Abstract
The aim of this work was to enhance the levels of fruity esters in spine grape (Vitis davidii Foёx) wine by goal-directed amino acid supplementation during fermentation. HPLC and GC-MS monitored the amino acids and fruity esters, respectively, during alcoholic fermentation of spine grape and Cabernet Sauvignon grape. HPLC was also used to determine the extracellular metabolites and precursors involved in the synthesis of fruity esters. Alanine, phenylalanine, and isoleucine levels in spine grape were less than those in Cabernet Sauvignon. Pearson correlation between amino acid profile and fruity ester content in the two systems indicated that deficiencies in alanine, phenylalanine, and isoleucine levels might have limited fruity ester production in spine grape wine. Supplementation of these three amino acids based on their levels in Cabernet Sauvignon significantly increased fruity ester content in spine grape wine. Interestingly, goal-directed amino acid supplementation might have led to changes in the distribution of carbon fluxes, which contributed to the increase in fruity ester production.
1. Introduction
Yeast assimilable nitrogen (YAN), composed of organic (amino acids) and inorganic (ammonium ions) forms, is an important macronutrient involved in yeast metabolism [1]. Their concentrations vary depending on variety, geographical location, climate, and viticulture practices [2,3]. During alcoholic fermentation, YAN is used to sustain growth and produce biomass as well as synthesize fermentative aroma compounds [4]. Esters are the major fermentative aroma compounds, and they can impart aroma even at concentrations below their odor threshold [5]. Fruity esters, including acetate esters and fatty acid ethyl esters (FAEEs), are considered target components to enhance wine aroma [6].
Studies on the correlation between amino acids and aroma compounds have revealed an association between amino acids and fruity ester production [7,8,9]. Amino acid supplementation in grape must enhanced the production of fermentation-derived aroma components, such as acetate esters and ethyl esters [10,11,12] and improved the scores for the descriptors “confectionary”, “red fruit”, and “dark fruit” [13]. These findings suggest that amino acid supplementation potentially improves wine aroma quality. The synthesis of fermentation-derived aroma compounds as well as their association with nitrogen and sugar metabolism in Saccharomyces cerevisiae has been extensively studied. α-keto acids have been considered as important intermediaries in the formation of higher alcohols and volatile acids. Either amino acid catabolism via the Ehrlich pathway [14] or glucose/fructose metabolism via central carbon metabolism [15] produces these precursors. Nevertheless, mechanisms underlying the increase in fruity esters under amino acid supplementation are still not clearly understood. Recent studies have shown that only a small fraction of higher alcohols is produced by catabolism of consumed amino acids [16,17]. The interaction between central carbon metabolism and nitrogen metabolism may significantly influence the production of volatile compounds in S. cerevisiae [2]. Traditionally, nitrogen was supplemented without the determination of amino acid content of grape must. Injudicious supplementation of nitrogen is wasteful and has negative impacts on wine aroma due to high acetic acid and ethyl acetate production [4]. Therefore, several studies have highlighted the need to determine the nutritional status of grape juice before nitrogen supplementation to increase aromatic compound production by yeast [11,18]. Further studies are necessary to evaluate the increase in wine aroma and explore the possible mechanisms of increase in fruity esters under goal-directed amino acid modulation.
Spine grape is a wild species widely distributed in southern China. Spine grape wine has high anthocyanin and polyphenol content [19] with poor fruity aroma. A study has shown that spine grape wine has weak aroma characteristics, which may be related to low levels of varietal aroma precursors and fermentative aroma compounds. In particular, fruity esters were positively correlated with wine fruity aroma [20]. On the contrary, Cabernet Sauvignon, a well-known Vitis vinifera grape, has elegant fruity aroma with high fruity ester content. Variations in the ester profiles of Shiraz and Cabernet Sauvignon wines were related to specific grape-juice nitrogen composition [21], and our previous study also indicated the significant correlation between amino acid composition and fruity ester production during fermentation of spine grapes [22]. Therefore, modulation based on the amino acid profile of Cabernet Sauvignon can be an effective approach to increasing the fruity ester content of spine grape wine.
In this study, we attempted to enhance the fruity ester content in spine grape wine by goal-directed amino acid supplementation. Pearson’s correlation was used to describe the association between amino acid profile and fruity ester content in spine grape and Cabernet Sauvignon grape during fermentation. Subsequently, we supplemented these amino acids before spine grape winemaking and evaluated the fruity ester content at different concentrations of the nitrogen supplement. We also analyzed the extracellular metabolites and precursors involved in fruity ester synthesis. These findings can provide novel insights into the mechanisms underlying the increase in fruity esters under goal-directed amino acid supplementation.
2. Materials and Methods
2.1. Grape Material
Cabernet Sauvignon grapes were harvested from Heyang, Shaanxi Province, China on 28 August 2019. Grape berries were disease-free, pest-free, and healthy and had 207.5 g/L reducing sugars, 5.0 g/L titratable acidity (tartaric acid), and a pH value of 3.34. Spine grape ‘Xiang Pearl’ was harvested from Huaihua, Hunan Province, China on 25 August 2019. Grape berries had 172.5 g/L reducing sugars, 4.3 g/L titratable acidity (tartaric acid), and a pH value of 3.57.
2.2. Chemicals, Standards, and Microorganisms
The analytical reagents glucose, sodium hydroxide (NaOH), sodium chloride (NaCl), potassium phosphate monobasic (KH2PO4), sodium carbonate (Na2CO3), sodium bicarbonate (NaHCO3), sodium acetate (NaAc), 2,4-dinitrofluorobenzene (DNFB), and diammonium phosphate (DAP) were purchased from Kermel (Tianjin, China).
Non-volatile standards (purity ≥ 98.0%), included L-aspartic acid, L-tryptophan, L-isoleucine, L-alanine, L-arginine, γ-aminobutyric acid (GABA), L-methionine, L-phenylalanine, L-tyrosine, and L-proline (Yuanye Shanghai, China); L-glutamic acid, L-valine, and L-leucine (Solarbio, Beijing, China); and L-malic acid, citric acid, acetic acid, succinic acid, and glycerol (DrE, Ratzeburg, Germany) (Table S1). Methanol and acetonitrile were obtained from Kermel (Tianjin, China). Water was obtained from a Milli-Q purification system (Millipore, Bedford, MA, USA).
Volatile chemical standards (purity ≥ 97.0%), including ethyl acetate, isobutyl acetate, isoamyl acetate, phenylethyl acetate, ethyl butyrate, ethyl hexanoate, ethyl octanoate, ethyl decanoate, isobutyl alcohol, isoamyl alcohol, 2-phenylethanol, 2-octanol, hexanoic acid, octanoic acid, and decanoic acid, were obtained from Sigma-Aldrich (Shanghai, China) (Table S2).
S. cerevisiae strain Angel RV171 (Angel Yeast, Yichang, China), a neutral wine yeast strain for dry wine fermentation, was used in this study.
2.3. Winemaking and Nitrogen Supplementation
Alcoholic fermentation was carried out as described by Kong et al. [20] Mature and healthy grape berries were manually selected, destemmed, and crushed. Sulfur dioxide (SO2; 60 mg/L) was added to the grape must to avoid oxidation. Subsequently, cold maceration was conducted at 4 °C for 24 h, and the must was divided into triplicates. Sucrose was added to the must to achieve a final alcohol concentration of 12% (w). Fermentation was performed in 20 L bottles after yeast inoculation. Fermentation temperature was maintained between 20 °C and 22 °C, and cap management to push the floating caps into the juice was done thrice daily. For the analysis of the nitrogen compounds, aroma compounds and other metabolites, fermented juice was sampled every 24 h. Each sample was centrifuged for 10 min at 8000 rpm to remove yeast cells, and 20 mL of the supernatant was collected and stored in a freezer at −20 °C until further analysis. After fermentation (reducing sugar level < 2 g/L), clear wine was separated from skin and lees, followed by sulfur dioxide addition (60 mg/L).
To determine the Pearson’s correlation coefficient between amino acids and fruity esters during fermentation, spine grape must was divided into six groups for different nitrogen supplements. Samples were analyzed before and after nitrogen addition to ensure similar YAN concentrations in spine grape must and Cabernet Sauvignon grape must (Table S3). Six different nitrogen supplements were used in the study, including IN, AA-Ala, AA-Phe, AA-Ile, MAA, and IN+MAA (Table 1). The treatment IN+MAA was used in an attempt to modulate the concentration of alanine, phenylalanine, and isoleucine in spine grape must to the same level as that in Cabernet Sauvignon must, and the difference in YAN was adjusted by DAP addition. MAA treatment was used to add the three key amino acids multiple times to attain a final proportion similar to IN+MAA without DAP. All supplements contained approximately 50 mg N/L YAN. After nitrogen supplementation, YAN in spine grape and Cabernet Sauvignon must was between 185.3 and 187.9 mg N/L. Similar alcoholic fermentation was carried out after nitrogen addition. All fermentation experiments were performed in triplicate. Wine samples were stored at −20 °C until further analysis.

Table 1.
Composition of each nitrogen supplement.
2.4. Amino Acid Analysis
Amino acids were analyzed by precolumn derivatization using DNFB as described by Li et al. [23] with some modifications. For precolumn derivatization, 100 μL of clarified wine or standard was mixed with 100 μL of 0.05 M NaHCO3 and 40 μL of DNFB solution (5 mg/mL; w). The mixture was incubated in a water bath at 60 °C for 60 min in the dark. After returning to room temperature, 760 μL of KH2PO4 buffer (0.01 M; pH 7.0) was added to the tube, vortexed, and kept in the dark for 15 min.
Amino acids were analyzed in a Waters Alliance 2695 HPLC system (Milford, MA, USA) using Agilent ZORBAX SB-C18 column (Analytical 4.6 × 250 mm; 5 micron). The solvent system consisted of mobile phase A (acetonitrile), mobile phase B (ultrapure water), and mobile phase C (NaAc buffer; pH = 6.4). Samples were filtered through a nylon filter (0.22 μm), injected (20 μL) onto the column, and eluted at 33 °C at a flow rate of 1 mL/min according to the following gradient: initial—8% A, 8% B, 84% C; 0–2 min/8–15% A, 8–15% B, 84–70% C; 2–4 min/15–7% A, 15–17% B, 70–66% C; 4–8 min/17–20% A, 17–20% B, 66–60% C; 8–14 min/20–21% A, 20–22% B, 60–57% C; 14–24 min/21–28% A, 22–27% B, 57–45% C; 24–27 min/28–28% A, 27–27%B, 45–45% C; 27–36 min/28–49% A; 27–49% B, 45–2% C; and 36–40 min/49–8% A, 49–8% B, 2–84% C. The separated amino acid derivatives were detected using Waters 2996 photodiode array detector at 360 nm. The external standard method was used to qualitatively and quantitatively analyze the separated amino acids.
2.5. Organic Acid and Glycerol Analyses
Malic acid, acetic acid, citric acid, and succinic acid were determined in a Waters Alliance 2695 HPLC system (Milford, MA, USA) with a Welch Ultimate AQ-C18 column (Analytical 4.6 mm × 250 mm; 5 µm) at 30 °C. The column was eluted with 98% 0.02 mM KH2PO4:2% methanol at 1 mL/min. The separated organic acids were detected using a Waters 2996 photodiode array detector at 210 nm. Glycerol was measured with a Welch Ultimate XB-NH2 column (Analytical 4.6 mm × 250 mm; 5 µm) at 30 °C. The column was eluted with 85% acetonitrile at 1 mL/min. Glycerol was detected using a Waters 2410 refractive index detector. Glycerol and organic acids were quantitated using the calibration curves.
2.6. Yeast Assimilable Nitrogen Analysis
Yeast assimilable nitrogen (YAN) was calculated using the following Equation (1):
where TAN indicates total amino nitrogen concentration, Nammonium indicates contribution of nitrogen corresponding to ammonium, and Nproline indicates contribution of nitrogen corresponding to proline.
YAN = TAN + Nammonium − Nproline
The formaldehyde titration method was used to analyze the concentration of TAN and Nammonium [24]. Briefly, 10 mL of filtered juice was titrated with NaOH solution until neutralized (pH = 8.0), and then 2 mL of neutral formaldehyde (pH = 8.0) was added. The mixture was titrated with NaOH solution until pH = 8.0. TAN and Nammonium were calculated using the following Equation (2):
where V1 indicates the volume of NaOH solution used in titration, C indicates the concentration of NaOH titrant, D indicates dilution factor of juice sample, and V2 indicates the volume of juice sample.
TAN + Nammonium = (V1 × C × 14 × D × 1000)/V2
2.7. Quantification of Volatiles
The volatiles were analyzed using headspace solid-phase microextraction (HS-SPME) coupled with GC–MS as described by Hu et al. [25]. A 50/30 μm DVB/CAR/PDMS fiber (Supelco, Bellefonte, PA, USA) was used to extract the volatiles. In a 20 mL gas-tight vial, 2 g of NaCl, 2 mL of wine, 6 mL of pure water, and 20 μL of internal standard (16 mg/L, 2-octanol) were added and incubated in a 40 °C water bath with stirring at 600 rpm for 15 min, extracted for 30 min, and desorbed in the GC injection port (230 °C) for 5 min using a Shimadzu QP2020 GC–MS (Shimadzu Corporation, Kyoto, Japan) and a DB-WAX column (60 m × 0.25 mm × 0.25 μm; Agilent Corporation, Santa Clara, CA, USA). The carrier gas used was helium (99.999%) at a flow rate of 1.5 mL/min. The GC program was as follows: 40 °C for 3 min, raised to 160 °C at 4 °C/min, followed by an increase to 220 °C at 7 °C/min, and hold for 8 min. MS transfer line and ion source temperatures were set to 220 °C and 200 °C, respectively. Electron ionization (EI) mass spectrometric data from m/z 35 to 350 were scanned at 0.2 s intervals. Esters were identified by comparing their retention time and mass spectra with those of pure standards using the NIST 17 mass spectral library. Target compound concentration was calculated by interpolation of relative areas in the calibration graphs obtained with the pure reference compound (2-octanol).
2.8. Statistical Analysis
All experiments were performed at least in duplicate. Data were expressed as mean ± standard deviation. One-way analysis of variance (ANOVA) was used to compare the means with Duncan’s test. Data correlation was evaluated using bivariate (two-tailed) Pearson correlation coefficient (r) in SPSS 23.0 (IBM Corp., Armonk, IL, USA).
3. Results
3.1. Evolution of Amino Acids and Fruity Esters during Alcoholic Fermentation
We investigated the dynamic evolution of 13 amino acids in spine grape and Cabernet Sauvignon during alcoholic fermentation (Table S4). Total amino acid content (except proline) of spine grape must was more than that of Cabernet Sauvignon must. In spine grape must, aspartic acid, valine, glutamic acid, GABA, and arginine levels were significantly (p < 0.01) more than those in Cabernet Sauvignon grape must, while proline, alanine, phenylalanine, and isoleucine levels were significantly less than those in Cabernet Sauvignon grape must (Figure 1). With progress in alcoholic fermentation, almost all amino acids gradually decreased, except proline and tyrosine. Proline was not assimilated and finally produced by yeast, and tyrosine content decreased slightly during Cabernet Sauvignon fermentation and increased during spine grape fermentation.
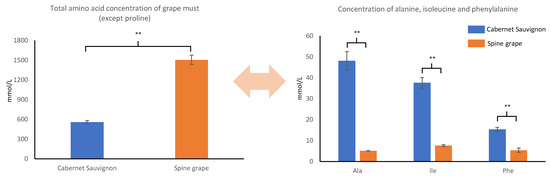
Figure 1.
Concentrations of total amino acid (except proline), alanine, isoleucine, and phenylalanine in Cabernet Sauvignon and spine grape must. Difference significant at 99% (**) confidence level.
Evolution of fruity esters during alcoholic fermentation was monitored by HPME-GC-MS (Figure 2, Tables S5 and S6). Acetates and ethyl esters rapidly increased once fermentation started, and the highest ethyl acetate and short-chain fatty acid ethyl ester (SCFAEE) levels were detected at the end of fermentation. In contrast, acetates of higher alcohols (AHAs) and medium-chain fatty acid ethyl esters (MCFAEEs) decreased during the later stage of fermentation (day 7 to day 12). The level of ethyl acetate in spine grape wine was 58% of that in Cabernet Sauvignon wine, AHA was 5%, SCFAEE was 89%, and MCFAEE was 67%.

Figure 2.
Evolution of fruity esters during Cabernet Sauvignon and spine grape alcoholic fermentation: (a) Ethyl acetate, (b) Acetate of higher alcohol, AHA, (c) Short-chain fatty acid ethyl ester, SCFAEE, (d) medium-chain fatty acid ethyl ester, MCFAEE. AHAs included isobutyl acetate, isoamyl acetate, hexyl acetate, and β-Phenylethyl acetate; SCFAEEs included ethyl butyrate; and MCFAEEs included ethyl hexanoate, ethyl octanoate, and ethyl decanoate.
3.2. Correlation Analysis and Identification of Goal-Directed Amino Acid
To estimate the goal-directed amino acid, Pearson correlation analysis was used to establish a fitting model between the amino acid profile and fruity ester levels in spine grape and Cabernet Sauvignon grape during fermentation (Table S7), and the differences in these Pearson’s correlation coefficients (r) were further assessed (Table 2). During fermentation, almost all amino acids were rapidly consumed and the fruity ester content gradually increased, thus all r values were negative. Correlation coefficients between these amino acids and AHAs were slightly less than those during Cabernet Sauvignon fermentation, nevertheless, a correlation coefficient similar to or higher than that of Cabernet Sauvignon fermentation was observed between the amino acids arginine, aspartic acid, glutamic acid, GABA, and valine and the fruity esters SCFAEEs and MCFAEEs during spine grape fermentation. In contrast, the correlation coefficients between the amino acids alanine, phenylalanine, and isoleucine, and the fruity esters AHAs, SCFAEEs, and MCFAEEs during fermentation of spine grape were less than those during fermentation of Cabernet Sauvignon. As mentioned in Section 3.1, arginine, aspartic acid, glutamic acid, GABA, and valine levels in spine grape must were more than those in Cabernet Sauvignon grape must, while alanine, phenylalanine, and isoleucine levels were less than those in Cabernet Sauvignon grape must. These findings indicate that the lesser abundance of alanine, phenylalanine, and isoleucine may have led to lower fruity ester production during spine grape fermentation. Therefore, alanine, phenylalanine, and isoleucine were used for nitrogen supplementation.

Table 2.
Difference in Pearson’s correlation coefficients (r) during Cabernet Sauvignon and spine grape fermentation. Data were calculated by rcabernet sauvignon − rspine grape, where rcabernet sauvignon indicates the correlation coefficient of Cabernet Sauvignon fermentation and rspine grape indicates the correlation coefficient of spine grape fermentation. Red cell means the correlation coefficient during spine grape fermentation was less than that during Cabernet Sauvignon fermentation. The darker the red, the weaker the correlation between the amino acids and fruity ester during spine grape fermentation. AHA, acetate of higher alcohol; SCFAEE, short-chain fatty acid ethyl ester; MCFAEE, medium-chain fatty acid ethyl ester.
3.3. Modification of Fruity Ester Production under Nitrogen Supplementation
Based on the results described in Section 3.2, different nitrogen sources were supplemented to the six groups of spine grape must with Cabernet Sauvignon control (CK-CS) and spine grape control (CK-SP) (Table S3). Fruity esters, especially ethyl acetate, AHAs, and MCFAEEs, in MAA and IN+MAA treatments were considerably more than that in CK-SP and their levels reached or even exceeded the ester levels in CK-CS (Table 3). A significant increase in ethyl acetate concentration was observed in MAA, which was 2.1-fold and 1.5-fold more than CK-SP and CK-CS, respectively, and even 1.6-fold more than that in the IN+MAA treatment. Meanwhile, the content of FAEEs was 1.2-fold less than that in IN+MAA treatment. These findings show that a comprehensive supplementation of alanine, phenylalanine, and isoleucine significantly influences the production of fruity esters.

Table 3.
Concentration of fruity esters and their precursors in spine grape wines obtained from different nitrogen nutrient treatments and controls. Data are mean ± standard deviation. Values displaying different letters within each row are significantly different according to the Duncan test at 95% confidence level. AHA, acetate of higher alcohol; SCFAEE, short-chain fatty acid ethyl ester; MCFAEE, medium-chain fatty acid ethyl ester; CK-CS, Cabernet Sauvignon control; CK-SP, spine grape control; IN, DAP supplement; AA-Ala, single alanine supplement; AA-Phe, single phenylalanine supplement; AA-Ile, single isoleucine supplement; MAA, mixed nitrogen supplement with alanine, phenylalanine, and isoleucine; IN+MAA, mixed nitrogen supplement with DAP, alanine, phenylalanine, and isoleucine.
With single nitrogen supplements, no significant difference was observed in the acetate and FAEE levels between inorganic nitrogen treatment (DAP addition, IN) and CK-SP. AA-Ala led to significantly higher levels of ethyl acetate, SCFAEEs, and MCFAEEs compared with other single amino acid supplements. AA-Phe and AA-Ile showed no significant increase in ethyl acetate content, while AA-Ile reduced the content. However, AHA production with AA-Ala was less than with AA-Phe and AA-Ile. AA-Phe significantly increased phenethyl acetate content, and AA-Ile significantly increased isobutyl acetate and isoamyl acetate levels. AA-Phe and AA-Ile increased FAEE content compared to CK-SP; no significant difference was observed compared with IN+MAA treatment.
3.4. Analysis of Extracellular Metabolites and Precursors Involved in Fruity Ester Synthesis
Fruity ester precursors and corresponding extracellular metabolites, including higher alcohols (isobutanol, isopentanol, 1-hexanol, and 2-phenylethanol), volatile linear fatty acids (hexanoic acid, octanoic acid, and decanoic acid), and carbon-metabolism-derived extracellular metabolites (L-malic acid, acetic acid, citric acid, succinic acid, and glycerol), under different treatments were analyzed (Table 3 and Table 4). Nitrogen supplementation significantly increased the levels of higher alcohols and fatty acids. AA-Phe and AA-Ile dramatically increased the higher alcohol level; AA-Phe increased 2-phenylethanol content (5-fold compared with CK-SP); and AA-Ile increased the isoamyl alcohol level (1.8-fold compared with CK-SP), whereas AA-Ala and IN+MAA did not contribute to higher alcohol production. In contrast, AA-Ala, AA-Phe, and IN+MAA treatments significantly enhanced the production of medium-chain fatty acids (MCFAs). Analysis of carbon-metabolism-derived extracellular metabolites indicated that CK-CS had the highest organic acid content while AA-Ile produced the highest amount of glycerol. All nitrogen treatments reduced citric acid content in spine grape wine. Compared with CK-SP, the treatments AA-Ala, AA-Phe, AA-Ile, and MAA led to a significant increase in glycerol content in spine grape wine while IN+MAA led to a significant decrease in glycerol, malic acid, and citric acid levels. Other nitrogen treatments, except AA-Phe, increased acetic acid content. In addition, IN+MAA produced the highest level of extracellular succinic acid, while other treatments had no significant effect on succinic acid production.

Table 4.
Concentrations of extracellular metabolites in wines obtained from different nitrogen nutrient treatments (g/L). Data are mean ± standard deviation. Values displaying different letters within each row are significantly different according to the Duncan test at 95% confidence level. The Duncan test was carried out in columns CK-SP, IN, MAA, and IN+MAA. CK-CS, Cabernet Sauvignon control; CK-SP, spine grape control; IN, DAP supplement; AA-Ala, single alanine supplement; AA-Phe, single phenylalanine supplement; AA-Ile, single isoleucine supplement; MAA, mixed nitrogen supplement with alanine, phenylalanine, and isoleucine; IN+MAA, mixed nitrogen supplement with DAP, alanine, phenylalanine, and isoleucine.
Furthermore, the differences in yeast extracellular metabolites involved in MCFA and acetate synthesis pathways are illustrated in Figure 3. Only MAA and IN+MAA treatments, which resulted in significant modification of fruity ester profile, were selected in Figure 3 while CK-SP and IN were used as controls. Compared with CK-SP and IN, IN+MAA treatment decreased glycerol and acetic acid levels and increased citric acid, succinic acid, ethyl octanoate, and ethyl decanoate levels. In contrast, IN had no significant influence compared with CK-SP.

Figure 3.
Redistribution of carbon fluxes in the central metabolic network by different nitrogen treatments. PYR, pyruvate; GA3P, glyceraldehyde 3-phosphate; ACTA, acetaldehyde. Data are mean ± standard deviation. Differences are significant at 95% (*), 99% (**)confidence level. AHA, acetate of higher alcohol; MCFA, medium-chain fatty acid; MCFAEE, medium-chain fatty acid ethyl ester; CK-SP, spine grape control; IN, DAP supplement; MAA, mixed nitrogen supplement with alanine, phenylalanine, and isoleucine; IN+MAA, mixed nitrogen supplement with DAP, alanine, phenylalanine, and isoleucine.
4. Discussion
Amino acids are important factors involved in the synthesis of fermentation-derived aroma compounds. Studies have correlated amino acid profiles with grape varieties [26]. In this study, we show differences in amino acid composition and consumption dynamics during fermentation between spine grape and Cabernet Sauvignon. S. cerevisiae has a nitrogen-catabolite repression (NCR) system that suppresses alanine assimilation until ammonium gets exhausted from the media. Therefore, alanine is always consumed late [27]. Here, the start of alanine consumption was delayed by more than 3 days during Cabernet Sauvignon fermentation, while it was consumed rapidly to depletion during spine grape fermentation. Meanwhile, tyrosine was not consumed in large amounts in either system. This observation is consistent with the findings of Gao et al. [28] who reported that tyrosine is not fully consumed during wine alcoholic fermentation due to the presence of aromatic amino acid biosynthetic pathway in certain S. cerevisiae [29]. However, Garde-Cerdán et al. [10] showed complete consumption of tyrosine during alcoholic fermentation of four different V. vinifera varieties. These contradictory results indicate that tyrosine evolution might be highly dependent on fermentation conditions. The levels of leucine, tryptophan, and methionine were not different between the two grape varieties. Alanine, isoleucine, and phenylalanine were lowest in spine grape must while their levels were still within the rational concentration range [1]. Moreover, yeast cannot assimilate proline [30], and therefore, its evolution and correlation with esters are not discussed.
Fruity esters are the main aroma compounds in wine [31]. Only a few studies have so far focused on aroma compounds in spine grape wine. Meng et al. analyzed the characteristic aroma components in spine grape berries instead of spine grape wine [32]. Kong et al. analyzed ester content in spine grape wine without comparing it with other types of wine and found that the fruity ester content in spine grape wine was significantly less than that in Cabernet Sauvignon wine [20]. Fruity esters, which are the fermentative volatiles, contributed significantly to the aroma of spine grape wine. This indicates that a low level of fruity esters is responsible for the weak fruity aroma of spine grape wine.
The amino acid profile of grapes and wine varied between varieties [33]. Different methods have been used to establish a model to determine the correlation between amino acids and aroma compounds. Procopio et al. [8] established a fingerprint of the amino acid and aroma compound profiles by partial least-squares regression (PLS) analysis, and Fairbairn et al. [7] established a linear model between amino acids and volatile aroma components of synthetic juice. In this study, Pearson’s correlation was used to determine the association between amino acid profile and fruity ester content during spine grape and Cabernet Sauvignon grape fermentation. The analysis revealed that alanine, phenylalanine, and isoleucine at low levels and with low correlation coefficients may have led to limited fruity ester production during spine grape fermentation, which is in accordance with our previous study that the low content of specific amino acids could negatively affect ester productions during alcoholic fermentation [22]. Accordingly, we speculate that the modification of amino acid profile in spine grape must by supplementing alanine, phenylalanine, and isoleucine has the potential to increase fruity ester production.
Studies have shown that amino acid addition affects wine ester concentration [7,12,34]. Generally, nitrogen management during winemaking is based on the catabolism of the amino acids combined with de novo synthesis of proteinogenic amino acids [16,17]. However, there are limited studies on amino acid supplementation based on the amino acid profile of grape must and on the correlation between amino acids and aroma compounds. We found that goal-directed supplementation of amino acids led to significant increase in the production of AHAs and MCFAEEs. In contrast, IN had no significant effect on fruity ester content. Earlier, Hernández-Orte et al. reported no effect of DAP supplementation on the ester content of synthetic juice [35]. Meanwhile, Vilanova et al. [4] showed an increase in ethyl ester content with DAP supplementation. These differences in findings may be due to the differences in nutrient availability and yeast strains [2]. Amino acid utilization via Ehrlich pathway had pronounced effects on the balance between higher alcohols and corresponding acetate esters. In the present study, AA-Phe and AA-Ile caused a higher production of β-phenylethyl acetate and isoamyl acetate due to increase in 2-phenylethanol and isoamyl alcohol precursors. However, AA-Ile had no marked effect on total MCFAEE content and, similar to AA-Phe, had reduced ethyl acetate production. In contrast to AA-Phe and AA-Ile, AA-Ala significantly enhanced ethyl acetate production. This may be because alanine is converted to pyruvate [36], a main precursor of acetyl-CoA, and the accumulation of acetyl-CoA could have led to increase in ethyl acetate and fatty acid biosynthesis [15,37]. To conclude, AA-Ala enhanced fruity ester production; however, AHA and MCFAEE levels in AA-Ala were less than that in IN+MAA.
Despite similar YAN content, differences in amino acid composition significantly influenced the ester content in wine. The production of fruity esters was considerably higher under both MAA and IN+MAA treatments; however, a higher (2.1-fold) increase in ethyl acetate content with MAA treatment may lead to off-flavor and finally decreased wine quality [38]. Meanwhile, IN did not contribute to fruity ester production. Single amino acid supplements led to an imbalance in the fruity ester profile. Therefore, IN+MAA, a balanced and goal-directed amino acid supplement, can be used to enhance fermentative aroma quality of spine grape wine.
In addition to the aroma compounds produced during fermentation, changes in metabolic intermediates also greatly affect wine ester profile. Amino acids act as precursors of fermentation-derived aroma compounds principally via the Ehrlich pathway [14]. Research has indicated that transamination of amino acids is essential for the subsequent redistribution of nitrogen for the de novo synthesis of proteinogenic amino acids [16], which consequently releases α-keto acid intermediates [39]. Crepin et al. [16] showed that the carbon skeletons of consumed amino acids contribute less to the production of volatile compounds. This is in contrast to the findings of Fairbairn et al. who reported that even minor changes in the amino acid profile of synthetic grape must significantly influence volatile production [7]. These contradicting results indicated an interaction between carbon and nitrogen metabolism for the production of aroma compounds. Accordingly, we analyzed fruity ester precursors and corresponding extracellular metabolites to illustrate the mechanism involved in fruity ester production. CK-SP, CK-CS, IN, and AA-Ile showed lower production of MCFAs, which are markers for limited acetyl-CoA levels [40]. Saerens et al. [41] showed that the level of fatty acids is the most limiting factor in ethyl ester production. An increase in MCFA production enhanced MCFAEE production [25]. Studies have shown that nitrogen management positively influences fatty acid biosynthesis via intensified glycolysis, impaired TCA cycle, and enhanced metabolic fluxes channeling pyruvate and acetyl-CoA [42]. Similarly, IN+MAA, the goal-directed supplementation of specific amino acids in moderate amounts based on the levels in Cabernet Sauvignon, seemingly triggered carbon-flux redistribution combined with an increase in α-keto acid precursors via the central carbon metabolism. These mechanisms led to higher acetyl-CoA and MCFA-CoA levels and enhanced MCFA production, which finally improved the contents of corresponding ethyl esters in spine grape wine. To conclude, IN+MAA may redistribute carbon flux and favor fruity ester production.
Other treatments also have led to an increase in MCFA and MCFAEE, which supports that amino acid supplements favor fruity ester production. AA-Ala improved MCFA production, while it resulted in lesser AHA production compared with IN+MAA. Acetate esters are synthesized from alcohols and acetyl-CoA by alcohol acetyltransferases (AATases) [43]. Amino acid profiles in grape juice influence the redox potential [44], which can modulate the enzymatic activity or gene expression [45] thereby affecting wine ester production. The variation in the formation of AHA derivatives from their precursors may be due to an imbalance in the amino acid profile that affects the intracellular redox potential. This might have led to limited activity or expression of AATases [45] under excessive supplementation of specific amino acids (MAA and AA-Ala). Besides, MAA treatment led to an increase in glycerol, acetic acid, citric acid, succinic acid, and ethyl acetate levels. This may be due to the increase in total carbon fluxes derived from the carbon skeletons of these specific amino acids and resultant increase in metabolites of carbon metabolism pathway [17]. However, in-depth studies are needed to reveal the transcriptomic and metabolomic differences under goal-directed amino acid supplementation.
5. Conclusions
This work demonstrates a change in fruity ester production with goal-directed amino acid supplementation during alcoholic fermentation of spine grape. Alanine, phenylalanine, and isoleucine may be the defect in the amino acid profile of spine grape must that lowered fruity ester production. Supplementation of these three amino acids based on the amino acid profile of Cabernet Sauvignon grape must dramatically enhanced the fruity ester content in spine grape wine. In addition, modification of the amino acid profile triggered carbon-flux redistribution, promoted MCFA production, and, finally, enhanced MCFAEE content. However, further work is needed to reveal the molecular mechanisms of fruity ester biosynthesis under nitrogen intervention.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/fermentation7040231/s1, Table S1: Qualitative and quantitative information of chromatographically pure standards in HPLC analysis, Table S2: Qualitative and quantitative information of chromatographically pure standards in SPME-GC-MS analysis, Table S3: Concentrations of yeast assimilable nitrogen in different nitrogen treatments and controls, Table S4: Evolution of amino acid content during alcoholic fermentation of spine grape and Cabernet Sauvignon grape (µmol/L), Table S5: Concentrations of wine fruity esters during alcoholic fermentation of Cabernet Sauvignon grape (μg/L), Table S6: Concentration of wine fruity esters during alcoholic fermentation of spine grape (μg/L), Table S7: Pearson’s correlation coefficients (r) between amino acids and wine fruity esters during alcoholic fermentation, Table S8 Physiochemical indices of wine samples.
Author Contributions
Conceptualization, Z.Z.; methodology, Z.Z. and K.H.; validation, K.H.; formal analysis, Z.Z., S.C., and S.X.; investigation, Z.Z., S.C., and S.X.; writing—original draft preparation, Z.Z.; writing—review and editing, Z.Z., K.H., and Y.T.; visualization, Z.Z.; supervision, Y.T.; project administration, Y.T.; funding acquisition, Y.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (31972199), the Shaanxi Science Fund for Distinguished Young Scholars (2020JC-22), and the Scientific and Technological Key Projects of Xinjiang production and Construction Corps (2019AB025).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data can be found within the manuscript and Supplementary Materials.
Acknowledgments
The authors would like to thank Aihua Li from the College of Food Science and Engineering, Northwest A&F University for the excellent technical assistance of instrumental analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bell, S.J.; Henschke, P.A. Implications of nitrogen nutrition for grapes, fermentation and wine. Aust. J. Grape Wine Res. 2005, 11, 242–295. [Google Scholar] [CrossRef]
- Gobert, A.; Tourdot-Marechal, R.; Sparrow, C.; Morge, C.; Alexandre, H. Influence of nitrogen status in wine alcoholic fermentation. Food Microbiol. 2019, 83, 71–85. [Google Scholar] [CrossRef]
- Gutierrez-Gamboa, G.; Carrasco-Quiroz, M.; Martinez-Gil, A.M.; Perez-Alvarez, E.P.; Garde-Cerdan, T.; Moreno-Simunovic, Y. Grape and wine amino acid composition from Carignan noir grapevines growing under rainfed conditions in the Maule Valley, Chile: Effects of location and rootstock. Food Res. Int. 2018, 105, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Vilanova, M.; Ugliano, M.; Varela, C.; Siebert, T.; Pretorius, I.S.; Henschke, P.A. Assimilable nitrogen utilisation and production of volatile and non-volatile compounds in chemically defined medium by Saccharomyces cerevisiae wine yeasts. Appl. Microbiol. Biotechnol 2007, 77, 145–157. [Google Scholar] [CrossRef] [Green Version]
- Lytra, G.; Tempere, S.; Le Floch, A.; de Revel, G.; Barbe, J.C. Study of sensory interactions among red wine fruity esters in a model solution. J. Agric. Food Chem. 2013, 61, 8504–8513. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Jin, G.J.; Mei, W.C.; Li, T.; Tao, Y.S. Increase of medium-chain fatty acid ethyl ester content in mixed H. uvarum/S. cerevisiae fermentation leads to wine fruity aroma enhancement. Food Chem. 2018, 239, 495–501. [Google Scholar] [CrossRef]
- Fairbairn, S.; McKinnon, A.; Musarurwa, H.T.; Ferreira, A.C.; Bauer, F.F. The Impact of Single Amino Acids on Growth and Volatile Aroma Production by Saccharomyces cerevisiae Strains. Front. Microbiol. 2017, 8, 2554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Procopio, S.; Krause, D.; Hofmann, T.; Becker, T. Significant amino acids in aroma compound profiling during yeast fermentation analyzed by PLS regression. LWT-Food Sci. Technol. 2013, 51, 423–432. [Google Scholar] [CrossRef]
- Hernández-Orte, P.; Cacho, J.F.; Ferreira, V. Relationship between varietal amino acid profile of grapes and wine aromatic composition. Experiments with model solutions and chemometric study. J. Agric. Food Chem. 2002, 50, 2891–2899. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Martínez-Gil, A.M.; Lorenzo, C.; Lara, J.F.; Pardo, F.; Salinas, M.R. Implications of nitrogen compounds during alcoholic fermentation from some grape varieties at different maturation stages and cultivation systems. Food Chem. 2011, 124, 106–116. [Google Scholar] [CrossRef]
- Torrea, D.; Varela, C.; Ugliano, M.; Ancin-Azpilicueta, C.; Leigh Francis, I.; Henschke, P.A. Comparison of inorganic and organic nitrogen supplementation of grape juice—Effect on volatile composition and aroma profile of a Chardonnay wine fermented with Saccharomyces cerevisiae yeast. Food Chem. 2011, 127, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Chia, J.Y.; Liu, S.Q. Impact of addition of aromatic amino acids on non-volatile and volatile compounds in lychee wine fermented with Saccharomyces cerevisiae MERIT.ferm. Int. J. Food Microbiol. 2014, 170, 12–20. [Google Scholar] [CrossRef]
- Ugliano, M.; Travis, B.; Francis, I.L.; Henschke, P.A. Volatile composition and sensory properties of Shiraz wines as affected by nitrogen supplementation and yeast species: Rationalizing nitrogen modulation of wine aroma. J. Agric. Food Chem. 2010, 58, 12417–12425. [Google Scholar] [CrossRef] [PubMed]
- Hazelwood, L.A.; Daran, J.M.; van Maris, A.J.; Pronk, J.T.; Dickinson, J.R. The Ehrlich pathway for fusel alcohol production: A century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 2008, 74, 2259–2266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malcorps, P.; Dufour, J.P. Short-chain and medium-chain aliphatic-ester synthesis in Saccharomyces cerevisiae. Eur. J. Biochem. 1992, 210, 1015–1022. [Google Scholar] [CrossRef]
- Crepin, L.; Truong, N.M.; Bloem, A.; Sanchez, I.; Dequin, S.; Camarasa, C. Management of Multiple Nitrogen Sources during Wine Fermentation by Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2017, 83. [Google Scholar] [CrossRef] [Green Version]
- Rollero, S.; Mouret, J.R.; Bloem, A.; Sanchez, I.; Ortiz-Julien, A.; Sablayrolles, J.M.; Dequin, S.; Camarasa, C. Quantitative (13) C-isotope labelling-based analysis to elucidate the influence of environmental parameters on the production of fermentative aromas during wine fermentation. Microb. Biotechnol. 2017, 10, 1649–1662. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.; Ye, D.; Duan, L.; Duan, C.; Yan, G. Effect of the addition of branched-chain amino acids to non-limited nitrogen synthetic grape must on volatile compounds and global gene expression during alcoholic fermentation. Aust. J. Grape Wine Res. 2018, 24, 197–205. [Google Scholar] [CrossRef]
- Meng, J.; Xu, T.; Qin, M.; Zhuang, X.; Fang, Y.; Zhang, Z. Phenolic characterization of young wines made from spine grape (Vitis davidii Foex) grown in Chongyi County (China). Food Res. Int. 2012, 49, 664–671. [Google Scholar] [CrossRef]
- Kong, C.; Li, A.; Su, J.; Wang, X.; Chen, C.; Tao, Y. Flavor modification of dry red wine from Chinese spine grape by mixed fermentation with Pichia fermentans and S. cerevisiae. LWT-Food Sci. Technol. 2019, 109, 83–92. [Google Scholar] [CrossRef]
- Antalick, G.; Suklje, K.; Blackman, J.W.; Meeks, C.; Deloire, A.; Schmidtke, L.M. Influence of Grape Composition on Red Wine Ester Profile: Comparison between Cabernet Sauvignon and Shiraz Cultivars from Australian Warm Climate. J. Agric. Food Chem. 2015, 63, 4664–4672. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, S.; Su, J.; Tao, Y. Correlation Analysis between Amino Acids and Fruity Esters during Spine Grape Fermentation. Sci. Agric. Sin. 2020, 53, 2272–2284. [Google Scholar] [CrossRef]
- Li, N.; Liu, Y.; Zhao, Y.; Zheng, X.; Lu, J.; Liang, Y. Simultaneous HPLC determination of amino acids in tea infusion coupled to pre-column derivatization with 2, 4-dinitrofluorobenzene. Food Anal. Methods 2016, 9, 1307–1314. [Google Scholar] [CrossRef]
- Zoecklein, B.; Fugelsang, K.; Gump, B.; Nury, F. Wine Analysis and Production; Chapman & Hall. Inc.: New York, NY, USA, 1995. [Google Scholar]
- Hu, K.; Jin, G.J.; Xu, Y.H.; Xue, S.J.; Qiao, S.J.; Teng, Y.X.; Tao, Y.S. Enhancing wine ester biosynthesis in mixed Hanseniaspora uvarum/Saccharomyces cerevisiae fermentation by nitrogen nutrient addition. Food Res. Int. 2019, 123, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Ough, C.S. Amino acid profiles of commercial grape juices and wines. Am. J. Enol. Vitic. 1991, 42, 261–267. [Google Scholar]
- Roca-Mesa, H.; Sendra, S.; Mas, A.; Beltran, G.; Torija, M.J. Nitrogen Preferences during Alcoholic Fermentation of Different Non-Saccharomyces Yeasts of Oenological Interest. Microorganisms 2020, 8, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, N.; Li, L.; Deng, X.; Song, L. Changes of free amino acids during wine fermentation. China Brew. 2011, 1, 28–33. [Google Scholar] [CrossRef]
- Braus, G.H. Aromatic amino acid biosynthesis in the yeast Saccharomyces cerevisiae: A model system for the regulation of a eukaryotic biosynthetic pathway. Microbiol. Rev. 1991, 55, 349–370. [Google Scholar] [CrossRef]
- Beltran, G.; Esteve-Zarzoso, B.; Rozès, N.; Mas, A.; Guillamón, J.M. Influence of the timing of nitrogen additions during synthetic grape must fermentations on fermentation kinetics and nitrogen consumption. J. Agric. Food Chem. 2005, 53, 996–1002. [Google Scholar] [CrossRef]
- Ebeler, S.E.; Thorngate, J.H. Wine chemistry and flavor: Looking into the crystal glass. J. Agric. Food Chem. 2009, 57, 8098–8108. [Google Scholar] [CrossRef]
- Meng, J.; Xu, T.; Song, C.; Li, X.; Yue, T.; Qin, M.; Fang, Y.; Zhang, Z.; Xi, Z. Characteristic free aromatic components of nine clones of spine grape (Vitis davidii Foex) from Zhongfang County (China). Food Res. Int. 2013, 54, 1795–1800. [Google Scholar] [CrossRef]
- Martínez-Pinilla, O.; Guadalupe, Z.; Hernández, Z.; Ayestarán, B. Amino acids and biogenic amines in red varietal wines: The role of grape variety, malolactic fermentation and vintage. Eur. Food Res. Technol. 2013, 237, 887–895. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Ancín-Azpilicueta, C. Effect of the addition of different quantities of amino acids to nitrogen-deficient must on the formation of esters, alcohols, and acids during wine alcoholic fermentation. LWT-Food Sci. Technol. 2008, 41, 501–510. [Google Scholar] [CrossRef]
- Hernández-Orte, P.; Ibarz, M.; Cacho, J.; Ferreira, V. Effect of the addition of ammonium and amino acids to musts of Airen variety on aromatic composition and sensory properties of the obtained wine. Food Chem. 2005, 89, 163–174. [Google Scholar] [CrossRef]
- Frick, O.; Wittmann, C. Characterization of the metabolic shift between oxidative and fermentative growth in Saccharomyces cerevisiae by comparative 13 C flux analysis. Microb. Cell Fact. 2005, 4, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Saerens, S.M.; Delvaux, F.R.; Verstrepen, K.J.; Thevelein, J.M. Production and biological function of volatile esters in Saccharomyces cerevisiae. Microb. Biotechnol. 2010, 3, 165–177. [Google Scholar] [CrossRef] [Green Version]
- Boutou, S.; Chatonnet, P. Rapid headspace solid-phase microextraction/gas chromatographic/mass spectrometric assay for the quantitative determination of some of the main odorants causing off-flavours in wine. J. Chromatogr. A 2007, 1141, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Magasanik, B.; Kaiser, C.A. Nitrogen regulation in Saccharomyces cerevisiae. Gene 2002, 290, 1–18. [Google Scholar] [CrossRef]
- Bloem, A.; Sanchez, I.; Dequin, S.; Camarasa, C. Metabolic Impact of Redox Cofactor Perturbations on the Formation of Aroma Compounds in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2016, 82, 174–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saerens, S.; Delvaux, F.; Verstrepen, K.; Van Dijck, P.; Thevelein, J.; Delvaux, F. Parameters affecting ethyl ester production by Saccharomyces cerevisiae during fermentation. Appl. Environ. Microbiol. 2008, 74, 454–461. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.; Wang, X.; Xu, Y. Palladium-Catalyzed Tandem Reactions of β-(2-Bromophenyl)-α,β-Unsaturated Carbonyl Compounds with 2-Hydroxyphenylboronic Acid: A New Route to Benzo[c]chromenes. Eur. J. Org. Chem. 2012, 2012, 1112–1114. [Google Scholar] [CrossRef]
- Mason, A.B.; Dufour, J.P. Alcohol acetyltransferases and the significance of ester synthesis in yeast. Yeast 2000, 16, 1287–1298. [Google Scholar] [CrossRef]
- Mauricio, J.C.; Valero, E.; Millán, C.; Ortega, J.M. Changes in nitrogen compounds in must and wine during fermentation and biological aging by flor yeasts. J. Agric. Food Chem. 2001, 49, 3310–3315. [Google Scholar] [CrossRef] [PubMed]
- Farina, L.; Medina, K.; Urruty, M.; Boido, E.; Dellacassa, E.; Carrau, F. Redox effect on volatile compound formation in wine during fermentation by Saccharomyces cerevisiae. Food Chem. 2012, 134, 933–939. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).