Solid State Fermentation of Shrimp Shell Waste Using Pseudonocardia carboxydivorans 18A13O1 to Produce Bioactive Metabolites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Isolation of Actinomycetes
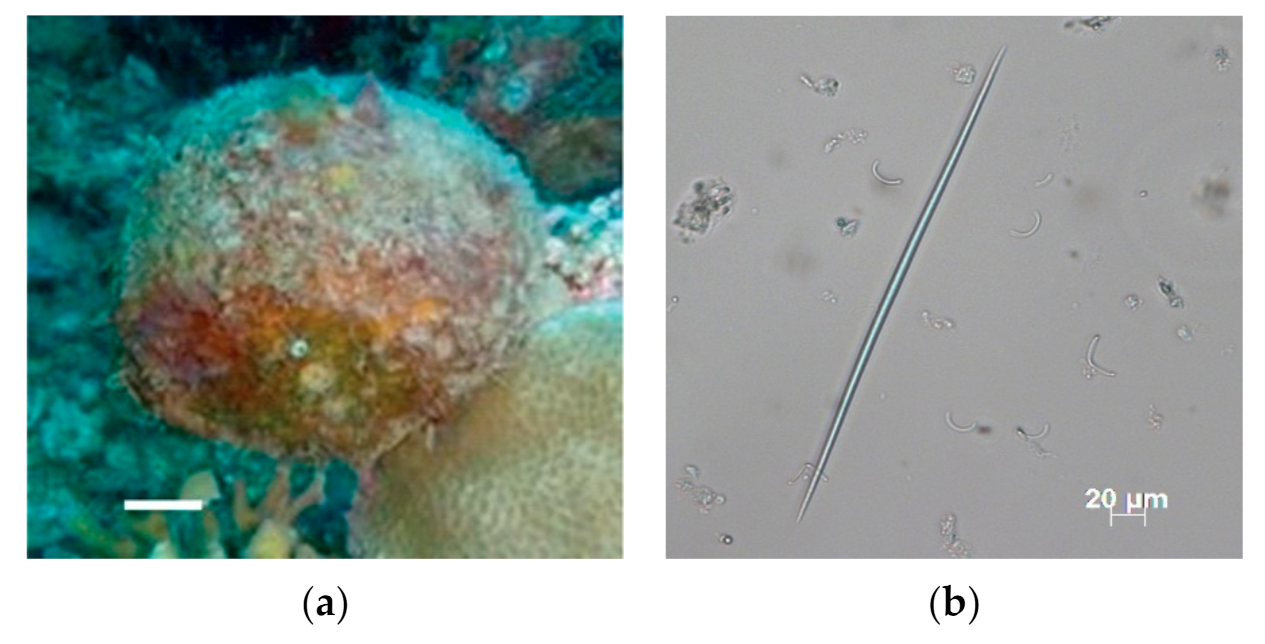
2.1.1. Sponge Collection
2.1.2. Isolation of Actinomycetes from Marine Sponges
2.2. Screening Antibacterial Activity
2.2.1. Clinical Pathogenic Bacteria
2.2.2. Resazurin Assay
2.3. Characterization of Selected Actinomycetes
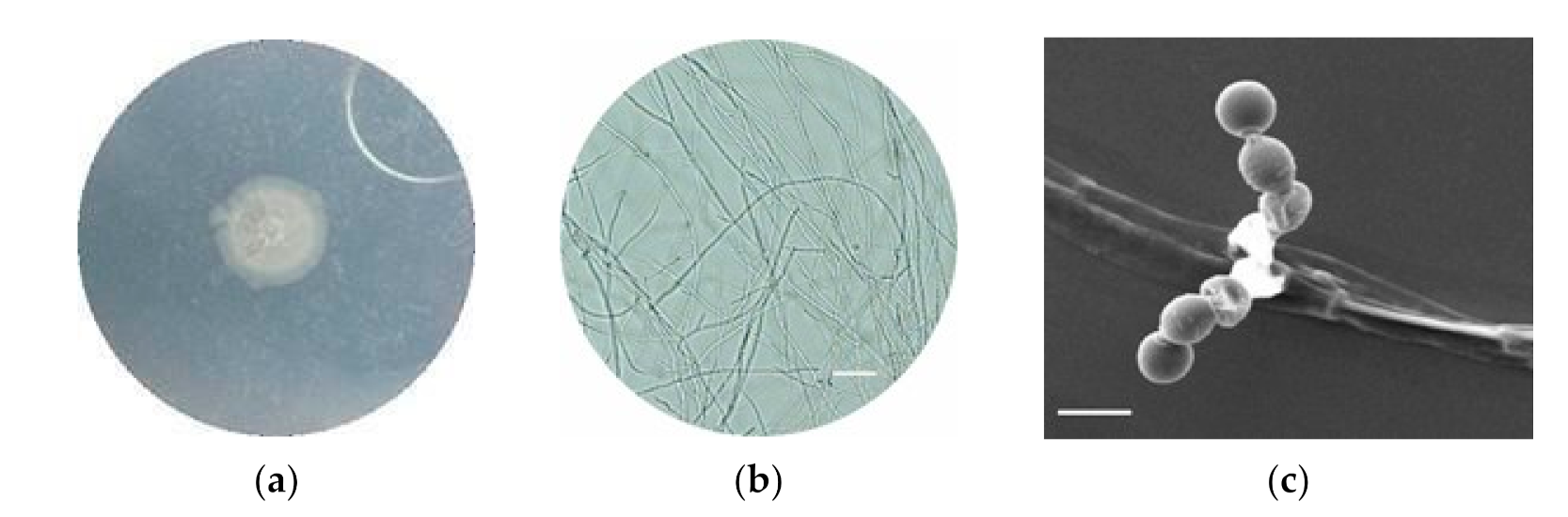
2.3.1. Analysis Morphology
2.3.2. Scanning Electron Microscope
2.3.3. Phylogenic Analysis of Isolate 18A13O1
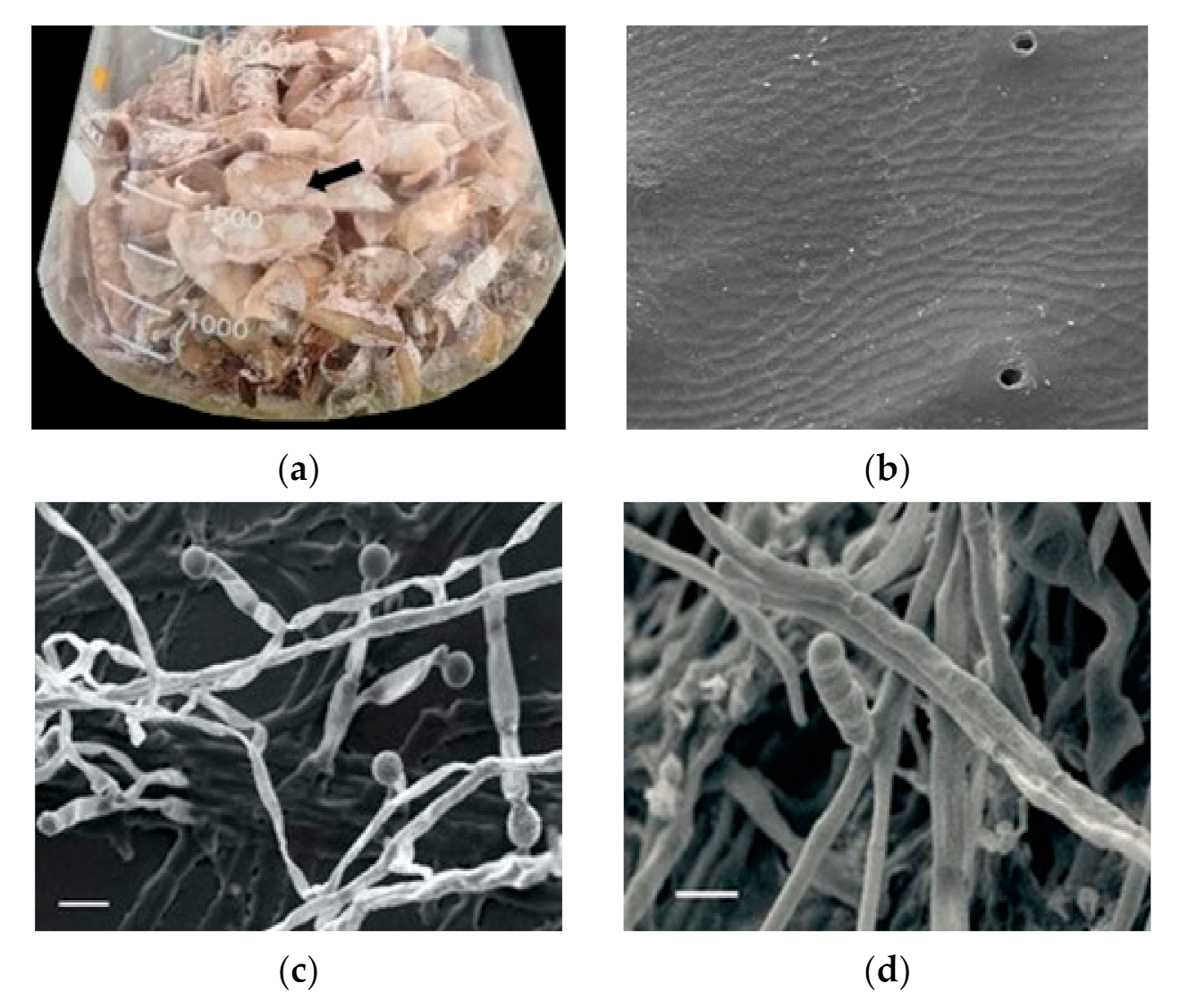
2.4. Solid State Fermentation
2.5. Extraction, Purification, and Characterization
3. Results and Discussions
3.1. Sample Collection and Isolation of Actinomycetes
3.2. Screening Antibacterial Activity
3.3. Characterization of Selected Actinomycetes
3.4. Phylogenetic Analysis of Isolate 18A13O1
3.5. Solid State Fermentation
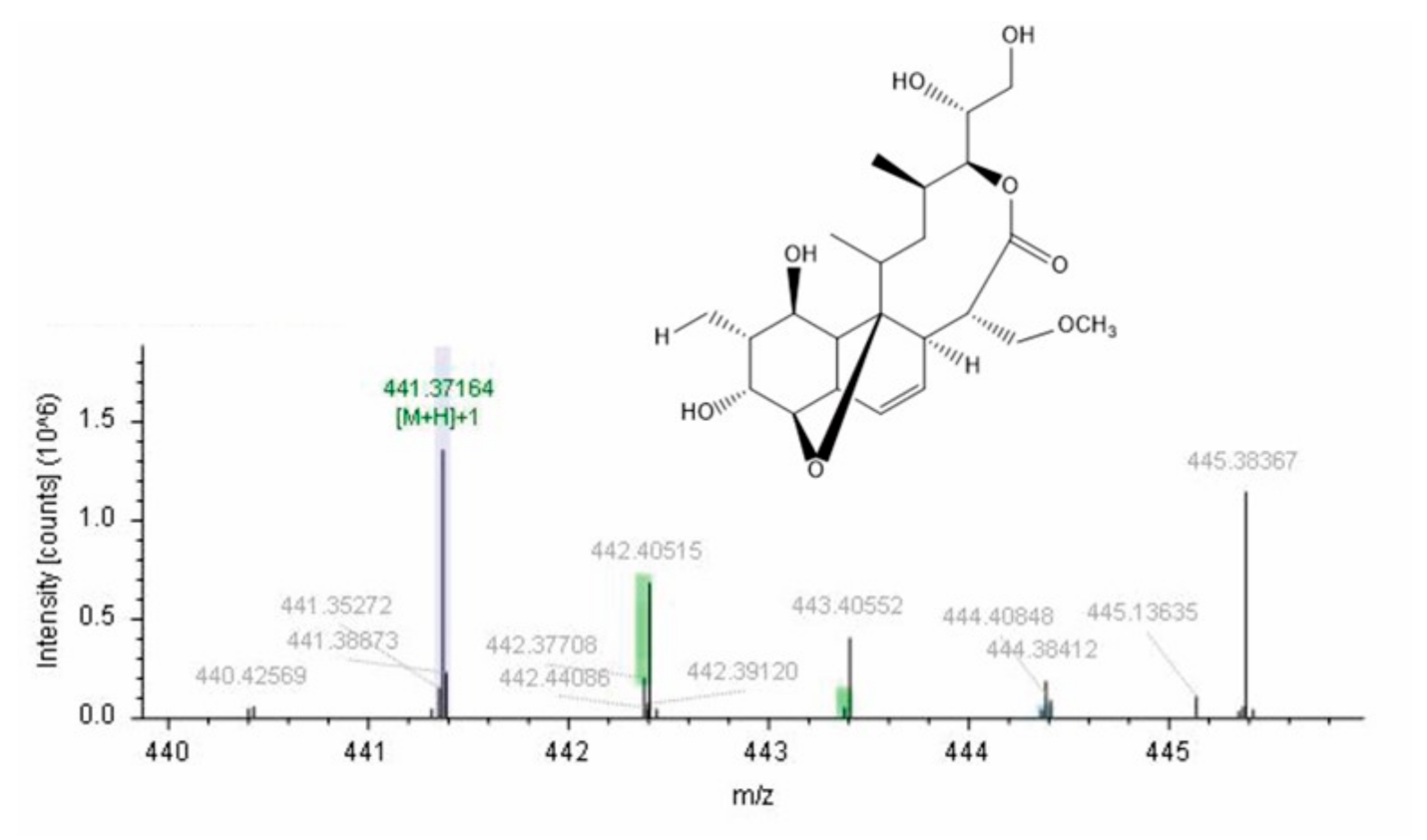
3.6. Extraction, Isolation, and Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santos, V.P.; Marques, N.S.S.; Maia, P.C.S.V.; Lima, M.A.B.D.; Franco, L.D.O.; Campos-Takaki, G.M.D. Seafood waste as atractive source of chitin and chitosan production and their applications. Int. J. Mol. Sci. 2020, 21, 4290. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Guo, N.; Sun, J.; Xue, C. Comprehensive utilization of shrimp waste based on biotechnological methods: A review. J. Clean. Prod. 2017, 143, 814–823. [Google Scholar] [CrossRef]
- Abu Yazid, N.; Barrena, R.; Komilis, D.; Sánchez, A. Solid-State Fermentation as a novel paradigm for organic waste valorization: A Review. Sustainability 2017, 9, 224. [Google Scholar] [CrossRef] [Green Version]
- Trejo-Hernandez, M.R.; Raimbault, M.; Roussos, S.; Lonsane, B.K. Potencial of solid state fermentation for production of ergot alkaloids. Lett. Appl. Microbiol. 1992, 15, 156–159. [Google Scholar] [CrossRef]
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Klenk HPClément, C.; Ouhdouch, Y.; van Wezel, G.P. Taxonomy, physiology, and natural products of actinobacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 1–43. [Google Scholar] [CrossRef] [Green Version]
- Subramani, R.; Sipkema, D. Marine rare actinomycetes: A promising source of structurally diverse and unique novel natural products. Mar. Drugs 2019, 17, 249. [Google Scholar] [CrossRef] [Green Version]
- Best, M.; Kenchington, E.; MacIsaac, K.; Wareham, V.; Fuller, S.D.; Thompson, A.B. Sponge identification guide NAFO area. NAFO Sci. Counc. Stud. 2010, 43, 1–49. [Google Scholar] [CrossRef]
- Hsu, S.C.; Lockwood, J.L. Powdered chitin agar as a selective medium for enumeration of actinomycetes in water and soil. Appl. Microbiol. 1975, 29, 422–426. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Truck, M. Antibiotic Susceptibility Testing by a Standardized Single Disk Method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 27th ed.; CLSI Supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- Coban, A.Y.; Bozdogan, B.; Cihan, C.C.; Cetinkaya, E.; Bilgin, K.; Darka, O.; Akgunes, A.; Durupinar, B.; Appelbaum, P.C. Two new colorimeteric methods for early detection of vancomycin and oxacillin resistance in Staphylococcus aureus. J. Clin. Microbiol. 2006, 44, 580–582. [Google Scholar] [CrossRef] [Green Version]
- Goodfellow, M.; Kämpfer, P.; Busse, H.-J.; Trujillo, M.E.; Suzuki, K.; Ludwig, W.; Whitman, W.B. (Eds.) Bergey’s Manual of Systematic Bacteriology, 2nd ed.; Springer: New York, NY, USA, 2012. [Google Scholar]
- Heuer, H.; Krsek, M.; Baker, P.; Smalla, K.; Wellington, E.M. Analysis of actinomycete communities by specific amplification of genes encoding 16S rRNA and gel-electrophoretic separation in denaturing gradients. Appl. Environ. Microbiol. 1997, 63, 3233–3241. [Google Scholar] [CrossRef] [Green Version]
- Baskaran, R.; Mohan, P.M.; Sivakumar, K.; Kumar, A. Antimicrobial Activity and Phylogenetic Analysis of Streptomyces Parvulus Dosmb-D105 Isolated from the Mangrove Sediments of Andaman Islands. Acta Microbiol. Immunol. Hung. 2016, 63, 27–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Nakeeb, M.A.; Lechevalier, H.A. Selective Isolation of Aerobic Actinomycetes. Appl. Microbiol. 1963, 11, 75–77. [Google Scholar] [CrossRef]
- Hooper, J.N.A.; Van Soest, R.W.M. Systema Porifera: A Guide to the Classification of Sponges; 2 Volumes; Kluwer Academic/Plenum Publishers: New York, NY, USA, 1718. [Google Scholar]
- Sun, W.; Zhang, F.; He, L.; Karthik, L.; Li, Z. Actinomycetes from the South China Sea sponges: Isolation, diversity, and potential for aromatic polyketides discovery. Front. Microbiol. 2015, 6, 1048. [Google Scholar] [CrossRef] [Green Version]
- Abdelmohsen, U.R.; Yang, C.; Horn, H.; Hajjar, D.; Ravasi, T.; Hentschel, U. Actinomycetes from Red Sea Sponges: Sources for Chemical and Phylogenetic Diversity. Mar. Drugs 2014, 12, 2771–2789. [Google Scholar] [CrossRef] [Green Version]
- Łukowiak, M. Utilizing sponge spicules in taxonomic, ecological and environmental reconstructions: A review. PeerJ 2020, 8, e10601. [Google Scholar] [CrossRef] [PubMed]
- Aoki, S.; Sanagawa, M.; Watanabe, Y.; Setiawan, A.; Arai, M.; Kobayashi, M. Novel isomarabarican triterpenes, exhibiting selective antiproliferative activity again vascular endothelial cells, from marine sponge Rhabdastrella globostellata. Bioorg. Med. Chem. 2007, 15, 4818–4828. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.-H.; Huang, Z.-H.; El-Shazly, M.; Peng, B.-R.; Wei, W.-C.; Su, J.-H. Isomalabaricane Triterpenes from the Marine Sponge Rhabdastrella sp. Mar. Drugs 2021, 19, 206. [Google Scholar] [CrossRef]
- Acharyabhatta, A.; Kandula, S.K.; Terli, R. Taxonomy and polyphasic characterization of alkaline amylase producing marine actinomycete Streptomyces rochei BTSS 1001. Int. J. Microbiol. 2013, 2013, 276921. [Google Scholar] [CrossRef] [Green Version]
- Deacon, J.W. Fungal Biology, 4th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2006; p. 48. [Google Scholar]
- Tanvir, R.; Sajid, I.; Hasnain, S.; Kulik, A.; Grond, S. Rare actinomycetes Nocardia caishijiensis and Pseudonocardia carboxydivorans as endophytes, their bioactivity and metabolites evaluation. Microbiol. Res. 2016, 185, 22–35. [Google Scholar] [CrossRef]
- Braña, A.F.; Sarmiento-Vizcaíno, A.; Pérez-Victoria, I.; Otero, L.; Fernández, J.; Palacios, J.J.; Martín, J.; de la Cruz, M.; Díaz, C.; Vicente, F.; et al. Branimycins B and C, antibiotics produced by the abyssal actinobacterium Pseudonocardia carboxydivorans M-227. J. Nat. Prod. 2017, 80, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.T.; Davies, F.L. Use of a scanning electron microscope for the examination of actinomycetes. Microbiology 1967, 48, 171–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brzezinska, M.S.; Walczak, M.; Lalke-Porczyk, E.; Donderski, W. Utilization of shrimp-shell waste as a substrate for the activity of chitinases produced by microorganisms. Pol. J. Environ. Stud. 2010, 19, 177–182. [Google Scholar]
- Marinelli, F.; Genilloud, O.; Fedorenko, V.; Ron, E.Z. Specialized bioactive microbial metabolites: From gene to product. BioMed Res. Int. 2015, 2015, 276964. [Google Scholar] [CrossRef] [Green Version]
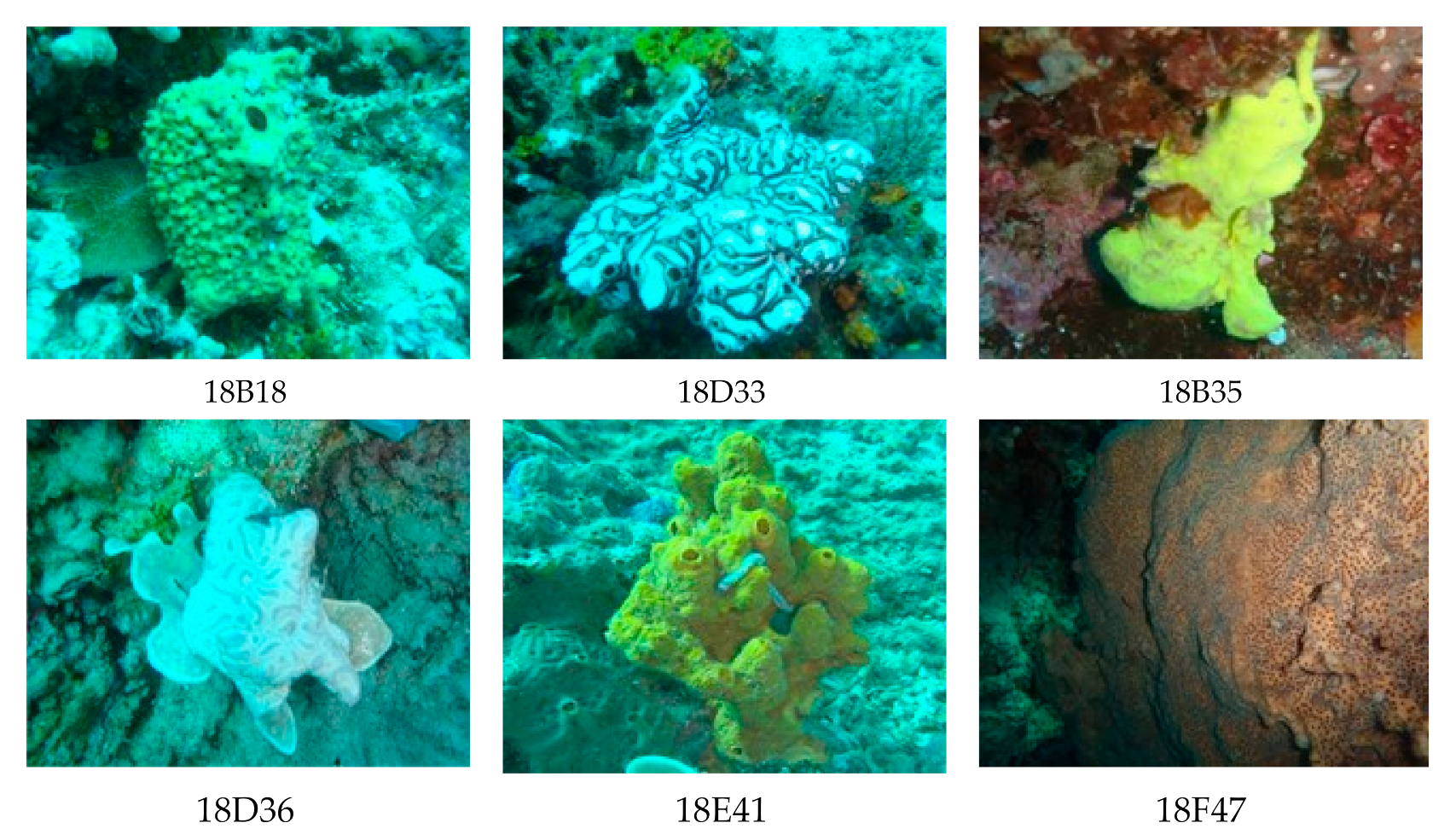

| No. | Sample Code | Phylum | Isolate Actinomycetes | Color | Inhibition Concentration (μg/mL) |
|---|---|---|---|---|---|
| 1 | 18A13 | Porifera | 18A13A1 | White | 500 |
| 18A13O1 | White | 250 | |||
| 2 | 18B18 | Porifera | 18B18A1 | White | 500 |
| 18B18A2 | White | 500 | |||
| 18B18A3 | White | - | |||
| 18B18A4 | White | 500 | |||
| 3 | 18D33 | Porifera | 18D33A1 | White | - |
| 18D33A2 | White | 500 | |||
| 4 | 18D35 | Porifera | 18D35A1 | Grey | 500 |
| 18D35A2 | White | - | |||
| 5 | 18D36 | Tunicate | 18D36A1 | Grey | 500 |
| 18D36A2 | Grey | - | |||
| 6 | 18E41 | Porifera | 18E41A1 | White | 500 |
| 7 | 18F47 | Porifera | 18F47A1 | White | - |
| 18F47A2 | White | 500 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Setiawan, A.; Widyastuti, W.; Irawan, A.; Wijaya, O.S.; Laila, A.; Setiawan, W.A.; Juliasih, N.L.G.R.; Nonaka, K.; Arai, M.; Hendri, J. Solid State Fermentation of Shrimp Shell Waste Using Pseudonocardia carboxydivorans 18A13O1 to Produce Bioactive Metabolites. Fermentation 2021, 7, 247. https://doi.org/10.3390/fermentation7040247
Setiawan A, Widyastuti W, Irawan A, Wijaya OS, Laila A, Setiawan WA, Juliasih NLGR, Nonaka K, Arai M, Hendri J. Solid State Fermentation of Shrimp Shell Waste Using Pseudonocardia carboxydivorans 18A13O1 to Produce Bioactive Metabolites. Fermentation. 2021; 7(4):247. https://doi.org/10.3390/fermentation7040247
Chicago/Turabian StyleSetiawan, Andi, Widyastuti Widyastuti, Arik Irawan, Oklis Syahrin Wijaya, Aspita Laila, Wawan Abdullah Setiawan, Ni Luh Gede Ratna Juliasih, Kenichi Nonaka, Masayoshi Arai, and John Hendri. 2021. "Solid State Fermentation of Shrimp Shell Waste Using Pseudonocardia carboxydivorans 18A13O1 to Produce Bioactive Metabolites" Fermentation 7, no. 4: 247. https://doi.org/10.3390/fermentation7040247
APA StyleSetiawan, A., Widyastuti, W., Irawan, A., Wijaya, O. S., Laila, A., Setiawan, W. A., Juliasih, N. L. G. R., Nonaka, K., Arai, M., & Hendri, J. (2021). Solid State Fermentation of Shrimp Shell Waste Using Pseudonocardia carboxydivorans 18A13O1 to Produce Bioactive Metabolites. Fermentation, 7(4), 247. https://doi.org/10.3390/fermentation7040247









