Abstract
The study reports the alternative use of non-edible fermented olives for the production of high-quality natural soaps with a fast production process, low environmental impact, and without preliminary treatments for the raw material. Damaged olives, not used as food, were fermented naturally and the oil was extracted by mechanical extraction. The product obtained was not for human consumption due to its high acidity, but it had a low content of peroxides. The non-edible olive oil obtained and an extra virgin olive oil, produced from the same olive cultivar, were subjected to saponification with sodium hydroxide. The soaps were produced with complete (0% of non-neutralized fatty acids) and incomplete (5% of non-neutralized fatty acids) saponification; the amount of sodium hydroxide to be used was determined with the saponification index. The soaps were aged for six months by monitoring pH, color, and behavior in an aqueous solution. The results show that the olives’ fermentation improves and speeds up the soap production and maturation process since the oil obtained from fermented non-edible olives is more suitable for the saponification process than the oil obtained from non-fermented edible olives. Non-edible fermented olives can be used for obtaining natural and high-quality soaps, reusing drupes classified as food waste.
1. Introduction
In ancient times, soap was produced with rancid oils and animal fat waste, involuntarily creating a circular economy with the requalification of products no longer used for human nutrition [1].
In recent times, the production of soaps, defined by law as cosmetic products, is regulated by the European Union [2]. The regulation determines and limits the substances to be used for the saponification processes (Regulation (EC) No. 1223/2009 article 14–17 [2]) and indirectly determines the chemical, physical, and mechanical treatments to obtain adequate raw materials for the cosmetic sector. Exhausted oils and fats, dangerous for human nutrition, are necessarily treated to eliminate all toxic and dangerous components such as heavy metals, microplastics, peroxides, alkyl esters, and suspended particles [3,4].
Companies that produce soaps buy large quantities of regenerated oils and fats, adding chemical compounds such as surfactants, parabens, silicones, and sulphates. These companies contribute to the recycling of regenerated oils and fats but add pollutants to improve certain products’ characteristics [5,6].
Often, artisanal companies use edible oils, such as olive oil, for obtaining natural and high-quality products. These soaps are obtained from food and not from food waste [7,8,9].
Scientific studies report the recycling of olive production waste, such as vegetation water, pomace, and plant parts of the tree, but there are relatively few studies in the literature on the recycling of damaged and overripe non-edible olives [10,11,12,13].
The authors were focused on the use of non-edible drupes (food waste) for the production of high-quality soaps through natural fermentation processes.
Non-edible olives are drupes with defects that develop negative and irreversible chemical, physical, and organoleptic alterations. Producers of fine oils eliminate these olives to obtain an excellent quality of the final product oils [14]. Furthermore, these drupes cannot be marketed because they do not have the minimum requirements defined by the European Community [15].
Non-edible olives are therefore used as fodder, natural fertilizer, and for the production of energy biomass [11,13].
The aim of this study was to produce and evaluate the different ripening stages of soaps produced with non-edible fermented olive oil (NEFOO soap) considering three parameters: pH, color, and solubility. The results obtained were compared with those obtained from soaps produced with extra virgin olive oil (EVOO soap).
2. Materials and Methods
2.1. Olives
The olives were harvested in the third week of October 2020, in Messina (Sicily, Italy). The soils had homogeneous composition and climatic conditions, and the olive cultivars were native of Sicily (Nocellara Etnea, Nocellara Messinese, and Cerasuola) [16,17]. The olives were harvested with mechanical systems that do not damage the plant or the fruit.
The olives were selected with machines and manually to remove overripe fruit with defects, the vegetative waste, and impurities.
Non-edible olives were collected in permeable bags and stored in a dark room with a temperature of 20 °C and constant humidity until the complete harvest of the olives.
2.2. Olives’ Fermentation
Non-edible olives, 24 kg, were cleaned with cold water and inserted into a plastic vessel with a non-pre-acidified brine and 5% m/m NaCl, in a final working volume of 75%. No sterilization procedures were adopted on the non-edible olives before starting the natural fermentation, and no starter strains of yeast or bacteria were inoculated. The olive-brine mixture was aerated manually every 3 days to facilitate the growth of aerobic microorganisms. The fermentation was carried out for 60 days and the pH was monitored during all the process. Neither sodium chloride nor acids or bases were added to the brine after fermentation starting [18,19,20,21].
Brine samples were withdrawn for analysis at regular time intervals. The samples (1 mL) were aseptically transferred to 9 mL sterile 1/4 Ringer’s solution. Decimal dilutions in the same Ringer’s solution were prepared and used for the enumeration of lactic acid bacteria, yeasts and molds, and for enterobacteria.
The lactic acid bacteria amount was estimated on de Man-Rogosa-Sharpe agar (MRS Agar, Oxoid, Basingstoke, UK) added with 50 mg/L of Nystatin (Sigma, Saint-Quentin Fallavier, France) at 25 °C for 72 h.
The population of yeasts and molds was estimated on Yeast Glucose Chloramphenicol Agar (YGC, bioMérieux, Lyon, France) at 25 °C for 48 h.
Violet Red Bile Glucose agar (VRBGA; Oxoid, Basingstoke, UK) was used for enterobacteria counts, incubated at 37 °C for 24 h.
The analyses were carried out in triplicate and the plates were subjected to microbiological numbering by CFU counting.
2.3. pH Determination
The pH of the brine solutions and the pH of the soap, after the maturation process, were determined with a digital benchtop pH meter (HANNA Instruments, Edge PH-HI2020, Smithfield, Rhode Island, USA) with glass immersion electrode.
The pH of the brine was determined by taking 50 mL of solution after mixing the fermentation mass.
The pH of the soaps was determined by solubilizing 1 g of soap, the inner part, in 50 mL of deionized water [7,8].
2.4. Oil Extraction
Non-edible fermented olive oil (NEFOO) was obtained with a cold mechanical extraction system. The extra virgin olive oil (EVOO) was kindly offered by the company that harvested the olives. It was obtained from the olives harvested but not discarded and stored in a dark room with a temperature of 20 °C.
2.5. Acidity Value of Oils
The acidity value (AV) of the olive oils was determined with the titration method reported in the Execution Regulation (EU) 2019/1604 and in the EN ISO 660: 2020 regulation.
The oil (1 g) was weighed into a flask and 50 mL of ethyl ether/ethanol mixture (1:1 ratio) and two drops of phenolphthalein ethanolic solution were added. The mixture was titrated with a KOH solution until the indicator changed color. The acidity was expressed as the weight percentage of free oleic acid present in 1 g of analyzed sample. The analyses were carried out in triplicate, and the average of the values are reported in Table 1 [22,23].

Table 1.
Saponification, acidity, and peroxide values of EVO and NEFO oils.
2.6. Peroxide Value of Oils
The peroxide value (PV) in olive oils was determined with iodometric titration, described in the Implementing Regulation (EU) 2019/1604 and in the ISO 3960: 2017 regulation. The oil (1 g) was weighed into a flask and 25 mL of a mixture of acetic acid and chloroform (ratio 3:2) with 0.5 mL of a saturated aqueous solution of KI were added. The solution was diluted with 25 mL of distilled water and stirred. The iodine formed into the solution was titrated with a sodium thiosulfate solution, and the starch was used as indicator [22,24].
The titrations were carried out in triplicate, and the average of the values, expressed in milliequivalents of oxygen per kg of sample, are reported in Table 1.
2.7. Saponification Value of Oils
The saponification value (SV) represents the quantity of sodium hydroxide (as an alternative to potassium hydroxide), expressed in milligrams, necessary to neutralize all the fatty acids present in one g of fat sample. The analytical determination of SV was carried out according with Wakita et al. [25].
The oil sample (2 g) was weighed into a 250 mL flask and 25 mL of NaOH 0.50 N ethanolic solution were added. The mixture was heated up to 50 °C, under constant magnetic stirring (300 rpm) for 90 min. Subsequently, the mixture was cooled to 30 °C and 0.5 mL of phenolphthalein solution was added.
The solution was titrated with HCl 0.50 N until the color of the indicator changed, the titration was also carried out without the sample, only with solution. The analyses were carried out in triplicate, obtaining an average of the volumes of HCl consumed; the values are reported in Table 1.
The saponification values were expressed in milligrams of sodium hydroxide needed to saponify 1 g of oil.
2.8. Oils’ Saponification
The fatty acids of the EVO and NEFO oils were totally (0% of non-neutralized fatty acids) and partially (5% of non-neutralized fatty acids) neutralized and four different types of soaps were obtained:
- EVOO soap 0%, obtained from EVOO with 0% of non-neutralized fatty acids
- EVOO soap 5%, obtained from EVOO with 5% of non-neutralized fatty acids
- NEFOO soap 0%, obtained from NEFOO with 0% of non-neutralized fatty acids
- NEFOO soap 5%, obtained from NEFOO with 5% of non-neutralized fatty acids.
The quantity of NaOH used for the production of the described soaps was determined by SV. The quantities of NaOH used and the SV values are shown in Table 1.
A classic saponification procedure was applied for soap produced with EVO oil, while for the saponification of NEFO oil it was necessary to make changes with respect to the standard procedures reported by Spitz [7,8].
In 30 mL of deionized water 13.541 g and 13.334 g of NaOH were dissolved to obtain, respectively, the soaps with 0% and 5% of non-neutralized fatty acids (Table 1). Each alkaline solution was mixed with 100 g of oil at a temperature of 35 °C. The oil-water mixture was shaken strongly to obtain a semi-liquid consistency. The dough was placed in a mold for the subsequent maturation process [7,8].
The saponification of NEFO oil was carried out by varying the “cold” method. In 30 mL of deionized water 12.864 g and 12.667 g of NaOH were dissolved to obtain, respectively, the soaps with 0% and 5% of non-neutralized fatty acids (Table 1).
Each NaOH solution was added with slow dripping to 100 g of oil in a thermostated system at 20 °C with continuous stirring. A warm, semi-solid soap paste formed quickly due to the exothermic acid-base reaction between NaOH and fatty acids. The soap paste was placed in a mold for the subsequent maturation process.
The saponification for EVO oil and NEFO oil was carried out considering the complete neutralization of the fatty acids and the non-neutralization of 5% of the fatty acids using the SV of the oils. The quantity of NaOH used for the production of the different soaps and the SV are shown in Table 1. The soaps were stored in a dark room with low humidity on filter paper to prevent rancidity.
The soaps considered (0% and 5% of non-neutralized fatty acids) are those most produced by industries and most consumed [7,8].
2.9. Colorimetric Determination
A colorimetric sensor system (LISUN GROUP, CD-200, Kowloon, Hong Kong) was used to determine the color variations of the soap samples at different maturation times. The instrument consists of a D65 light source, a blue LEDs light source and a photoelectric diode detector, and an electronic system for determining the electrical impulses.
The color determination was carried out with the RGB model (red, green, and blue) and the CIELAB color space, which considers the parameters l*, a*, and b* that represent the brightness and the four unique colors of human vision (red, yellow, green, and blue) [26,27].
2.10. Solubility Determination
The solubility of the soaps was determined by solubilizing 1 g of soap, not in contact with air, in 50 mL of deionized water. Each solution, after the complete dissolution of the soap, was stirred constantly for 5 min and, subsequently, its transparency was observed.
3. Results
3.1. Olives’ Fermentation
Initial brine pH was 8.21, after 5 days it showed a pH of 7.22, representing an optimal condition for the proliferation of initial lactic bacteria but also enterobacteria.
In fact, enterobacteria counts showed a rapid increase within the first 10 days, followed by a sharp decline thereafter. No viable counts were enumerated after 25 days of fermentation, according with Sanchez et al. [28] (Figure 1).
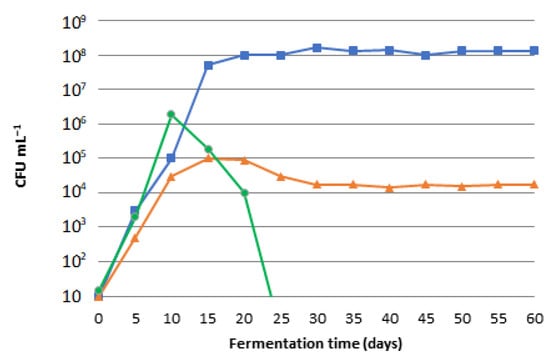
Figure 1.
Lactic bacteria (square), yeast and molds (triangle), enterobacteria (circle) concentration, reported as CFU per mL.
The pH decreasing was due to lactic bacteria, which ferment a wide variety of water-soluble carbohydrates and produce lactic acid [20,29,30]. In this phase the olives lost their classic hardness: this indicates the presence of yeasts and molds that degrade pectin and cellulosic substances, important for the compactness of the olives [31,32].
Lactic acid bacteria (LAB) and yeasts increased steadily and became the dominant members of the microflora during fermentation [33]. Lactic acid bacteria reached their maxima within the first 30 days (1.6 × 108 CFU mL−1), and their enumeration was stable until the end of the fermentation process. Yeasts coexisted with lactic acid bacteria throughout the whole fermentation period. Their counts were lower than those of the LAB by approximately 3–4.5 log cycles. They grew in similar populations with lactic acid bacteria during the first week, and their presence was stable until the end of fermentation reaching 1.7 × 104 CFU mL−1 (Figure 1).
Subsequently, the pH of the brine after 25 days was 5.87. In this phase, no viable counts of enterobacteria were enumerated.
At the end of the fermentation process, after 60 days, the olives were very soft and the epicarp was easily detached from the pulp, and the pH was 4.52.
The pH, throughout the fermentation phase, decreased slowly compared to the common fermentation processes of table olives (Spanish and Greek method) [19,34]; moreover, anomalous phenomena such as the loss of hardness of the olives were observed [21,35,36].
3.2. Oil Analysis
The comparison of the values of acidity and peroxides of the NEFO and EVO oils are shown in Table 1. NEFO oil showed a low number of peroxides (produced by oxidative rancidity processes) but a high amount of free fatty acids (produced by hydrolytic rancidity processes) [37].
The comparison of the results obtained is interesting; the acidity of NEFO oil is 255 times higher than the EVO oil, while the quantity of peroxides of the first oil is only 4.7 times higher than the second.
The high acidity of NEFO oil is caused by the lipases present in the pulp of the drupes but also by lipolytic enzymes produced by bacteria, yeasts, and molds developed in natural fermentation [38,39]
The oxidative rancidity, which forms peroxides, was probably limited by the brine because it blocked the direct contact of the drupes with oxygen. Furthermore, some species of lactobacilli degrade hydrogen peroxides and prevent the formation of peroxide radicals and hydroperoxide radicals, thus limiting the formation of peroxides and stabilizing the drupes in brine [38,40,41].
Natural fermentation with aerobic conditions determines the high AV and low PV of non-edible olive oil, these results are in agreement with the results of Girgis A. Y. and Alajtal A. I. et al. [40,41].
3.3. Soap Analysis
Saponification with EVOO developed normally, as described in Spitz L. [8]; the soap paste, initially semi-liquid, slowly solidified.
NEFOO soaps have different characteristics from EVOO soaps. The sodium salts of fatty acids are formed very quickly in NEFO oil due to the high acidity, so the soap paste hardens very quickly. The saponification process was developed at low temperature and with slow addition of the basic solution to obtain a homogeneous product.
The maturation times of the soaps were determined considering three parameters: pH, color, and solubility.
Initially, the pH of NEFOO soaps was lower than EVOO soaps (Table 2), probably due to the high amount of free fatty acids that neutralize part of the sodium hydroxide very quickly [41].

Table 2.
pH of the soap solutions produced for different maturation times.
The pH of NEFOO soaps was constant from the thirtieth day of ripening, while the pH of EVOO soaps was constant after the ninetieth day of ripening. The pH values shown in Table 2 are the average of the results obtained in triplicate.
EVO oil (Figure 2A) showed a more intense color than NEFO oil (Figure 2B) due to the greater quantity of chlorophylls, carotenoids, polyphenols, and flavonoids, which determine the initial color of the soaps [16,17,42].

Figure 2.
Comparison of EVO oil (A) and NEFO oil (B) used for the production of soaps.
Soaps whiten in the ripening phase due to oxidative processes that act on the pigments. The color of the soaps depends on the type of oil initially used and the maturation time, generally, the soaps marketed have a natural color between soft yellow and white.
The colors of the soaps produced are shown in Table 3 with the models RGB (model that uses red, green, and blue as base colors) and CIELAB (color space l* a* b*).

Table 3.
Report colors of soaps with RGB and CIELAB models (l* brightness and a* b* mixture of red, yellow, green, and blue).
NEFOO soaps after 30 days of maturation were white and this color was maintained up to 180 days of maturation, while EVOO soaps tended to soft yellow after 90 days of maturation and to white at 180 days (Table 3).
Considering the CIELAB parameters, EVOO soap with 0% free fatty acids had a more intense yellow-green color than EVOO soap with 5% free fatty acids, this difference was not observable for NEFOO soaps.
Soaps’ solubility was determined considering the degree of clarity of the solutions with complete dissolution of the soap.
EVOO soaps, after 5 days of maturation, showed different turbidity (Figure 3A). After 15 and 30 days of maturation (Figure 3B,C) the turbidity rapidly decreased for soap with 0% of non-neutralized fatty acids, while it slowly decreased for soap with 5% of non-neutralized fatty acids.
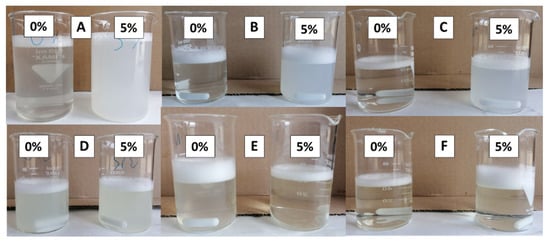
Figure 3.
Images of the solutions of EVOO (A–C) and NEFOO (D–F) soaps in different ripening days 5 days (A,D) 15 days (B,E) and 30 days (C,F).
NEFOO soaps, after 5 days of maturation, had very similar turbidity (Figure 3D). After 15 and 30 days of maturation (Figure 3E,F) the turbidity of both soaps decreased until clear solutions were obtained.
After 90 and 180 days, all soaps showed a slight turbidity: this was more present in EVOO soaps (Figure 4A,B) than in NEFOO soaps (Figure 4C,D). This low turbidity for very long maturation times could be due to the initial rancidity of the soaps.
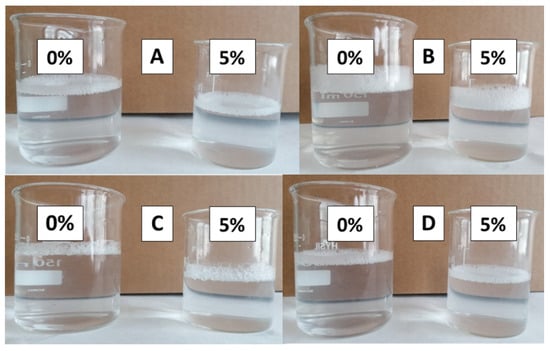
Figure 4.
Images of the solutions of EVOO (A,B) and NEFOO (C,D) soaps after different ripening days: 90 days (A,C) and 180 days (B,D).
It is interesting to observe the hardness of the soaps: NEFOO soaps tended to be harder and more compact than EVOO soaps, especially in the early stages of maturation, with a consequent difficulty in their solubilization in water.
Considering the pH, color, and solubility, NEFOO soaps showed a correct maturation after 30 days from their production, while EVOO soaps had an optimal maturation after 90–180 days. Soaps with maturation after 30 and 180 days are shown in Figure 5.

Figure 5.
Soaps matured 30 days and 180 days.
There were no substantial differences, for the parameters considered in this study, between soaps with 0% and 5% of free fatty acids produced with the same oil.
The stage of maturation is not strictly linked to the use of the product but represents an important parameter for its marketing.
4. Conclusions
This study highlighted that non-edible fermented olives can be used for soaps’ production.
The high acidity of NEFOO determines different and shorter saponification processes than the normal procedures reported by Spitz L. [7,8].
Considering the pH, color, and solubility data, NEFOO soaps ripen faster than EVOO soaps, while there are no substantial differences between soaps with 0% and 5% free fatty acids obtained with the same oil.
The fermentation of non-edible olives makes it possible to easily and quickly recycle an undervalued food waste to produce a widely used product, such as olive oil soaps, with lower costs, production times, and maturation.
The study described could be used to produce “alternative” olive oils on a large scale, exploiting non-edible drupes currently used to produce fodder, natural fertilizer, and energy biomass.
Author Contributions
Conceptualization, A.T., A.F. and F.S.; Methodology, A.T., A.F. and F.S.; Formal Analysis, A.T., A.F. and F.S.; Investigation, A.T., A.F. and F.S.; Data Curation, A.T., A.F. and F.S.; Writing-Review and Editing, A.T., A.F. and F.S.; Supervision, A.T., A.F. and F.S.; Project Administration, A.F., A.T. and F.S. All authors provided critical feedback and helped shape the research, analysis, and manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Konkol, K.L.; Rasmussen, S.C. An Ancient Cleanser: Soap Production and Use in Antiquity. ACS J. 2015, 9, 245–266. [Google Scholar] [CrossRef]
- European Commission. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products; European Commission: Brussels, Belgium, 2009. [Google Scholar]
- Mannu, A.; Garroni, S.; Porras, J.I.; Mele, A. Available Technologies and Materials for Waste Cooking Oil Recycling. Processes 2020, 8, 366. [Google Scholar] [CrossRef] [Green Version]
- Kajdas, C. Major pathways for used oil disposal and recycling. Part 1. Lubr. Sci. 2006, 7, 61–74. [Google Scholar] [CrossRef]
- Abdel-Gawad, S.; Abdel-Shafy, M. Pollution control of industrial wastewater from soap and oil industries: A case study. Water Sci. Technol. 2002, 46, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Bettley, F.R. Some Effects of Soap on the Skin. Br. Med. J. 1960, 1, 1675–1679. [Google Scholar] [CrossRef]
- Spitz, L. Sodeopec: Soaps, Detergents, Oleochemicals, and Personal Care Products, 3rd ed.; American Oil Chemists’ Society: Champaign, IL, USA, 2004. [Google Scholar] [CrossRef]
- Spitz, L. Soap Manufacturing Technology, 2nd ed.; Academic Press: Cambridge, MA, USA; AOCS Press: Urbana, IL, USA, 2016. [Google Scholar]
- Félix, S.; Araújo, J.; Piresa, A.M.; Sousa, C. Soap production: A green prospective. Waste Manag. 2017, 66, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Azbar, N.; Bayram, A.; Filibeli, A.; Muezzinoglu, A.; Sengul, F.; Ozer, A. A Review of Waste Management Options in Olive Oil Production. Crit. Rev. Environ. Sci. Technol. 2004, 34, 209–247. [Google Scholar] [CrossRef]
- Charis, M.; Galanakis, C.M. Olive Mill Waste: Recent Advances for Sustainable Management, 1st ed.; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- García Martín, J.F.; Cuevas, M.; Feng, C.H.; Mateos, P.Á.; García, M.T.; Sánchez, S. Energetic valorisation of olive biomass: Olive-tree pruning, olive stones and pomaces. Processes 2020, 8, 511. [Google Scholar] [CrossRef]
- García Martín, J.F.; Cuevas, M.; Bravo, V.; Sánchez, S. Ethanol production from olive prunings by autohydrolysis and fermentation with Candida tropicalis. Renew. Energy 2010, 35, 1602–1608. [Google Scholar] [CrossRef]
- Cabrera, E.R.; Arriaza, M.; Rodríguez-Entrena, M. Is the extra virgin olive oil market facing a process of differentiation? A hedonic approach to disentangle the effect of quality attributes. Grasas Aceites 2015, 66, 4. [Google Scholar] [CrossRef] [Green Version]
- European Comission. Commission Regulation: Commission Implementing Regulation (EU) 2017/2470 of 20 December 2017 Establishing the Union List of Novel Foods in Accordance with Regulation (EU) 2015/2283 of the European Parliament and of the Council on Novel Foods; European Commission: Brussels, Belgium, 2017. [Google Scholar]
- Rozanska, A.; Russo, M.; Cacciola, F.; Salafia, F.; Polkowska, Z.; Dugo, P.; Mondello, L. Concentration of Potentially Bioactive Compounds in Italian Extra Virgin Olive Oils from Various Sources by Using LC-MS and Multivariate Data Analysis. Foods 2020, 9, 1120. [Google Scholar] [CrossRef] [PubMed]
- Dugo, L.; Russo, M.; Cacciola, F.; Mandolfino, F.; Salafia, F.; Vilmercati1, A.; Fanali, C.; Casale, M.; De Gara, L.; Dugo, P.; et al. Determination of the Phenol and Tocopherol Content in Italian High-Quality Extra-Virgin Olive Oils by Using LC-MS and Multivariate Data Analysis. Food Anal. Methods 2020, 13, 1027–1041. [Google Scholar] [CrossRef]
- Poiana, M.; Romeo, F.V. Changes in chemical and microbiological parameters of some varieties of Sicily olives during natural fermentation. Grasas Aceites 2006, 57, 402–408. [Google Scholar] [CrossRef]
- Panagou, E.Z.; Katsaboxakis, C.Z. Effect of different brining treatments on the fermentation of cv. Conservolea green olives processed by the Spanish-method. Food Microbiol. 2006, 23, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Lanza, B. Abnormal fermentations in table-olive processing: Microbial origin and sensory evaluation. Front. Biol. 2013, 4, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiain, H.; Hafidi, A. Chemical composition changes in four green olive cultivars during spontaneous fermentation. LWT-Food Sci. Technol. 2014, 57, 663–670. [Google Scholar] [CrossRef]
- Commission Regulation. Commission Implementing Regulation (EU) 2019/1604 of 27 September 2019 Amending Regulation (EEC) No 2568/91 on the Characteristics of Olive Oil and Olive-Residue Oil and on the Relevant Methods of Analysis; European Commission: Brussels, Belgium, 2019. [Google Scholar]
- International Organization for Standardization. ISO 660:2020-Animal and Vegetable Fats and Oils-Determination of Acid Value and Acidity; ISO: Geneva, Switzerland, 2020. [Google Scholar]
- International Organization for Standardization. ISO 3960:2017-Animal and Vegetable Fats and Oils-Determination of Peroxide Value-Iodometric (Visual) Endpoint Determination; ISO: Geneva, Switzerland, 2017. [Google Scholar]
- Wakita, K.; Kuwabara, H.; Furusho, N.; Tatebe, C.; Sato, K.; Akiyama, H. A Comparative Study of the Hydroxyl and Saponification Values of Polysorbate 60 in International Food Additive Specifications. Am. J. Anal. Chem. 2014, 5, 199–204. [Google Scholar] [CrossRef] [Green Version]
- Conesa, A.; Manera, F.C.; Brotons, J.M.; Fernandez-Zapata, J.C.; Simón, I.; Simón-Grao, S.; Alfosea-Simón, M.; Martínez Nicolás, J.J.; Valverde, J.M.; García-Sanchez, F. Changes in the content of chlorophylls and carotenoids in the rind of Fino 49 lemons during maturation and their relationship with parameters from the CIELAB color space. Sci. Hortic. 2019, 243, 252–260. [Google Scholar] [CrossRef]
- Gatti, E.; Bordegoni, M.; Spence, C. Investigating the influence of colour, weight, and fragrance intensity on the perception of liquid bath soap: An experimental study. Food Qual. Prefer. 2014, 31, 56–64. [Google Scholar] [CrossRef]
- Sanchez, A.H.; de Castro, A.; Rejano, L.; Montano, A. Comparative study on chemical changes in olive juice and brine during green olive fermentation. J. Agric. Food Chem. 2000, 48, 5975–5980. [Google Scholar] [CrossRef]
- Tofalo, R.; Schiron, M.; Perpetuini, G.; Angelozzi, G.; Suzzi, G.; Corsetti, A. Microbiological and chemical profiles of naturally fermented table olives and brines from different Italian cultivars. Antonie Van Leeuwenhoek 2012, 102, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Perpetuini, G.; Prete, R.; Garcia-Gonzalez, N.; Khairul Alam, M.; Corsetti, A. Table Olives More than a Fermented Food. Foods 2020, 9, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arroyo-López, F.N.; Querol, A.; Bautista-Gallego, J.; Garrido-Fernándezc, A. Role of yeasts in table olive production. Int. J. Food Microbiol. 2008, 128, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Giannoutsou, E.P.; Meintanis, C.; Karagouni, A.D. Identification of yeast strains isolated from a two-phase decanter system olive oil waste and investigation of their ability for its fermentation. Bioresour. Technol. 2004, 93, 301–306. [Google Scholar] [CrossRef]
- Hurtado, A.; Reguant, C.; Bordons, A.; Rozès, N. Lactic acid bacteria from fermented table olives. Food Microbiol. 2012, 31, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Alexandraki, V.; Georgalaki, M.; Papadimitriou, K.; Anastasiou, R.; Zoumpopoulou, G.; Chatzipavlidis, I.; Papadelli, M.; Vallis, N.; Moschochoritis, K.; Tsakalido, E. Determination of triterpenic acids in natural and alkaline-treated Greek table olives throughout the fermentation process. LWT-Food Sci. Technol. 2014, 58, 609–613. [Google Scholar] [CrossRef]
- Sherahi, M.H.A.; Shahidi, F.; Yazdi, F.T.; Hashemi, S.M.B. Effect of Lactobacillus plantarum on olive and olive oil quality during fermentation process. LWT-Food Sci. Technol. 2018, 89, 572–580. [Google Scholar] [CrossRef]
- Borràs, E.; Mestres, M.; Aceña, L.; Busto, O.; Ferré, J.; Boqué, R.; Calvo, A. Identification of olive oil sensory defects by multivariate analysis of mid infrared spectra. Food Chem. 2015, 187, 197–203. [Google Scholar] [CrossRef]
- Harzalli, U.; Rodrigues, N.; Veloso, A.C.A.; Dias, L.G.; Pereira, A.J.; Oueslati, S.; Peres, A.M. A taste sensor device for unmasking admixing of rancid or winey-vinegary olive oil to extra virgin olive oil. Comput. Electron. Agric. 2018, 144, 222–231. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.K.; Mukhopadhyay, M. Overview of Fungal Lipase: A Review. Biotechnol. Appl. Biochem. 2012, 166, 486–520. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, S.C.; Shoemakera, C.F. Volatile constituents in sensory defective virgin olive oils. Flavour Fragr. J. 2016, 31, 22–30. [Google Scholar] [CrossRef]
- Alajtal, A.I.; Sherami, F.E.; Elbagermi, M.A. Acid, Peroxide, Ester and Saponification Values for Some Vegetable Oils Before and After Frying. AASCIT J. Mater. 2018, 4, 43–47. [Google Scholar] [CrossRef]
- Girgis, A.Y. Production of high quality castile soap from high rancid olive oil. Grasas Aceites 2003, 54, 226–233. [Google Scholar] [CrossRef]
- Kachouri, F.; Hamdi, M. Enhancement of polyphenols in olive oil by contact with fermented olive mill wastewater by Lactobacillus plantarum. Process Biochem. 2004, 39, 841–845. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).