Abstract
The core objective of this work was to take advantage of the unexploited wheat straw biomass, currently considered as a broadly available waste stream from the Greek agricultural sector, towards the integrated valorization of sugar streams for the microbial production of polyunsaturated omega-3 fatty acids (PUFAs). The OxiOrganosolv pretreatment process was applied using acetone and ethanol as organic solvents without any additional catalyst. The results proved that both cellulose-rich solid pulp and hemicellulosic oligosaccharides-rich aqueous liquid fraction after pretreatment can be efficiently hydrolyzed enzymatically, thus resulting in high yields of fermentable monosaccharides. The latter were supplied as carbon sources to the heterotrophic microalga Crypthecodinium cohnii for the production of PUFAs, more specifically docosahexaenoic acid (DHA). The solid fractions consisted mainly of hexose sugars and led to higher DHA productivity than their pentose-rich liquid counterparts, which can be attributed to the different carbon source and C/N ratio in the two streams. The best performance was obtained with the solid pulp pretreated with ethanol at 160 °C for 120 min and an O2 pressure of 16 bar. The total fatty acids content reached 70.3 wt% of dried cell biomass, of which 32.2% was DHA. The total DHA produced was 7.1 mg per g of untreated wheat straw biomass.
1. Introduction
Lignocellulosic biomass has become the subject of a great deal of attention from researchers in order to mitigate the diminution of fossil reserves stemming from growing energy requirements worldwide. Biomass is considered one of the most low-cost and largest sources of carbon, rendering it the most important raw material with great potential to support the development of a bio-based economy. This renewable feedstock can contribute to the production of versatile and value-added products in either stand-alone processors or biorefineries [1]. Lignocellulose is encountered in hardwood, softwood, grasses, agricultural and forest residues, domestic and municipal solid wastes, as well as food/feed industry residues. The main components of these materials are cellulose, hemicellulose and lignin which form a compact and recalcitrant structure [2]. The present study focuses on agricultural residues and specifically on wheat straw from Greek wheat fields, aiming to exploit the maximum potential of lignocellulosic-derived sugar streams valorization. Towards this direction, the choice of integrated processes for the conversion of all sugar streams is considered to be of paramount importance. Hence, not only the pretreatment step of biomass but also the performance of a successful fractionation of biomass to its constituents holds a prominent role in the biorefinery concept.
Due to the recalcitrant structure of lignocellulosic biomass, pretreatment and hydrolysis are deemed necessary, in order not to rate-limit the subsequent fermentation [3]. Therefore, aiming for the adequate disruption of the compact structure of biomass, an initial pretreatment procedure is required in order to remove hemicellulose/lignin and expand the accessible surface area of cellulose for enzymes [4]. Among all pretreatment processes that have been thoroughly examined throughout the recent years, organosolv stands out due to the numerous advantages it offers such as the high purity of cellulose and the isolation of high-quality, sulfur-free lignin [5,6]. The organosolv treatment uses a liquid phase reaction medium of organic solvents or their aqueous solutions and allows for the separation and the simultaneous recovery of three streams, namely a cellulose-rich solid pulp, an aqueous hemicellulose liquid fraction and a dry solid lignin that can be obtained after evaporation of the organic solvent [7]. Moreover, organosolv pretreatment contributes to high lignin dissolution, thus resulting in high delignification rates [8] and preservation of β-O-4 linkages for downstream lignin utilization [9] while maintaining the formation of sugar degradation products at very low levels. It is worth noting that despite the costs of the chemicals that are used through the organosolv pretreatment, there are many reports in the literature that highlight the recyclability of these solvents, thus reducing the economic constraints as much as possible [7].
Microalgae include both photosynthetic and heterotrophic unicellular organisms that commonly live in freshwater or marine aquatic environments, and they can be of a high value or can be used as a green feedstock for many important products [10]. Nowadays, microalgae are already cultivated for commercial purposes, adding value to the market by producing microalgae-based products, such as health food supplements (nutraceuticals), pharmaceuticals, cosmetics, lubricants and feed for aquaculture hatcheries in agriculture and in many other applications [11]. In this context, the cultivation of heterotrophic microalgae on sustainable, abundant and available carbon sources, such as lignocellulosic residues, not only provides an option to take advantage of these streams but also significantly reduces the cost and carbon footprint compared to autotrophic or conventional microalgae production systems that use pure sugars in the growth medium. This is achieved by converting biomass-derived hydrolysates as low-value resources into value-added bioproducts, including compounds of nutritional value, such as omega-3 fatty acids (FA) and carotenoids [12,13]. Poly-unsaturated fatty acids (PUFAs), especially those with very long chains (LC-PUFAs) such as eicosapentaenoic acid (20:5n-3, EPA) and docosahexaenoic acid (22:6n-3, DHA), are bioactive compounds of great importance within the food and nutraceutical industry, where they are used as ingredients in functional foods, fulfilling the appropriate specifications of high value-added products [14]. Crypthecodinium cohnii is a heterotrophic microalga widely known for its ability to produce high percentages of DHA [15], a necessary provider for the proper cardiovascular and neural development of humans. This microorganism has been shown to be able to utilize a variety of different carbon sources apart from glucose, including volatile fatty acids from anaerobic digestion [16], ethanol [17], liquor produced after exhausted olive pomace pretreatment [18] as well as sugar-rich hydrolysates obtained after enzymatic hydrolysis of organosolv-pretreated beechwood [19,20]. In this study, we attempted to expand the range of substrates that can support the growth of C. cohnii and lead to accumulation of DHA by assessing wheat straw from Greek agricultural residues as a feedstock. Both pentose and hexose streams yielded after organosolv pretreatment were evaluated as carbon sources. The present work reports an efficient holistic approach for the integrated valorization of all sugar-containing fractions of biomass towards the production of this valuable product through fermentation.
2. Materials and Methods
2.1. Material and OxiOrganosolv Pretreatment Process
The wheat straw feedstock used in this work was provided by Flourmills Thrakis S.A. It originated from soft white wheat and it was collected from wheat fields in Northern Greece in 2019. The wheat straw was analyzed according to the methods described in Section 2.2.
The fractionation of the wheat straw feedstock was carried out with an acid-free oxidative organosolv fractionation (OxiOrganosolv), as described previously [6]. An amount of 25 g of wheat straw and an organic solvent/water mixture (solid: liquid ratio 1:20, organic solvent: H2O ratio 1:1) were added into an autoclave reactor, which was pressurized with 100% O2 gas to 16 bar and then heated to the desired temperature. The organic solvents investigated in this work were acetone (ACO) and ethanol (EtOH), which were chosen to comply with food industry regulations. Three reaction temperatures were studied, 150, 160 and 175 °C, while the reaction time was kept constant at 120 min. The starting time of the reaction (t0) was considered as the time when the system reached the desired temperature. After the reaction, the cellulose-rich solid fraction was separated from the liquid with vacuum filtration. The liquid fraction contained water, organic solvent, dissolved lignin and hydrolyzed hemicellulose fragments. The organic solvent was separated from the liquid fraction in a rotary evaporator under vacuum, which caused the lignin to precipitate. The lignin was then separated from the liquid fraction by vacuum filtration. The solid fraction, referred to as pulp, was washed first with 500 mL of the organic solvent and then with 250 mL of distilled water before it was finally air dried to a moisture content of around 5–8 wt%.
2.2. Analysis Methods
2.2.1. Moisture Content
The moisture content was determined by weighing ca. 2 g of as-received wheat straw in a pre-weighed crucible and placing it in a muffle furnace at 100 °C for 3 h. The crucible was then placed in a desiccator to cool down to room temperature, and then it was weighed. The moisture content was calculated from the weight difference of the sample before and after drying.
2.2.2. Ash Content
The ash content was determined by weighing 1 g of as-received or extractives-free wheat straw in a pre-weighed crucible and placing it in a muffle furnace at 575 °C for 3 h, in the presence of ambient air. The crucible was then removed from the furnace and placed in a desiccator to cool down to room temperature, and then it was weighed. The ash content was calculated based on the final sample weight, which was considered to be the ash in the sample.
2.2.3. Elemental Analysis
The carbon and hydrogen content of the as-received wheat straw feedstock was determined by analysis in a CHN 628 elemental analyzer from Leco Corporation, according to ASTM D5291. The oxygen content was determined by difference (O% = 100% − C% − H% − Ash%).
2.2.4. Compositional Analysis of Lignocellulosic Samples
The extractives content of the wheat straw feedstock was determined according to the method described by the National Renewable Energy Laboratory [21]. The hemicellulose, a-cellulose and lignin content of the wheat straw feedstock and the wheat straw pulp were determined after hydrolysis with 4 wt% H2SO4 in an autoclave (121 °C for 60 min) according to the procedures described by the National Renewable Energy Laboratory [22]. The hydrolyzed samples were filtered to remove any solids and analyzed by Ion Chromatography (IC) on an ICS5000 (Dionex, Sunnyvale, CA, USA) to quantify the content of sugars. The quantification was based on external calibration, using standard solutions of sugars (glucose, mannose, xylose, fructose, galactose, arabinose and rhamnose) and sugar alcohols (sorbitol and mannitol). The analysis was performed using a CarboPac PA1 (10 μm, 4 × 250 mm) column and guard column (10 μm, 4 × 30 mm) connected to a pulsed amperometric detector (PAD). The eluent was 20 mM NaOH at a 0.6 mL/min flow rate, and the total analysis time was 75 min. The compositional analyses were carried out twice, and the mean values are reported.
2.3. Enzymatic Hydrolysis of Solid Pulps
Both the cellulose-rich solid fraction and the hemicellulose-rich liquid fraction of the OxiOrganosolv pretreatment were exploited for the production of DHA with C. cohnii. An SHF approach (separated hydrolysis and fermentation) was employed because the conditions of these operations are different.
The solid fraction was enzymatically hydrolyzed to fermentable sugars following a procedure previously reported [19]. The reaction took place by employing a commercial enzyme cocktail, namely Cellic® CTec2 (Novozymes A/S, Bagsværd, Denmark), with a protein content of 95.6 mg/mL, as determined by Bradford assay [23]. Hydrolysis was performed in 250 mL glass flasks, at 50 °C, under agitation (160 rpm) and at an initial dry matter (DM) of 9% (w/v), in order to obtain a hydrolysate with high sugar concentration, suitable for C. cohnii cultivation. An enzyme loading of 9 mg/g of biomass was used, and the pH levels were maintained constant at 5.5 upon addition of 80 mM MES (2-N-morpholino-ethanesulfonic acid) buffer solution. The final ratio of the reaction volume to shake flask volume was 1/10.
In order to track down the glucose and total reducing sugars (TRS) production, the glucose oxidase/peroxidase (GOD/POD) [24] and 3,5-dinitrosalicylic acid (DNS) method [25] were used, respectively. Samples were taken at different time intervals (8, 24, 48 and 72 h), centrifuged before each analysis, and the supernatant was filtered with 0.22 μΜ pore size filters. As a complement, in order to accurately determine the monosaccharide (glucose, xylose) and acetic acid concentration, a chromatographic analysis was performed (HPLC) by isocratic ion-exchange chromatography using an Aminex HPX-87H column with a micro-guard column, at 50 °C (Bio-Rad Laboratories, Hercules, CA, USA), using 3 mM H2SO4 as a mobile phase at a flow rate of 0.6 mL/min. After 72 h of hydrolysis and analysis of released products, all supernatants containing fermentable sugars were collected after vacuum filtration and kept in the freezer (−20 °C) for future use.
2.4. Detoxification and Enzymatic Hydrolysis of Liquid Fractions
Detoxification and enzymatic hydrolysis of liquid fractions were required before the evaluation of pretreatment aqueous liquors as carbon source for C. cohnii. Even after the initial removal of the OxiOrganosolv organic solvent, as described in Section 2.1, some residual organic solvent with potential to alter the microalga metabolism was still present (as detected by HPLC analysis), so an additional evaporation step was necessary. After the removal of the organic solvent, the liquid fraction was filled with water up to its original volume in order to maintain the same sugar concentration as the corresponding sample prior to evaporation. Moreover, the OxiOrganosolv-derived liquid fractions contained phenolic compounds that are toxic to the microalgae used. Therefore, 5% (w/v) active carbon was used for the removal of phenol compounds, whose levels were determined with the Folin−Ciocalteu method [26].
The liquid fractions are richer in hemicelluloses in comparison with the solid fractions. However, most of the compounds are found in the form of oligosaccharides, which cannot be metabolized by C. cohnii. As a result, hydrolysis with Cellic® HTec2 enzyme cocktail (Novozymes A/S, Bagsværd, Denmark) was carried out in order to produce fermentable monosaccharides. The protein content was 75 mg/mL, as calculated by the Bradford method [23]. Hydrolysis experiments were performed in 500 mL glass flasks at 45 °C under agitation (100 rpm) with an enzyme loading of 10 mg/g of oligosaccharides at pH 5.5 (self pH, no addition of buffer). The total reaction volume to shake flask volume was 1/10. Samples were analyzed with HPLC by isocratic ion-exchange chromatography using an Aminex HPX-87P column with a micro-guard column at 85 °C (Bio-Rad Laboratories, Hercules, CA, USA) and using water as a mobile phase at a flow rate of 0.6 mL/min. With this HPLC analysis, the levels of glucose, xylose, mannose, galactose and arabinose were determined. The hydrolysis yield was calculated based on the increase of monosaccharides after enzymatic reaction.
2.5. Microalga Cultures
The heterotrophic marine microalga C. cohnii AΤCC® 30772™ (American Type Culture Collection) was used to study the production of omega-3 fatty acids. Stock cultures and growth procedure were performed according to ATCC guidelines and protocols, as previously described [16]. Initially, the microalgal cells were incubated in standing pre-cultures at 27 °C in the dark, on a medium containing 9 g/L glucose, 25 g/L sea salts and 2 g/L yeast extract. The pH of the medium was set at 6.5. After 4 days of growth, the standing cultures were used as inoculum for shaken cultures.
The medium for the shaken cultures contained 25 g/L sea salts and 2 g/L yeast extract, while hydrolysates after enzymatic treatment of the solid pulps (6 samples) or the liquid fractions (3 samples) were employed as a carbon source. In order to increase the total sugar content of the hydrolysates from solid pulps, enzymatic reactions took place at 12% (w/v) initial solids, while all other parameters (temperature, enzyme loading, pH, agitation) were the same as those mentioned in Section 2.3. The total culture volume was 30 mL in 100 mL shake flasks, 27 mL of either 2-times diluted enzymatic hydrolysate from solid fraction or undiluted enzymatically treated liquid fraction and 3 mL inoculum, as it had to consist of 10% (v/v) of the final culture volume. The pH of the culture was set at 6.5 with NaOH solution. The temperature of the culture was set at 27 °C and agitation was essential for homogeneity, namely 160 rpm [27]. Each experiment was performed in duplicate. The growth of cells was supervised on a daily basis by measuring the optical density (OD) at 685 nm and the TRS concentration with the DNS method. Moreover, to track carbon consumption more accurately, an HPLC analysis was performed with an Aminex HPX-87H column, as mentioned above. At the end of cultivation, cells were harvested by centrifugation, washed thoroughly with dH2O contacting 25 g/L sea salts, lyophilized and weighed, in order to determine the biomass concentration.
2.6. Extraction of Lipids, Quantification and Evaluation of Fatty Acids Profile
For the extraction of lipids, the Folch method was utilized [28] with some alterations [19]. Namely, 50 mg of dried cells were mixed with 10 mL of chloroform: MeOH 2:1 mixture and left overnight at room temperature. Then, distilled H2O was added at a volume equal to 20% of that of the organic mixture, and the mixture was centrifuged. After centrifugation, the lower phase was collected in glass tubes and washed with a MeOH: dH2O 1:1 mixture. The organic solvents used were evaporated in a vacuum oven for a maximum of 24 h in order to minimize the risk of sample oxidation. After the evaporation of the solvent, the total lipids were weighted. Following this, the collected fatty lipids had to be transformed into fatty acid methyl esters (FAME). For this reason, they were diluted in 1 mL pure chloroform and mixed with 2.5 mL solution of MeOH: HCl 92:8. The samples at that point were incubated at 60 °C for 15 min for the esterification to take place. The reaction was halted with the addition of 2.5 mL of CaCl2 5% (v/v). The methyl esters were extracted from the mixture using hexane, which proved to be a very good solvent, and analyzed by GC–MS, as described by Chalima et al. [19]. The quantification of fatty acids took place by employing a commercially accessible FAME combination (Supelco® 37, Sigma-Aldrich, St. Louis, MI, USA), whereas estimation of wt% DHA was considered out of the whole sum of five fatty acids that were primarily distinguished (C14:0, C16:0, C18:0, C18:1 and DHA).
3. Results
3.1. OxiOrganosolv Fractionation of Wheat Straw with ACO and EtOH as Organic Solvents
Initially, wheat straw biomass was organosolv-pretreated by following the OxiOrganosolv process [6] with an H2O/ACO 50:50 or an H2O/EtOH 50:50 co-solvent system. The pretreatment time was kept constant at 120 min in all cases upon addition of 16 bar O2 as a catalyst. The only parameter shifting was the temperature that was regulated at 150, 160 and 175 °C for both solvent mixtures, resulting in six different experimental runs. Pretreatment conditions as well as compositional analysis, cellulose/hemicellulose recovery and lignin removal of solid fraction are shown in Table 1. The results showed that, with a constant residence time of 120 min, increasing the pretreatment temperature results in a corresponding increase in the % biomass solubilization in the case of both ACO and EtOH. This was attributed to the removal of the hemicellulose and lignin fractions from the wheat straw substrate. Delignification peaked at 175 °C of pretreatment both in the case of ACO and EtOH, reaching 87.5 ± 0.6% and 86.9 ± 0.1% for sample S3 and S6, correspondingly. Notably, cellulose recovery in the solid fraction was almost 100% in almost all cases in both solvents, indicating only very limited hydrolysis of cellulose in the pre-treatment conditions that were studied. On the other hand, hemicellulose recovery in the solid fraction reached the highest values of 82.6 ± 0.8% in the case of ACO (sample S1) and 84.6 ± 0.1% in the case of EtOH (sample S4) in the lowest temperature of 150 °C, while increasing temperature resulted in increased hemicellulose removal and also combined with increasing lignin removal. As such, both solvents achieved very good delignification of the wheat straw, while ACO was also more effective for the removal of hemicellulose, resulting in the most cellulose-rich pulps.

Table 1.
Pretreatment conditions, compositional analysis of solid pulps, lignin removal and % recovery of cellulose and hemicellulose. Standard error is ≤2.5% in all measurements.
The aqueous liquid fraction resulting from the organosolv pretreatment was examined for hemicellulose recovery after organic solvent evaporation and lignin removal by vacuum filtration. The results, presented in Figure 1 and in Table 2, demonstrate that the highest % of hemicellulose recovery in the form of monosaccharides and polysaccharides was achieved when the temperature was the highest for both organic solvents, reaching up to 32.7 ± 1.2% for ACO, 175 °C, 120 min (sample L3) and 34.4 ± 0.9% for EtOH, 175 °C, 120 min (sample L6). These values accounted for 119 and 88.3 mg/g of untreated wheat straw biomass after ACO or EtOH pretreatment, respectively. The aforementioned results are in agreement with the % hemicellulose recovery values in solid pulps, as depicted in Table 1, where the samples that underwent pretreatment at higher temperatures stood out for their high cellulose and low hemicellulose content. As such, the results in Table 2 demonstrated a dwindling trend in the oligosaccharides/monosaccharides ratio in the aqueous phase when the pretreatment conditions became more severe in the case of ACO. More specifically, the ratio was 10.5 for 150 °C, 120 min (sample L1) noting a remarkable decline to 2.2 for 175 °C, 120 min (sample L3), which was linked to the degradation of oligosaccharides in severe conditions. Contrariwise, the corresponding data in the case of EtOH did not follow the same trend, instead exhibiting a similar ratio regardless of the pretreatment temperature, and more specifically, 10.8 for 150 °C, 120 min (sample L4) and 10.5 for 175 °C, 120 min (sample L6).
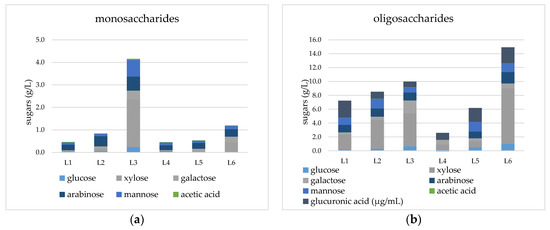
Figure 1.
Compositional analysis and hemicellulose sugar profile of liquid fractions. The concentration of sugars in the form of (a) monosaccharides and (b) oligosaccharides is presented. Standard error is ≤2.5% in all measurements.

Table 2.
Compositional analysis of monosaccharides/oligosaccharides in the liquid fraction. Standard error is ≤2.5% in all measurements.
The optimal temperature for efficient % hemicellulose recovery in the liquid fraction was 160 °C (sample L2) in the case of ACO, resulting in 25.4 ± 2.1% hemicellulose recovery in the form of oligosaccharides and only 2.8 ± 0.2% in the form of monosaccharides. In the case of EtOH, the optimal temperature for high hemicellulose recovery was 175 °C (sample L6), achieving 31.4 ± 1.4% hemicellulose recovery in the form of oligosaccharides and only 3.0% in the form of monosaccharides. Furthermore, no presence of sugar degradation products was detected, while phenolic compounds were found only in low quantities, as identified by the Folin–Ciocâlteu method (Supplementary Table S1).
3.2. Saccharification of Solid Fraction towards the Production of a C6 Sugar-Rich Hydrolysate
Cellulose-rich solid pulps obtained after pretreatment were enzymatically hydrolyzed with a commercial cellulase cocktail in order to produce a glucose-rich syrup to be utilized as carbon source for the subsequent fermentation. The time course data of hydrolysis and overall % cellulose conversion to glucose are presented in Figure 2, while the TRS released after enzymatic treatment are described in Supplementary Table S2.
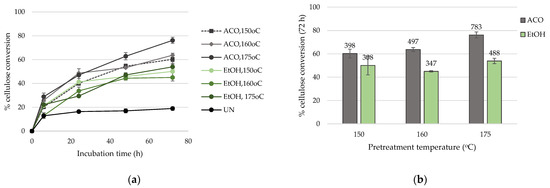
Figure 2.
(a) Time course of hydrolysis (solid fraction) as function of incubation time and (b) effect of temperature and solvent on the % cellulose conversion. Labels in (b) represent the mg of glucose/g of pretreated biomass.
Even though the total number of pretreatment runs was relatively small, a correlation between pretreatment conditions and cellulose conversion to glucose can be extracted. Broadly, wheat straw samples pretreated with ACO showed better hydrolysability that their EtOH counterparts, while there was an upsurge in cellulose conversion as pretreatment temperature increased. More specifically, pretreatment at 150 °C with ACO yielded a solid pulp that exhibited 60.2 ± 3.7% cellulose conversion to glucose after 72 h of hydrolysis, while pretreatment with EtOH at 150 °C resulted in 50.1 ± 8.0% conversion. The advantageous effect of ACO over EtOH was also profound at higher temperatures (160 and 175 °C), while at 175 °C, cellulose conversion reached the highest values, namely 76.2 ± 2.6% and 53.9 ± 2.3% for ACO and EtOH pulps, respectively. These values corresponded to 783 ± 26 and 488 ± 21 mg of glucose/g of pretreated biomass. Regarding the amount of TRS, similar trends were observed, with the highest titer achieved at 175 °C with ACO, reaching 64.9 ± 1.1 mg/mL (721 ± 13 mg/g of pretreated biomass).
3.3. Enzymatic Hydrolysis of Liquid Fraction for the Enrichment in C5 Fermentable Sugars
Taking into account that a substantial amount of sugars is required for C. cohnii cultivation, L2, L3 and L6 samples were selected to be hydrolyzed and further evaluated as carbon sources, due to the higher concentration of total sugars compared to other samples and due to the presence of xylose. Cellic® HTec2 was used in order to hydrolyze hemicellulose-derived oligosaccharides to monomers that can be utilized by C. cohnii. The concentration of monosaccharides was increased after hydrolysis, and the results are presented in Table 3, while the sugar profile is described in Figure 3. The data show that the hydrolysis was efficient, resulting in more than a three-fold increase in the concentration of monosaccharides, especially for the L3 sample (ACO, 175 °C) which reached a hydrolysis yield of 73.4 ± 1.8%. Moreover, all monosaccharides were increased, especially xylose, thus confirming the results from sugar analysis prior to hydrolysis that showed the presence of xylo-oligosaccharides (Figure 1). However, the sample population is rather low to extract a statistically significant correlation between hydrolysis yield and pretreatment conditions, both in terms of solvents and temperature. The enzymatically treated liquid fractions were further utilized in C. cohnii cultures, as described below.

Table 3.
Hydrolysis yield in terms of monosaccharide increase. The fraction of oligosaccharides refers to the sugar streams only (the presence of acetic or uronic acids was not considered). Standard errors are given in parenthesis.
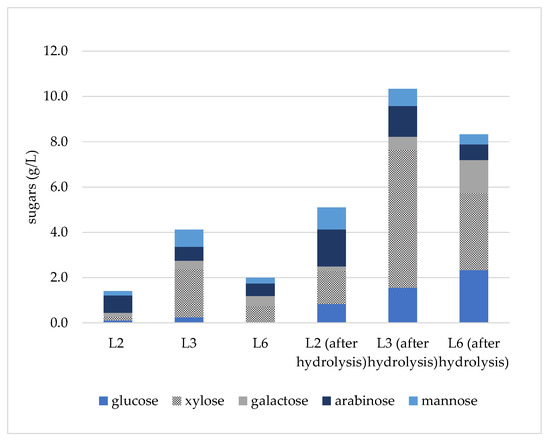
Figure 3.
Sugar profile of monosaccharide fraction before and after enzymatic hydrolysis of liquid fraction. Standard error is ≤2.5% in all measurements.
3.4. Lipid Accumulation and DHA Production by C. cohnii Growing on Biomass-Derived Enzymatic Hydrolysates
Hydrolysates after enzymatic treatment of solid and liquid fractions were evaluated as carbon sources for C. cohnii cultures towards the production of a DHA-rich oil. The results are presented in Table 4, showing that both cellulose and hemicellulose-derived streams were able to support the growth of microalgal cells and accumulation of fatty acids. What can be observed is that solid fractions showed a better performance than liquids in terms of growth, TFA accumulation and % DHA concentration. Regarding the solid fractions, EtOH pretreated samples showed higher TFA synthesis (60.5–70.3% of dried cell weight) than their ACO pretreated counterparts (37–39%), while the cell biomass was also slightly higher (Supplementary Figure S1). ACO pretreated samples reached the highest peak for cell biomass productivity at 150 °C pretreatment temperature, where the cell biomass concentration was 6.72 ± 0.67 g/L. On the other hand, EtOH-pretreated solid fraction reached a peak at production at 160 °C, namely 6.23 ± 0.25 g/L. The highest TFA concentration observed was 4.38 ± 0.52 and 2.49 ±0.38 g/L of culture medium for EtOH and ACO pretreatment, respectively. No significant differences were observed in the % DHA content of the oil extracted from C. cohnii cells in different pretreatment conditions; in fact, only a slight increase could be observed in samples S1–S3 (ACO) compared to S4–S6 (EtOH). As far as the DHA concentration is concerned, the highest values were observed at 150 °C, 120 min with 0.97 ± 0.19 g/L in ACO pretreated samples and at 160 °C, 120 min with 1.41 ± 0.45 g/L % in EtOH pretreated samples, respectively. No correlation was observed between the TFA accumulation, the % DHA and the cellulose content of the initial solid pulps. Moreover, solid fractions pretreated with the same solvent have produced similar levels of fatty acids, indicating that pretreatment temperature was of little significance for % TFA yields.

Table 4.
Cell biomass growth and accumulation of total fatty acid (TFA) by C. cohnii cell biomass. The carbon source used was hydrolysates from both solid and liquid fraction of organosolv process at wheat straw biomass. Standard errors are given in parenthesis. % TFA represents g of lipids per 100 g of cell biomass, while % DHA refers to the weight percentagewise concentration of DHA in the C. cohnii oil extracted. Pure glucose (4.5 wt%) was also used as a carbon source for comparison.
All enzymatic hydrolysates from pretreated solid pulps showed higher cell growth, TFA accumulation and % DHA compared to the untreated one, which is indicative of the efficiency of the OxiOrganosolv pretreatment in order to enhance lipid production from biomass. However, it is worth mentioning that C. cohnii cell growth on pure glucose resulted in lower biomass production, which was attributed to the high initial concentration of the carbon source (4.5 wt%), which hinders microalga growth [17]. Similar results regarding low cell growth were also observed in samples S3 and S6, where the initial sugar concentration was 42.3 ± 0.8 and 43.2 ± 0.5 g/L, respectively (Supplementary Table S3), which may be attributed to the high concentration of the carbon source. When pure glucose was used as the carbon source, cells accumulated 40.61 ± 3.29% of dry weight TFA, which was comparable to hydrolysates from ACO pretreated samples, resulting in a concentration of 1.08 g/L of culture medium. % DHA was significantly higher (47.2 ± 1.75% of total lipids), which corresponded to 0.51 ± 0.06 g/L. As far as the profile of fatty acids is concerned (Figure 4), one can observe that a low % DHA content is accompanied by a higher accumulation of C18:1, such as in samples S2 and S4, or C16:0 such as in sample S3.
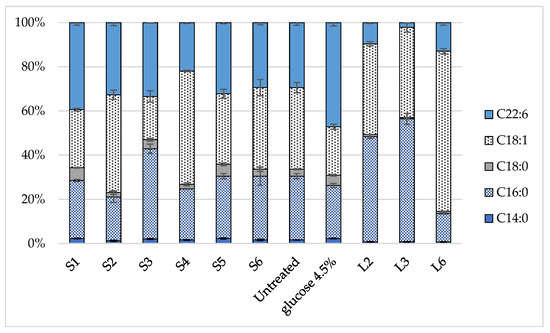
Figure 4.
Fatty acid profile of oil extracted by C. cohnii cells after 120 h of cultivation on biomass-derived sugars.
C. cohnii cultivation using enzymatically treated liquid fractions resulted in lower yields compared to those from the solid fractions, regarding not only the TFA accumulation (with the exception of L2) but also % DHA content; the latter reached up to 13.03 ± 1.41% of total lipids in the L6 sample. One possible explanation would be the presence of sugar degradation products such as furans or phenolic compounds that inhibit the cells biomass; however, after detoxification with activated carbon, the concentration of phenolic compounds was negligible, while no presence of HMF or furfural was detected on HPLC, indicating that the low amounts of DHA could arise from the different sugar profile. The profile of fatty acids, as presented in Figure 4, reveals that hydrolysates from liquid fractions have produced far less DHA and more C18:1 and C16:0 than their solid counterparts. More specifically, C16:0 was 47.8 ± 0.9% and 55.6 ± 2.4% in L2 and L3, respectively, while C18:1 reached 72.7 ± 1.2% of total fatty acids in L6.
In an attempt to provide a clear view of the efficiency of the suggested process for the production of TFAs and, more specifically, DHA, the results are expressed in mg/g of pretreated and untreated biomass, as described in Table 5. Regarding the results from the solid fractions, EtOH pretreatment favored the production of TFA and DHA when considering the pulp recovery yield after pretreatment, the saccharification efficiency, C. cohnii cell growth, TFA accumulation and % DHA content. When hydrolysate from biomass pretreated with EtOH at 160 °C was used as carbon source (sample S5), 20.3 ± 1.17 mg TFA/g of untreated biomass were produced, corresponding to 7.1 ± 1.2 mg DHA/g of untreated biomass, which was the highest achieved. The above results, when compared to those of the untreated sample (3.8 ± 1.09 mg TFA and 1.1 ± 0.05 mg DHA/g of untreated biomass), were significantly higher, thus demonstrating the efficiency of the pretreatment and the fractionation for the downstream process yields. The liquid fractions resulted in lower yields, with the highest being 10.71 ± 1.08 mg TFA and 1.41 ± 0.39 mg DHA/g of untreated biomass, providing the first documented evidence that hemicellulose-rich streams can be valorized as carbon sources for C. cohnii.

Table 5.
Summary of total TFA and DHA yields per g of pretreated solid pulp or mL of aqueous liquid fraction and overall yields per g of untreated biomass. Standard errors are given in parenthesis.
4. Discussion
Wheat straw was preferred in this case study since high amounts are made available in Greece as side streams from the agricultural sector. The term “agricultural residues” refers to any kind of by-products or agricultural derivatives without any economic value for the enterprise or their further management or any profitable utilization [29]. In Europe, agricultural waste consists mainly of wheat straw, while its production equals about 32% of the worldwide production [30]. In particular, according to the Centre for Renewable Energy Sources & Saving (CRES) in Greece for the year 2017, wheat straw residues were estimated to be 1,150,738 tonnes of dry matter, thus rendering the valorization of these feedstocks of pivotal importance.
The OxiOrganosolv pretreatment was applied in our previous studies and was proven optimal for the maximum delignification efficiency [6,19,20]. The use of ACO and EtOH as organic solvents in the pretreatment of various lignocellulosic biomass residues has been widely reported in the literature, since they are milder and green solvents that can be easily recovered and reused, making the whole process sustainable and applicable on an industrial scale [31,32,33]. Moreover, they have the ability not only to cleave the bonds between lignin and hemicellulose during the organosolv pretreatment but also to remove hemicellulose and solubilize the lignin, thereby causing an increase in surface area and pore volume of cellulose, which renders cellulose more accessible to enzymatic hydrolysis [34]. ACO is reported as the most favored ketone used for delignification [7]. However, the prevalence of EtOH is probably linked to its low cost and lack of toxicity, while also leading to the efficient deconstruction of lignocellulosic biomass [33]. The advantageous use of EtOH is supported by recent approaches, such as the integration of first- and second-generation ethanol, to enhance the commercialization of cellulose-derived EtOH [34]. In addition, the concentration of EtOH in the reaction medium of organosolv processes has been mainly within 30–60% (v/v) [35,36,37], which is in concert with the concentration acquired after the first distillation at first-generation EtOH industries. Both ACO and EtOH have aroused research interest on account of their distinct properties: they dissolve lignin and enable recovery of solid lignin after solvent removal, since the latter is insoluble in aqueous solutions [35]; they enable fractionation of hemicellulose-derived oligosaccharides from cellulose because the former exhibit some solubility in the organic solvent: H2O mixture [36]; they result in cellulose structure swelling, causing the crystal structure of cellulose fibers to be unfolded and thus rendering cellulose more eligible for hydrolysis [38]; and they can be both collected and recycled, thus reducing operating costs. This process seems to be promising for lignocellulose fractionation in order to obtain a solid and a liquid fraction that can be further processed separately, provided that a techno economical study including the solvent recycling is carried out prior to setting up larger scale units.
In our previous works, the advantages of OxiOrganosolv both for the efficient fractionation and increased enzymatic digestibility of hardwoods (beechwood) and softwoods (pine) has been demonstrated [6,20], thus highlighting the advantages of this process compared to the traditional organosolv pretreatment upon addition of sulfuric acid. In the present study, we attempted to further apply the process in crops, showing that it is also efficient for delignification of this type of substrate and the production of sugar-rich streams. Salapa et al. [39] reported the organosolv treatment of wheat straw in the presence of sulfuric acid as catalyst by evaluating five different organic solvents; the results showed that ACO was the most efficient in biomass delignification, achieving 76.4% lignin removal after pretreatment at 180 °C for 40 min. However, enzymatic hydrolysis of the produced solid pulp reached only 49.97% cellulose conversion, while in the case of the present study, the yield was 76.2%. In another work, organosolv fractionation of wheat straw upon addition of acid catalyst resulted in 75.8% delignification at 190 °C for 60 min, while in the absence of acid at 160 and 170 °C (conditions comparable to those in this study), lignin removal was 4.7 and 14.4% respectively, and the enzymatic digestibility of the pulps was only 30.5% and 31.7% [40].
For the development of an efficient organosolv process taking advantage of all sugar streams, valorization of the pentose-rich liquid fraction together with the hexose-rich solid pulp is a prerequisite. Hemicellulose streams have been already studied as a potential substrate for the production of valuable chemicals, such as xylitol produced from corncob [41], biobutanol from birch kraft black liquor [42] and lactic acid from corn stover [43]. Moreover, the produced aqueous fraction contains a high number of xylo-oligosaccharides which have be studied as prebiotic compounds [20,44]. However, no work has yet been made towards the utilization of the liquid fraction for the production of fatty acids from microalga. The great advantage of C. cohnii is that this microorganism is able to utilize not only hexoses but also pentoses, accumulating high amounts of DHA-rich oil, as it has been originally reported from Karnaouri et al. using pure xylose and arabinose [19]. In this study, we further evaluated the ability of the microalga to grow on a true hydrolysate from wheat straw pretreatment. Pentose utilization is still halted because hemicellulose-rich fraction originating from biomass pretreatment may be contaminated with sugar degradation products that act as inhibitors [45]. However, the OxiOrganosolv pretreatment method yields an inhibitor-free solid fraction [6], as was also shown in the present study, which is the great advantage of this process. In the process that is suggested in this work, tuning the pretreatment for the removal of hemicellulose and the release of pentose sugars in the form of monosaccharides would be an option in order to eliminate the necessity for enzymatic treatment. Additionally, concerning wheat straw, no studies have taken place regarding fatty acids production, despite the fact that wheat straw is a widely available agricultural waste.
Regarding the growth of C. cohnii and the production of fatty acids, all enzymatic hydrolysates from solid pulps efficiently served as carbon sources in this work. Notably, higher biomass pretreatment temperatures with ACO or EtOH produced very cellulose-rich pulps that progressively lowered the cell biomass productivity when used as carbon source for cell growth. This was attributed to high initial concentration of glucose in the growth medium, which was confirmed with the pure glucose sample tests and is also supported by the literature [46]. Since high initial concentration of carbon source hinders C. cohnii growth, employing a fed-batch strategy could improve cell productivity yields and reduce incubation time. As far as the % DHA content is concerned, the results are in accordance with the previous reports in the literature; a variety of different substrates, such as carob pulp syrup, resulting in 48% DHA of total lipids (however with a much lower accumulation of TFA 9.2% of cell biomass) [47], rapeseed meal hydrolysate mixed with crude molasses, yielding 22–34% DHA [48], and cheese whey with corn steep liquor [49] have been used as carbon sources in media formulations for C. cohnii fatty acid production. Cultivation on liquid fractions in this work produced far less DHA and more C18:1 when compared to solid fraction hydrolysates, corroborating the idea that the initial sugar concentration and the type of carbon source affected not only the amount of accumulated TFAs but also the quality of the oil and the % DHA content. It has been already reported that when pure xylose and arabinose were used as carbon sources, the oil accumulated in C. cohnii cells contained more C18:1 than DHA [19]. Moreover, the initial concentration of sugars in liquid fractions was much lower than that of their solid counterparts (as shown in Table S3), leading to a lower C/N ratio in the culture medium, since the initial concentration of the nitrogen source was constant in all experiments. In addition, although strain-specific, it has been demonstrated that reduced degree of unsaturation and chain-length is related to lower specific growth rate and higher TFA accumulation yields [50].
Cellular stress and nutrient deprivation can affect not only the lipid accumulation but also the fatty acid profile. A lower cell growth has been correlated with a higher lipid content, and this has been observed for many microalgal strains [51]. The differences observed in this study regarding the TFA accumulation and DHA content can be attributed to the fact that C. cohnii responds differently to multi-stress factors that are related to the addition of hemicellulosic fraction as carbon source, even after detoxification. Moreover, the difference in the lipid profiles when using either the solid or the liquid fractions might be also correlated with the difference in their sugar profiles; the hydrolysates derived from solid fraction are richer in glucose, whereas the hydrolysis of liquid fractions produced more xylose. Despite the lower yields observed with liquid fractions, this is the first report of the cultivation of C. cohnii on the pentose-rich fraction and the valorization of this fraction for the production of DHA.
5. Conclusions
In the present work, hexose- and pentose-rich streams after organosolv pretreatment and fractionation of wheat straw with ACO and EtOH were used as carbon sources for C. cohnii cells for the production of fatty acids and, more specifically, DHA. Enzymatic hydrolysates from solid fractions have shown great potential, achieving up to 70.3 wt.% TFA accumulation and 32.2% DHA of total lipids. Pentose-rich liquid fractions resulted in lower DHA yield (up to 13%), indicating the presence of compounds in the hydrolysate that may affect the lipid synthesis and alter the fatty acids profile. Moreover, the type of carbon source affects not only the amount of lipids but also the relative proportion of DHA that is accumulated in the cells. It is worth mentioning that this is the first report demonstrating, as a proof of concept, the valorization of all sugar streams towards the production of omega-3 fatty acids. Despite the challenges of the liquid fraction utilization, the suggested process is a promising approach towards the production of omega-3 fatty acids from non-edible sources and opens up new routes, considering the availability of wheat straw residues that are made available from the Greek agricultural sector, as well as worldwide.
Supplementary Materials
The following are available online at www.mdpi.com/article/10.3390/fermentation7040219/s1, Figure S1: Time course of growth of C. cohnii cells on enzymatic hydrolysate from solid pulps as carbon source, Table S1: Concentration of phenolic compounds in the liquid fraction before and after detoxification, Table S2: Total reducing sugars released after enzymatic hydrolysis of solid fraction, Table S3. Initial sugar concentration and consumption after 120 h of fermentation of C. cohnii.
Author Contributions
Conceptualization, A.K. and K.G.K.; methodology, G.A., A.K., S.S., K.G.K. and S.D.S.; validation, G.A., A.K. and S.D.S.; formal analysis, G.A., A.K., S.S. and S.D.S.; investigation, G.A., S.S. and S.D.S.; resources, A.A.L. and E.T.; data curation, G.A., A.K. and S.D.S.; writing—original draft preparation, G.A., S.S., A.K. and S.D.S.; writing—review and editing, A.K. and S.D.S.; visualization, A.K. and S.D.S.; supervision, A.K., A.A.L. and E.T.; project administration, A.K., A.A.L. and E.T.; funding acquisition, K.G.K., A.A.L. and E.T. All authors have read and agreed to the published version of the manuscript.
Funding
The research was financed by the European Regional Development Fund of the European Union and Greek national funds through the Operational Program Competitiveness, Entrepreneurship and Innovation, under the call RESEARCH—CREATE—INNOVATE (project code: T2EDK-00468). G. Asimakopoulou would like to thank the State Scholarship Foundation (IKY) of Greece for providing a PhD fellowship (NSRF 2014–2020) through the program “Development of human resources, education and lifelong learning”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Solarte-Toro, J.C.; Chacon-Perez, Y.; Cardona-Alzate, C.A. Evaluation of biogas and syngas as energy vectors for heat and power generation using lignocellulosic biomass as raw material. Electron. J. Biotechnol. 2018, 33, 52–62. [Google Scholar] [CrossRef]
- da Costa Lopes, A.M.; João, K.G.; Rubik, D.F.; Bogel-Łukasik, E.; Duarte, L.C.; Andreaus, J.; Bogel-Łukasik, R. Pre-treatment of lignocellulosic biomass using ionic liquids: Wheat straw fractionation. Bioresour. Technol. 2013, 142, 198–208. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Zhang, J.; Xie, J.; Qin, Y. Effects of NaOH-catalyzed organosolv pretreatment and surfactant on the sugar production from sugarcane bagasse. Bioresour. Technol. 2020, 312, 123601. [Google Scholar] [CrossRef]
- Alvira, P.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef]
- Jørgensen, H.; Kristensen, J.B.; Felby, C. Enzymatic conversion of lignocellulose into fermentable sugars: Challenges and opportunities. Biofuel Bioprod. Biorefin. 2007, 1, 119–134. [Google Scholar] [CrossRef]
- Kalogiannis, K.G.; Karnaouri, A.; Michailof, C.; Tzika, A.M.; Asimakopoulou, G.; Topakas, E.; Lappas, A.A. OxiOrganosolv: A novel acid free oxidative organosolv fractionation for lignocellulose fine sugar streams. Bioresour. Technol. 2020, 313, 123599. [Google Scholar] [CrossRef]
- Zhao, X.; Cheng, K.; Liu, D. Organosolv pretreatment of lignocellulosic biomass for enzymatic hydrolysis. Appl. Microbiol. Biotechnol. 2009, 82, 815–827. [Google Scholar] [CrossRef]
- Zhao, X.; Li, S.; Wu, R.; Liu, D. Organosolv fractionating pre-treatment of lignocellulosic biomass for efficient enzymatic saccharification: Chemistry, kinetics, and substrate structures. Biofuel Bioprod. Biorefin. 2017, 11, 567–590. [Google Scholar] [CrossRef]
- Dong, C.; Meng, X.; Yeung, C.S.; Ho-Yin, T.S.E.; Ragauskas, A.J.; Leu, S.Y. Diol pretreatment to fractionate a reactive lignin in lignocellulosic biomass biorefineries. Green Chem. 2019, 21, 2788–2800. [Google Scholar] [CrossRef]
- Tang, D.Y.Y.; Khoo, K.S.; Chew, K.W.; Tao, Y.; Ho, S.H.; Show, P.L. Potential utilization of bioproducts from microalgae for the quality enhancement of natural products. Bioresour. Technol. 2020, 304, 122997. [Google Scholar] [CrossRef]
- Levasseur, W.; Perré, P.; Pozzobon, V. A review of high value-added molecules production by microalgae in light of the classification. Biotechnol. Adv. 2020, 41, 107545. [Google Scholar] [CrossRef]
- Silambarasan, S.; Logeswari, P.; Sivaramakrishnan, R.; Incharoensakdi, A.; Cornejo, P.; Kamaraj, B.; Chi, N.T.L. Removal of nutrients from domestic wastewater by microalgae coupled to lipid augmentation for biodiesel production and influence of deoiled algal biomass as biofertilizer for Solanum lycopersicum cultivation. Chemosphere 2021, 268, 129323. [Google Scholar] [CrossRef]
- Brasil, B.D.S.A.F.; de Siqueira, F.G.; Salum, T.F.C.; Zanette, C.M.; Spier, M.R. Microalgae and cyanobacteria as enzyme biofactories. Algal Res. 2017, 25, 76–89. [Google Scholar] [CrossRef]
- Khan, R.S.; Grigor, J.; Winger, R.; Win, A. Functional food product development–Opportunities and challenges for food manufacturers. Trends Food Sci. Technol. 2013, 30, 27–37. [Google Scholar] [CrossRef]
- Chalima, A.; Oliver, L.; Fernández de Castro, L.; Karnaouri, A.; Dietrich, T.; Topakas, E. Utilization of volatile fatty acids from microalgae for the production of high added value compounds. Fermentation 2017, 3, 54. [Google Scholar] [CrossRef] [Green Version]
- Chalima, A.; Hatzidaki, A.; Karnaouri, A.; Topakas, E. Integration of a dark fermentation effluent in a microalgal-based biorefinery for the production of high-added value omega-3 fatty acids. Appl. Energy 2019, 241, 130–138. [Google Scholar] [CrossRef]
- de Swaaf, M.E.; Pronk, J.T.; Sijtsma, L. Fed-batch cultivation of the docosahexaenoic-acid-producing marine alga Crypthecodinium cohnii on ethanol. Appl. Microbiol. Biotechnol. 2003, 61, 40–43. [Google Scholar] [CrossRef]
- Paz, A.; Karnaouri, A.; Templis, C.C.; Papayannakos, N.; Topakas, E. Valorization of exhausted olive pomace for the production of omega-3 fatty acids by Crypthecodinium cohnii. Waste Manag. 2020, 118, 435–444. [Google Scholar]
- Karnaouri, A.; Chalima, A.; Kalogiannis, K.; Varamogianni-Mamatsi, D.; Lappas, A.; Topakas, E. Utilization of organosolv pretreated lignocellulosic biomass for the production of omega-3 fatty acids by the heterotrophic marine microalga Crypthecodinium cohnii. Bioresour. Technol. 2020, 303, 122899. [Google Scholar] [CrossRef]
- Karnaouri, A.; Asimakopoulou, G.; Kalogiannis, K.G.; Lappas, A.A.; Topakas, E. Efficient production of nutraceuticals and lactic acid from lignocellulosic biomass by combining organosolv fractionation with enzymatic/fermentative routes. Bioresour. Technol. 2021, 341, 125846. [Google Scholar] [CrossRef]
- Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Extractives in Biomass; TP-510-42619; NREL: Golden, CO, USA, 2008. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D.L.A.P. Determination of structural carbohydrates and lignin in biomass. Lab. Anal. Proced. 2008, 1617, 1–16. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Raba, J.; Mottola, H.A. Glucose Oxidase as an Analytical Reagent. Crit. Rev. Anal. Chem. 1995, 25, 1–42. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar]
- de Swaaf, M.E.; de Rijk, T.C.; Eggink, G.; Sijtsma, L. Optimisation of docosahexaenoic acid production in batch cultivations by Crypthecodinium cohnii. J. Biotechnol. 1999, 70, 185–192. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.S. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Alatzas, S.; Moustakas, K.; Malamis, D.; Vakalis, S. Biomass potential from agricultural waste for energetic utilization in Greece. Energies 2019, 12, 1095. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Dale, B.E. Global potential bioethanol production from wasted crops and crop residues. Biomass Bioenerg. 2004, 26, 361–375. [Google Scholar] [CrossRef]
- Zhang, K.; Pei, Z.; Wang, D. Organic solvent pretreatment of lignocellulosic biomass for biofuels and biochemicals: A review. Bioresour. Technol. 2016, 199, 21–33. [Google Scholar] [CrossRef]
- Zhang, Y.P. Reviving the carbohydrate economy via multi-product lignocellulose biorefineries. J. Ind. Microbiol. Biotechnol. 2008, 35, 367–375. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Brancoli, P.; Agnihotri, S.; Bolton, K.; Taherzadeh, M.J. A review of integration strategies of lignocelluloses and other wastes in 1st generation bioethanol processes. Process Biochem. 2018, 75, 173–186. [Google Scholar] [CrossRef]
- Li, X.; Luo, Y.; Daroch, M.; Hou, J.; Gui, W. Oxygen-assisted ethanol organosolv pretreatment of sugarcane bagasse for efficient removal of hemicellulose and lignin. Cellulose 2018, 25, 5511–5522. [Google Scholar] [CrossRef]
- Martín-Sampedro, R.; Eugenio, M.E.; Fillat, Ú.; Martín, J.A.; Aranda, P.; Ruiz-Hitzky, E.; Ibarra, D.; Wicklein, B. Biorefinery of lignocellulosic biomass from an elm clone: Production of fermentable sugars and lignin-derived biochar for energy and environmental applications. Energy Technol. 2019, 7, 277–287. [Google Scholar] [CrossRef]
- Park, Y.C.; Kim, J.S.; Kim, T.H. Pretreatment of corn stover using organosolv with hydrogen peroxide for effective enzymatic saccharification. Energies 2018, 11, 1301. [Google Scholar] [CrossRef] [Green Version]
- Alves, L.A.; Almeida e Silva, J.B.; Giulietti, M. Solubility of D-glucose in water and ethanol/water mixtures. J. Chem. Eng. Data 2007, 52, 2166–2170. [Google Scholar] [CrossRef]
- Del Rio, L.F.; Chandra, R.P.; Saddler, J.N. The effects of increasing swelling and anionic charges on the enzymatic hydrolysis of organosolv-pretreated softwoods at low enzyme loadings. Biotechnol Bioeng. 2011, 108, 1549–1558. [Google Scholar] [CrossRef]
- Salapa, I.; Katsimpouras, C.; Topakas, E.; Sidiras, D. Organosolv pretreatment of wheat straw for efficient ethanol production using various solvents. Biomass Bioenerg. 2017, 100, 10–16. [Google Scholar] [CrossRef]
- Wildschut, J.; Smit, A.T.; Reith, J.H.; Huijgen, W.J. Ethanol-based organosolv fractionation of wheat straw for the production of lignin and enzymatically digestible cellulose. Bioresour. Technol. 2013, 135, 58–66. [Google Scholar] [CrossRef]
- Kumar, V.; Krishania, M.; Sandhu, P.P.; Ahluwalia, V.; Gnansounou, E.; Sangwan, R.S. Efficient detoxification of corn cob hydrolysate with ion-exchange resins for enhanced xylitol production by Candida tropicalis MTCC 6192. Bioresour. Technol. 2018, 251, 416–419. [Google Scholar] [CrossRef]
- Kudahettige-Nilsson, R.L.; Helmerius, J.; Nilsson, R.T.; Sjöblom, M.; Hodge, D.B.; Rova, U. Biobutanol production by Clostridium acetobutylicum using xylose recovered from birch Kraft black liquor. Bioresour. Technol. 2015, 176, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Lin, Y.; Zhang, Z.; Xiang, T.; Mei, Y.; Zhao, S.; Liang, Y.; Peng, N. High-titer lactic acid production by Lactobacillus pentosus FL0421 from corn stover using fed-batch simultaneous saccharification and fermentation. Bioresour. Technol. 2016, 214, 74–80. [Google Scholar] [CrossRef]
- Boonchuay, P.; Techapun, C.; Leksawasdi, N.; Seesuriyachan, P.; Hanmoungjai, P.; Watanabe, M.; Takenaka, S.; Chaiyaso, T. An integrated process for xylooligosaccharide and bioethanol production from corncob. Bioresour. Technol. 2018, 256, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Chen, F. Effects of medium glucose concentration and pH on docosahexaenoic acid content of heterotrophic Crypthecodinium cohnii. Process Biochem. 2000, 35, 1205–1209. [Google Scholar] [CrossRef]
- Mendes, A.; Guerra, P.; Madeira, V.; Ruano, F.; da Silva, T.L.; Reis, A. Study of docosahexaenoic acid production by the heterotrophic microalga Crypthecodinium cohnii CCMP 316 using carob pulp as a promising carbon source. World J. Microbiol. Biotechnol. 2007, 23, 1209–1215. [Google Scholar] [CrossRef]
- Gong, Y.; Liu, J.; Jiang, M.; Liang, Z.; Jin, H.; Hu, X.; Wan, X.; Hu, C. Improvement of omega-3 docosahexaenoic acid production by marine dinoflagellate Crypthecodinium cohnii using rapeseed meal hydrolysate and waste molasses as feedstock. PLoS ONE 2015, 10, e0125368. [Google Scholar] [CrossRef] [Green Version]
- Isleten-Hosoglu, M.; Elibol, M. Bioutilization of cheese whey and corn steep liqour by heterotrophic microalgae Crypthecodinium cohnii for biomass and lipid production. Acad. Food J. Akad. GIDA 2017, 15, 233–241. [Google Scholar]
- Gachelin, M.; Boutoute, M.; Carrier, G.; Talec, A.; Pruvost, E.; Guihéneuf, F.; Bernard, O.; Sciandra, A. Enhancing PUFA-rich polar lipids in Tisochrysis lutea using adaptive laboratory evolution (ALE) with oscillating thermal stress. Appl. Microbiol. Biotechnol. 2021, 105, 301–312. [Google Scholar] [CrossRef]
- Roleda, M.Y.; Slocombe, S.P.; Leakey, R.J.G.; Day, J.G.; Bell, E.M.; Stanley, M.S. Effects of temperature and nutrient regimes on biomass and lipid production by six oleaginous microalgae in batch culture employing a two-phase cultivation strategy. Bioresour. Technol. 2013, 129, 439–449. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).