Abstract
In order to obtain a high-protein-content supplement for aquaculture feeds, rich in healthy microorganisms, in this study, Saccharomyces cerevisiae American Type Culture Collection (ATCC) 4126 and Lactobacillus reuteri ATCC 53608 strains were used as starters for fermenting fish waste supplemented with lemon peel as a prebiotic source and filler. Fermentation tests were carried out for 120 h until no further growth of the selected microorganisms was observed and the pH value became stable. All the samples were tested for proteins, crude lipids, and ash determination, and submitted for fatty acid analysis. Moreover, microbiological analyses for coliform bacteria identification were carried out. At the end of the fermentation period, the substrate reached a concentration in protein and in crude lipids of 48.55 ± 1.15% and 15.25 ± 0.80%, respectively, representing adequate levels for the resulting aquafeed, whereas the ash percentage was 0.66 ± 0.03. The main fatty acids detected were palmitic, oleic, and linoleic acids. Saturated fatty acids concentration was not affected by the fermentation process, whereas monounsaturated and polyunsaturated ones showed an opposite trend, increasing and decreasing, respectively, during the process. Coliform bacteria were not detected in the media at the end of the fermentation, whereas the amount of S. cerevisiae and L. reuteri were around 1011 and 1012 cells per g, respectively.
1. Introduction
World capture fishery production reached a peak of approximately 96 million tons in 2018; the most recent estimates suggest that 52% of marine stocks are fully exploited, 17% are overexploited, and 7% are totally depleted, while human population and the demand for marine and other aquatic resources continue to increase [1]. Global aquaculture would make a considerable contribution toward bridging the gap between supply and demand. Unfortunately, its development is hampered by an inadequate supply of feed, particularly fishmeal, which is scarce and expensive [2]. This has stimulated the evaluation of a variety of alternative dietary protein sources with the objective of partially, or totally, replacing fishmeal protein in aquafeeds [3]. The use of food industry waste as animal feed is an alternative of high interest because it stands to bring both environmental and public benefits, besides reducing the costs of animal production [4,5,6]. In particular, with reference to the fishing industry, the cost of waste management for aquaculture is typically in the range of USD 0.05 to USD 0.065 per pound of fish produced, representing concerns from both an economic and an environmental point of view. Waste management, in fact, contributes to the overall costs of production and reduces farmers’ net income. Moreover, the improper management of fish wastes could have a negative environmental impact, such as eutrophication effects, on natural aquatic ecosystems [7].
By-products coming from the fishing industry, such as viscera, skin, scales and bones, representing up to 30–80% of the fish body weight, are discarded as solid wastes by industrial fish-processing operations [8] but, due to their composition, they have great potential to be used as protein supplements in aquaculture feeds [9,10]. Their conversion to aquafeed is also encouraged by the significant advantage that they do not require any thermal–chemical and/or enzymatic hydrolysis pretreatment steps. Since the pretreatment step is neither economically favorable nor environment-friendly, its elimination from the process makes the utilization of fish waste economic and more environmentally friendly [7].
Biotechnological methods like fermentation with microbe cultures are gaining more popularity for the treatment of waste [11,12].
Among the different microbes used, especially for the fermentation of animal/fish processing wastes, lactic bacteria have advantages over other microbes, as they are generally recognized as safe (GRAS) [13]. In addition, the products obtained upon fermentation with Lactobacillus are also reported to have additional beneficial effects on various aquatic animal intestines (anti-microbial properties, antioxidative properties), making them suitable for food/feed applications. In fact, they easily adapt to the intestinal environment of both aquatic and domestic animals, making them favorable for use in probiotic aquaculture feeds [14,15,16,17,18].
Among the microorganisms applied, yeasts have also been used as inoculum, along with lactic bacteria, to ferment fish waste [19] for converting it to a useful product that can be used as an ingredient to balance the food rations of animals. Yeast has many different immunostimulatory compounds, e.g., nucleic acid, b-glucans, and mannan oligosaccharides [20,21]. These compounds may enhance the growth of different fish species and therefore can be considered as the best health promoters for fish culture [22].
Feed nutritional composition is important; the major growth-promoting factors are proteins and lipids, since they are known to influence the growth and the body composition of fish [23,24].
Fermented fish waste is a liquid product, obtained by the liquefaction of tissues carried out by the enzymes already present in the fish and accelerated by an acid pH [10]. Natural fillers, such as agricultural by-products, can also be added to the substrate [25].
Citrus peel can be used as filler [26] during fermentation, playing at the same time an important role as a prebiotic source [27,28,29]. Among its beneficial effects, it has been reported that prebiotics can elevate fish resistance to pathogens and improve growth performance, feed utilization and lipid metabolism, as well as stimulating the immune response through the modulation of intestinal microbiota [18,30,31,32].
The aim of this research was to process non-sterilized fish wastes, supplemented with lemon peel as a filler and prebiotic source, by biological fermentation using combined starter cultures of Saccharomyces cerevisiae American Type Culture Collection (ATCC)4126 and Lactobacillus reuteri ATCC 53608 for bio-transforming these by-products into a high protein content supplement, rich in healthy microorganisms, for aquaculture feeds.
For this purpose, and to verify the optimum nutritional composition of aquafeed, proteins, crude lipids, ash and lipid content percentages were monitored throughout the process. The influence of the fermentation process on fatty acid concentrations was also evaluated. Finally, microbiological analyses of the starters and total and fecal coliform bacteria quantification were carried out, to evaluate the healthiness of the final product.
2. Materials and Methods
2.1. Substrate
Fish by-products (non-edible parts) of Dicentrarchus labrax, represented by the head, viscera, skin and bones, were provided by Acqua Azzurra S.p.a. (Pachino, Italy). Samples were collected directly at the farm, forwarded to the laboratory under refrigerated conditions, and stored at −20 °C until tests were performed. Lemon peel was provided by Simone Gatto S.r.l. (San Pier Niceto, Italy) and stored at −20 °C until use.
2.2. Microorganisms
Saccharomyces cerevisiae ATCC 4126 was maintained on yeast medium (YM) agar (yeast extract 3 g/L, malt extract 3 g/L, peptone 5 g/L, glucose 10 g/L, agar 20 g/L; Oxoid, Basingstoke, UK) and Lactobacillus reuteri ATCC 53608, then maintained on MRS (de Man, Rogosa, Sharpe) agar (peptone 10 g/L, “Lab-Lemco” powder 8 g/L, yeast extract 4 g/L, sorbitan mono-oleate 1 mL, di-potassium hydrogen phosphate 2 g/L, sodium acetate tri-hydrate 5 g/L, tri-ammonium citrate 2 g/L, magnesium sulfate heptahydrate 0.2 g/L, manganese sulfate tetrahydrate 0.05 g/L, agar 10 g/L; Oxoid, Basingstoke, UK) at 4 °C. To carry out the tests, S. cerevisiae and L. reuteri were cultured overnight at 35 °C and 37 °C, respectively, on a rotary shaker (INNOVA 44, Incubator Shaker Series, New Brunswick Scientific, Edison, NJ, USA) at 200 rpm, in tubes containing 20 mL YM medium for the yeast and MRS broth for the bacteria.
2.3. Experimental Set-Up
Fermentation tests were carried out in a 5 L batch fermenter (Biostat Biotech B, Sartorius Stedim Biotech, Goettingen, Germany. The fermenter was equipped with one four-bladed Rushton turbine and the usual control systems: temperature, pH, pO2 and a foam detector. Fish waste and lemon peel (2:1 w/w) were homogenized in a blender for 5 min.
The resulting homogenate, with a dry matter content of 40% (w/w) was supplemented with 20 mL of S. cerevisiae (108 cells per mL) and 20 mL of L. reuteri culture (108 cells per mL), simultaneously. No sterilization procedures were adopted.
Fermentation parameters were 35 °C, with pH 5.0 and constant stirring at 200 rpm, and a final working volume of 3.5 L.
All fermentations were carried out for 120 h until no further growth of the selected microorganisms was observed, and the pH value became stable. The pH was not controlled by alkali addition during cultivation.
Medium samples were withdrawn daily from the reaction vessel using a sterile 20 mL syringe and immediately frozen at −20 °C until analysis.
2.4. Yeast Cell, Lactic Acid Bacteria and Coliform Bacteria Numbers
Suspended yeast cells and lactic acid bacteria were counted via the dilution plating method, whereas coliform bacteria were counted via the MPN (most probable number) method.
The CFU (colony-forming unit) of suspended yeast was counted by culturing at 35 °C in yeast media agar (pH 5.8) containing 100 mg/mL chloramphenicol (Oxoid, Basingstoke, UK). Lactic acid bacterial colonies were counted by assessing acid formation at 37 °C in the MRS agar (pH 6.8) containing 50 mg/mL bromocresol purple (Oxoid, Basingstoke, UK). Coliform bacteria grown at 37 °C in lauryl tryptose broth (Oxoid, Basingstoke, UK) (pH 6.8) and the gas-forming bacteria were confirmed on green bile medium (Oxoid, Basingstoke, UK) (pH 7.4).
2.5. Protein, Moisture, and Ash Determination
Representative samples were drained off daily for protein content testing, using the method suggested by the AOAC (Association of Official Agricultural Chemists, Rockville, MD, USA) [33]. The protein percentage was calculated considering a conversion factor of 6.25. The dry weights, both of the fresh waste and fermentation samples, were calculated as steady weights after 2 h at 110 °C, using a Mettler PM 200 equipped with a Mettler LP16 IR balance (Mettler-Toledo GmbH, Laboratory & Weighing Technologies, Greifensee, Switzerland). Ash determination was carried out according to the AOAC method [33]. All samples were analyzed in triplicate.
2.6. Crude Fat and Fatty Acid Determination
Samples were extracted with a mixture of chloroform and methanol (2:1). The mixture was allowed to stand overnight and the lower lipid layer, transferred into a pretreated and weighed flask, was dried off. The difference in the two weights established the weight of the fat [24].
The fatty acid analysis was performed by gas chromatography after transmethylation with 2% H2SO4 in methanol at 80 °C. The separation and quantification of fatty acid methyl esters were conducted with a Dani Master GC 1000, equipped with an FID detector (Dani Instruments, Milan, Italy) and a capillary column Supelco SLB-IL100 60 m × 0.25 mm, film 0.20 μm(Merck KGaA, Darmstadt, Germany), using the following experimental conditions: injector temperature 220 °C; oven temperature from 120 °C to 200 °C (10 min hold) at a rate of 1 °C/min; detector temperature 240 °C; carrier gas He at a constant velocity rate of 34 cm/sec; and a split ratio of 1:50.
Fatty acids were identified by comparing the samples with reference standards, using Supelco 37 component Fatty Acid Methyl Esters (FAME) mix in methylene chloride. All samples were analyzed in triplicate.
All chemicals were provided by Merk Life Science (Merck KGaA, Darmstadt, Germany).
2.7. Statistical Analysis
The studies of significant differences were carried out via Kruskal–Wallis tests, using the SPSS 13.0 software package for Windows (SPSS Inc., Chicago, IL, USA). The H statistic and asymptotic significance are the Kruskal–Wallis test output values that allow the significance evaluation of differences when more than two groups are considered.
3. Results and Discussion
3.1. Substrate Fermentation
The time course of fermentation by yeast and lactobacilli is shown in Figure 1, as well as the pH trend.
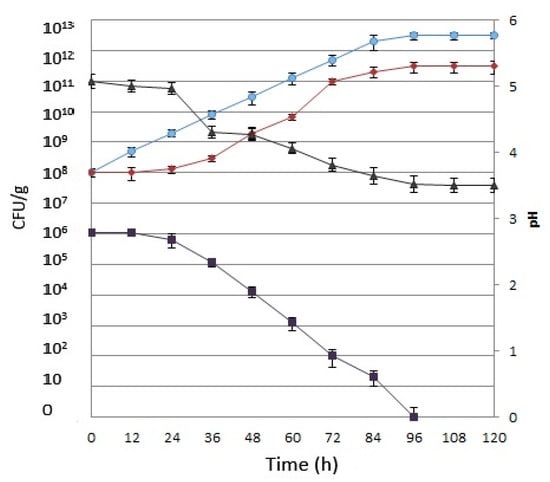
Figure 1.
Lactobacillus reuteri (circle), Saccharomyces cerevisiae (diamond), and coliform (square) concentrations, reported as colony-forming unit (CFU) per g, and pH (triangle) values recorded during the fermentation.
The growth of S. cerevisiae was slow during the first 24 h of fermentation, maintaining a concentration of 108 CFU/g. The amount of S. cerevisiae reached a concentration of 1011 CFU/g after 72 h, remaining stable until the end of the process.
L. reuteri increased constantly from the beginning of the fermentation until after 96 h, rising from 108 CFU/g up to 1012 CFU/g, reaching a steady state until the end of the process. According to Giraffa et al. [17] and Hoseinifar et al. [18], this represents an added value for the resulting aquafeed.
The reduction in pH was slow during the first 24 hours of fermentation because of microorganism adaptations at the beginning of the process [19,34]. In the presence of acid lactic bacteria and yeast, after 24 h the pH of the mixture became stable at 3.5 after 96 h. The decrease in pH in the substrate offers evidence of good acidification through lactic fermentation by the starter cultures and represents the most important factor to control in biotransformation. Acidification must be achieved as quickly as possible, in order to inhibit the growth of pathogenic and spoilage microorganisms in the substrate, increasing the shelf life of the resulting fermented substrate [10,35,36]. Moreover, considering that no sterilization procedures were carried out, the quick drop in pH was found to be necessary for maintaining microbial hygiene, along with retaining the quality of the product as an aquaculture feed [37].
In fact, the amount of initial substrate total coliforms was 106 CFU/g, whereas no fecal coliforms were detected. The microbiological analysis for total coliform determination showed a net decrease during the fermentation, to reach a complete absence after 96 h (Figure 1).
The reduction in coliform numbers could be due to some inhibitory compounds (bacteriocins) formed by the microorganisms employed during lactic acid fermentation and/or to the acidification of the medium [19]. Moreover, the decrease in coliforms may ensure good biopreservation against undesirable and/or hazardous microorganisms.
The final fermented products were low in spoilage microorganisms and rich in healthy microorganisms, representing a healthy final substrate enriched by added value.
The starter cultures’ capability of growing at low pH can be ascribed to the lemon peel supplementation since polysaccharides, such as pectins, show a protective effect on lactic acid bacteria LAB against low pH [38,39]. Their ability to achieve this on fermenting fish waste supplemented by lemon peel was confirmed by the protein level’s increasing during the process, up to 48.55%, making these wastes an excellent raw material for aquafeed production with Lactobacillus reuteri and Saccharomyces cerevisiae.
3.2. Substrate Protein, Ash and Crude Lipids Concentration
In Table 1, the protein, crude lipid, and ash percentages at different fermentation times are reported. All statistical evaluations were performed at α = 0.05.

Table 1.
Protein, crude lipid, and ash percentages, after different fermentation times.
The substrate’s initial protein content was 11.68 ± 0.48%. It increased slowly by 72 h, reaching up to 32.09 ± 0.77%. The highest protein percentage in the substrate, 48.55 ± 1.15%, was reached after 96 h. This value remained stable until the end of the fermentative process, allowing to obtain a substrate rich in protein, achieving a suitable percentage for aquafeed formulation according to Nasseri et al. [34]. The protein content against the CFU of the yeast and LAB is reported in Figure 2.
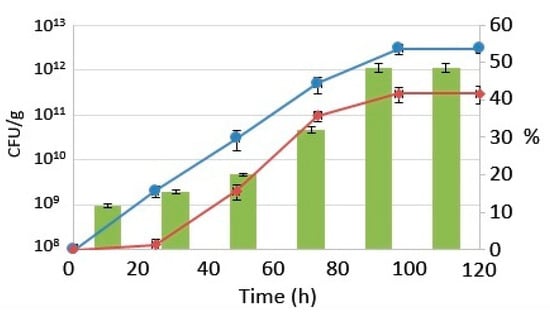
Figure 2.
Lactobacillus reuteri (circle), Saccharomyces cerevisiae (diamond), reported as colony-forming unit (CFU) per g, and protein (bars) levels increasing during the fermentation process, reported as percentages.
During all fermentations, ash concentrations decreased significantly, from 0.83 ± 0.04% to 0.66 ± 0.03%. This could be due to partial ash utilization by the yeast as a source of minerals [40].
The crude lipid content calculated on the initial substrate was 13.74 ± 0.72%. Throughout the process, this value did not increase significantly; at the end of the fermentation period, it reached just 15.25 ± 0.80%.
According to the literature [8,35,41], final protein and lipid contents had reached adequate levels in the resulting aquafeed, offering a way to ameliorate the problem of a lack of protein sources in aquaculture by encouraging the conversion of fish waste into feed, using a low-cost process such as lactic fermentation.
3.3. Fatty Acid Composition
Fatty acid contents at different fermentation times are shown in Table 2. At the end of the fermentation period, as confirmed by statistical tests with significances higher than 95%, MUFAs (monounsaturated fatty acids) increased significantly (+23.5%) and PUFAs (polyunsaturated fatty acids) decreased (−22.7%), whereas the SFA (saturated fatty acids) content was not affected by the fermentation process. Changes in fatty acid composition during the fermentation process are shown in Table 3. The main fatty acids detected throughout the process were C18:1 n-9 cis (33.02%), C18:2 n-6 cis (20.49%) and C16:0 (17.53%). C14:1, C17:0, C17:1, C20:0, and C21:0 were detected, starting after 72 h of the process. A significant increase in concentration for C20:1 n-9, C 20:2 n-6 and C 24:1 n-9 was observed, whereas C18:2 n-6 cis, C20:3 n-6, C20:4 n-6, C20:5 n-3, C22:2, C23:0 and C24:0 showed a significant opposite trend. According to Nadège et al. [23] and Babalola and Apata [42], this fatty-acid composition is suitable for aquafeed formulations in which the shelf life could be extended because of the decrease in polyunsaturated fatty acids.

Table 2.
Fatty acid contents at different fermentation times.

Table 3.
Fatty acid contents (%) at different fermentation times.
Previous studies carried out by Fickers et al. [43] and Yano et al. [44] reported similar fatty acid behavior. This trend can be ascribed to the yeast and lactic bacteria activities that degrade fats for single-cell protein production [44] and to obtain the energy necessary for metabolic activities during the fermentation process [45]. The fatty acids resulting from the degradation of lipids are subsequently degraded through the β-oxidation system in the yeast cells [44], resulting in a reduction in polyunsaturated fatty acids.
4. Conclusions
This study demonstrated an effective approach to utilizing the demonstrated fermented substrate for aquafeed, starting with fish and lemon peel wastes, allowing the conversion of both animal and vegetable food wastes into an added-value product.
The final fermented product is low in spoilage microorganisms and rich in healthy microorganisms, representing a healthy final substrate enriched by added value.
The microorganisms’ ability to feed on fermenting fish waste that is supplemented by lemon peel was confirmed by the protein level’s increasing during the process, up to 48.55%, making these wastes an excellent raw material for aquafeed production via Lactobacillus reuteri and Saccharomyces cerevisiae. In fact, the final protein and lipid contents represent adequate levels in the resulting aquafeed, reducing the problem of a lack of protein sources for aquaculture by encouraging the conversion of fish waste and lemon peel into feed.
This study pointed out the possibility of setting up a fermentation process based on the simultaneous addition of two different microorganisms, reaching a plateau after 96 h. Further studies are in progress for converting the resulting fermentation product into pellets and for testing the effect of the final product on the growth and immune response of fish from aquaculture and, consequently, in human consumers.
Finally, additional work will be needed to further optimize production to facilitate future larger-scale production, also evaluating it from an economic point of view.
Author Contributions
Conceptualization, A.T., V.L.T., A.G.P. and G.D.B.; methodology, A.T., V.L.T., A.G.P. and G.D.B.; validation, A.T., V.L.T., A.G.P. and G.D.B.; formal analysis, A.T., V.L.T., A.G.P. and G.D.B.; investigation, A.T., V.L.T., A.G.P., E.R., R.V. and R.R.; resources, A.T., V.L.T., A.G.P. and G.D.B.; data curation, A.T., V.L.T., A.G.P., E.R., R.V., R.R. and G.D.B.; writing—original draft preparation, A.T., V.L.T., A.G.P., E.R., R.R. and G.D.B.; writing—review and editing, A.T., V.L.T., A.G.P. and G.D.B.; supervision, A.T. and G.D.B.; project administration, A.T. and G.D.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Agriculture Organization of the United Nations. The State of World Fisheries and Aquaculture; FAO: Rome, Italy, 2020; ISSN 2410-5902. [Google Scholar]
- Tacon, A.G.J.; Metian, M. Global overview on the use of fishmeal and fish oil in industrially compounded aquafeeds: Trends and future prospects. Aquaculture 2008, 285, 146–158. [Google Scholar] [CrossRef]
- Nwanna, L.C. Nutritional Value and Digestibility of Fermented Shrimp Head Waste Meal by African Catfish Clariasgariepinus. Pak. J. Nutr. 2003, 2, 339–345. [Google Scholar] [CrossRef]
- Samuels, W.A.; Fontenot, J.P.; Allen, V.G.; Abazinge, M.D. Seafood processing wastes ensiled with straw: Utilization and intake by sheep. J. Anim. Sci. 1991, 69, 4983–4992. [Google Scholar] [CrossRef]
- Westendorf, M.L.; Dong, Z.C.; Schoknecht, P.A. Recycled cafeteria food waste as a feed for swine: Nutrient content digestibility, growth, and meat quality. J. Anim. Sci. 1998, 76, 2976–2983. [Google Scholar] [CrossRef] [PubMed]
- Westendorf, M.L. Food Waste as Animal Feed: An Introduction. In Food Waste to Animal Feed; Michael, L., Ed.; Iowa State University Press: Ames, IA, USA, 2000; pp. 3–16, 69–90. [Google Scholar]
- Suan, S.; Jing, L.; Wenjian, G.; David, B. Nutrient value of fish manure waste on lactic acid fermentation by Lactobacillus pentosus. R. Soc. Chem. 2018, 8, 31267–31274. [Google Scholar] [CrossRef]
- Zhiwen, Z.; Baiyu, Z.; Qinhong, C.; Jingjing, L.; Kenneth, L.; Bing, C. Fish Waste Based Lipopeptide Production and the Potential Application as a Bio-Dispersant for Oil Spill Control. Front. Bioeng. Biotechnol. 2020, 8, 734. [Google Scholar] [CrossRef]
- Hua, K.; Cobcroft, J.M.; Andrew, C.; Condon, K.; Jerry, D.R.; Mangott, A.; Praeger, C.; Vucko, M.J.; Zeng, C.; Zenger, K.; et al. The Future of Aquatic Protein: Implications for Protein Sources in Aquaculture Diets. One Earth 2019, 1, 316–329. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Kassaveti, A. Fish industry waste: Treatments, environmental impacts, current and potential uses. Int. J. Food Sci. Technol. 2008, 43, 726–745. [Google Scholar] [CrossRef]
- Coello, N.; Montiel, E.; Concepcion, M.; Christen, P. Optimization of a culture medium containing fish silage for L-lysine production by Corynebacterium glutamicum. Bioresour. Technol. 2002, 85, 207–211. [Google Scholar] [CrossRef]
- Tropea, A.; Wilson, D.; La Torre, G.L.; Lo Curto, R.B.; Saugman, P.; Troy-Davies, P.; Dugo, G.; Waldron, K.W. Bioethanol Production From Pineapple Wastes. J. Food Res. 2014, 3, 60–70. [Google Scholar] [CrossRef]
- Amit, K.R.; Swapna, H.C.; Bhaskar, N.; Halami, P.M.; Sachindra, N.M. Effect of fermentation ensilaging on recovery of oil from fresh water fish viscera. Enzym. Microb. Technol. 2010, 46, 9–13. [Google Scholar] [CrossRef]
- Bernardeau, M.; Guguen, M.; Vernoux, J.P. Beneficial lactobacilli in food and feed: Long-term use biodiversity and proposal for specific and realistic safety assessments. FEMS Microbiol. 2006, 30, 487–513. [Google Scholar] [CrossRef] [PubMed]
- Bucio, A.; Hartemink, R.; Schrama, J.W.; Verreth, J.; Rombouts, F.M. Presence of lactobacilli in the intestinal content of freshwater fish from a river and from a farm with a recirculation system. Food Microbiol. 2006, 23, 476–482. [Google Scholar] [CrossRef]
- Balcazar, J.L.; Venderll, D.; de Blas, I.; Ruiz-Zarzuela, I.; Muzquiz, J.L.; Girones, O. Characterization of probiotic properties of lactic acid bacteria isolated from intestinal microbiota of fish. Aquaculture 2008, 278, 188–191. [Google Scholar] [CrossRef]
- Giraffa, G.; Chanishvili, N.; Widyastuti, Y. Importance of lactobacilli in food and feed biotechnology. Res. Microbiol. 2010, 161, 480–487. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Sharifian, M.; Vesaghi, M.J.; Khalili, M.; Esteban, M.Á. The effects of dietary xylooligosaccharide on mucosal parameters, intestinal microbiota and morphology and growth performance of Caspian white fish (Rutilus frisiikutum) fry. Fish Shellfish. Immunol. 2014, 39, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Ennouali, M.; Elmoualdi, L.; Labioui, H.; Ouhsine, M.; Elyachioui, M. Biotransformation of the fish waste by fermentation. Afr. J. Biotechnol. 2006, 5, 1733–1737. [Google Scholar] [CrossRef]
- White, L.A.; Newman, M.C.; Cromwell, G.L.; Lindemann, M.D. Brewers dried yeast as a source of mannan oligosaccharides for weanling pigs. J. Anim. Sci. 2002, 80, 2619–2628. [Google Scholar] [CrossRef]
- Tropea, A.; Wilson, D.; Cicero, N.; Potortì, A.G.; La Torre, G.L.; Dugo, G.; Richardson, D.; Waldron, K.W. Development of minimal fermentation media supplementation for ethanol production using two Saccharomyces cerevisiae strains. Nat. Prod. Res. 2016, 30, 1009–1016. [Google Scholar] [CrossRef]
- Lara-Flores, M.; Olvera-Novoa, M.A.; Guzma’n-Me’ndez, B.E.; Lo’-pez-Madrid, W. Use of the bacteria Streptococcus faecium and Lactobacillus acidophilus, and the yeast Saccharomyces cerevisiae as growth promoters in Nile tilapia (Oreochromis niloticus). Aquaculture 2002, 216, 193–201. [Google Scholar] [CrossRef]
- Nadège, R.; Mourente, G.; Sadasivam, K.; Corraze, G. Replacement of a large portion of fish oil by vegetable oils does not affect lipogenesis, lipid transport and tissue lipid uptake in European seabass (Dicentrarchus labrax L.). Aquaculture 2006, 261, 1077–1087. [Google Scholar] [CrossRef]
- Mahmud, N.A.; Robiul Hasan, M.D.; Hossain, M.B.; Minar, M.H. Proximate Composition of Fish Feed Ingredients Available in Lakshmipur Region, Bangladesh. Am.-Eurasian J. Agric. Environ. Sci. 2012, 12, 556–560. [Google Scholar]
- Soltan, M.A.; El-Laithy, S.M. Evaluation of fermented silage made from fish, tomato and potato by-products as a feed ingredient for Nile tilapia. Oreochromis niloticus. Egypt. J. Aquat. BioiFish 2008, 12, 25–41. [Google Scholar] [CrossRef][Green Version]
- Soltan, M.A.; Hanafy, M.A.; Wafa, M.I.A. An evaluation of fermented silage made from fish by-products as a feed ingredient for african catfish (Clariasgariepinus). Glob. Vet. 2008, 2, 80–86. [Google Scholar]
- Manderson, K.; Pinart, M.; Tuohy, K.M.; Grace, W.E.; Hotchkiss, A.T.; Widmer, W.; Yadhav, M.P.; Gibson, G.R.; Rastall, R.A. In Vitro Determination of Prebiotic Properties of Oligosaccharides Derived from an Orange Juice Manufacturing By-Product Stream. Appl. Environ. Microbiol. 2005, 71, 8383–8389. [Google Scholar] [CrossRef] [PubMed]
- Roberfroid, M. Prebiotics: The concept revisited. J. Nutr. 2007, 137, 830–837. [Google Scholar] [CrossRef]
- Gomez, B.; Gullon, B.; Remoroza, C.; Schols, H.A.; Parajo, J.C.; Alonso, J.L. Purification, Characterization, and Prebiotic Properties of Pectic Oligosaccharides from Orange Peel Wastes. J. Agric. Food Chem. 2014, 62, 9769–9782. [Google Scholar] [CrossRef]
- Gibson, G.R. Fibre and effects on probiotics (the prebiotic concept). Clin. Nutr. Suppl. 2004, 1, 25–31. [Google Scholar] [CrossRef]
- Ringø, E.; Hoseinifar, S.H.; Ghosh, K.; Doan, H.V.; Beck, B.R.; Song, S.K. Lactic acid bacteria in finfish—An update. Front. Microbiol. 2018, 9, 1818. [Google Scholar] [CrossRef]
- Ringø, E.; Dimitroglou, A.; Hoseinifar, S.H.; Davies, S.J. Prebiotics in finfish: An update. In Aquaculture Nutrition: Gut Health, Probiotics and Prebiotics; Ringø, E., Merrifield, D.L., Eds.; Wiley-Blackwell Scientific Publication: London, UK, 2014. [Google Scholar] [CrossRef]
- Latimer, G.W. Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 2016. [Google Scholar]
- Nasseri, A.T.; Rasoul-Amini, S.; Morowvat, M.H.; Ghasemi, Y. Single Cell Protein: Production and Process. Am. J. Food Technol. 2011, 6, 103–116. [Google Scholar] [CrossRef]
- Shigeaki, I.; Kyoko, S.U.; Yukako, K.; Akemi, K.; Isao, Y.; Koudai, T.; Ayumi, M.; Kunimasa, K. Fermentation of non-sterilized fish biomass with a mixed culture of film-forming yeasts and lactobacilli and its effect on innate and adaptive immunity in mice. J. Biosci. Bioeng. 2013, 116, 682–687. [Google Scholar] [CrossRef]
- García-Díez, J.; Saraiva, C. Use of Starter Cultures in Foods from Animal Origin to Improve Their Safety. Int. J. Environ. Res. Public Health 2021, 18, 2544. [Google Scholar] [CrossRef] [PubMed]
- Ayan, S. A review of fish meal replacement with fermented biodegradable organic wastes in aquaculture. Int. J. Fish. Aquat. Stud. 2018, 6, 203–208. [Google Scholar]
- Nadja, L.; Thiago, B.C.; Susana, M.I.S.; Andreas, B.; Lene, J. The effect of pectins on survival of probiotic Lactobacillus spp. in gastrointestinal juices is related to their structure and physical properties. Food Microbiol. 2018, 74, 11–20. [Google Scholar] [CrossRef]
- Huan, L.; Mingyong, X.; Shaoping, N. Recent trends and applications of polysaccharides for microencapsulation of probiotics. Food Front. 2020, 1, 45–59. [Google Scholar] [CrossRef]
- Araya-Cloutier, C.; Rojas-Garbanzo, C.; Velàzquez-Carrillo, C. Effetct of initial sugar concentration on the production of L(+) lactic acid by simultaneous enzymatic hydrolysis and fermentation of an agro-industrial waste product of pineapple (Ananas comosus) using Lactobacillus casei subspeies rhamnosus. Int. J. Wellness Ind. 2012, 1, 91–100. [Google Scholar] [CrossRef][Green Version]
- Craig, S.; Helfrich, L.A. Understanding Fish Nutrition, Feeds and Feeding. Va. Coop. Ext. 2002. Available online: https://vtechworks.lib.vt.edu/bitstream/handle/10919/80712/FST-269.pdf (accessed on 19 November 2021).
- Babalola, T.O.O.; Apata, D.F. Chemical and quality evaluation of some alternative lipid sources for aqua feed production. Agric. Biol. J. N. Am. 2011, 2, 935–943. [Google Scholar] [CrossRef]
- Fickers, P.; Benetti, P.H.; Wache, Y.; Marty, A.; Mauersberger, S.; Smit, M.S.; Nicaud, J.M. Hydrophobic substrate utilization by the yeast Yarrowia lipolytica, and its potential applications. FEMS Yeast Res. 2005, 5, 527–543. [Google Scholar] [CrossRef]
- Yano, Y.; Oikawa, H.; Satomi, M. Reduction of lipids in fishmeal prepared from fish waste by a yeast Yarrowia lipolytica. Int. J. Food Microbiol. 2007, 121, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Oseni, O.A.; Akindahunsi, A.A. Some phytochemical properties and effect of fermentation on the seed of Jatropha curcas L. J. Food Technol. 2011, 6, 158–165. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).