Sourdough Fermentation as a Tool to Improve the Nutritional and Health-Promoting Properties of Its Derived-Products
Abstract
:1. Introduction
2. Cereal Prolamins: Celiac Disease and Wheat Sensitivity
3. FODMAPs: Non-Celiac Gluten Sensitivity and Irritable Bowel Symptoms
| FODMAPs Contents [g/100 g DM] | |||||||
|---|---|---|---|---|---|---|---|
| Products | Fructans | GOS | Fructose (FEG) | Lactose | Polyols Sorbitol | Polyols Mannitol | Reference |
| 1. Gluten-containing cereal | |||||||
| Whole wheat | 1.88 | 0.14 | - | na | 0.04 | 0.01 | |
| Whole barley | 1.38 | 0.56 | - | na | nd | nd | |
| Rye | 3.61 | 0.13 | - | na | 0.01 | nd | Ispiryan et al. [43] |
| Spelt | 0.85 | 0.13 | - | na | nd | nd | |
| 2. Gluten-free cereals and pseudocereals | |||||||
| Corn starch | nd | nd | - | na | nd | nd | |
| Potato starch | nd | nd | - | na | nd | nd | |
| Quinoa | nd | 0.09 | - | na | 0.28 | nd | Ispiryan et a. [43] |
| Buckwheat | nd | 0.01 | - | na | 0.17 | nd | |
| 3. Seeds from pulses | |||||||
| Lentil | 3.98 | 1.44 | - | na | 0.95 | nd | |
| Chickpea | nd | 2.11 | - | na | nd | nd | |
| Soy | nd | 3.55 | - | na | 0.06 | nd | Ispiryan et al. [43] |
| Faba bean | nd | 3.45 | - | na | 0.03 | nd | |
| 4. Fruits | |||||||
| Pear | nd | nd | 2.3–5.0 | na | 2.3–60 | nd | |
| Apple | nd | nd | 0.14–0.76 | na | 0.70–0.83 | nd | |
| Peach | nd | nd | 0.0–4.2 | na | 0.68–0.99 | nd | Muir et al. [1] |
| Blackberries | nd | nd | nd | na | 4.6 | nd | |
| 5. Dairy products | |||||||
| Yoghurt | na | na | na | 2.9–4.2 | na | na | |
| Curd cheese | na | na | na | 1.8 | na | na | Gille et al. [51] |
| Bovine milk | na | na | na | 4.1–5.0 | na | na | |
| 6. Cereal products and gluten-free alternatives [g/100 g FW] | |||||||
| White wheat bread | 0.44 | 0.01 | 0.19 | nd | 0.01 * | ||
| Wheat sourdough bread | 0.11 | nd | nd | nd | 0.21 * | Ispiryan et al. [43] | |
| Gluten-free white bread | nd | nd | nd | nd | 0.03 * | ||
4. Effect of Sourdough Fermentation in Alleviating Symptoms of Celiac Disease and Wheat Sensitivity
4.1. Proteolytic Enzymes from Dormant and Germinated Wheat Grains
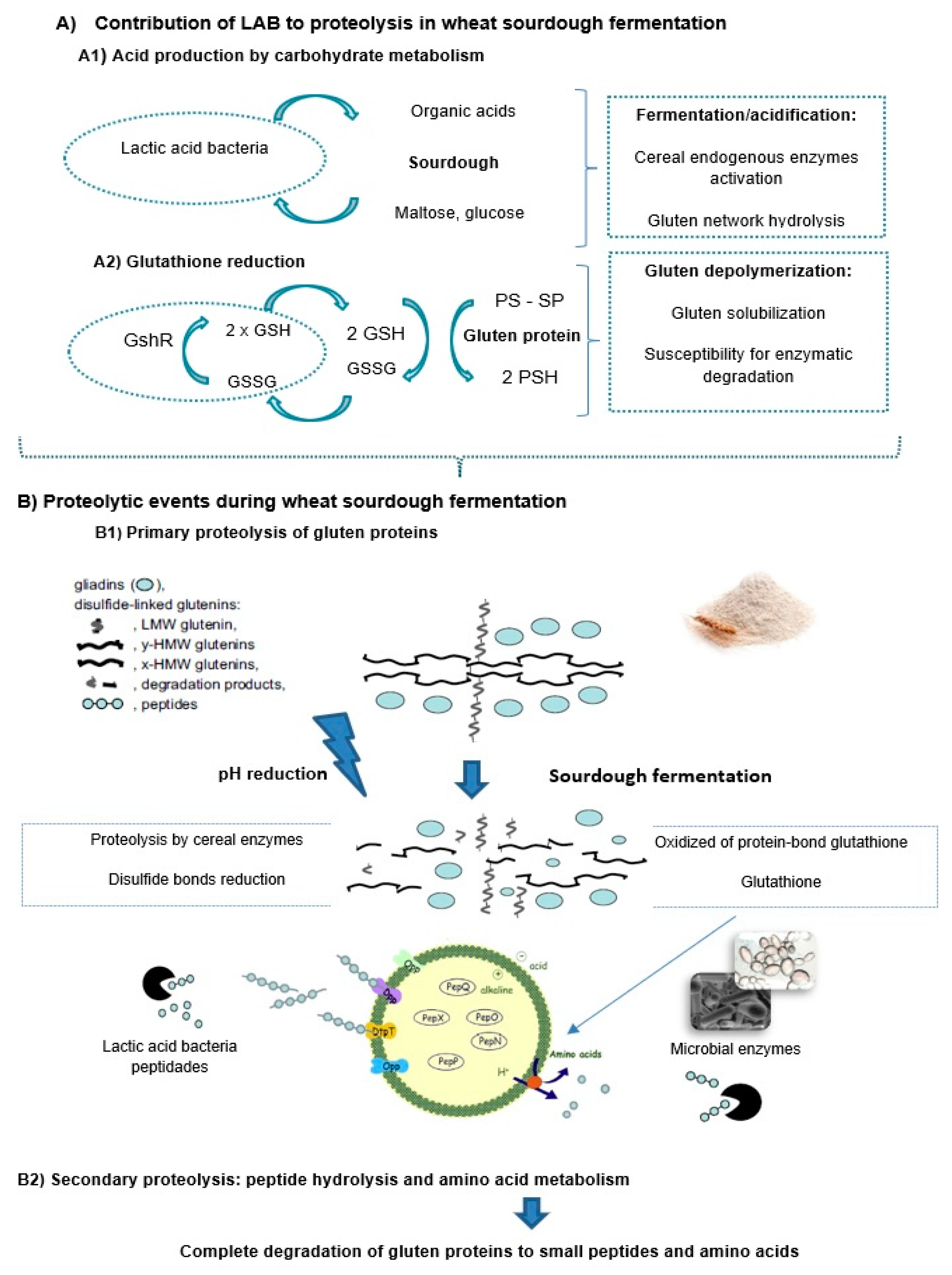
4.2. Prolamin Proteolysis in Wheat Sourdough Fermentation
4.3. Combining Cereal Endogenous Enzymes and LAB Sourdough Fermentation
4.4. Contribution of Sourdough Fermentation to Nutritional, Functional, and Human Health-Promoting Benefits
5. The Role of Sourdough to Reduce FODMAPs Compounds
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muir, J.G.; Varney, J.E.; Ajamian, M.; Gibson, P.R. Gluten-free and low-FODMAP sourdoughs for patients with coeliac disease and irritable bowel syndrome: A clinical perspective. Int. J. Food Microbiol. 2019, 290, 237–246. [Google Scholar] [CrossRef]
- Dickey, W.; Kearney, N. Overweight in celiac disease: Prevalence, clinical characteristics, and effect of a gluten-free diet. Am. J. Gastroent. 2006, 101, 2356–2359. [Google Scholar] [CrossRef]
- Green, P.; Jabri, B. Coeliac Disease. Lancet 2003, 362, 383–391. [Google Scholar] [CrossRef]
- Catassi, C.; Fabiani, E.; Iacono, G.; D’Agate, C.; Francavilla, R.; Biagi, F.; Volta, U.; Accomando, S.; Picarelli, A.; De Vitis, I.; et al. A prospective, double-blind, placebo-controlled trial to establish a safe gluten threshold for patients with celiac disease. Amer. J. Clin. Nut. 2007, 85, 160–166. [Google Scholar] [CrossRef]
- De Giorgio, R.; Volta, U.; Gibson, P.R. Sensitivity to wheat, gluten and FODMAPs in IBS: Facts or fiction? Rec. Adv. Clin. Pract. 2015, 65, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Biesiekierski, J.R.; Peters, S.L.; Newnham, E.D.; Rosella, O.; Muir, J.G.; Gibson, P.R. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology 2013, 145, 320–328. [Google Scholar] [CrossRef]
- Zevallos, V.F.; Raker, V.; Tenzer, S.; Jimenez-Calvente, C.; Schuppan, D. Nutritional Wheat Amylase-Trypsin Inhibitors Promote Intestinal Inflammation via Activation of Myeloid Cells. Gastroenterology 2017, 152, 1100–1113. [Google Scholar] [CrossRef] [Green Version]
- Laatikainen, R.; Koskenpato, J.; Hongisto, S.-M.; Loponen, J.; Poussa, T.; Huang, X. Pilot study: Comparison of sourdough wheat bread and yeast-fermented wheat bread in individuals with wheat sensitivity and irritable bowel syndrome. Nutrients 2017, 9, 1215. [Google Scholar] [CrossRef] [Green Version]
- Poutanen, K.; Flander, L.; Katina, K. Sourdough and cereal fermentation in a nutritional perspective. Food Microbiol. 2009, 26, 693–699. [Google Scholar] [CrossRef]
- Reale, A.; Di Renzo, T.; Succi, M.; Tremonte, P.; Coppola, R.; Sorrentino, E. Microbiological and Fermentative Properties of Baker’s Yeast Starter Used in Breadmaking. J. Food Sci. 2013, 78, 8. [Google Scholar] [CrossRef] [PubMed]
- Sapone, A.; Bai, J.C.; Ciacci, C.; Dolinsek, J.; Green, P.H.R.; Hadjivassiliou, M.; Fasano, A. Spectrum of gluten-related disorders: Consensus on new nomenclature and classification. BMC Med. 2012, 10, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gobbetti, M.; Rizzello, C.G.; Di Cagno, R.; De Angelis, M. How the sourdough may affect the functional features of leavened baked goods. Food Microbiol. 2014, 37, 30–40. [Google Scholar] [CrossRef]
- Wang, H.; Xia, X.; Yu, H.; Zhao, X.; Zhong, X.; Li, Q.; Tang, J.; Zhao, Y. Effect of liquid fermentation on bread fortified with Lycium ruthenicum: A quality attribute and in vitro digestibility study. Food Chem. 2019, 299, 125131. [Google Scholar] [CrossRef] [PubMed]
- Ganzle, M.G.; Loponen, J.; Gobbetti, M. Proteolysis in sourdough fermentations: Mechanisms and potential for improved bread quality. Trends Food Sci. Technol. 2008, 19, 513–521. [Google Scholar] [CrossRef]
- Gänzle, M.G. Enzymatic and bacterial conversions during sourdough fermentation. Food Microbiol. 2014, 37, 2–10. [Google Scholar] [CrossRef]
- Coda, R.; Di Cagno, R.; Gobbetti, M.; Rizzello, C.G. Sourdough lactic acid bacteria: Exploration of non-wheat cereal-based fermentation. Food Microbiol. 2014, 37, 51–58. [Google Scholar] [CrossRef]
- Coda, R.; Varis, J.; Verni, M.; Rizzello, C.G.; Katina, K. Improvement of the protein quality of wheat bread through faba bean sourdough addition. LWT—Food Sci. Technol. 2017, 82, 296–302. [Google Scholar] [CrossRef] [Green Version]
- Fekri, A.; Torbati, M.; Khosrowshahi, A.Y.; Shamloo, H.B.; Azadmard-Damirchi, S. Functional effects of phytate-degrading, probiotic lactic acid bacteria and yeast strains isolated from Iranian traditional sourdough on the technological and nutritional properties of whole wheat bread. Food Chem. 2020, 306, 125620. [Google Scholar] [CrossRef]
- Arte, E.; Rizzello, C.G.; Verni, M.; Nordlund, E.; Katina, K.; Coda, R. Impact of Enzymatic and Microbial Bioprocessing on Protein Modification and Nutritional Properties of Wheat Bran. J. Agric. Food Chem. 2015, 63, 8685–8693. [Google Scholar] [CrossRef] [PubMed]
- Verspreet, J.; Hemdane, S.; Dornez, E.; Cuyvers, S.; Delcour, J.A.; Courtin, C.M. Maximizing the concentrations of wheat grain fructans in bread by exploring strategies to prevent their yeast (Saccharomyces cerevisiae)-mediated degradation. J. Agric. Food Chem. 2013, 61, 1397–1404. [Google Scholar] [CrossRef]
- Longin, C.F.H.; Beck, H.; Gütler, A.; Gütler, H.; Heilig, W.; Zimmermann, J.; Bischoff, S.C.; Würschum, T. Influence of wheat variety and dough preparation on FODMAP content in yeast-leavened wheat breads. J. Cereal Sci. 2020, 95, 103021. [Google Scholar] [CrossRef]
- Huang, X.; Schuppan, D.; Rojas Tovar, L.E.; Zevallos, V.F.; Loponen, J.; Gänzle, M. Sourdough Fermentation Degrades Wheat Alpha-Amylase/Trypsin Inhibitor (ATI) and Reduces Pro-Inflammatory Activity. Foods 2020, 9, 943. [Google Scholar] [CrossRef] [PubMed]
- Gobbetti, M.; De Angelis, M.; Di Cagno, R.; Calasso, M.; Archetti, G.; Rizzello, C. Novel insights on the functional/nutritional features of the sourdough fermentation. Int. J. Food Microbiol. 2019, 302, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Compaoré-Sérémé, D.; Sawadogo-Lingani, H.; Coda, C.; Katina, K.; Maina, N. Influence of dextran synthesized in situ on the rheological, technological, and nutritional properties of whole-grain pearl millet bread. Food Chem. 2019, 285, 221–230. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, X.; Xu, H.; Sun, Y.; Zhang, Y.; Wang, Y. Improvement of the nutritional, antioxidant and bioavailability properties of corn gluten-wheat bran mixture fermented with lactic acid bacteria and acid protease. LWT—Food Sci. Technol. 2021, 144, 111161. [Google Scholar] [CrossRef]
- Montemurro, M.; Pontonio, E.; Gobbetti, M.; Giuseppe, C. Investigation of the nutritional, functional and technological effects of the sourdough fermentation of sprouted flours. Int. J. Food Microbiol. 2019, 302, 47–58. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Cassone, A.; Di Cagno, R.; Gobbetti, M. Synthesis of angiotensin I-converting enzyme (ACE)-inhibitory peptides and g-aminobutyric acid (GABA) during sourdough fermentation by selected lactic acid bacteria. J. Agric. Food Chem. 2008, 56, 6936–6943. [Google Scholar] [CrossRef] [PubMed]
- Coda, R.; Rizzello, C.G.; Pinto, D.; Gobbetti, M. Selected lactic acid bacteria to synthesize antioxidant peptides during sourdough fermentation of cereal flours. Appl. Environ. Microbiol. 2012, 78, 1087–1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malaguti, M.; Dinelli, G.; Leoncini, E.; Bregola, V.; Bosi, S.; Cicero, A.F.; Hrelia, S. Bioactive peptides in cereals and legumes: Agronomical, biochemical, and clinical aspects. Int. J. Mol. Sci. 2014, 15, 21120–21135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, T.B.; He, T.-P.; Li, H.B.; Tang, H.W.; Xia, E.Q. The structure-activity relationship of the antioxidant peptides from natural proteins. Molecules 2016, 21, 72. [Google Scholar] [CrossRef]
- Settanni, L.; Ventimiglia, G.; Alfonzo, A.; Corona, O.; Miceli, A.; Moschetti, G. An integrated technological approach to the selection of lactic acid bacteria of flour origin for sourdough production. Food Res. Int. 2013, 12, 1569–1578. [Google Scholar] [CrossRef] [Green Version]
- Engström, N.; Sandberg, A.S.; Scheers, N. Sourdough fermentation of wheat flour does not prevent the interaction of transglutaminase 2 with α (2)—gliadin or gluten. Nutrients 2015, 7, 2134–2144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gänzle, M.G.; Zheng, J. Lifestyles of sourdough lactobacilli—Do they matter for microbial ecology and bread quality? Inter. J. Food Microbiol. 2019, 302, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Koning, F. Celiac disease: Caught between a rock and a hard place. Gastroenterology 2005, 129, 1294–1301. [Google Scholar] [CrossRef]
- Lionetti, E.; Gatti, S.; Pulvirenti, A.; Catassi, C. Celiac disease from a global perspective. Best Pract. Res. Clin. Gastroenterol. 2015, 29, 365–379. [Google Scholar] [CrossRef]
- Schalk, K.; Lang, C.; Wieser, H.; Koehler, P.; Scherf, K.A. Quantitation of the immunodominant 33-mer peptide from α-gliadin in wheat flours by liquid chromatography tandem mass spectrometry. Sci. Rep. 2017, 7, 45092. [Google Scholar] [CrossRef]
- Osborne, T.B. The Protein of the Wheat Kernel; Publication No. 84; Carnegie Institute of Washington: Washington, DC, USA, 1907. [Google Scholar]
- Shewry, P.R.; Tatham, A.S.; Forde, J.; Kreis, M.; Miflin, B.J. The classification and nomenclature of wheat gluten proteins: A reassessment. J. Cereal Sci. 1986, 4, 97–106. [Google Scholar] [CrossRef]
- Shewry, P.R.; Tatham, A.S. Disulphide bonds in wheat gluten proteins. J. Cereal Sci. 1997, 25, 207–227. [Google Scholar] [CrossRef]
- Loponen, J. Prolamins Degradation in Sourdoughs. Ph.D. Dissertation, University of Helsinki, Helsinki, Finland, 2006. [Google Scholar]
- Makki, K.; Deehan, E.C.; Walter, J.; Backhed, F. The impact of dietary fibre on gut microbiota in host health and disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varney, J.; Barrett, J.; Scarlata, K.; Catsos, P.; Gibson, P.R.; Muir, J.G. FODMAPs: Food composition, defining cutoff values and international application. J. Gastroent. Hepatol. 2017, 32, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Ispiryan, L.; Zannini, E.; Arendt, E.K. Characterization of the FODMAP-profile in cereal-product ingredients. J. Cereal Sci. 2020, 92, 102916. [Google Scholar] [CrossRef]
- Halmos, E.P.; Gibson, P.R. Controversies and reality of the FODMAP diet for patients with irritable bowel syndrome. J. Gastroent. Hepatol. 2019, 34, 1134–1142. [Google Scholar] [CrossRef] [Green Version]
- Gibson, P.R.; Newnham, E.; Barrett, J.S.; Shepherd, S.J.; Muir, J.G. Review article: Fructose malabsorption and the bigger picture. Aliment. Pharmacol. Ther. 2006, 25, 349–363. [Google Scholar] [CrossRef]
- Nyyssola, A.; Ellila, S.; Nordlund, E.; Poutanen, K. Reduction of FODMAP content by bioprocessing. Trends Food Sci. Technol. 2020, 99, 257–272. [Google Scholar] [CrossRef]
- Gerbault, P.; Liebert, A.; Itan, Y.; Powell, A.; Currat, M.; Burger, J.; Swallow, D.M.; Thomas, M.G. Evolution of lactase persistence: An example of human niche construction. Philos. Trans. R. Soc. Lond. Ser. B. Biol. Sci. 2011, 366, 863–877. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, J.U.; Steiner, D.; Longin, C.F.H.; Würschum, T.; Schweiggert, R.M.; Carle, R. Wheat and the irritable bowel syndrome—FODMAP levels of modern and ancient species and their retention during bread making. J. Funct. Foods 2016, 25, 257–266. [Google Scholar] [CrossRef]
- Sahin, A.W.; Rice, T.; Zannini, E.; Axel, C.; Coffey, A.; Lynch, K.M.; Arendt, E.K. Leuconostoc citreum TR116: In-situ production of mannitol in sourdough and its application to reduce sugar in burger buns. Int. J. Food Microbiol. 2019, 302, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Loponen, J.; Ganzle, M. Use of sourdough in low FODMAP baking. Foods 2018, 7, 96. [Google Scholar] [CrossRef] [Green Version]
- Gille, D.; Schmid, A.; Walther, B.; Vergères, G. Fermented Food and Non-Communicable Chronic Diseases: A Review. Nutrients 2018, 10, 448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brijs, K.; Bleukx, W.; Delcour, J.A. Proteolytic activities in dormant rye (Secale cereale L.) grain. J. Agric. Food Chem. 1999, 47, 3572–3578. [Google Scholar] [CrossRef]
- Loponen, J.; Strohm, T.S.; Venalainen, J.; Salovaara, H. Prolamin Hydrolysis in Wheat Sourdoughs with Differing Proteolytic Activities. J. Agric. Food Chem. 2007, 55, 978–984. [Google Scholar] [CrossRef]
- Capocchi, A.; Cinollo, M.; Galleschi, L.; Saviozzi, F.; Calucci, L.; Pinzino, C.; Zandomeneghi, M. Degradation of gluten by proteases from dry and germinating wheat (Triticum durum) seeds: An in vitro approach to storage protein mobilization. J. Agric. Food Chem. 2000, 48, 6271–6279. [Google Scholar] [CrossRef]
- Loponen, J.; Kanerva, P.; Zhang, C.; Sontag-Strohm, T.; Salovaara, H.; Ganzle, M. Prolamin hydrolysis and pentosane solubilization in germinated-rye sourdoughs determined by chromatography and immunological methods. J. Agric. Food Chem. 2009, 57, 746–753. [Google Scholar] [CrossRef]
- Schwalb, T.; Wieser, H.; Koehler, P. Studies on the gluten-specific peptidase activity of germinated grains from different cereal species and cultivars. Eur. Food Res. Technol. 2012, 235, 1161–1170. [Google Scholar] [CrossRef]
- Wiess, H. Chemistry of gluten proteins. Food Microbiol. 2007, 24, 115–119. [Google Scholar]
- Alvarez-Sieiro, P.; Montalban-Lopez, M.; Mu, D.; Kuipers, O.P. Bacteriocins of lactic acid bacteria: Extending the family. Appl. Microbiol. Biotechnol. 2016, 100, 2939–2951. [Google Scholar] [CrossRef] [Green Version]
- Bottari, A.; Capocchi, A.; Fontanini, D.; Galleschi, L. Major proteinase hydrolyzing gliadins during wheat germination. Phytochemistry 1996, 43, 39–44. [Google Scholar] [CrossRef]
- Di Cagno, R.; De Angelis, M.; Lavermicocca, P.; De Vincenzi, M.; Giovannini, C.; Faccia, M. Proteolysis by sourdough lactic acid bacteria: Effects on wheat flour protein fractions and gliadin peptides involved in human cereal intolerance. Appl. Microbiol. Biotechnol. Appl. Environ. Microbiol. 2002, 68, 623–633. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, G.; Koehler, P.; Wieser, H. Rapid degradation of gliadin peptides toxic for coeliac disease patients by proteases from germinating cereals. J. Cereal Sci. 2006, 44, 368–371. [Google Scholar] [CrossRef]
- Mandile, R.; Picascia, S.; Parrella, C.; Camarca, A.; Gobbetti, M.; Greco, L.; Troncone, R.; Gianfrani, C.; Auricchio, R. Lack of immunogenicity of hydrolysed wheat flour in patients with coeliac disease after a short-term oral challenge. Aliment. Pharmacol. Ther. 2017, 46, 440–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzello, C.G.; De Angelis, M.; Di Cagno, R.; Camarca, A.; Silano, M.; Losito, I. Highly efficient gluten degradation by lactobacilli and fungal proteases during food processing: New perspectives for celiac disease. Appl. Environ. Microbiol. 2007, 73, 4499–4507. [Google Scholar] [CrossRef] [Green Version]
- Reale, A.; Luigia Di Stasio, L.; Tiziana Di Renzo, T.; Salvatore De Caro, S.; Pasquale Ferranti, P.; Gianluca Picariello, G.; Francesco Addeo, F.; Mamone, G. Bacteria do it better! Proteomics suggests the molecular basis for improved digestibility of sourdough products. Food Chem. 2021, 359, 129955. [Google Scholar] [CrossRef]
- Ganzle, M.G.; Vermeulen, N.; Vogel, R.F. Carbohydrate peptide, and lipid metabolism of lactic acid bacteria in sourdough. Food Microbiol. 2007, 24, 12–138. [Google Scholar] [CrossRef] [PubMed]
- Grosch, W.; Wieser, H. Redox Reactions in Wheat Dough as Affected by Ascorbic Acid. J. Cereal Sci. 1999, 29, 1–16. [Google Scholar] [CrossRef]
- Vermeulen, N.; Kretzer, J.; Machalitza, H.; Vogel, R.F.; Ganzle, M.G. Influence of redox-reactions catalyzed by homo- and heterofermentative lactobacilli on gluten in wheat sourdoughs. J. Cereal Sci. 2006, 43, 137–143. [Google Scholar] [CrossRef]
- Jansch, A.; Korakli, M.; Vogel, R.F.; Ganzle, M.G. Glutathione reductase from Lactobacillus sanfranciscensis DSM20451T: Contribution to oxygen tolerance and thiol-exchange reactions in wheat sourdoughs. Appl. Environ. Microbiol. 2007, 73, 4469–4476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Angelis, M.; Cassone, A.; Rizzello, C.G.; Gagliardi, F.; Minervini, F.; Calasso, M.; Di Cagno, R.; Gobbetti, M. Mechanism of degradation of immunogenic gluten epitopes from Triticum turgidum L. var. durum by sourdough lactobacilli and fungal proteases. Appl. Environ. Microbiol. 2010, 76, 508–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perri, G.; Coda, R.; Rizzello, C.G.; Celano, G.; Ampollini, M.; Gobbetti, G.; De Angelis, M.; Calasso, M. Sourdough fermentation of whole and sprouted lentil flours: In situ formation of dextran and effects on the nutritional, texture and sensory characteristics of white bread. Food Chem. 2021, 355, 129638. [Google Scholar] [CrossRef]
- Curiel, J.A.; Coda, R.; Limitone, A.; Katina, K.; Raulio, M.; Giuliani, G.; Rizzello, C.G.; Gobbetti, M. Manufacture and characterization of pasta made with wheat flour rendered gluten-free using fungal proteases and selected sourdough lactic acid bacteria. J. Cereal Sci. 2014, 59, 79–87. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Tagliazucchi, D.; Babini, E.; Rutella, G.S.; Saa, D.L.T.; Gianotti, A. Bioactive peptides from vegetable food matrices: Research trends and novel biotechnologies for synthesis and recovery. J. Funct. Foods 2016, 27, 549–569. [Google Scholar] [CrossRef]
- Canesin, R.M.; Cazarin, C.C.B. Nutritional quality and nutrient bioaccessibility in sourdough bread. Curr. Opin. Food Sci. 2021, 40, 81–86. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, S. Bioactive components and functional properties of biologically activated cereal grains: A bibliographic review. Crit. Ver. Food Sci. Nutr. 2017, 57, 3051–3071. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Lorusso, A.; Russo, V.; Pinto, D.; Marzani, B.; Gobbetti, M. Improving the antioxidant properties of quinoa flour through fermentation with selected autochthonous lactic acid bacteria. Int. J. Food Microbiol. 2017, 241, 252–326. [Google Scholar] [CrossRef]
- Siragusa, S.; De Angelis, M.; Di Cagno, R.; Rizzello, C.G.; Coda, R.; Gobbetti, M. Synthesis of gamma-aminobutyric acid by lactic acid bacteria isolated from a variety of Italian cheeses. Appl. Environ. Microbiol. 2007, 73, 7283–7290. [Google Scholar] [CrossRef] [Green Version]
- Coda, R.; Rizzello, C.G.; Gobbetti, M. Use of sourdough fermentation and pseudo-cereals and leguminous flours for the making of a functional bread enriched of g-aminobutyric acid (GABA). Int. J. Food Microbiol. 2010, 137, 236–245. [Google Scholar] [CrossRef]
- Lorusso, A.; Coda, C.; Montemurro, M.; Rizzello, C.G. Use of Selected Lactic Acid Bacteria and Quinoa Flour for Manufacturing Novel Yogurt-Like Beverages. Foods 2018, 7, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koistinen, V.M.; Mattila, O.; Katina, K.; Poutanen, K.; Aura, A.M.; Hanhineva, K. Metabolic profiling of sourdough fermented wheat and rye bread. Sci. Rep. 2018, 8, 5684–5692. [Google Scholar] [CrossRef] [PubMed]
- Leroy, F.; De Vuyst, L. Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci. Technol. 2004, 15, 67–78. [Google Scholar] [CrossRef]
- Korhonen, H.; Pihlanto, A. Technological options for the production of health-promoting proteins and peptides derived from milk and colostrum. Curr. Pharm. Des. 2007, 13, 829–843. [Google Scholar] [CrossRef]
- Hu, Y.; Stromeck, A.; Loponen, J.; Lopes-Lutz, D.; Schieber, A.; Gänzle, M.G. LCMS/ MS quantification of bioactive antiotensin I-converting enzyme inhibitory peptides in rye malt sourdoughs. J. Agric. Food Chem. 2011, 59, 11983–11989. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Guha, S.; Majumder, K. Food-derived bioactive peptides in human health: Challenges and opportunities. Nutrients 2018, 10, 1738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cicero, A.F.G.; Fogacci, F.; Colletti, A. Potential role of bioactive peptides in prevention and treatment of chronic diseases: A narrative review. Br. J. Pharmacol. 2017, 174, 1378–1394. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Nionelli, L.; Coda, R.; Gobbetti, M. Synthesis of the cancer-preventive peptide lunasin by lactic acid bacteria during sourdough fermentation. Nutr. Cancer 2012, 64, 111–120. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Hernández-Ledesma, B.; Fernández-Tomé, S.; Curiel, J.A.; Pinto, D.; Marzani, B.; Coda, R.; Gobbetti, M. Italian legumes: Effect of sourdough fermentation on lunasin-like polypeptides. Microb. Cell Factories 2015, 14, 168–188. [Google Scholar] [CrossRef] [Green Version]
- Rizzello, C.G.; Lorusso, A.; Montemurro, M.; Gobbetti, M. Use of sourdough made with quinoa (Chenopodium quinoa) flour and autochthonous selected lactic acid bacteria for enhancing the nutritional, textural and sensory features of white bread. Food Microbiol. 2016, 56, 1–13. [Google Scholar] [CrossRef]
- Reale, A.; Zotta, T.; Ianniello, R.G.; Mamone, G.; Di Renzo, T. Selection criteria of lactic acid bacteria to be used as a starter for sweet and salty leavened baked products. LWT—J. Food Sci. Technol. 2020, 133, 110092. [Google Scholar] [CrossRef]
- Di Renzo, T.; Reale, A.; Boscaino, F.; Messia, M.C. Flavoring Production in Kamut, Quinoa and Wheat Doughs Fermented by Lactobacillus paracasei, Lactobacillus plantarum, and Lactobacillus brevis. Front. Microbiol. 2018, 9, 429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graça, C.; Mota, J.; Lima, A.; Ferreira, R.B.; Raymundo, A.; Sousa, I. Glycemic Response and Bioactive Properties of Gluten-Free Bread with Yoghurt or Curd-Cheese Addition. Foods 2020, 9, 1410. [Google Scholar] [CrossRef]
- Graça, C.; Raymundo, A.; Sousa, I. Yoghurt and curd cheese addition to wheat bread dough: Impact on in vitro starch digestibility and estimated glycemic index. Food Chem. 2021, 339, 127887. [Google Scholar] [CrossRef]
- Fardet, A.; Leenhardt, F.; Lioger, D.; Scalbert, A.; Rémésy, C. Parameters controlling the glycaemic response to bread. Nutr. Res. Rev. 2006, 19, 18–25. [Google Scholar] [CrossRef]
- Fois, S.; Piu, P.P.; Sanna, M.; Roggio, T.; Catzeddu, P. In Vivo and In Vitro Starch Digestibility of Fresh Pasta Produced Using Semolina-Based or Wholemeal Semolina-Based Liquid Sourdough. Foods 2021, 10, 2507. [Google Scholar] [CrossRef]
- Nionelli, L.; Montemurro, M.; Pontonio, E.; Verni, M.; Gobbetti, M.; Rizzello, C.G. Pro-technological and functional characterization of lactic acid bacteria to be used as starters for hemp (Cannabis sativa L.) sourdough fermentation and wheat bread fortification. Int. J. Food Microbiol. 2018, 279, 14–25. [Google Scholar] [CrossRef]
- Novotni, D.; Cukelj, N.; Smerdel, B.; Bituh, M.; Dujmic, F.; Curic, D. Glycemic index and firming kinetics of partially baked frozen gluten-free bread with sourdough. J. Cereal Sci. 2012, 55, 120–125. [Google Scholar] [CrossRef]
- Scazzina, F.; Del Rio, D.; Pellegrini, N.; Brighenti, F. Sourdough bread: Starch digestibility and postprandial glycemic response. J. Cereal Sci. 2009, 49, 419–421. [Google Scholar] [CrossRef]
- Messia, M.C.; Reale, A.; Maiuro, L.; Candigliota, T.; Sorrentino, E.; Marconi, E. Effects of pre-fermented wheat bran on dough and bread characteristics. J. Cereal Sci. 2016, 36, 138–144. [Google Scholar] [CrossRef]
- Shumoy, H.; Gabaza, M.; Vandevelde, J.; Raes, K. Soluble and bound phenolic contents and antioxidant capacity of tef injera as affected by traditional fermentation. J. Food Compos. Anal. 2017, 58, 52–59. [Google Scholar] [CrossRef]
- Bei, Q.; Chen, G.; Liu, Y.; Zhang, Y.; Wu, Z. Improving phenolic compositions and bioactivity of oats by enzymatic hydrolysis and microbial fermentation. J. Funct. Foods 2018, 47, 512–520. [Google Scholar] [CrossRef]
- Xiao, T.; Guo, Z.; Sun, B.; Zhao, Y. Identification of anthocyanins from four kinds of berries and their inhibition activity to alpha-glycosidase and protein tyrosine phosphatase 1B by HPLC-FT-ICR MS/MS. J. Agric. Food Chem. 2017, 65, 6211–6221. [Google Scholar] [CrossRef]
- Lal, M.K.; Singh, B.; Sharma, S.; Pal Singh, M.; Kumar, A. Glycemic index of starchy crops and factors affecting its digestibility: A review. Trends Food Sci. Technol. 2021, 111, 741–755. [Google Scholar] [CrossRef]
- Corsetti, A.; Settanni, L. Lactobacilli in sourdough fermentation. Food Res. Int. 2007, 40, 539–558. [Google Scholar] [CrossRef]
- Luti, S.; Galli, V.; Venturi, M.; Granchi, L.; Paoli, P.; Pazzagli, L. Bioactive Properties of Breads Made with Sourdough of Hull-Less Barley or Conventional and Pigmented Wheat Flours. Appl. Sci. 2021, 11, 3291. [Google Scholar] [CrossRef]
- Andersson, R.; Fransson, G.; Tietjen, M.; Aman, P. Content, and molecular weight distribution of dietary fibre components in whole-grain rye flour and bread. J. Agric. Food Chem. 2009, 57, 2004–2008. [Google Scholar] [CrossRef]
- Gelinas, P.; McKinnon, C.; Gagnon, F. Fructans, water-soluble fibre and fermentable sugars in bread and pasta made with ancient and modern wheat. Int. J. Food Sci. Technol. 2016, 51, 555–564. [Google Scholar] [CrossRef]
- Struyf, N.; Laurent, J.; Verspreet, J.; Verstrepen, K.J.; Courtin, C.M. Saccharomyces cerevisiae and Kluyveromyces marxianus cocultures allow reduction of fermentable oligo-, di-, and monosaccharides and polyols levels in whole wheat bread. J. Agric. Food Chem. 2017, 65, 8704–8713. [Google Scholar] [CrossRef] [Green Version]
- Granito, M.; Frias, J.; Doblado, R.; Guerra, M.; Champ, M.; Vidal-Valverde, C. Nutritional improvement of beans (Phaseolus vulgaris) by natural fermentation. Eur. Food Res. Technol. 2002, 214, 226–231. [Google Scholar] [CrossRef]
- Granito, M.; Alvarez, G. Lactic acid fermentation of black beans (Phaseolus vulgaris): Microbiological and chemical characterization. J. Sci. Food Agric. 2006, 86, 1164–1171. [Google Scholar] [CrossRef]
- Liu, D.M.; Li, L.; Yang, X.Q.; Liang, S.Z.; Wang, J.S. Survivability of Lactobacillus rhamnosus during the preparation of soy cheese. Food Technol. Biotechnol. 2006, 44, 417–422. [Google Scholar]
- Bau, T.R.; Garcia, S.; Ida, E.I. Changes in soymilk during fermentation with kefir culture: Oligosaccharide’s hydrolysis and isoflavone aglycone production. Int. J. Food Sci. Nutr. 2015, 66, 845–850. [Google Scholar] [CrossRef]
- Battistini, C.; Gullon, B.; Ichimura, E.S.; Gomes, A.M.P.; Ribeiro, E.P.; Kunigk, L. Development and characterization of an innovative synbiotic fermented beverage based on vegetable soybean. Braz. J. Microbiol. 2018, 49, 303–309. [Google Scholar] [CrossRef]
- Rizzello, C.G. Characterization of indigenous Pediococcus pentosaceus, Leuconostoc kimchii, Weissella cibaria and Weissella confusa for faba bean bioprocessing. Int. J. Food Microbiol. 2019, 302, 24–34. [Google Scholar] [CrossRef]
- De Vuyst, L.; Van Kerrebroeck, S.; Harth, H.; Huys, G.; Daniel, H.-M.; Weckx, S. Microbial ecology of sourdough fermentations: Diverse or uniform? Food Microbiol. 2014, 37, 11–29. [Google Scholar] [CrossRef] [PubMed]
- Loponen, J.; Mikola, M.; Sibakov, J. An enzyme exhibiting fructan hydrolase activity. U.S. Patent 10,716,308, 21 July 2020. [Google Scholar]
- Griffin, L.; Dean, L. Nutrient composition of raw, dry-roasted, and skin-on cashew nuts. J. Food Res. 2017, 6, 13–28. [Google Scholar] [CrossRef] [Green Version]
- Gu, J.; Liu, T.; Hou, J.; Pan, L.; Sadiq, F.A.; Yuan, L. Analysis of bacterial diversity and biogenic amines content during the fermentation processing of stinky tofu. Food Res. Int. 2018, 111, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Coda, R.; Shi, Q.; Katina, K.; Tenkanen, M. Exopolysaccharides production during the fermentation of soybean and fava bean flours by Leuconostoc mesenteroides DSM 20343. J. Agric. Food Chem. 2017, 65, 2805–2815. [Google Scholar] [CrossRef] [PubMed]
- Francesca, N.; Gaglio, R.; Alfonzo, A.; Corona, O.; Moschetti, G.; Settanni, L. Characteristics of sourdoughs and baked pizzas as affected by starter culture inoculums. Int. J. Food Microbiol. 2019, 293, 114–123. [Google Scholar] [CrossRef] [PubMed]
| Prolamins of the Cereals Grains | |||
|---|---|---|---|
| Wheat | Rye | Barley | |
| HMW prolamins | HMW glutenins | HMW secalins | D-hordeins |
| S-rich prolamins | LMW glutenins | - | B-hordeins |
| S-rich prolamins | α- and γ-gliadins | γ-secalins | γ-hordeins |
| S-poor prolamins | ꞷ-gliadins | ꞷ-secalins | C-hordeins |
| Gluten proteins | Secalins | Hordeins | |
| Product/Subtract | Method Applied | FODMAP Reduction | Reference |
|---|---|---|---|
| Whole wheat bread | Fermentation of 4.5 h, 30 °C using bakery´s yeast (Saccharomyces cerevisiae) | 90% of fructans and raffinose | Ziegler et al. [50] |
| Whole rye bread | Sourdough fermentation rye bread (not specified) Traditional bakery´s yeast rye bread | 62% in fructans 32% in fructans | Andersson et al. [104] |
| Wheat bread | Bakey´s yeast fermentation of 180 min, 35 °C | 40% in fructans | Gélinas et al. [105] |
| Whole wheat bread | Bakery´s yeats and K. marxianus fermentation of 180 min, 30 °C | 95% in fructans | Struyf et al. [106] |
| Seed Beans flour (Phaseolus vulgaris) | Natural fermentation | 100% Raffinose | Granito et al. [107] |
| Black Beans flour (Phaseolus vulgaris) | Fermentation by Lactobacillus casei and Lactobacillus plantarum | 88.6% raffinose | Granito and Álvarez [108] |
| Soy milk (Glycine max) | Fermentation by Lactobacillus rhamnosus 6013 | 100% raffinose | Liu et al. [109] |
| Soy milk (Glycine max) | Fermentation by Kefir starter culture (Clerici Sacco) | 100% raffinose | Bau et al. [110] |
| Soy milk (Glycine max) | Fermentation by Lactobacillus acidophilus, Bifidobacterium animalis and Streptococcus thermophilus | 40% raffinose | Battistine et al. [111] |
| Faba bean flour (Vicia faba) | Fermentation by Weissella cibaria, Weissella confusa, Pediococcus pentosaceus Leuconostoc kimchi | 100% raffinose, 84% verbascose | Rizzello [112] |
| Chickpea flour (Cicer arietinum), Sprouted Lentil flour (Lens culinaris) | Fermentation by Lactobacillus rossiae, Lactobacillus plantarum and Lactobacillus sanfrancensis | 95% raffinose | Montemurro et al. [65] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graça, C.; Lima, A.; Raymundo, A.; Sousa, I. Sourdough Fermentation as a Tool to Improve the Nutritional and Health-Promoting Properties of Its Derived-Products. Fermentation 2021, 7, 246. https://doi.org/10.3390/fermentation7040246
Graça C, Lima A, Raymundo A, Sousa I. Sourdough Fermentation as a Tool to Improve the Nutritional and Health-Promoting Properties of Its Derived-Products. Fermentation. 2021; 7(4):246. https://doi.org/10.3390/fermentation7040246
Chicago/Turabian StyleGraça, Carla, Ana Lima, Anabela Raymundo, and Isabel Sousa. 2021. "Sourdough Fermentation as a Tool to Improve the Nutritional and Health-Promoting Properties of Its Derived-Products" Fermentation 7, no. 4: 246. https://doi.org/10.3390/fermentation7040246
APA StyleGraça, C., Lima, A., Raymundo, A., & Sousa, I. (2021). Sourdough Fermentation as a Tool to Improve the Nutritional and Health-Promoting Properties of Its Derived-Products. Fermentation, 7(4), 246. https://doi.org/10.3390/fermentation7040246








