Xylitol Production by Candida Species from Hydrolysates of Agricultural Residues and Grasses
Abstract
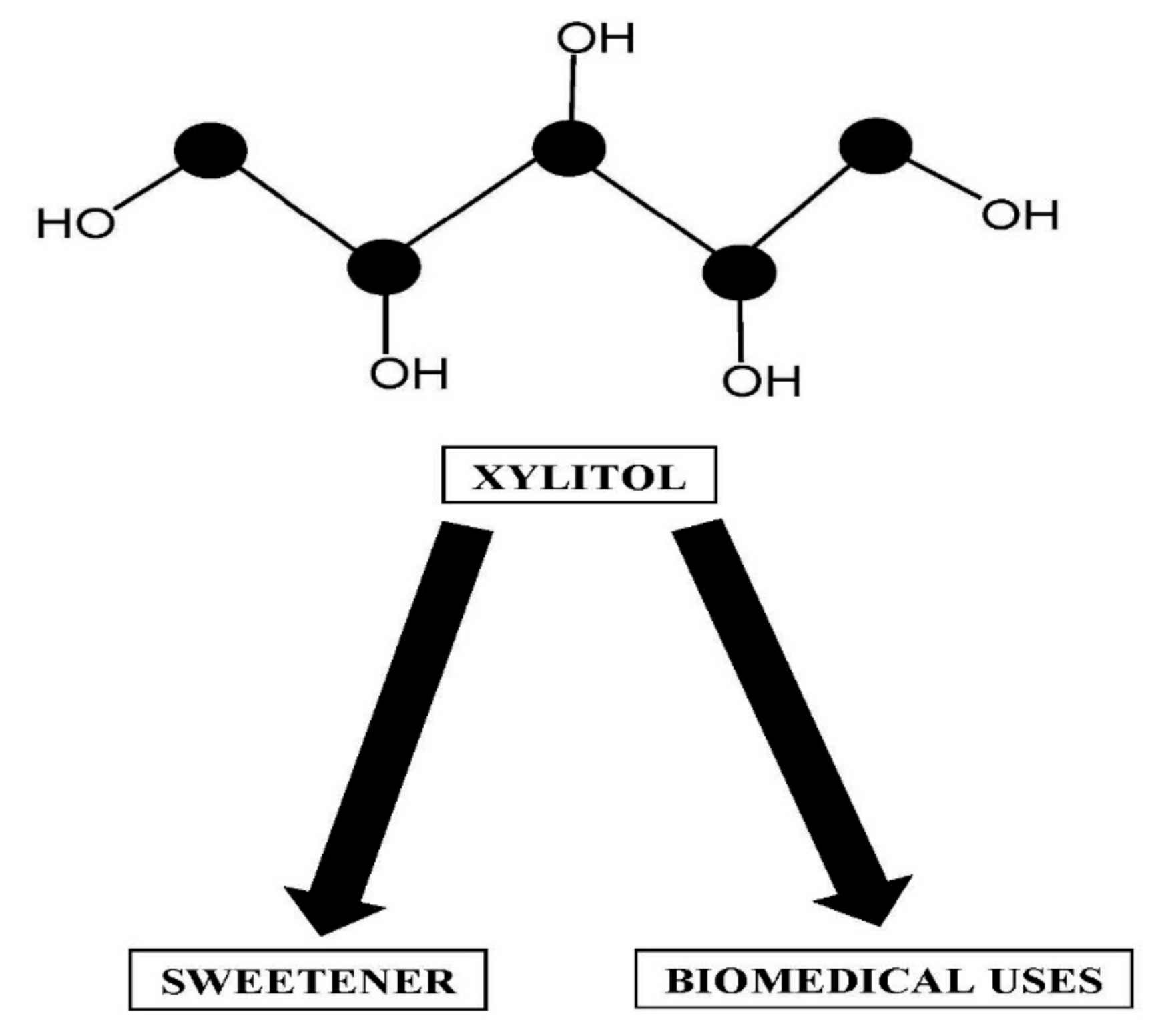
:1. Introduction
2. Pathway of Xylitol Biosynthesis in the Yeast Candida
3. Xylitol Production by Candida Species from Agricultural Residues
| Agricultural Residue | Candida Species/ Strain | Growth Conditions | Xylitol Level (g/L) 1 | Yield (g/g) 1 | Reference |
|---|---|---|---|---|---|
| Apple pomace | C. guilliermondii ATCC 201935 | 96 h, 30 °C | 9.4 | 0.38 | [33] |
| Banana leaves | C. tropicalis MTCC 230 | 48 h, 30 °C | 12.4 | 0.47 | [34] |
| Chestnut shells | C. tropicalis M43 | 120 h, 30 °C | 6.3 | 0.19 | [35] |
| Cocoa pod husks | C. boidinii XM02G | 372 h, 30 °C | 11.3 | 0.52 | [36] |
| Corncob | C. tropicalis CCTCC M2012462 | 84 h, 35 °C | 38.8 | 0.70 | [38] |
| Corncob | C. tropicalis MTCC 6192 | 90 h, 30 °C | 29.6 | 0.60 | [39] |
| Corncob | C. tropicalis MTCC 6192 | 48 h, 30 °C | 40.0 | 0.62 | [40] |
| Corncob | C. tropicalis PNL3 | 24 h, 35 °C | 30.5 | 0.61 | [41] |
| Corncob | C. tropicalis NCIM 3123 | 72 h, 30 °C | 41.0 | 0.73 | [43] |
| Cotton stalk | C. tropicalis KUEN 1022 | 96 h, 30 °C | 3.5 | 0.36 | [44] |
| Olive pomace | C. boidinii NCAIM Y.01308 | 96 h, 30 °C | 6.0 | 0.43 | [45] |
| Olive pruning | C. tropicalis NBRC 0618 | 96 h, 30 °C | ND | 0.23 | [46] |
| Rapeseed straw | C. guilliermondii ATCC 201935 | 144 h, 30 °C | 14.4 | 0.42 | [47] |
| Rice straw | C. tropicalis MTCC 6192 | 96 h, 30 °C | 25.8 | 0.60 | [48] |
| Rice straw | C. tropicalis ATCC 9968 | 50 h, 30 °C | 26.5 | 0.58 | [49] |
| Sisal fiber | C. tropicalis CCT 1516 | 60 h, 30 °C | ND | 0.32 | [50] |
| Sugarcane bagasse | C. guilliermondii ATCC 201935 | 72 h, 30 °C | 17.0 | 0.42 | [51] |
| Sugarcane bagasse | C. magnoliae TISTR 5663 | 288 h, 30 °C | 4.8 | 0.30 | [52] |
| Sugarcane bagasse | C. tropicalis Y-27290 | 40 h, 30 °C | 34.5 | 0.86 | [53] |
| Sugarcane bagasse | C. tropicalis MTCC 184 | 36 h, 30 °C | 10.2 | 0.56 | [54] |
| Sugarcane bagasse | C. tropicalis JA2 | 39 h, 30 °C | 109.5 | 0.86 | [55] |
| Sugarcane bagasse | C. tropicalis UFMGBX12 | 96 h, 30 °C | 12.0 | 0.61 | [56] |
| Sugarcane straw | C. guilliermondii ATCC 201935 | 48 h, 30 °C | 8.7 | 0.67 | [57] |
| Water hyacinth leaves | C. tropicalis MTCC 230 | 48 h, 30 °C | 9.9 | 0.47 | [34] |
| Wheat bran | C. boidinii NCAIM Y.01308 | 24 h, 30 °C | 14.2 | 0.72 | [61] |
4. Xylitol Production by Candida Species from Grasses
5. Conclusions
Funding
Conflicts of Interest
References
- Gränstrom, T.B.; Izumori, K.; Leisola, M. A rare sugar xylitol. Part II: Biotechnological production and future applications of xylitol. Appl. Microbiol. Biotechnol. 2007, 74, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Edomi, A. Xylitol: Its properties and food applications. Food Technol. 1978, 32, 20–32. [Google Scholar]
- Petch, D.; Butler, M. The effect of alternative carbohydrates on the growth and antibody production of a murine hybridoma. Appl. Biochem. Biotechnol. 1996, 59, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Uhari, M.; Kontiokari, T.; Niemela, M. A novel use of xylitol sugar in preventing acute otitis media. Pediatrics 1998, 102, 879–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subroto, E.; Hayati, F. Chemical and biotechnological methods for the production of xylitol: A review. IJETER 2020, 8, 2508–2512. [Google Scholar] [CrossRef]
- Xiang, S.; Ye, K.; Li, M.; Ying, J.; Wang, H.; Han, J.; Shi, L.; Xiao, J.; Shen, Y.; Feng, X.; et al. Xylitol enhances synthesis of propionate in the colon via cross-feeding of gut microbiota. Microbiome 2021, 9, 62. [Google Scholar] [CrossRef]
- Xu, Y.; Chi, P.; Bilal, M.; Cheng, H. Biosynthetic strategies to produce xylitol: An economical venture. Appl. Microbiol. Biotechnol. 2019, 103, 5143–5160. [Google Scholar] [CrossRef]
- Silva, S.S.; Matos, Z.R.; Carvalho, W. Effects of sulfuric acid loading and resident time on the composition of sugarcane bagasse hydrolysate and its use as a source of xylose for xylitol bioproduction. Biotechnol. Prog. 2005, 21, 1449–1452. [Google Scholar] [CrossRef]
- Saha, B.C.; Kennedy, G.J. Production of xylitol from mixed sugars of xylose and arabinose without co-producing arabitol. Biocatal. Agric. Biotechnol. 2020, 29, 101786. [Google Scholar] [CrossRef]
- Zhang, Y.-H.P. Reviving the carbohydrate economy via multiproduct lignocellulose biorefineries. J. Ind. Microbiol. Biotechnol. 2008, 35, 367–375. [Google Scholar] [CrossRef]
- Mulkey, V.R.; Owens, V.N.; Lee, D.K. Management of warm-season grass mixtures for biomass production in South Dakota USA. Bioresour. Technol. 2008, 99, 609–617. [Google Scholar] [CrossRef]
- West, T.P.; Peterson, J.L. Production of the polysaccharide curdlan by an Agrobacterium strain grown on a plant biomass hydrolysate. Can. J. Microbiol. 2014, 60, 53–56. [Google Scholar] [CrossRef] [PubMed]
- West, T.P. Effect of nitrogen source concentration on curdlan production by Agrobacterium sp. ATCC 31749 grown on prairie cordgrass hydrolysates. Prep. Biochem. Biotechnol. 2016, 46, 85–90. [Google Scholar] [CrossRef]
- West, T.P. Fungal production of the polysaccharide pullulan from a plant hydrolysate. Z. Naturforsch. C 2017, 72, 491–496. [Google Scholar] [CrossRef]
- Kennedy, D.E., II; West, T.P. Effect of yeast extract addition to a mineral salts medium containing hydrolyzed plant xylan on fungal pullulan production. Z. Naturforsch. C 2018, 73, 319–323. [Google Scholar] [CrossRef] [PubMed]
- West, T.P. Production of the polysaccharide curdlan by Agrobacterium species on processing coproducts and plant lignocellulosic hydrolysates. Fermentation 2020, 6, 16. [Google Scholar] [CrossRef] [Green Version]
- Onishi, H.; Suzuki, T. Microbial production of xylitol from glucose. Appl. Microbiol. 1969, 18, 1031–1035. [Google Scholar] [CrossRef]
- Barbosa, M.F.S.; de Medeiros, M.B.; de Mancilha, I.M.; Schneider, H.; Lee, H. Screening of yeasts for production of xylitol from D-xylose and some factors which affect xylitol yield in Candida guilliermondii. J. Ind. Microbiol. 1988, 3, 241–251. [Google Scholar] [CrossRef]
- Mayerhoff, Z.D.V.L.; Roberto, I.C.; Silva, S.S. Xylitol production from rice straw hemicellulose using different yeast strains. Biotechnol. Lett. 1997, 19, 407–409. [Google Scholar] [CrossRef]
- Guo, C.; Zhao, C.; He, P.; Shen, A.; Jiang, N. Screening and characterization of yeasts for xylitol production. J. Appl. Microbiol. 2006, 101, 1096–1104. [Google Scholar] [CrossRef]
- Kwaka, S.; Job, J.H.; Yuna, E.J.; Jina, Y.-S.; Seo, J.-H. Production of biofuels and chemicals from xylose using native and engineered yeast strains. Biotechnol. Adv. 2019, 37, 219–283. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, B.S.; Forte, M.B.S. Purification of biotechnological xylitol from Candida tropicalis fermentation using activated carbon in fixed-bed adsorption columns with continuous feed. Food Bioprod. Process. 2021, 126, 73–80. [Google Scholar] [CrossRef]
- Neuhauser, W.; Haltrich, D.; Kulbe, K.D.; Nideetzky, B. NAD(P)H-dependent aldose reductase from the xylose-assimilating yeast Candida tenuis: Isolation, characterization and biochemical properties of the enzyme. Biochem. J. 1997, 326, 683–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Handumrongkul, C.; Ma, D.-P.; Silva, J.L. Cloning and expression of Candida guilliermondii xylose reductase gene (xyl1) in Pichia pastoris. Appl. Microbiol. Biotechnol. 1998, 49, 399–404. [Google Scholar] [CrossRef]
- Sene, L.; Felipe, M.G.A.; Silva, S.S.; Vitolo, M. Preliminary kinetic characterization of xylose reductase and xylitol dehydrogenase extracted from Candida guilliermondii FTI 20037 cultivated in sugarcane bagasse hydrolysate for xylitol production. Appl. Biochem. Biotechnol. 2001, 91–93, 671–680. [Google Scholar] [CrossRef]
- Kavanagh, K.L.; Klimacek, M.; Nidetzky, B.; Wilson, D.K. The Structure of apo and holo forms of xylose reductase, a dimeric aldo-keto reductase from Candida tenuis. Biochemistry 2002, 41, 8785–9795. [Google Scholar] [CrossRef]
- Mayr, P.; Bruggler, K.; Kulbe, K.D.; Nidetzky, B. D-Xylose metabolism by Candida intermedia: Isolation and characterisation of two forms of aldose reductase with different coenzyme specificities. J. Chromatogr. B Biomed. Sci. Appl. 2000, 737, 195–202. [Google Scholar] [CrossRef]
- Lee, J.-K.; Koo, B.S.; Kim, S.-Y. Cloning and characterization of the xyl1 gene, encoding an NADH-preferring xylose reductase from Candida parapsilosis, and its functional expression in Candida tropicalis. Appl. Environ. Microbiol. 2003, 69, 6179–6188. [Google Scholar] [CrossRef] [Green Version]
- Kratzer, R.; Wilson, D.K.; Nidetzky, B. Catalytic mechanism and substrate selectivity of aldo-keto reductases: Insights from structure-function studies of Candida tenuis xylose reductase. Life 2006, 58, 499–507. [Google Scholar] [CrossRef]
- Chen, L.-C.; Huang, S.-C.; Chuankhayan, P.; Chen, C.-D.; Huang, Y.-H.; Jeyakanthan, J.; Pang, H.-F.; Men, L.-C.; Chen, Y.-C.; Wang, Y.-K.; et al. Purification, crystallization and preliminary X-ray crystallographic analysis of xylose reductase from Candida tropicalis. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2009, F65, 419–421. [Google Scholar] [CrossRef] [PubMed]
- Rafiqul, I.S.M.; Sakinah, A.M.M. Biochemical properties of xylose reductase prepared from adapted strain of Candida tropicalis. Appl. Biochem. Biotechnol. 2015, 175, 387–399. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.; Sung, B.H. Isolation and characterization of the stress-tolerant Candida tropicalis YHJ1 and evaluation of its xylose reductase for xylitol production from acid pre-treatment wastewater. Front Bioeng. Biotechnol. 2019, 7, 138. [Google Scholar] [CrossRef]
- Leonel, L.V.; Sene, L.; da Cunha, M.A.A.; Dalanhol, K.C.F.; Felipe, M.G.A. Valorization of apple pomace using bio-based technology for the production of xylitol and 2G ethanol. Bioproc. Biosyst. Eng. 2020, 43, 2153–2163. [Google Scholar] [CrossRef] [PubMed]
- Shankar, K.; Kulkarnia, N.S.; Sajjanshettya, R.; Jayalakshmib, S.K.; Sreeramulua, K. Co-production of xylitol and ethanol by the fermentation of the lignocellulosic hydrolysates of banana and water hyacinth leaves by individual yeast strains. Ind. Crop. Prod. 2020, 155, 112809. [Google Scholar] [CrossRef]
- Eryasar-Orer, K.; Karasu-Yalcin, S. Optimization of activated charcoal detoxification and concentration of chestnut shell hydrolysate for xylitol production. Biotechnol. Lett. 2021, 43, 1195–1209. [Google Scholar] [CrossRef] [PubMed]
- Santana, N.B.; Dias, J.C.T.; Rezende, R.P.; Franco, M.; Oliveira, L.K.S.; Souza, L.O. Production of xylitol and bio-detoxification of cocoa pod husk hemicellulose hydrolysate by Candida boidinii XM02G. PLoS ONE 2018, 13, e0195206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irmak, S.; Canisag, H.; Vokoun, C.; Meryemoglu, B. Xylitol production from lignocellulosics: Are corn biomass residues good candidates? Biocatal. Agric. Biotechnol. 2017, 11, 220–223. [Google Scholar] [CrossRef]
- Ping, Y.; Ling, H.-Z.; Song, G.; Ge, J.-P. Xylitol production from non-detoxified corncob hemicellulose acid hydrolysate by Candida tropicalis. Biochem. Eng. J. 2013, 75, 86–91. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, R.; Li, Z.; Dai, D.; Li, C.; Zhou, X. A novel pathway construction in Candida tropicalis for direct xylitol conversion from corncob xylan. Bioresour. Technol. 2013, 128, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Sandhu, P.P.; Ahluwalia, V.; Mishra, B.B.; Yada, S.K. Efficient detoxification of corn cob hydrolysate with ion-exchange resins for enhanced xylitol production by Candida tropicalis MTCC 6192. Bioresour. Technol. 2018, 251, 416–419. [Google Scholar] [CrossRef]
- Kumar, V.; Sandhu, P.P.; Ahluwalia, V.; Mishra, B.B.; Yada, S.K. Improved upstream processing for detoxification and recovery xylitol produced from corncob. Bioresour. Technol. 2019, 291, 121931. [Google Scholar] [CrossRef]
- Li, Z.; Guo, X.; Feng, X.; Li, C. An environment friendly and efficient process for xylitol bioconversion from enzymatic corncob hydrolysate by adapted Candida tropicalis. Chem. Eng. J. 2015, 263, 249–256. [Google Scholar] [CrossRef]
- Yewale, T.; Panchwagh, S.; Rajagopalan, S.; Dhamole, P.B.; Jain, R. Enhanced xylitol production using immobilized Candida tropicalis with non-detoxified corn cob hemicellulosic hydrolysate. 3 Biotech 2016, 6, 75. [Google Scholar] [CrossRef] [Green Version]
- Sapcı, B.; Akpinar, O.; Bolukbasi, U.; Yilmaz, L. Evaluation of cotton stalk hydrolysate for xylitol production. Prep. Biochem. Biotechnol. 2016, 46, 474–482. [Google Scholar] [CrossRef]
- López-Linares, J.C.; Ruiz, E.; Romero, I.; Castro, E.; Manzanares, P. Xylitol production from exhausted olive pomace by Candida boidinii. Appl. Sci. 2020, 10, 6966. [Google Scholar] [CrossRef]
- Mateo, S.; Puentes, J.G.; Moya, A.J.; Sánchez, S. Ethanol and xylitol production by fermentation of acid hydrolysate from olive pruning with Candida tropicalis NBRC 0618. Bioresour. Technol. 2015, 190, 1–6. [Google Scholar] [CrossRef] [PubMed]
- López-Linaresa, J.C.; Romeroa, I.; Caraa, C.; Castroa, E.; Solange, I. Mussatto SI. Xylitol production by Debaryomyces hansenii and Candida guilliermondii from rapeseed straw hemicellulosic hydrolysate. Bioresour. Technol. 2018, 247, 736–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Dalveer, D.; Yadav, S.K.; Krishania, M. Process scale-up of an efficient acid-catalyzed steam pretreatment of rice straw for xylitol production by C. tropicalis MTCC 6192. Bioresour. Technol. 2021, 320, 124422. [Google Scholar]
- Zahed, O.; Jouzani, G.S.; Abbasalizadeh, S.; Khodaiyan, F.; Tabatabaei, M. Continuous co-production of ethanol and xylitol from rice straw hydrolysate in a membrane bioreactor. Folia Microbiol. 2016, 61, 179–189. [Google Scholar] [CrossRef]
- Xavier, F.D.; Bezerra, G.S.; Santos, S.F.M.; Oliveira, L.S.C.; Silva, F.L.H.; Silva, A.J.O.; Conceição, M.M. Evaluation of the simultaneous production of xylitol and ethanol from sisal fiber. Biomolecules 2018, 8, 2. [Google Scholar]
- Santos, J.C.; Carvalho, W.; Silva, S.S.; Converti, A. Xylitol production from sugarcane bagasse hydrolyzate in fluidized bed reactor. Effect of air flowrate. Biotechnol. Prog. 2003, 19, 1210–1215. [Google Scholar] [CrossRef] [PubMed]
- Wannawilai, S.; Sirisansaneeyakul, S. Economical production of xylitol from Candida magnolia TISTR 5663 using sugarcane bagasse hydrolysate. Kasetsart J. Nat. Sci. 2015, 49, 583–596. [Google Scholar]
- Raj, K.; Krishnan, C. Improved co-production of ethanol and xylitol from low-temperature aqueous ammonia pretreated sugarcane bagasse using two-stage high solids enzymatic hydrolysis and Candida tropicalis. Renew. Energy 2020, 153, 392–403. [Google Scholar] [CrossRef]
- Tizazu, B.Z.; Roy, K.; Moholkar, V.S. Mechanistic investigations in ultrasound-assisted xylitol fermentation. Ultrason. Sonochem. 2018, 48, 321–328. [Google Scholar] [CrossRef]
- Morais, W.G., Jr.; Pacheco, T.F.; Trichez, D.; Almeida, J.R.M.; Gonçalves, S.B. Xylitol production on sugarcane biomass hydrolysate by newly identified Candida tropicalis JA2 strain. Yeast 2019, 36, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Antunes, F.A.F.; Thome, L.C.; Santos, J.C.; Ingle, A.P.; Costa, C.B.; Anjo, V.D.; Bell, M.J.V.; Rosa, C.A.; Da Silva, S.S. Multi-scale study of the integrated use of the carbohydrate fractions of sugarcane bagasse for ethanol and xylitol production. Renew. Energy 2021, 163, 1343–1355. [Google Scholar] [CrossRef]
- Hernández-Pérez, A.F.; de Arruda, P.V.; de Almeida Felipe, M.G. Sugarcane straw as a feedstock for xylitol production by Candida guilliermondii FTI 20037. Braz. J. Microbiol. 2016, 47, 489–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, J.C.; Pinto, I.R.G.; Carvalho, W.; Mancilha, I.M.; Felipe, M.G.A.; Silva, S.S. Sugarcane bagasse as raw material and immobilization support for xylitol production. Appl. Biochem. Biotechnol. 2005, 121–124, 673–683. [Google Scholar] [CrossRef]
- Silva, S.S.; Solange, I.; Mussatto, S.I.; Santos, J.C.; Santos, D.T.; Polizel, J. Cell immobilization and xylitol production using sugarcane bagasse as raw material. Appl. Biochem. Biotechnol. 2007, 141, 215–227. [Google Scholar] [CrossRef]
- Dorantes-Landa, D.N.; Cocotle-Ronzón, Y.; Morales-Cabreraa, M.A.; Hernández-Martíneza, E. Modeling of the xylitol production from sugarcane bagasse by immobilized cells. J. Chem. Technol. Biotechnol. 2020, 95, 1936–1945. [Google Scholar] [CrossRef]
- Bedό, S.; Fehér, A.; Khunnonkwao, P.; Jantama, K.; Fehér, C. Optimized bioconversion of xylose derived from pre-treated crop residues into xylitol by using Candida boidinii. Agronomy 2021, 11, 79. [Google Scholar] [CrossRef]
- West, T.P. Xylitol production by Candida species grown on a grass hydrolysate. World J. Microbiol. Biotechnol. 2009, 25, 913–916. [Google Scholar] [CrossRef]
- Rudrangi, S.R.R.; West, T.P. Effect of pH on xylitol production by Candida species from a prairie cordgrass hydrolysate. Z. Naturforsch. C 2020, 75, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Dien, B.S.; Mitchell, R.B.; Bowman, R.J.; Jin, V.L.; Quarterman, J.; Schmer, M.R.; Singh, V.; Sliminger, P.J. Bioconversion of pelletized big bluestem, switchgrass and low-diversity grass mixtures into sugars and bioethanol. Front. Energy Res. 2018, 6, 129. [Google Scholar] [CrossRef] [Green Version]

| Grass | Candida Species/ Strain | Growth Conditions | Xylitol Level (g/L) 1 | Yield (g/g) 1 | Reference |
|---|---|---|---|---|---|
| Big bluestem | C. guilliermondii ATCC 20216 | 120 h, 30 °C | 15.6 | 0.46 | [62] |
| Big bluestem | C. guilliermondii ATCC 201935 | 120 h, 30 °C | 14.6 | 0.38 | [62] |
| Big bluestem | C. mogii ATCC 18365 | 120 h, 30 °C | 13.8 | 0.42 | [62] |
| Big bluestem | C. tropicalis ATCC 750 | 120 h, 30 °C | 16.5 | 0.43 | [62] |
| Big bluestem | C. tropicalis ATCC 20215 | 120 h, 30 °C | 11.9 | 0.31 | [62] |
| Prairie cordgrass | C. mogii ATCC 18365 | 120 h, 30 °C | 12.8 | 0.75 | [63] |
| Prairie cordgrass | C. guilliermondii ATCC 20216 | 120 h, 30 °C | 13.7 | 0.81 | [63] |
| Prairie cordgrass | C. guilliermondii ATCC 201935 | 120 h, 30 °C | 10.9 | 0.76 | [63] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
West, T.P. Xylitol Production by Candida Species from Hydrolysates of Agricultural Residues and Grasses. Fermentation 2021, 7, 243. https://doi.org/10.3390/fermentation7040243
West TP. Xylitol Production by Candida Species from Hydrolysates of Agricultural Residues and Grasses. Fermentation. 2021; 7(4):243. https://doi.org/10.3390/fermentation7040243
Chicago/Turabian StyleWest, Thomas P. 2021. "Xylitol Production by Candida Species from Hydrolysates of Agricultural Residues and Grasses" Fermentation 7, no. 4: 243. https://doi.org/10.3390/fermentation7040243
APA StyleWest, T. P. (2021). Xylitol Production by Candida Species from Hydrolysates of Agricultural Residues and Grasses. Fermentation, 7(4), 243. https://doi.org/10.3390/fermentation7040243