme activity assays were purchased from Sigma-Aldrich (St. Louis, MO, USA).
The inoculum for the fermentor was prepared in six 750 mL Erlenmeyer flasks containing 100 mL of cultivation medium (see below) inoculated with a yeast colony grown on the agar medium. The flasks were incubated on an orbital shaker (130 rpm) at 29 °C during 48 h. Twice a day, 0.3 mL of EAF were added to each flask. pH of the medium was regularly adjusted to a value of 4.5–6.0 by 10% KOH. The inoculum (600 mL) was transferred to a fermentor; the dry weight concentration of the inoculum was 3–4 g/L.
To study the effect of itaconic acid, iron, and aeration, Y. lipolytica VKM Y-2373 was cultivated in a 10 L ANKUM-2M fermentor (Institute of Biological Instrumentation of RAS, Pushchino, Moscow region, Russia) of 6 L. The medium contained (g/L): (NH4)2SO4, 3.0; MgSO4·7H2O, 1.4; Ca(NO3)2, 0.8; NaCl, 0.5; KH2PO4, 2.0; K2HPO4, 0.2; the microelements (in mg/L): ZnSO4·7H2O, 0.04; KJ, 0.1; Na2B4O7·10H2O, 0.08; MnSO4·5H2O, 0.05; CuSO4, 0.04; Na2MoO4, 0.03; the yeast autolysate, 8 mL/L. The concentration of iron ions (as a salt FeSO4(NH4)2SO4·6 H2O) and itaconic acid were varied as indicated in Section 3. Itaconic acid was added at 12 h of cultivation to avoid the delay of growth of the producer, and iron concentration of 1.2 mg/L was chosen according to earlier experiments with pure ethanol [27]. Experiments on the influence of iron ions and itaconic acid were carried out at 60% of air saturation by dissolved oxygen (pO2) and the agitation rate of 800 rpm. In aeration experiments the concentration of pO2 (5%, 20%, 40%, 60%, 80% of air saturation) was controlled by changing the air inflow rate from 1 to 10 L/min per fermentor and agitation rate from 200 to 1000 rpm. pH of the medium during fermentation was maintained at 6.0 by adding the necessary volume of 20% KOH solution. The cultivation temperature was 29 °C. In all experiments, unsterilized EAF was added in portions (from 10 to 60 mL) at the moments when oxygen concentration rose by 10% from the basal level. Cultivations lasted 4 days.
For biomass assay, the cells were separated from the cultural broth through a paper filter and dried at 105 °C to a constant weight. Concentration of NH4+ was determined potentiometrically as described earlier [36]. The concentration of residual ethanol was analyzed by gas–liquid chromatography as described earlier [36]. Isocitric and citric acids were analyzed by HPLC and enzymatically as described earlier [36].
Yeast cells grown for 48 h (active phase of ICA synthesis) were separated from the culture liquid by centrifugation at 5000× g for 10 min (4 °C) and washed twice with ice-cold 0.9% NaCl solution. Cells were resuspended in 100 mM potassium phosphate buffer, pH = 7.4, containing 1 mM EDTA, and disrupted using Ballotini glass beads (d = 100–150 μm, BDH Chemicals LtD, Poole, UK) on a planetary mill at 1000 rpm for 3 min. To remove intact cells, the homogenate was centrifuged at 10,000× g for 30 min (4 °C), and the supernatant was used for enzyme analysis.
Citrate synthase (EC 2.3.3.1), aconitate hydratase (EC 4.2.1.3), NAD-dependent isocitrate dehydrogenase (EC 1.1.1.41), isocitrate lyase (EC 4.1.3.1) were assayed as described earlier [30]. NAD-dependent alcohol dehydrogenase (E.C. 1.1.1.1) and malate synthase (EC 4.1.3.2) were assayed as described [1]. Catalase was measured at λ = 240 nm in reaction mixtures containing 50 mM potassium phosphate buffer (pH 7.0) and 10 mM hydrogen peroxide. Protein concentration in the cell-free extract was determined as described by Bradford [40]. Enzyme activity (U) was expressed in micromoles of product formed per minute. Specific activity was expressed as units per mg of protein (U/mg protein).
The mass yield (in g/g) was calculated using the formula: YICA = P/S; where P is the total amount of ICA in the culture liquid at the end of cultivation (g), and S is the total amount of ethanol (not EAF) consumed (g). The volumetric productivity (in g/(L·h)) was calculated using the formula: QICA = P/(V·t), where V is the initial volume of culture liquid (L), and t—time of incubation (h). The specific productivity (mg/(g·h)) was calculated using the formula: qICA = P/(X·t), where X is the average working biomass in the fermentor (g).
All the data presented represents the mean ± standard deviation of three experiments and two measurements for each experiment. Data related to ICA biosynthesis was subjected to analysis of variance using Student’s t-test (p < 0.05); the differences between the values were statistically significant if the confidence intervals did not overlap. The amount of total acids, the ICA/CA ratio, values of YICA, qICA, and QICA were calculated using the mean value of biomass, ICA, and ethanol consumed.
3. Results
The effect of itaconic acid was studied in the range of 10–80 mM, and as a control, Y. lipolytica VKM Y-2373 was grown without the addition of itaconic acid. The data on the effect of itaconic acid on the accumulation of biomass and acids production are presented in Figure 1. As seen from Figure 1, the concentration of biomass of Y. lipolytica VKM Y-2373 reached a maximum (13.1 g/L) in control experiment and decreased with the addition of itaconic acid by an average of 27%. The addition of 15 mM itaconic acid resulted in an increase in isocitric acid (ICA) production from 65.3 to 73 g/L with a simultaneous decrease in citric acid (CA) synthesis from 31.3 to 17.0 g/L. A further increase in itaconic acid to 80 mM led to a decrease in ICA synthesis to 52.3 g/L.
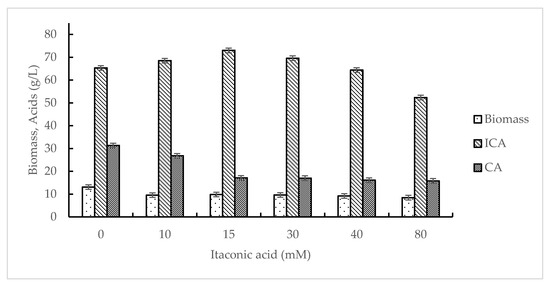
Figure 1.
Effect of itaconic acid on the growth of Y. lipolytica VKM Y-2373 and production of isocitric acid (ICA) and citric acid (CA).
The calculated data about the effect of itaconic acid on ratio between ICA and CA, as well as on mass yield (YICA), the specific productivity (qICA), and volume productivity (QICA) are given in Table 1.

Table 1.
Effect of itaconic acid on indices of ICA production by Y. lipolytica VKM Y-2373.
As seen from Table 1, at 15 mM itaconic acid, the ratio between ICA and CA shifted to 4.3:1 compared to 2.1:1 in control experiment. The maximal values of YICA (0.97 g/g), qICA (0.078 g/g·h), and QICA (1.18 g/L·h) were observed at 15 mM itaconic acid. It coincided with the highest ICA production.
The study of enzyme activities (Table 2) revealed that, in control experiment (without itaconic acid), the activities of alcohol dehydrogenase (ADH), aldehyde dehydrogenase (ALDH) and catalase (enzymes involved in the primary oxidation of EAF), citrate synthase (CS) and aconitate hydratase (AH) (involved in the formation of CA and ICA in the tricarboxylic acid cycle (TCA cycle)), NAD-dependent isocitrate dehydrogenase (NAD-ICDH) (involved in the oxidation ICA in TCA cycle), isocitrate lyase (ICL) and malate synthase (MS) (enzymes of glyoxylate cycle) were high (0.075, 0.072, 310, 2.875, 0.405, 0.100, 0.231, and 0.068 U/mg protein, respectively). The addition of itaconic acid up to 15 mM had practically no effect on enzymes involved in the primary oxidation of EAF and ICA synthesis in TCA cycle. A further increase of itaconic acid concentration from 15 to 80 mM resulted in a decrease in the activities of ADH (by 20%), catalase (by 16%), CS (by 30%), AH (by 31%), and NAD-ICDH (by 31%). It should be noted that itaconic acid concentration had a powerful effect only on ICL. In its presence, the specific activity of ICL decreased by 9–23 times as a function of increased inhibitor concentration (from 10 to 30 mM).

Table 2.
Effect of itaconic acid on the enzyme activities (U/mg protein) of Y. lipolytica VKM Y-2373.
The effect of iron concentrations on the growth of Y. lipolytica VKM Y-2373 and ICA production was studied in the range of 0.001–5 mg/L at 15 mM itaconic acid. The data on the effect of iron on the accumulation of biomass and acids production are presented in Figure 2.
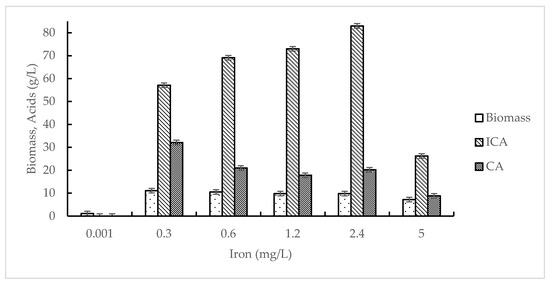
Figure 2.
Effect of iron concentration on the growth of Y. lipolytica VKM Y-2373 and production of isocitric acid (ICA) and citric acid (CA). The medium contained 15 mM itaconic acid.
As seen from Figure 2, the iron concentration of 0.001 mg/L limited the cell growth (biomass of 1.1 g/L), and ICA and CA production was completely abolished while acetic acid was produced (1.25 g/L). In these conditions, the cell growth was limited by iron deficiency that confirmed by a high concentration of residual nitrogen (336 mg/L). Under other iron concentrations, the growth of Y. lipolytica VKM Y-2373 was limited by nitrogen (the residual nitrogen consisted of 31.25 mg/L). Under conditions of nitrogen limitation, an increase in iron concentration from 0.3 to 2.4 mg/L enhanced ICA production from 57.1 to 83.0 g/L and decreased CA production from 32.1 to 20.2 g/L. A further increase in iron concentration from 2.4 to 5 mg/L caused a decrease in ICA production by 3.2 times.
The calculated data about the effect of iron on ratio between ICA and CA, as well as values of YICA, qICA, and QICA are given in Table 3.

Table 3.
Effect of iron concentration on indices of ICA production by Y. lipolytica VKM Y-2373.
As seen from Table 3, the maximum shift of acids towards the highest accumulation of isocitrate (4.1:1) was observed at iron concentrations of 1.2 and 2.4 mg/L. The value of YICA was the highest (1.1 g/g) at 2.4 mg/L Fe and decreased by 1.7 and 3.1 times with a decrease in iron to 0.3 mg/L or its increase to 5 mg/L, respectively. The maximum values of qICA (0.088 g/g·h) and QICA (1.25 g/L·h) were observed at iron concentration of 2.4 mg/L.
The study of enzyme activities (Table 4) revealed that under iron deficiency (0.001 mg/L), the specific activities of all enzymes studied were low. The increase in iron concentration from 0.001 to 2.4 mg/L resulted in a considerable rise in the activity of AH (15 times), AlDH (7 times), and catalase (7 times), while other enzymes increased slightly: ADH (1.8 times), CS (1.8 times), and NAD-ICDH (4 times). A further increase in iron concentration up to 5 mg/L caused a slight increase in activities of AH, AlDH, and catalase, while the activities of ADH, CS and NAD-ICDH were reduced by 2, 1.5, and 1.6 times, respectively. The activities of ICL and MS were maintained at a constant low level (0.010–0.015 and 0.025–0.030 U/mg protein, respectively) in all experimental variants.

Table 4.
Effect of iron concentration on the enzyme activities (U/mg protein) of Y. lipolytica VKM Y-2373.
The influence of aeration on Y. lipolytica VKM Y-2373 growth and ICA synthesis was studied from 5 to 80% of pO2, at 15 mM itaconic acid and 2.4 mg/L iron concentrations. As seen from Figure 3, at pO2 of 5%, the level of biomass and ICA production were low, at 4.3 and 25.2 g/L, respectively. A rise in pO2 from 5 to 60% of air saturation enhanced ICA synthesis 3.3 times. A further increase in pO2 up to 80% decreased ICA synthesis by 1.5-fold.
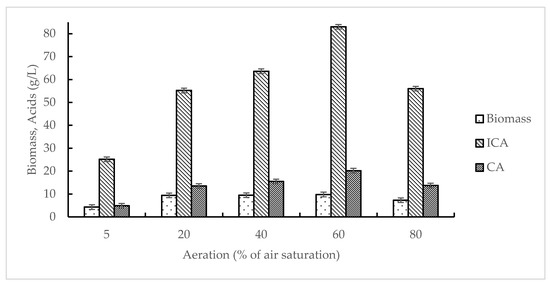
Figure 3.
Effect of aeration on the growth of Y. lipolytica VKM Y-2373 and production of isocitric acid (ICA) and citric acid (CA).
As seen from Table 5, the maximum shift of produced acids towards the highest accumulation of isocitrate (5.1:1) was observed at pO2 of 5%. The maximum value of YICA (1.1 g/g) was observed at pO2 of 60% and decreased by 1.9 and 1.5 times, respectively, with a decrease in pO2 to 5% or its increase to 80%. The maximum values of qICA (0.088 g/g·h) and QICA (1.25 g/L·h) were observed at pO2 of 60%.

Table 5.
Effect of aeration on indices of ICA production by Y. lipolytica VKM Y-2373.
As seen from Table 6, the extremely low aeration (pO2 of 5%) maintained all enzymes at a low level. An increase in pO2 from 5 to 60% caused an increase in activities of ADH (for 20%), AlDH (for 15%), catalase (for 20%), CS (for 30%), AH (for 30%), and NAD-ICDH (for 30%), and ICL and MS were increased—5 times. The extremely high aeration (pO2 of 80%) resulted in a twofold decrease in activities of ADH, CS, AH, and NAD-ICDH and slightly influenced in AlDH, catalase, ICL, and MS.

Table 6.
Effect of aeration on the enzyme activities (U/mg protein) of Y. lipolytica VKM Y-2373.
4. Discussion
The putative mechanism of isocitric acid (ICA) synthesis from ether-aldehyde fraction (EAF) by Y. lipolytica is presented in Figure 4.
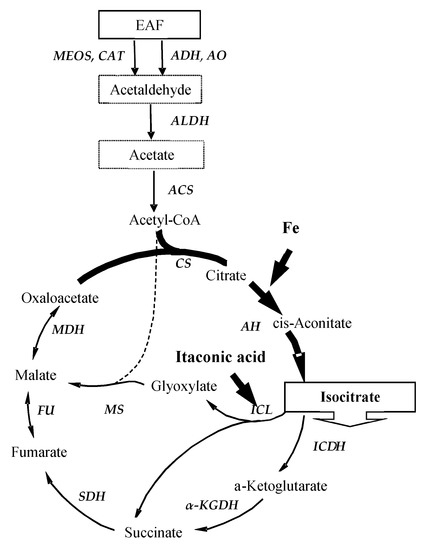
Figure 4.
Putative scheme of oxidation of EAF and ICA production in Y. lipolytica. ADH—alcohol dehydrogenase; AlDH—aldehyde dehydrogenase; MEOS—microsomal ethanol-oxidizing system (including cytochrome P-450); CAT—catalase; ACS—acetyl-CoA synthase; CS—citrate synthase; AH—aconitate hydratase; ICDH—NAD-dependent isocitrate dehydrogenase; ICL—isocitrate lyase; MS—malate synthase; α-KGDH—α-ketoglutarate dehydrogenase; Fu—fumarase; MDH—dehydrogenase.
As seen from Figure 4, the primary oxidation of ethanol (the main component of EAF) to acetaldehyde occurs in the cytosol via alcohol dehydrogenase (ADH). Y. lipolytica has one NAD-dependent ADH (I) and three NADP-dependent ADHs (ADH II, ADH III, ADH IV). The induction of various forms of ADH depends on the carbon source in the medium [2]. Along with ADH, other enzyme systems also participate in the oxidation of ethanol to acetaldehyde in Y. lipolytica. These include alcohol oxidase (AO), catalase, and microsomal ethanol-oxidizing system (including cytochrome P-450), induced by various cultivation conditions [41,42]. In the above papers, it is noted that AO and catalase were induced at high alcohol concentration, while ADH was active at low alcohol. The resulting acetaldehyde enters the mitochondrial matrix, where aldehyde dehydrogenase (AlDH) catalyzes its oxidation, coupled with oxidative phosphorylation, to acetate [41,42]. Until now, the mechanism of acetate transport into Y. lipolytica cells has not been studied in detail. Considering the exclusively cytosolic localization of acetyl-CoA synthetase (ACS), it remains to assume that acetate penetrates to the peroxisome, where it is included in the glyoxylate cycle or converted into acetyl-carnitine and is transferred to mitochondria, entering the tricarboxylic acid cycle (TCA cycle) [43]. Molecular genetic studies have shown that Y. lipolytica has eight alcohol dehydrogenase (ADH) genes and one alcohol oxidase gene (FAO1), and only ADH1, ADH2, or ADH3 are required for ethanol utilization in Y. lipolytica [44]. In addition to ADH1, ADH2, and ADH3, an acetyl-CoA synthetase encoding gene ACS1 is essential for oxidation of ethanol [44].
As seen from Figure 4, the acetyl-CoA generated during the primary oxidation of ethanol is involved through citrate synthase (CS) reaction and subsequent steps of TCA cycle to form ICA and its further oxidation via TCA cycle. Moreover, there is the evidence that metabolism of carbon sources in Y. lipolytica occurred not only via the TCA cycle, but also through glyoxylate cycle [45,46,47,48]. It seems it can provide the metabolic flexibility of cells in the formation of ICA from EAF, and hence, the amount of excreted ICA is determined not only by enzymatic activities of CS, aconitate hydratase (AH), NAD-dependent isocitrate dehydrogenase (NAD-ICDH) but isocitrate lyase (ICL) (a key enzyme of glyoxylate cycle).
Thus, we propose that the maintenance of high activity of CS provided ICA production and activation/inhibition of key enzymes of metabolism of ICA by metabolic regulators of AH and ICL will shift the citrate–isocitrate equilibrium toward preferential synthesis of ICA.
It was shown that intensive ICA production from EAF by Y. lipolytica VKM Y-2373 occurred at high activity of CS in different conditions, and the synthesis was decreased due to reduced activity of CS (in experiment with low iron concentration (Table 4) and extremely low and ultra-high aeration (Table 6)). Hapeta et al. reported that transformants of Y. lipolytica A101.1.31, overexpressing CIT1 or CIT2 gene (encoding proteins with citrate synthase activity) produced acids in a ratio close to 1 while the CA/ICA ratio for wild-type strain was 4.12 [29].
The results obtained revealed that the addition of itaconic acid stimulated high ICA synthesis with reduced CA production (Figure 1). In the presence of 15 mM itaconic acid, when ICA production by Y. lipolytica VKM Y-2373 was maximal, the inhibition of ICL was 94% of the control while other enzymes involved in ICA synthesis (CS and AH) were kept at constant level (Table 2). These findings are comparable with our data obtained for Y. lipolytica grown in media with rapeseed oil [27,39] and sunflower oil [30]. At the same time, the transformant Y. lipolytica H222-41 (JMP5) Z123 with the inactivated ICL1 gene, encoding isocitrate lyase, cultivated in media with glucose and glycerol exhibited only a small increase in the ICA production in comparison with the parent strain Y. lipolytica H222 (by 5 and 4%, respectively) [29].
It should be noted that studies on the use of itaconic acid and other inhibitors of ICL for production of valuable metabolites are rare. It was reported that ICL is strongly inhibited by oxalate and itaconate in different yeast, fungi, bacteria [38,49,50]. The review of Krátký and Vinšová [51] summarizes information on ICL inhibitors, such as 3-nitropropionate, 3-bromopyruvate, itaconate, itaconic anhydride, peptide inhibitors, and recently developed inhibitors with various chemical structures. However, according to our recent data, strong inhibitors of ICL are glucose-6-P, fructose 1,6-bisphosphate, pyruvate, citrate, α-ketoglutarate, succinate, fumarate that are quickly assimilated and included in the metabolism of Y. lipolytica and, hence, cannot be considered as specific inhibitors of ICL in growing yeast cultures; Y. lipolytica VKM Y-2373 does not metabolize only itaconic and oxalic acids [30].
All the above suggests that ICL may play an important role in the preferential syn-thesis of ICA in the cleavage of carbon sources with a high contribution of the glyoxylate cycle. On the contrary, NAD-ICDH plays a decisive role for CA production in Y. lipolytica cells cultivated in a medium with glycerol-containing wastes; its activity decreased to minimum levels during acidification [5].
The other important factor ensuring ICA production from EAF is an iron concentration. Our results revealed that iron-limitation (0.001 mg/L) resulted in insignificant cell growth without ICA and CA production; the good growth of Y. lipolytica VKM Y-2373 and intensive ICA production required increased iron concentration (2.4 mg/L) (Figure 2). It can be assumed that the real limiting factor of cell growth under iron deficiency conditions is the metabolically available energy because the activity of all enzymes involved in production of ICA drastically reduced (Table 4). Similar data have been reported for Candida utilis grown on glucose under iron limitation conditions [52]. Authors of the above article observed that the iron-deficiency cells lose the first site of phosphorylation (from the mitochondrial NADH to cytochromes); the molar growth yields for the organic substrate and ammonium drastically falls; the adenylate charge drops to values of 0.4–0.5, which were previously considered only in nonproliferating cells or even dead cells; the specific rate of oxygen consumption increases. In addition, a significant amount of acetyl-CoA formed from glucose does not enter the TCA cycle but condenses with ethanol to form ethyl acetate. A similar effect has been also shown for yeast Kluyveromyces marxianus, which produce ethyl acetate in significant quantities under iron-limited conditions [53].
The results also revealed a clear influence of iron concentration on the ICA/CA ratio (Table 3) which was determined by the activity of AH. These findings are comparable with those obtained for genetically modified strains of Y. lipolytica. It was shown that transformant Y. lipolytica H222-S4 (p67ACO1) T1 with ACO1 gene encoding the aconitate hydratase, cultivated on sunflower oil, showed a higher content of ICA (66–71%) in comparison with the wild-type strain Y. lipolytica H222 (35–49% ICA) [32]. This transformant after 144 h of cultivation in the fermentor produced 68.4 g/L ICA (75.6% of total acids) with the product yield (YICA) of 0.64 g/g substrate consumed and the process productivity of 0.47 g/L·h [19]. The enhanced expression of the ACO1 gene in another wild-type strain Y. lipolytica 672 shifted the acid balance toward ICA to 75.6% and increased its accumulation to 72.6 g/L [54]. It should be noted that the transformant overexpressing ACO1 gene showed only a small increase in the synthesis of ICA from glycerol and glucose (by 3–5 and 2–3%, respectively) [32].
The results revealed that the aeration is one of the most important environmental factors affecting ICA production from EAF by Y. lipolytica VKM Y-2373. ICA production increased significantly with high level of aeration (pO2 of 60%) and decreased with higher level of aeration (pO2 80%). Low level of aeration (pO2 5%) inhibited both yeast growth and acid production (Figure 3) which correlated with a decrease in activities of enzymes (ADH, catalase, CS, AH, NAD-ICDH, ICL, and MS) (Table 6).
The data available in the literature on the effect of aeration on the synthesis of organic acids by yeast Y. lipolytica are rather contradictory. It was shown that the maximum production of ICA in Y. lipolytica grown on n-alkanes was observed only at high level of aeration (pO2 85–90%) [55], while with purified ethanol, yeast predominantly produce ICA at medium level of aeration (pO2 60–65%) [26]. However, there is information that oxygen supply had no marked effect on CA synthesis by Y. lipolytica grown on pure glycerol [48]. Papanikolaou et al. reported that the level of oxygen supply affects the direction of metabolic processes: at low oxygen saturation Y. lipolytica produced sugar-alcohols (mannitol, arabitol, and erythritol), while with the increase of pO2 values, almost exclusively organic acids (mostly CA and to lesser extent ICA) [10]. Our studies carried out with Y. lipolytica VKM Y-2373 grown on sunflower oil showed that the oxygen requirement of cells depended considerably on the concentration of iron ions in the medium and sharply increased under iron deficiency [30]. The inconsistency of the literature data concerning the effect of aeration on production of ICA and other acids may be possibly due to the use of nonoptimal media and to the limited availability of iron ions.
The developed process of ICA production from EAF by Y. lipolytica VKM Y-2373 leads to the accumulation of 83 g/L ICA with YICA of 1.1 g/g. It should be noted that the same strain grown on pure ethanol produced 90.5 g/L ICA with YICA of 0.77 g/g under conditions of batch culture [27] and 109.6 g/L ICA with YICA of 0.80 g/g when cultivated in repeated-batch culture [24]. It seems that the decrease in ICA production concentration when using EAF is associated with the presence of harmful impurities (aldehydes, esters) in this carbon source. At the same time, the value of YICA on a medium with EAF is higher in comparison with that on pure ethanol, which may be due to the fact that Y. lipolytica VKM Y-2373 assimilates simultaneously ethanol and other impurities (for example, methanol). According to Babel (2009), the simultaneous consumption of physiologically similar substrates can increase the yield of the target product [56]. In the yeast Hansenula polymorpha, the mixed consumption of methanol and glucose resulted in an increase in biomass yield of up to 25% [57].
5. Conclusions
The novelty of the work lies in the study of the regulation of the biosynthesis of isocitric acid (ICA) from a poorly studied substrate, ether-aldehyde fraction (EAF) in the yeast Y. lipolytica. Results indicated that the process of ICA production can be improved by the regulation of key enzymes (aconitate hydratase and isocitrate lyase) by metabolic regulators (iron and itaconic acid) and aeration. It is recommended to cultivate Y. lipolytica VKM Y-2373 under nitrogen deficiency conditions, a high aeration (60% of air saturation), an addition of 15 mM itaconic acid and 2.4 mg/L iron. Under optimal conditions, Y. lipolytica VKM Y-2373 produced 83 g/L ICA with isocitrate to citrate ratio of 4.1:1 and mass yield of 1.1 g/g. This is the first time that Y. lipolytica grown on ethanol-containing waste was shown to produce ICA at high concentration comparable to ones on pure ethanol [27]. It should be noted that the utilization of EAF to obtain ICA by Y. lipolytica is of great practical importance because this by-product is subject to further recycling or use for technical purposes due to the presence of impurities of ethers and aldehydes which cause a strong irritant effect on the mucosa of the eyes and upper respiratory tract.
Author Contributions
Conceptualization, I.G.M.; investigation, S.V.K., I.G.M.; methodology, S.V.K.; resources, I.G.M.; supervision, I.G.M.; writing—original draft, S.V.K., I.G.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Barth, G.; Gaillardin, C. Yarrowia lipolytica. In Nonconventional Yeasts in Biotechnology: A Handbook; Wolf, K., Ed.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 313–388. [Google Scholar] [CrossRef]
- Barth, G.; Gaillardin, C. Physiology and genetics of the dimorphic fungus Yarrowia lipolytica. FEMS Microbiol. Rev. 1997, 19, 219–237. [Google Scholar] [CrossRef]
- Zinjarde, S.S. Food-related applications of Yarrowia lipolytica. Food Chem. 2014, 152, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Groenewald, M.; Boekhout, T.; Neuvéglise, C.; Gaillardin, C.; van Dijck, P.V.; Wyss, M. Yarrowia lipolytica: Safety assessment of an oleaginous yeast with a great industrial potential. Crit. Rev. Microbiol. 2014, 40, 187–206. [Google Scholar] [CrossRef] [PubMed]
- Makri, A.; Fakas, S.; Aggelis, G. Metabolic activities of biotechnological interest in Yarrowia lipolytica grown on glycerol in repeated batch cultures. Bioresour. Technol. 2010, 101, 2351–2358. [Google Scholar] [CrossRef] [PubMed]
- Blazeck, J.; Hill, A.; Liu, L.; Knight, R.; Miller, J.; Pan, A.; Otoupal, P.; Alper, H.S. Harnessing Yarrowia lipolytica lipogenesis to create a platform for lipid and biofuel production. Nat. Commun. 2014, 5, 3131. [Google Scholar] [CrossRef] [Green Version]
- Rakicka, M.; Rywińska, A.; Cybulski, K.; Rymowicz, W. Enhanced production of erythritol and mannitol by Yarrowia lipolytica in media containing surfactants. Braz. J. Microbiol. 2016, 47, 417–423. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.; Wang, S.; Bilal, M.; Ge, X.; Zhang, C.; Fickers, P.; Cheng, H. Identification, characterization of two NADPH-dependent erythrose reductases in the yeast Yarrowia lipolytica and improvement of erythritol productivity using metabolic engineering. Microb. Cell Fact. 2018, 17, 133. [Google Scholar] [CrossRef]
- Diamantopoulou, P.; Filippousi, R.; Antoniou, D.; Varfi, E.; Xenopoulos, E.; Sarris, D.; Papanikolaou, S. Production of added-value microbial metabolites during growth of yeast strains on media composed of biodiesel-derived crude glycerol and glycerol/xylose blends. FEMS Microbiol. Lett. 2020, 367, fnaa063. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Diamantopoulou, P.; Blanchard, F.; Lambrinea, E.; Chevalot, I.; Stoforos, N.G.; Rondags, E. Physiological characterization of a novel wild-type Yarrowia lipolytica strain grown on glycerol: Effects of cultivation conditions and mode on polyols and citric acid production. Appl. Sci. 2020, 10, 7373. [Google Scholar] [CrossRef]
- Imandi, S.B.; Bandaru, V.V.R.; Somalanka, S.R.; Bandaru, S.R.; Garapati, H.R. Application of statistical experimental designs for the optimization of medium constituents for the production of citric acid from pineapple waste. Bioresour. Technol. 2008, 99, 4445–4450. [Google Scholar] [CrossRef]
- Morgunov, I.G.; Kamzolova, S.V.; Lunina, J.N. Citric acid production by Yarrowia lipolytica yeast on different renewable raw materials. Fermentation 2018, 4, 36. [Google Scholar] [CrossRef] [Green Version]
- Finogenova, T.V.; Morgunov, I.G.; Kamzolova, S.V.; Chernyavskaya, O.G. Organic acid production by the yeast Yarrowia lipolytica: A review of prospects. Appl. Biochem. Microbiol. 2005, 41, 418–425. [Google Scholar] [CrossRef]
- Kamzolova, S.V.; Morgunov, I.G. Microbial production of (2 R,3 S)-isocitric acid: State of the arts and prospects. Appl. Microbiol. Biotechnol. 2019, 103, 9321–9333. [Google Scholar] [CrossRef]
- Fickers, P.; Cheng, H.; Sze, K.; Lin, C. Sugar alcohols and organic acids synthesis in Yarrowia lipolytica: Where Are We? Microorganisms 2020, 8, 574. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kim, M.J.; Yoon, W.; Kim, E.Y.; Kim, H.; Lee, Y.; Min, B.; Kang, K.S.; Son, J.H.; Park, H.T.; et al. Isocitrate protects DJ-1 null dopaminergic cells from oxidative stress through NADP+-dependent isocitrate dehydrogenase (IDH). PLoS Genet. 2017, 13, e1006975. [Google Scholar] [CrossRef] [PubMed]
- Heretsch, P.; Thomas, F.; Aurich, A.; Krautscheid, H.; Sicker, D.; Giannis, A. Syntheses with a chiral building block from the citric acid cycle: (2R,3S)-isocitric acid by fermentation of sunflower oil. Angew. Chem. Int. Ed. Engl. 2008, 47, 1958–1960. [Google Scholar] [CrossRef] [PubMed]
- Aurich, A.; Specht, R.; Müller, R.A.; Stottmeister, U.; Yovkova, V.; Otto, C.; Holz, M.; Barth, G.; Heretsch, P.; Thomas, F.A.; et al. Microbiologically produced carboxylic acids used as building blocks in organic synthesis. In Reprogramming Microbial Metabolic Pathways. Subcellular Biochemistry; Wang, X., Chen, J., Quinn, P., Eds.; Springer: Dordrecht, The Netherlands, 2012; Volume 64, pp. 391–423. [Google Scholar] [CrossRef]
- Aurich, A.; Hofmann, J.; Oltrogge, R.; Wecks, M.; Glaser, R.; Blömer, L.; Mauersberger, S.; Roland, A.; Müller, R.A.; Sicker, D.; et al. Improved isolation of microbiologically produced (2R,3S)-isocitric acid by adsorption on activated carbon and recovery with methanol. Org. Process Res. Dev. 2017, 21, 866–870. [Google Scholar] [CrossRef] [Green Version]
- Bullin, K.; Hennig, L.; Herold, R.; Krautscheid, H.; Richter, K.; Sicker, D. An optimized method for an (2R,3S)-isocitric acid building block. Mon. Chem. 2019, 150, 247–253. [Google Scholar] [CrossRef]
- Kulkarni, M.G.; Shaikh, Y.B.; Borhade, A.S.; Dhondge, A.P.; Chavhan, S.W.; Desai, M.P.; Birhade, D.R.; Dhatrak, N.R.; Gannimani, R. The efficient synthesis of (3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-ol and its isomers. Tetrahedron Asymmetry 2010, 21, 2394–2398. [Google Scholar] [CrossRef]
- Moore, G.L.; Stringham, R.W.; Teager, D.S.; Yue, T.Y. Practical synthesis of the bicyclic darunavir side chain: (3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-ol from monopotassium isocitrate. Org. Process Res. Dev. 2017, 21, 98–106. [Google Scholar] [CrossRef]
- Morgunov, I.G.; Karpukhina, O.V.; Kamzolova, S.V.; Samoilenko, V.A.; Inozemtsev, A.N. Investigation of the effect of biologically active threo-Ds-isocitric acid on oxidative stress in Paramecium caudatum. Prep. Biochem. Biotechnol. 2018, 48, 1–5. [Google Scholar] [CrossRef]
- Morgunov, I.G.; Kamzolova, S.V.; Karpukhina, O.V.; Bokieva, S.V.; Inozemtsev, A.N. Biosynthesis of isocitric acid in repeated-batch culture and testing of its stress-protective activity. Appl. Microbiol. Biotechnol. 2019, 103, 3549–3558. [Google Scholar] [CrossRef]
- Morgunov, I.G.; Kamzolova, S.V.; Karpukhina, O.V.; Bokieva, S.B.; Lunina, J.N.; Inozemtsev, A.N. Microbiological Production of Isocitric Acid from Biodiesel Waste and Its Effect on Spatial Memory. Microorganisms 2020, 8, 462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finogenova, T.V.; Shishkanova, N.V.; Fausek, E.A.; Eremina, S.S. Biosynthesis of isocitric acid from ethanol by yeasts. Appl. Microbiol. Biotechnol. 1991, 36, 231–235. [Google Scholar] [CrossRef]
- Kamzolova, S.V.; Shamin, R.V.; Stepanova, N.N.; Morgunov, G.I.; Lunina, J.N.; Allayarov, R.K.; Samoilenko, V.A.; Morgunov, I.G. Fermentation conditions and media optimization for isocitric acid production from ethanol by Yarrowia lipolytica. Biomed Res. Int. 2018, 2018, e2543210. [Google Scholar] [CrossRef] [Green Version]
- Kamzolova, S.V.; Dedyukhina, E.G.; Samoilenko, V.A.; Lunina, J.N.; Puntus, I.F.; Allayarov, R.K.; Chiglintseva, M.N.; Mironov, A.A.; Morgunov, I.G. Isocitric acid production from rapeseed oil by Yarrowia lipolytica yeast. Appl. Microbiol. Biotechnol. 2013, 97, 9133–9144. [Google Scholar] [CrossRef] [PubMed]
- Hapeta, P.; Rakicka-Pustułka, M.; Juszczyk, P.; Robak, M.; Rymowicz, W.; Lazar, Z. Overexpression of citrate synthase increases isocitric acid biosynthesis in the yeast Yarrowia lipolytica. Sustainability 2020, 12, e7364. [Google Scholar] [CrossRef]
- Kamzolova, S.V.; Samoilenko, V.A.; Lunina, J.N.; Morgunov, I.G. Effects of medium components on isocitric acid production by Yarrowia lipolytica yeast. Fermentation 2020, 6, 112. [Google Scholar] [CrossRef]
- Förster, A.; Jacobs, K.; Juretzek, T.; Mauersberger, S.; Barth, B. Overexpression of the ICL1 gene changes the product ratio of citric acid production by Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2007, 77, 861–869. [Google Scholar] [CrossRef]
- Holz, M.; Förster, A.; Mauersberger, S.; Barth, G. Aconitase overexpression changes the product ratio of citric acid production by Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2009, 81, 1087–1096. [Google Scholar] [CrossRef]
- Yuzbasheva, E.Y.; Scarcia, P.; Yuzbashev, T.V.; Messina, E.; Kosikhina, I.M.; Palmieri, L.; Shutov, A.V.; Taratynova, M.O.; Amaro, R.L.; Palmieri, F.; et al. Engineering Yarrowia lipolytica for the selective and high-level production of isocitric acid through manipulation of mitochondrial dicarboxylate-tricarboxylate carriers. Metab. Eng. 2021, 65, 156–166. [Google Scholar] [CrossRef]
- Da Silva, L.V.; Tavares, C.B.; Amaral, P.F.F.; Coehlo, M.A.Z. Production of citric acid by Yarrowia lipolytica in different crude oil concentrations and in different nitrogen sources. Chem. Eng. Trans. 2012, 27, 199–204. [Google Scholar]
- Rzechonek, D.A.; Dobrowolski, A.; Rymowicz, W.; Mirończuk, A.M. Aseptic production of citric and isocitric acid from crude glycerol by genetically modified Yarrowia lipolytica. Bioresour. Technol. 2019, 271, 340–344. [Google Scholar] [CrossRef]
- Kamzolova, S.V.; Samoilenko, V.A.; Lunina, J.N.; Morgunov, I.G. Isocitric acid production from ethanol industry waste by Yarrowia Lipolytica. Fermentation 2021, 7, 146. [Google Scholar] [CrossRef]
- Tsygankov, P.S.; Tsygankov, S.P. Handbook on Alcohol Rectification; Pishchepromizdat: Moscow, Russia, 2002; p. 400. (In Russian) [Google Scholar]
- Karklin, R.; Peltzmane, I.; Raminya, L.; Korde, G. Overproduction of isocitric acid by wild strain of Candida lipolytica. In Metabolism of n-Alkanes and Oversynthesis of Products by Microorganisms; USSR Academy of Sciences, Institute of Biochemistry and Physiology of Microorganisms: Pushchino, Russia, 1991; pp. 143–146. (In Russian) [Google Scholar]
- Kamzolova, S.V.; Allayarov, R.K.; Lunina, J.N.; Morgunov, I.G. The effect of oxalic and itaconic acids on threo-Ds-isocitric acid production from rapeseed oil by Yarrowia lipolytica. Bioresour. Technol. 2016, 206, 128–133. [Google Scholar] [CrossRef]
- Bradford, M.M. Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Il’chenko, A.P.; Chernyavskaya, O.G.; Shishkanova, N.V.; Finogenova, T.V. The Induction of cytochrome P-450 and ethanol oxidation in Yarrowia lipolytica cells. Microbiology 2003, 72, 138–143. [Google Scholar] [CrossRef]
- Il’chenko, A.P.; Chernyavskaya, O.G.; Finogenova, T.V. Ethanol metabolism in the yeasts Yarrowia and Torulopsis: A Review. Appl. Biochem Microbiol. 2005, 41, 426–432. [Google Scholar] [CrossRef]
- Kujau, M.; Weber, H.; Barth, G. Characterization of mutants of the yeast Yarrowia lipolytica defective in acetyl-coenzyme A synthetase. Yeast 1992, 8, 193–203. [Google Scholar] [CrossRef]
- Gatter, M.; Ottlik, S.; Kövesi, Z.; Bauer, B.; Matthäus, F.; Barth, G. Three alcohol dehydrogenase genes and one acetyl-CoA synthetase gene are responsible for ethanol utilization in Yarrowia lipolytica. Fungal Genet. Biol. 2016, 95, 30–38. [Google Scholar] [CrossRef]
- Holdsworth, J.E.; Veenhuis, H.; Ratledge, C. Enzyme activities in oleaginous yeasts accumulating and utilizing exogenous or endogenous lipids. J. Gen. Microbiol. 1988, 134, 2907–2915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fickers, P.; Benetti, P.-H.; Waché, Y.; Marty, A.; Mauersberger, S.; Smit, M.S.; Nicaud, J.-M. Hydrophobic substrate utilisation by the yeast Yarrowia lipolytica, and its potential applications. FEMS Yeast Res. 2005, 5, 527–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papanikolaou, S.; Aggelis, G. Yarrowia lipolytica: A model microorganism used for the production of tailor-made lipids. Eur. J. Lipid Sci. Technol. 2010, 112, 639–654. [Google Scholar] [CrossRef]
- Sabra, W.; Bommareddy, R.R.; Maheshwari, G.; Papanikolaou, S.; Zeng, A.-P. Substrates and oxygen dependent citric acid production by Yarrowia lipolytica: Insights through transcriptome and fluxome analyses. Microb. Cell Fact. 2017, 16, 78. [Google Scholar] [CrossRef]
- Stahmann, K.P.; Revuelta, J.L.; Seulberger, H. Three biotechnical processes using Ashbya gossypii, Candida famata, or Bacillus subtilis compete with chemical riboflavin production. Appl. Microbiol. Biotechnol. 2000, 53, 509–516. [Google Scholar] [CrossRef]
- Berg, I.A.; Filatova, L.V.; Ivanovsky, R.N. Inhibition of acetate and propionate assimilation by itaconate via propionyl-CoA carboxylase in isocitrate lyase-negative purple bacterium Rhodospirillum rubrum. FEMS Microbiol. Lett. 2002, 216, 49–54. [Google Scholar] [CrossRef]
- Krátký, M.; Vinšová, J. Advances in mycobacterial isocitrate lyase targeting and inhibitors. Curr. Med. Chem. 2012, 19, 6126–6137. [Google Scholar] [CrossRef]
- Thomas, K.C.; Dawson, P.S.S. Relationship between iron-limited growth and energy limitation during phased cultivation of Candida utilis. Can. J. Microbiol. 1978, 24, 440–447. [Google Scholar] [CrossRef]
- Hoffmann, A.; Kupsch, C.; Walther, T.; Löser, C. Synthesis of ethyl acetate from glucose by Kluyveromyces marxianus, Cyberlindnera jadinii and Wickerhamomyces anomalus depending on the induction mode. Eng. Life Sci. 2021, 21, 154–168. [Google Scholar] [CrossRef]
- Laptev, I.A.; Filimonova, N.A.; Allayarov, R.K.; Kamzolova, S.V.; Samoilenko, V.A.; Sineoky, S.P.; Morgunov, I.G. New recombinant strains of the yeast Yarrowia lipolytica with overexpression of the aconitate hydratase gene for the obtainment of isocitric acid from rapeseed oil. Appl. Biochem. Microbiol. 2016, 52, 699–704. [Google Scholar] [CrossRef]
- Finogenova, T.V. Biosynthesis of Organic Acids by Yeast Organisms and its Regulation. Ph.D. Thesis, USSR Academy of Sciences, Institute of Biochemistry and Physiology of Microorganisms, Pushchino, Russia, 1982. [Google Scholar]
- Babel, W. The auxiliary substrate concept: From simple considerations to heuristically valuable knowledge. Eng. Life Sci. 2009, 9, 285–290. [Google Scholar] [CrossRef]
- Müller, R.; Markuske, K.D.; Babel, W. Improvement of Y-values of Hansenula polymorpha growth on methanol by simultaneous utilization of glucose. Z. Allg. Mikrobiol. 1983, 23, 375–384. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).