The Effects of Soil Application of Digestate Enriched with P, K, Mg and B on Yield and Processing Value of Sugar Beets
Abstract
:1. Introduction
2. Material and Methods
3. Results and Discussion
3.1. Suitability of by-Products of Anaerobic Digestion as a Soil Amendment for Sugar Beet Field
3.2. The Effects of Studied Digestate Fractions on Sugar Beet Growth and Yield
3.3. The Effects of Digestate Fractions Studied on the Quality of Sugar Beets as a Stock Material
3.4. Determination of the Effects of Soil Application of Digestate on Technological Quality of Stock Material
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hutnan, M.; Drtil, M.; Derco, J.; Mrafkova, L.; Hornak, M.; Mico, S. Two-Step Pilot-Scale Anaerobic Treatment of Sugar Beet Pulp. Pol. J. Environ. Stud. 2001, 10, 237–243. [Google Scholar]
- Brooks, L.; Parravicini, V.; Svardal, K.; Kroiss, H.; Prendl, L. Biogas from sugar beet press pulp as substitute of fossil fuel in sugar beet factories. Water Sci. Technol. 2008, 58, 1498–1504. [Google Scholar] [CrossRef] [PubMed]
- Khanna, M.; Dhunganna, B.; Clifton-Brown, J. Cost of producing miscanthus and switchgrass for bioenergy in Illinois. Biomass Bioenergy 2008, 32, 482–493. [Google Scholar] [CrossRef]
- Seppala, M.; Paavola, T.; Lehtomaki, A.; Pakarinen, O.; Rintala, J. Biogas from energy crops—Optimal pre-treatments and storage, co-digestion and energy balance in boreal conditions. Water Sci. Technol. 2008, 58, 1857–1863. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.D.; Power, N. Technical and economic analysis of biogas production in Ireland utilizing three different crop rotations. Appl. Energy 2009, 86, 25–36. [Google Scholar] [CrossRef]
- Herrmann, C.; Prochnow, A.; Heiermann, M.; Idler, C. Biomass from landscape management of grassland used for biogas production: Effects of harvest date and silage additives on feedstock quality and methane yield. Grass Forage Sci. 2013, 69, 549–566. [Google Scholar] [CrossRef]
- Dale, B.E.; Bozzetto, S.; Couturier, C.; Fabbri, C.; Hilbert, J.A.; Ong, R.; Richard, T.; Rossi, L.; Thelen, K.D.; Woods, J. The potential for expanding sustainable biogas production and some possible impacts in specific countries. Biofuels Bioprod. Biorefin. 2020, 14, 1335–1347. [Google Scholar] [CrossRef]
- Abubaker, J.; Risberg, K.; Pell, M. Biogas residues as fertilisers—Effect on wheat growth and soil microbial activities. Appl. Energy 2012, 99, 126–134. [Google Scholar] [CrossRef]
- Nkoa, R. Agricultural benefits and environmental risks of soil fertilization with anaerobic digestate: A review. Agron. Sustain. Dev. 2014, 34, 473–492. [Google Scholar] [CrossRef] [Green Version]
- Khakbaz, A.; De Nobili, M.; Mainardis, M.; Contin, M.; Aneggi, E.; Mattiussi, M.; Cabras, I.; Busut, M.; Daniele Goi, D. Monitoring of heavy metals, EOX and LAS in sewage sludge for agricultural use: A case study. Detritus 2020, 12, 160–168. [Google Scholar] [CrossRef]
- Orzi, V.; Riva, C.; Scaglia, B.; Di Imperzano, G.; Tambone, F.; Adani, F. Anaerobic digestion coupled with digestate injection reduced odour emissions from soil during manure distribution. Sci. Total Environ. 2018, 621, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, K.; Lal, R.; Preston, C.M.; Nierop, K.G. Strengthening the soil organic carbon pool by increasing contribution from recalcitrant aliphatic bio (macro) molecules. Geoderma 2007, 142, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Ros, C.D.; Libralato, G.; Ghirardini, A.V.; Radaelli, M. Assessing the potential phytotoxicity of digestate from winery waste. Ecotoxicol. Environ. Saf. 2018, 150, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Solé-Bundó, M.; Cucina, M.; Folch, M.; Tàpias, J.; Gigliotti, G.; Garfi, M.; Ferrer, I. Assessing the agricultural reuse of the digestate from microalgae anaerobic digestion and co-digestion with sewage sludge. Sci. Total Environ. 2017, 586, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Alburquerque, J.A.; de la Fuente, C.; Ferrer-Costa, A.; Carrasco, I.; Cegarra, J.; Abad, M.; Bernal, M.P. Assessment of the fertiliser potential of digestate from farm and agroindustrial residues. Biomass Bioenergy 2012, 4, 181–189. [Google Scholar] [CrossRef]
- Evangelisti, S.; Lettieri, P.; Borello, D.; Clift, R. Life cycle assessment of energy from waste via anaerobic digestion: A UK case study. Waste Manag. 2014, 34, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Arthurson, V. Closing the global energy and nutrient cycle through application of biogas residues to agriculture land—Potential benefits and drawbacks. Energies 2009, 2, 226–242. [Google Scholar] [CrossRef] [Green Version]
- Odlare, M.; Arthurson, V.; Pell, M.; Svensson, K.; Nehrenheim, E.; Abubaker, J. Land application of organic waste—Effects on the soil ekosystem. Appl. Energy 2011, 88, 2210–2218. [Google Scholar] [CrossRef]
- Odlare, M.; Pell, M.; Svensson, K. Changes in soil chemical and microbiological properties Turing 4 years of application of various organic residues. Waste Manag. 2008, 28, 1246–1253. [Google Scholar] [CrossRef]
- Hupfauf, S.; Bachmann, S.; Fernández-Delgado Juárez, M.; Insam, H.; Eichler-Löbermann, B. Biogas digestate affect crop P uptake and soil microbial community composition. Sci. Total Environ. 2016, 542, 1144–1154. [Google Scholar] [CrossRef]
- Galvez, A.; Sinicco, T.; Cayuela MLMingorance, M.D.; Fornasier, F.; Mondini, C. Short term effects of bioenergy by-products on soil C and N Dynamics, nutrients availability and biochemical properties. Agric. Ecosyst. Environ. 2012, 160, 3–14. [Google Scholar] [CrossRef]
- Różyło, K.; Oleszczuk, P.; Kraska, P.; Kiecińska-Poppe, E.; Andruszczak, S. An ecotoxicological evaluation of soil fertilized with biogas residues or mining waste. Environ. Sci. Pollut. Res. 2015, 22, 7833–7842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nabel, M.; Schrey, S.D.; Poorter, H.; Koller, R.; Jablonowski, A.D. Effects of digestate fertilization on Sida hermaphrodita: Boosting biomass fields on marginal soils by increasing soil fertility. Biomass Bioenergy 2017, 107, 207–213. [Google Scholar] [CrossRef]
- Tambone, F.; Orzi, V.; D’Imporzano, G.; Adani, F. Solid and liquid fractionation of digestate: Mass balance, chemical characterization, and agronomic and environmental value. Bioresour. Technol. 2017, 243, 1251–1256. [Google Scholar] [CrossRef] [PubMed]
- Tambone, F.; Terruzzi, L.; Scaglia, B.; Adani, F. Composting of the solid fraction of digestate derived from pig slurry. Biological processes and compost properties. Waste Manag. 2015, 35, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Tampio, E.; Marttinen, S.; Rintala, J. Liquid fertilizer products from anaerobic digestion of food waste: Mass, nutrient and energy balance of four digestate liquid treatment systems. J. Clean. Prod. 2016, 125, 22–32. [Google Scholar] [CrossRef] [Green Version]
- Sigurnjak, I.; Vaneeckhaute, C.; Michels, E.; Ryckaert, B.; Ghekiere, G.; Tack, F.M.G.; Meers, E. Fertilizer performance of liquid fraction of digestate as synthetic nitrogen substitute in silage maize cultivation for three consecutive years. Sci. Total Environ. 2017, 599, 1885–1894. [Google Scholar] [CrossRef]
- Tambone, F.; Orzi, V.; Zilio, M.; Adani, F. Measuring the organic amendment properties of the liquid fraction of digestate. Waste Manag. 2019, 88, 21–27. [Google Scholar] [CrossRef]
- Finzi, A.; Guido, V.; Riva, E.; Ferrari, O.; Quilez, D.; Herrero, E.; Provolo, G. Performance and sizing of filtration equipment to replace mineral fertilizer with digestate in drip and sprinkler fertigation. J. Clean. Prod. 2021, 317, 128431. [Google Scholar] [CrossRef]
- Gunnarsson, A.; Bengtsson, F.; Caspersen, S. Use efficiency of nitrogen from biodigested plant material by ryegrass. J. Plant Nutr. Soil Sci. 2010, 173, 113–119. [Google Scholar] [CrossRef]
- Jamison, J.; Khanal, S.K.; Nguyen, N.H.; Deenik, J.L. Assessing the Effects of Digestates and Combinations of Digestates and Fertilizer on Yield and Nutrient Use of Brassica juncea (Kai Choy). Agronomy 2021, 11, 509. [Google Scholar] [CrossRef]
- Gunnarsson, A.; Lindén, B.; Gertsson, U. Biodigestion of plant material can improve nitrogen use efficiency in a red beet crop sequence. HortScience 2011, 46, 765–775. [Google Scholar] [CrossRef] [Green Version]
- Berruto, R.; Busato, P.; Bochtis, D.D.; Sørensen, C.G. Comparison of distribution systems for biogas plant residues. Biomass Bioenergy 2013, 52, 139–150. [Google Scholar] [CrossRef]
- Stinner, W.; Möller, K.; Leithold, G. Effects of biogas digestion of clover/grass-leys, cover crops and crop residues on nitrogen cycle and crop field in organic stockless farming systems. Eur. J. Agron. 2008, 29, 125–134. [Google Scholar] [CrossRef]
- Šimon, T.; Kunzová, E.; Friedlová, M. The effect of digestate, cattle slurry and mineral fertilization on the winter wheat yield and soil quality parameters. Plant Soil Environ. 2015, 61, 522–527. [Google Scholar] [CrossRef] [Green Version]
- Cavalli, D.; Cabassi, G.; Borrelli, L.; Geromel, G.; Bechini, L.; Degano, L.; Gallina, P.M. Nitrogen fertilizer replacement value of undigested liquid cattle manure and digestates. Eur. J. Agron. 2016, 73, 34–41. [Google Scholar] [CrossRef]
- Lošák, T.; Hlušek, J.; Válka, T.; Elbl, J.; Vitěz, T.; Běliková, H.; Von Bennewitz, E. The effect of fertilization with digestate on kohlrabi yields and quality. Plant Soil Environ. 2016, 62, 274–278. [Google Scholar] [CrossRef] [Green Version]
- Panuccio, M.R.; Papalia, T.; Attinà, E.; Giuffrè AMuscolo, A. Use of digestate as an alternative to mineral fertilizer: Effects on growth and crop quality. Arch. Agron. Soil Sci. 2019, 65, 700–711. [Google Scholar] [CrossRef]
- Barłóg, P.; Hlisnikovský, L.; Kunzová, E. Yield, content and nutrient uptake by winter wheat and spring barley in response to applications of digestate, cattle slurry and NPK mineral fertilizers. Arch. Agron. Soil Sci. 2020, 66, 1481–1496. [Google Scholar] [CrossRef]
- Kolář, L.; Kužel, S.; Peterka, J.; Plát, V. Agrochemical value of organic matter of fermenter wastes in biogas production. Plant Soil Environ. 2008, 54, 321–328. [Google Scholar] [CrossRef] [Green Version]
- Kolář, L.; Kužel, S.; Peterka, J.; Borová-Batt, J. Agrochemical value of the liquid phase of wastes from fermenters during biogas production. Plant Soil Environ. 2010, 56, 23–27. [Google Scholar] [CrossRef] [Green Version]
- Kupper, T.; Bucheli, T.D.; Brandi, R.C.; Ortelli, D.; Edder, P. Dissipation of pesticides during composting and anaerobic digestion of source-separated organic waste at full-scale plants. Bioresour. Technol. 2008, 99, 7988–7994. [Google Scholar] [CrossRef] [PubMed]
- Pivato, A.; Vanin, S.; Raga, R.; Lavagnolo MCBarausse, A.; Rieple, A.; Laurent, A.; Cossu, R. Use of digestate from decentralized on-farm biogas plant as fertilizer in 29 soil: An ecotoxicological study for future indicators in risk and life cycle assessment. Waste Manag. 2016, 49, 378–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tigini, V.; Franchino, M.; Bona, F.; Varese, G.C. Is digestate safe? A study on its ecotoxicity and environmental risk on a pig manure. Sci. Total Environ. 2016, 551, 127–132. [Google Scholar] [CrossRef]
- Baryga, A.; Połeć, B. Studies on technological quality of sugar beets and soil parameters in relation to method of soil fertilization. Int. J. Environ. Agric. Res. 2016, 2, 42–53. [Google Scholar]
- Baryga, A.; Połeć, B.; Skibniewska, K.; Seciu, E.; Grabara, J. Utilisation of residual waste from sugar beet. J. Environ. Prot. Ecol. 2016, 17, 1048–1057. [Google Scholar]
- Baryga, A. Studia nad Wartością Technologiczną Buraka Cukrowego i Jakością Cukru w Aspekcie Wykorzystania w Uprawie Pofermentu z Biogazowni; Praca habilitacyjna; UWM: Olsztyn, Polska, 2019. [Google Scholar]
- Baryga, A.; Połeć, B.; Klasa, A. Quality of Sugar Beets under the Effects of Digestate Application to the Soil. Processes 2020, 8, 1402–1414. [Google Scholar] [CrossRef]
- Hoffmann, C.M. Root Quality of Sugarbeet. Sugar Tech. 2010, 12, 276–287. [Google Scholar] [CrossRef]
- Campbell, L.G.K.K.; Fugate, K.K. Relationships Among Impurity Components, Sucrose, and Sugarbeet Processing Quality. J. Sugar Beet Res. 2015, 52, 3–21. [Google Scholar] [CrossRef]
- Hassan, I.; Mostafa, S. Influence of Sugar Beet Nitrogen Content on Quality and Efficiency of Sugar Extraction. J. Food Dairy Sci. 2018, 9, 111–116. [Google Scholar] [CrossRef] [Green Version]





| Parameter | Apr | May | June | Jul | Aug | Sept | Oct | Nov |
|---|---|---|---|---|---|---|---|---|
| Daily temperature, °C | 6.7 | 13.1 | 17.1 | 17.7 | 18.6 | 13.2 | 9.7 | 4.4 |
| Number of hours with direct sunlight | 146 | 252 | 256 | 231 | 250 | 125 | 93 | 49 |
| Precipitation, mm·m−2 | 58 | 56 | 129 | 61 | 54 | 185 | 89 | 46 |
| Parameter | Units | Fraction of Digestate: | Permissible Level for Soil Application in Crop Production | |
|---|---|---|---|---|
| Solid | Liquid | |||
| pH | pH-H2O | 7.7 | 7.6 | – |
| Dry matter | % fresh weight | 20.1 | 4.0 | – |
| Organic substances | % fresh weight | 68.6 | 76.8 | – |
| Cadmium (Cd) | mg·kg DM−1 | 1.23 | 1.35 | ≤20 |
| Chromium (Cr) | mg·kg DM−1 | ˂25 | ˂25 | ≤750 |
| Copper (Cu) | mg·kg DM−1 | 8.10 | 8.40 | ≤300 |
| Nickel (Ni) | mg·kg DM−1 | 8.35 | 7.40 | ≤500 |
| Lead (Pb) | mg·kg DM−1 | ˂5.0 | ˂5.0 | ≤16 |
| Zinc (Zn) | mg·kg DM−1 | 250 | 320 | ≤1000 |
| Mercury (Hg) | mg·kg DM−1 | 0.066 | <0.050 | ≤2.5 |
| Manganese (Mn) | mg·kg DM−1 | 24 | 22 | – |
| Sulfur (S) | % DM | 7.1 | 7.2 | – |
| Calcium (Ca) | % DM | 10.2 | 4.77 | – |
| Magnesium (Mg) | % DM | 0.845 | 0.305 | – |
| Total nitrogen Kjeldahl’s (N) | % DM | 9.34 | 4.68 | – |
| Total phosphorous (P) | % DM | 0.650 | 0.456 | – |
| Potassium (K) | % DM | 3.13 | 0.607 | – |
| Boron (B) | % DM | 0.008 | 0.011 | – |
| Salmonella bacteria | cfu in 100 g | not present | not present | 0 |
| Living eggs of parasites Atrichuris sp., Trichuris sp., Toxocara sp. | number in 1 kg DM−1 | not present | not present | 0 |
| Treatment | Parameter (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sucrose | Dry Residues | Poma-ce | Inverted Sugar | α-amino Acid N | Amide N | ash | Na | K | |
| Solid fraction of digestate | 13.5 a | 18.6 ab | 3.4 ab | 0.04 a | 0.003 ab | 0.014 ab | 0.60 | 0.021 a | 0.150 a |
| Solid fraction of digestate + P, K, Mg and B | 13.9 a | 19.9 b | 3.5 ab | 0.08 b | 0.007 c | 0.016 b | 0.54 | 0.036 b | 0.173 ab |
| Liquid fraction of digestate | 13.9 a | 18.4 ab | 3.5 ab | 0.04 a | 0.005 b | 0.013 ab | 0.58 | 0.033 ab | 0.145 a |
| Liquid fraction of digestate + P, K, Mg and B | 14.9 b | 19.3 b | 3.6 b | 0.06 ab | 0.007 c | 0.014 ab | 0.48 | 0.042 b | 0.152 a |
| Parameter | Modification of Parameters under Effect of Supplementation with P, K, Mg and B | Technological Effects of Modification of Digestate with P, K, Mg and B | Significance of the Difference between Treatments with and Without Supplementation with P, K, Mg and B | |||
|---|---|---|---|---|---|---|
| Liquid Fraction | Solid Fraction | Liquid Fraction | Solid Fraction | Liquid Fraction | Solid Fraction | |
| Expected purity of thick juice (%) | increase by 0.1% | increase by 0.2% | + | + | ns | ns |
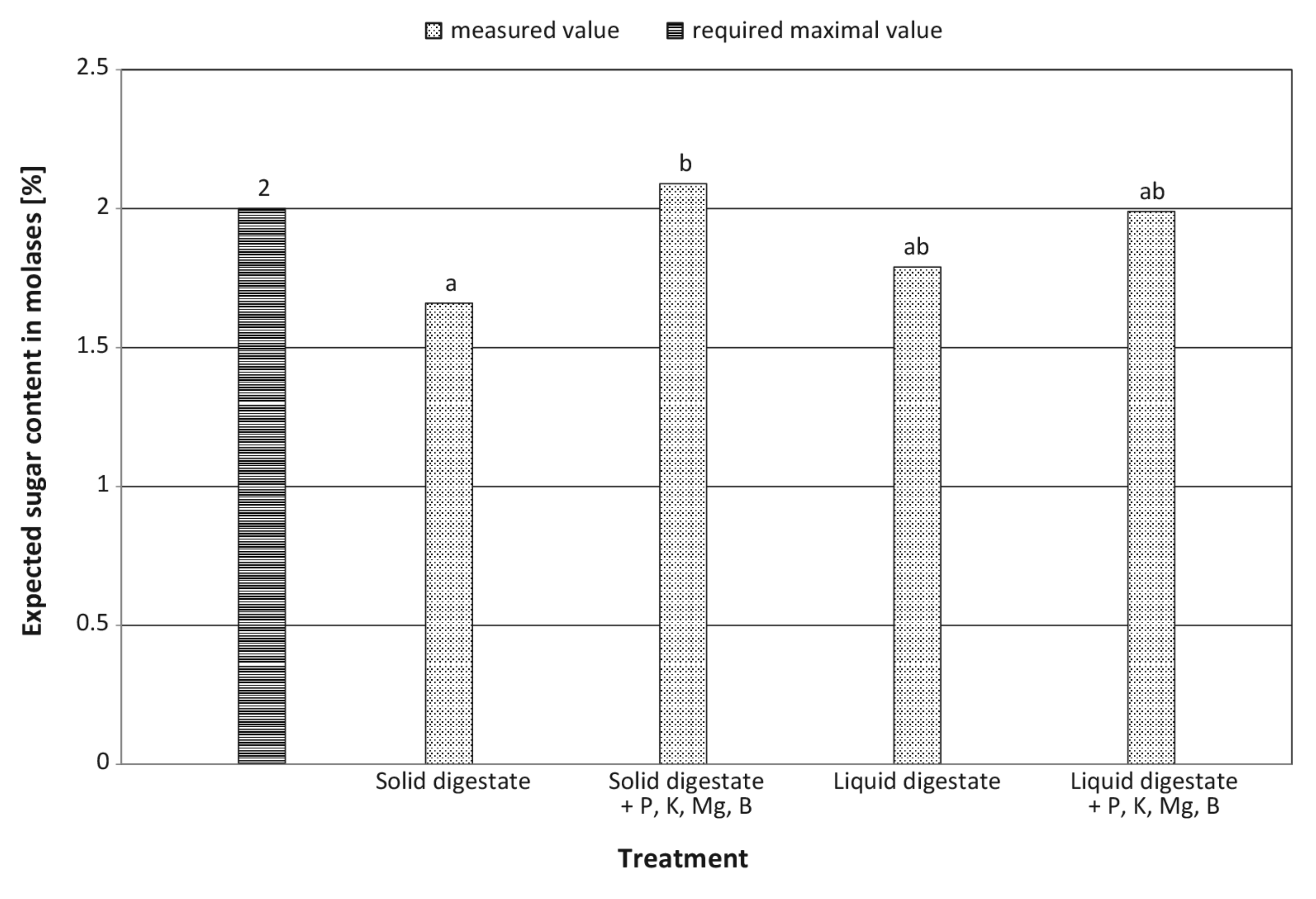
| Expected concentration of sugar in molasses (%) | increase by 20.6% | increase by 11.1% | – | – | ** | ns |
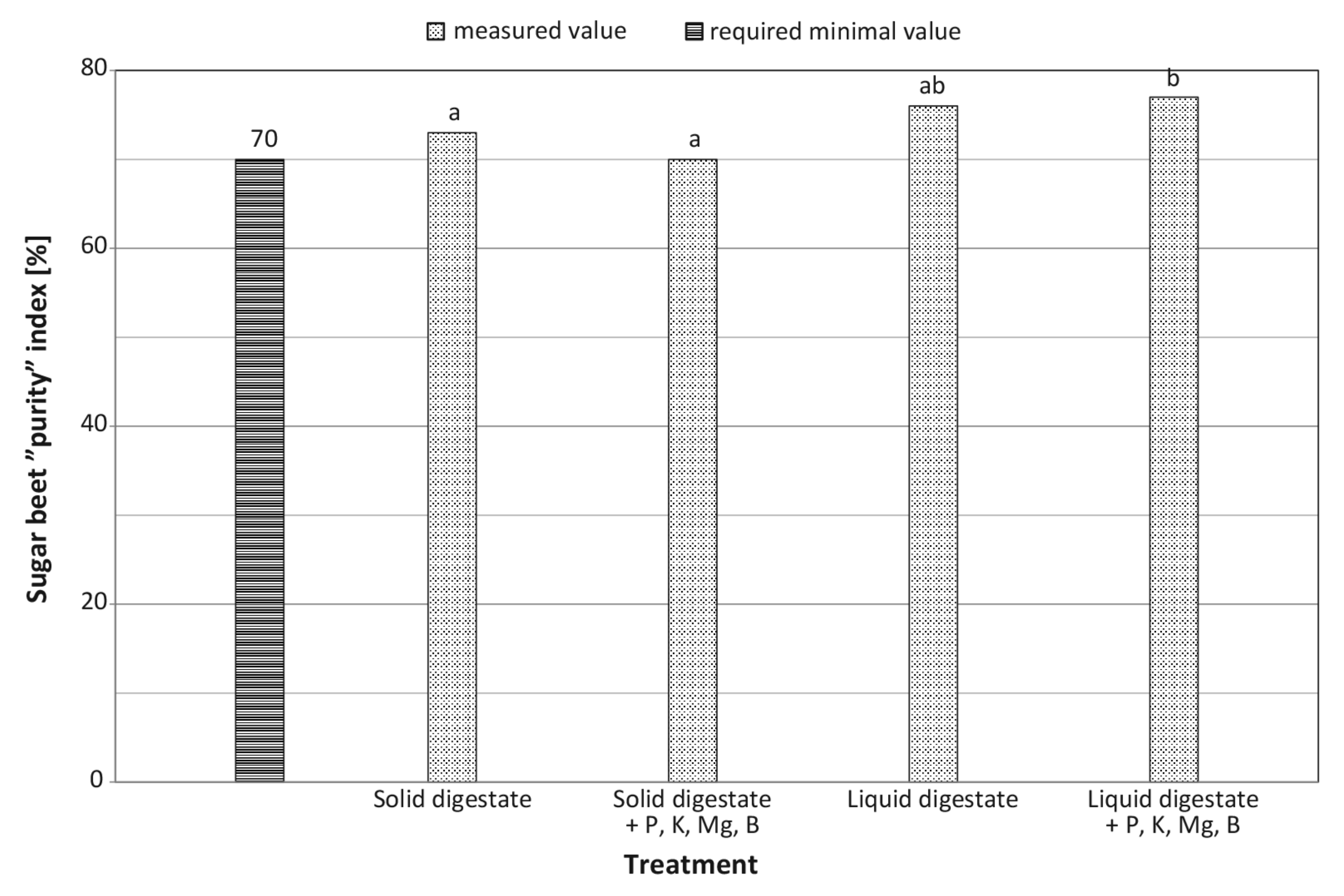
| “Purity” of stock material (%) | decrease by 4.1% | increase by 1.3% | + | – | ** | ns |
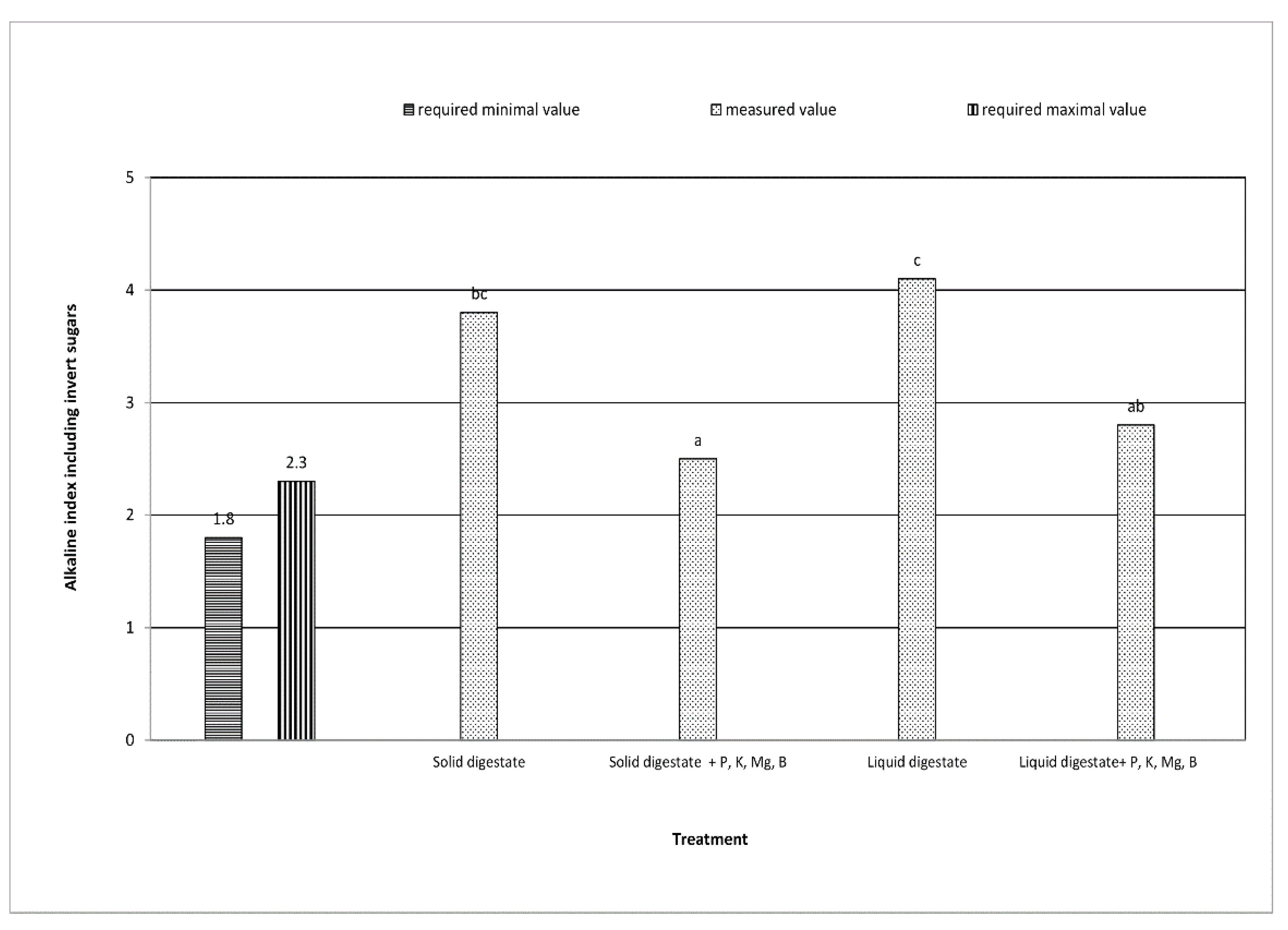
| Alkaline coefficient including inverted sugars | decrease by 34.2% | decrease by 31.7% | + | + | ** | ** |
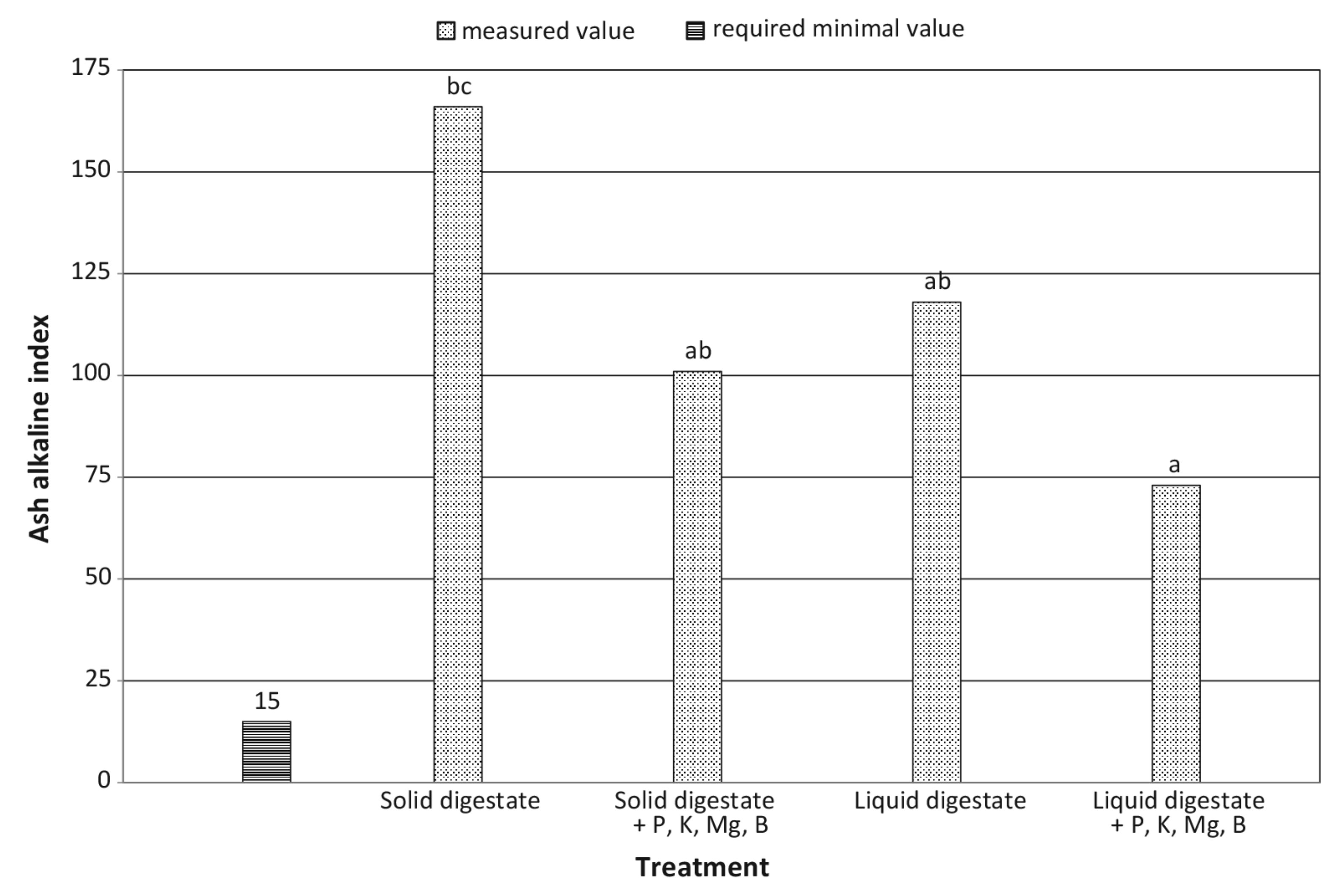
| Ash coefficient | decrease by 8.0% | increase by 22.5% | + | – | ** | ** |
| α-amino acid coefficient | decrease by 43.3% | decrease by 20.4% | + | + | ** | ** |
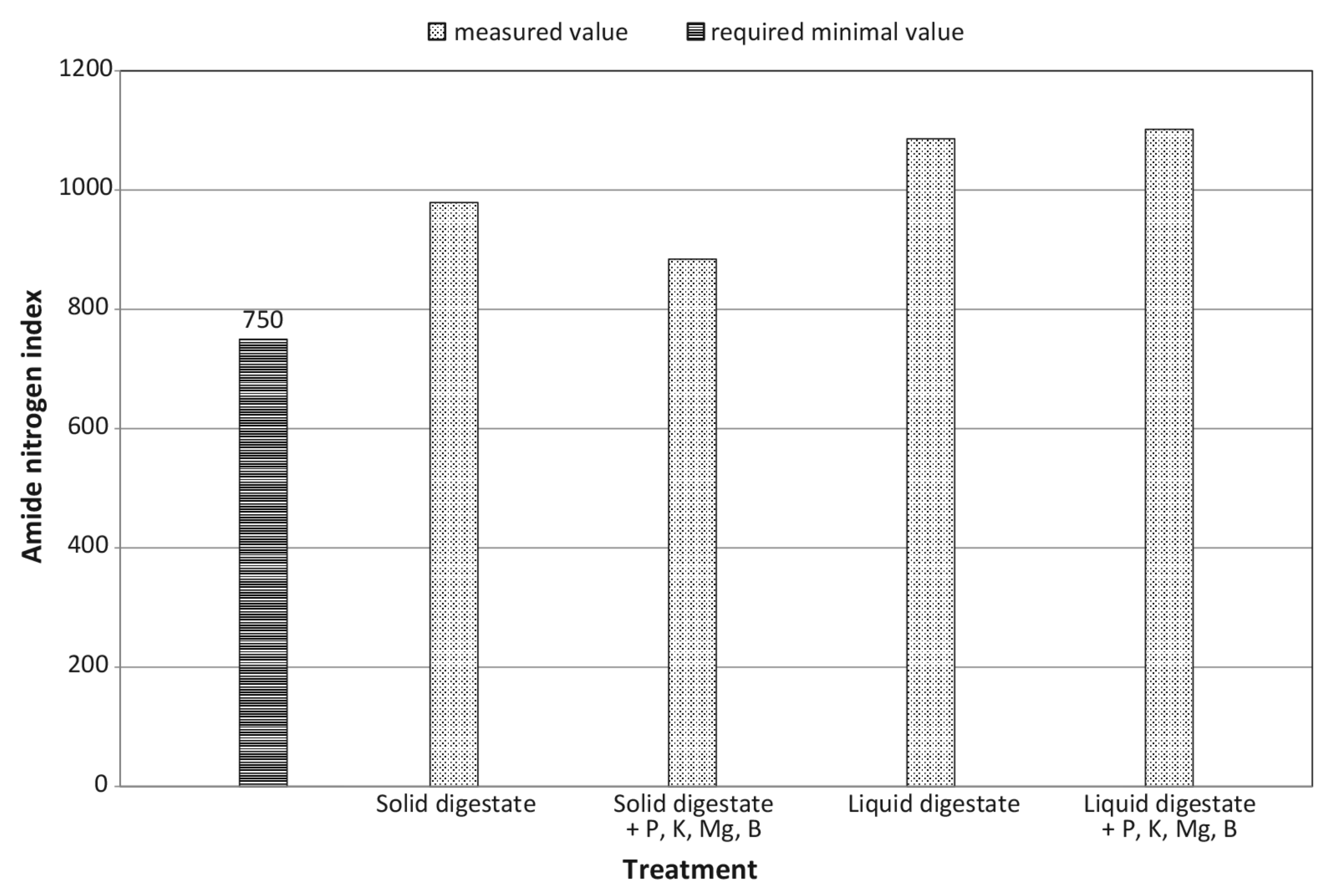
| Amide nitrogen coefficient | decrease by 9.7% | increase by 1.5% | + | – | ns | ns |
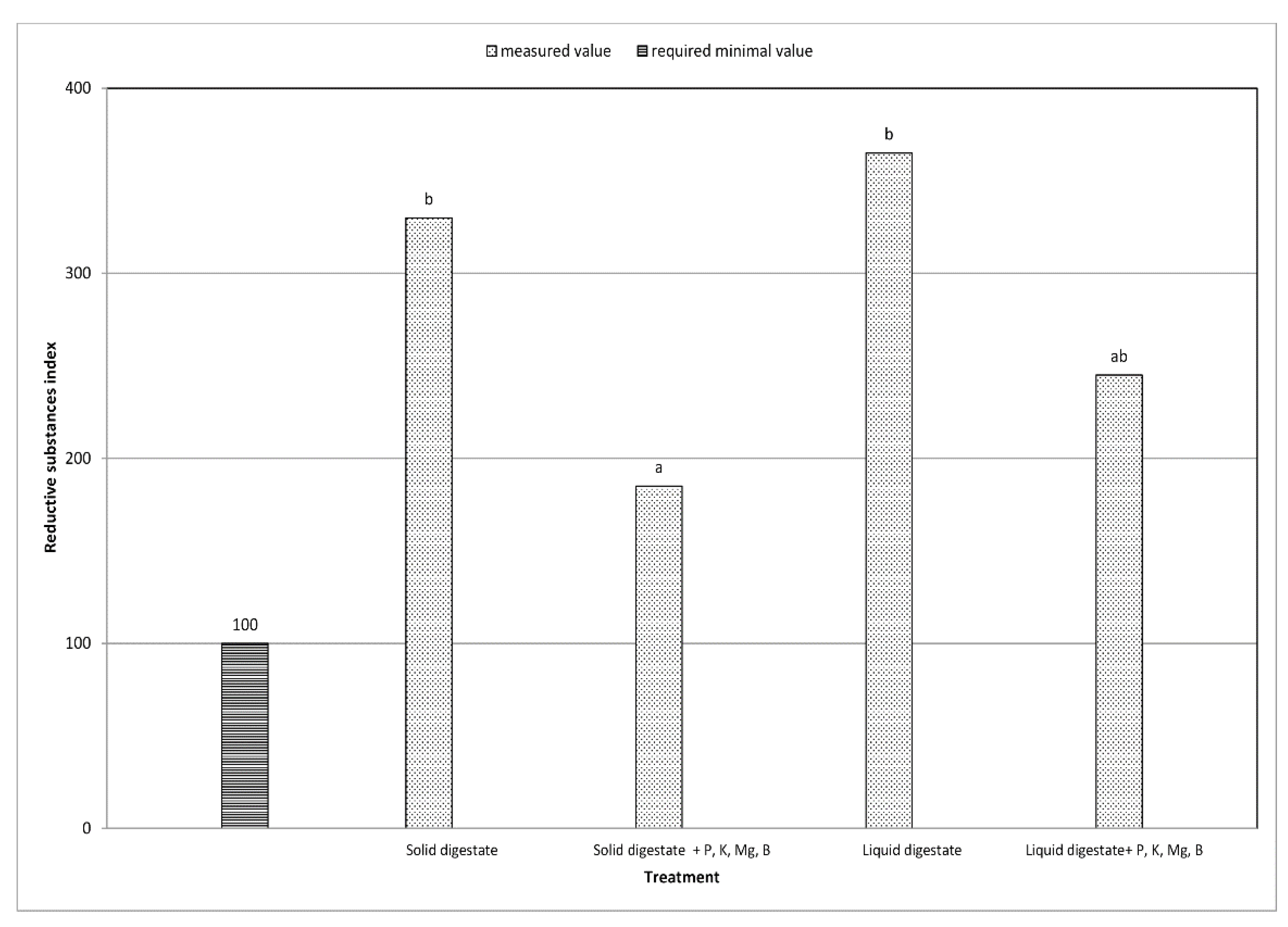
| Coefficient of reductive substances | decrease by 43.9% | decrease by 32.9% | + | + | ** | ** |
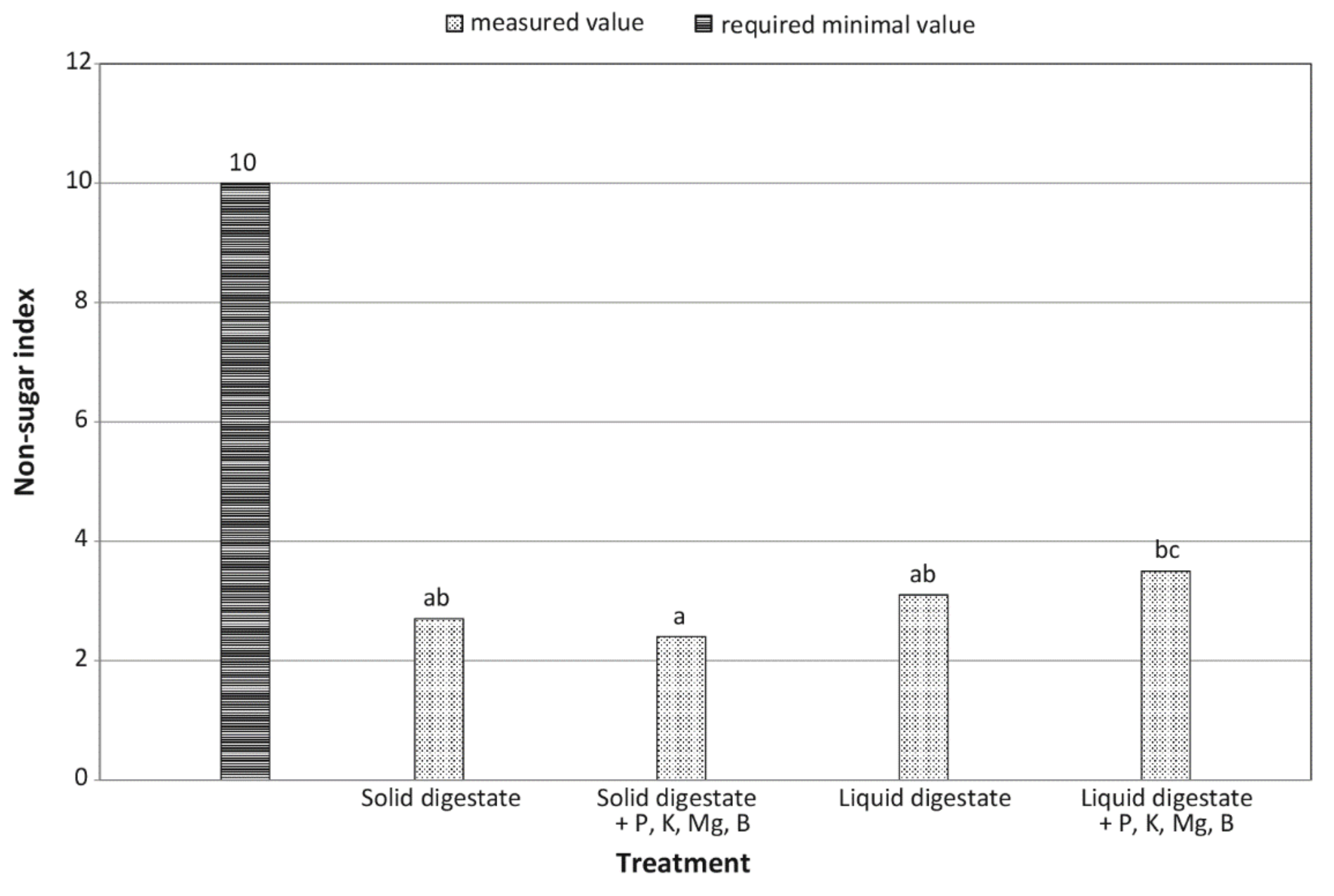
| Non-sugar coefficient | decrease by 11.1% | increase by 11.4% | + | – | ** | ** |
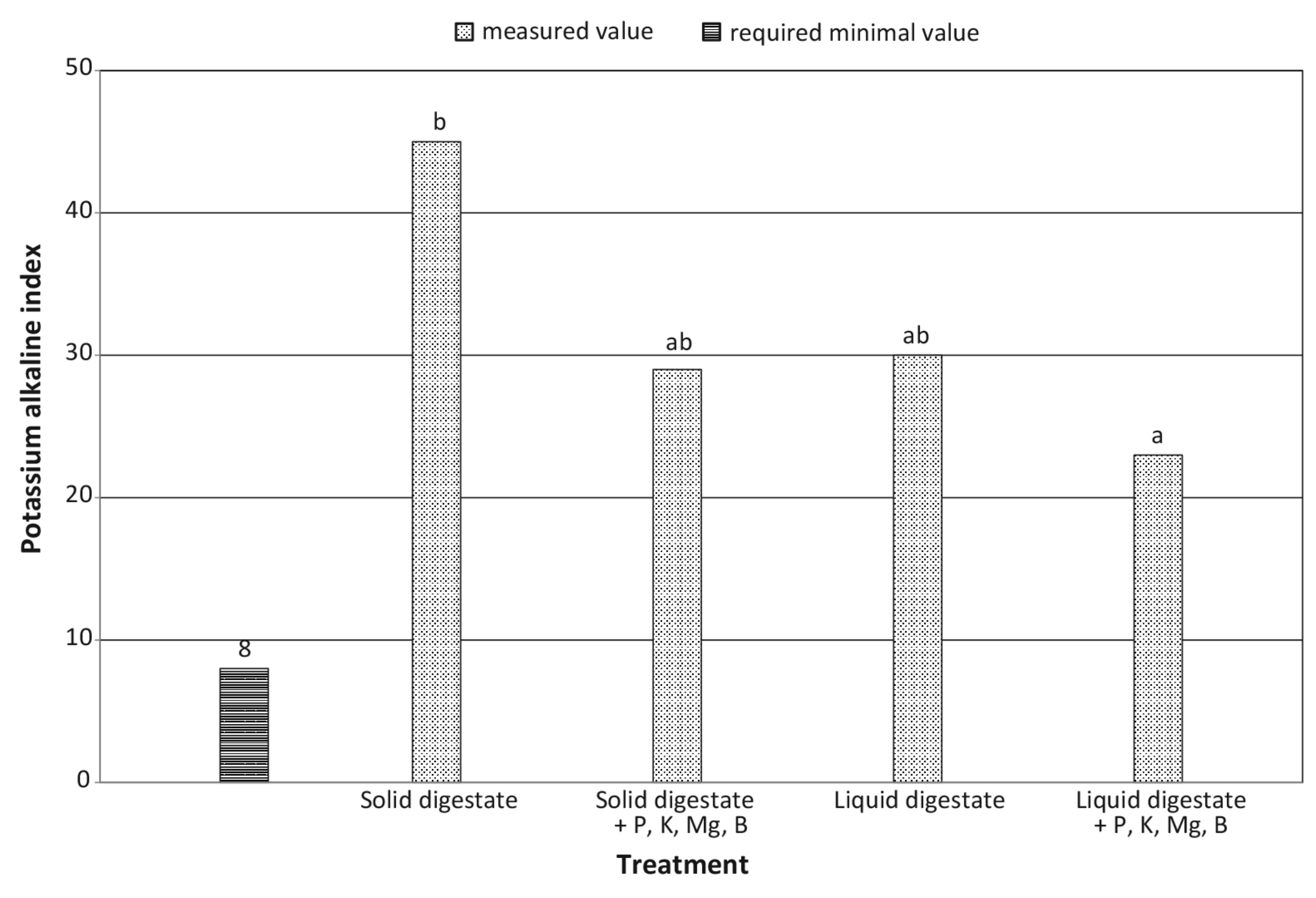
| Alkaline potassium coefficient | decrease by 35.6% | decrease by 23.3% | + | + | ** | ** |
| Alkaline ash coefficient | decrease by 39.2% | decrease by 38.1% | + | + | ** | ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baryga, A.; Połeć, B.; Klasa, A. The Effects of Soil Application of Digestate Enriched with P, K, Mg and B on Yield and Processing Value of Sugar Beets. Fermentation 2021, 7, 241. https://doi.org/10.3390/fermentation7040241
Baryga A, Połeć B, Klasa A. The Effects of Soil Application of Digestate Enriched with P, K, Mg and B on Yield and Processing Value of Sugar Beets. Fermentation. 2021; 7(4):241. https://doi.org/10.3390/fermentation7040241
Chicago/Turabian StyleBaryga, Andrzej, Bożenna Połeć, and Andrzej Klasa. 2021. "The Effects of Soil Application of Digestate Enriched with P, K, Mg and B on Yield and Processing Value of Sugar Beets" Fermentation 7, no. 4: 241. https://doi.org/10.3390/fermentation7040241
APA StyleBaryga, A., Połeć, B., & Klasa, A. (2021). The Effects of Soil Application of Digestate Enriched with P, K, Mg and B on Yield and Processing Value of Sugar Beets. Fermentation, 7(4), 241. https://doi.org/10.3390/fermentation7040241







