Silver Ion-Complexation High-Speed Countercurrent Chromatography Coupled with Prep-HPLC for Separation of Sesquiterpenoids from Germacrene A Fermentation Broth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Apparatus
2.3. Preparation of the Crude Extract for HSCCC Isolation
2.4. Selection of Two-Phase Solvent System for HSCCC
2.5. Preparation of Solvent System and Sample Solutions
2.6. HSCCC Separation
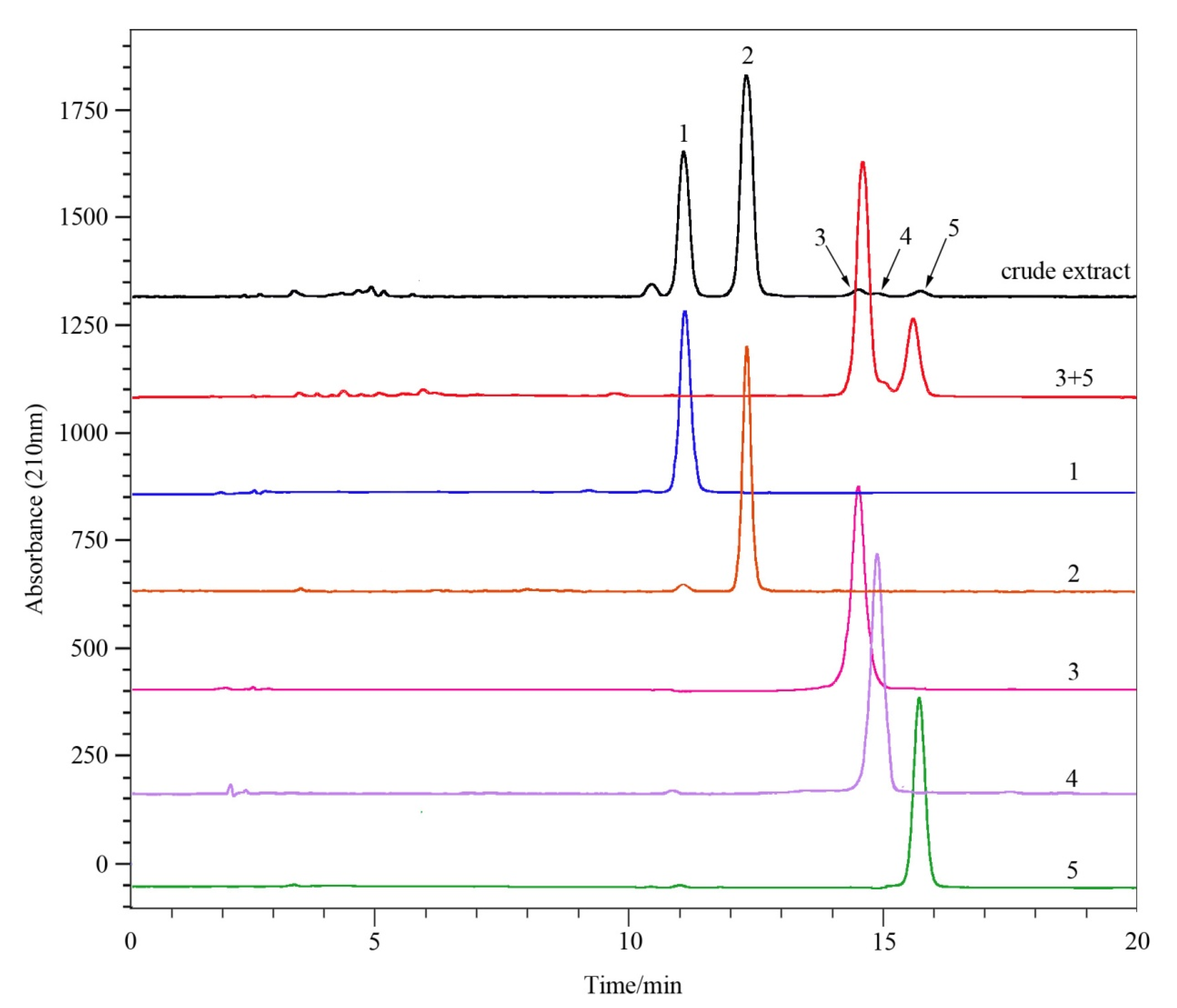
2.7. Sample Analysis and Structural Identification
3. Results
3.1. Two-Phase Solvent System in HSCCC
3.2. Scheme of Complexation via Computational Study
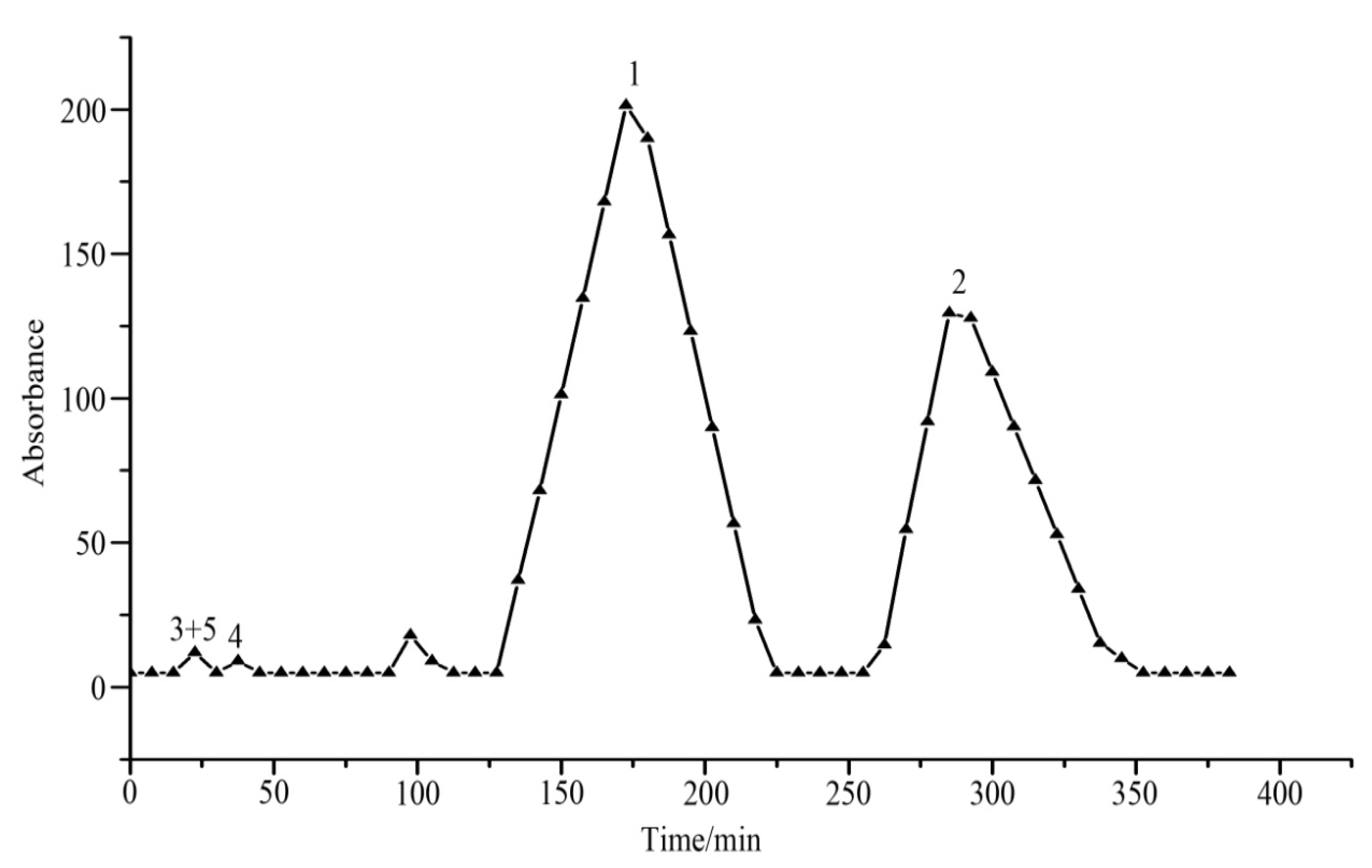
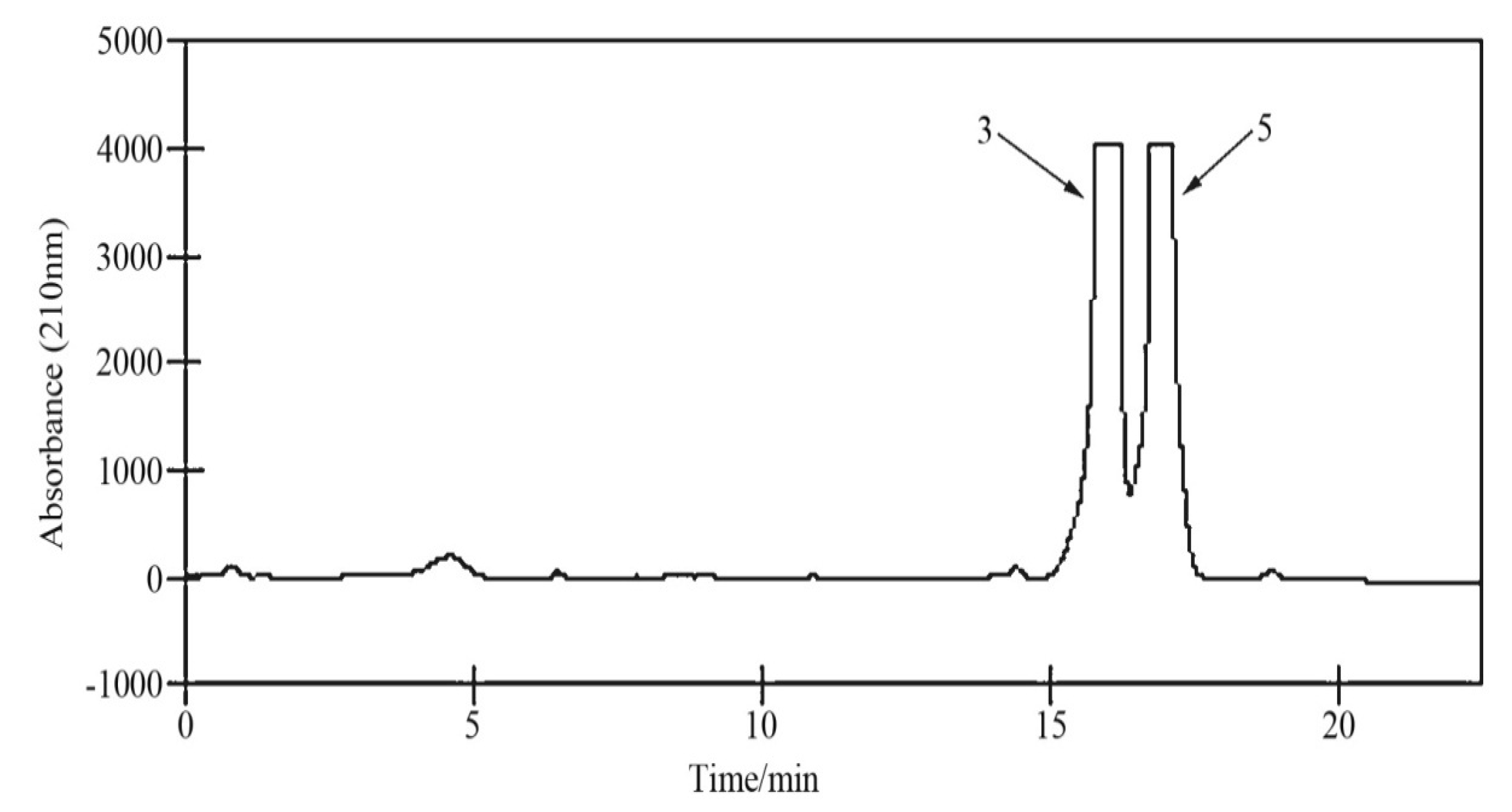
3.3. HSCCC Procedure
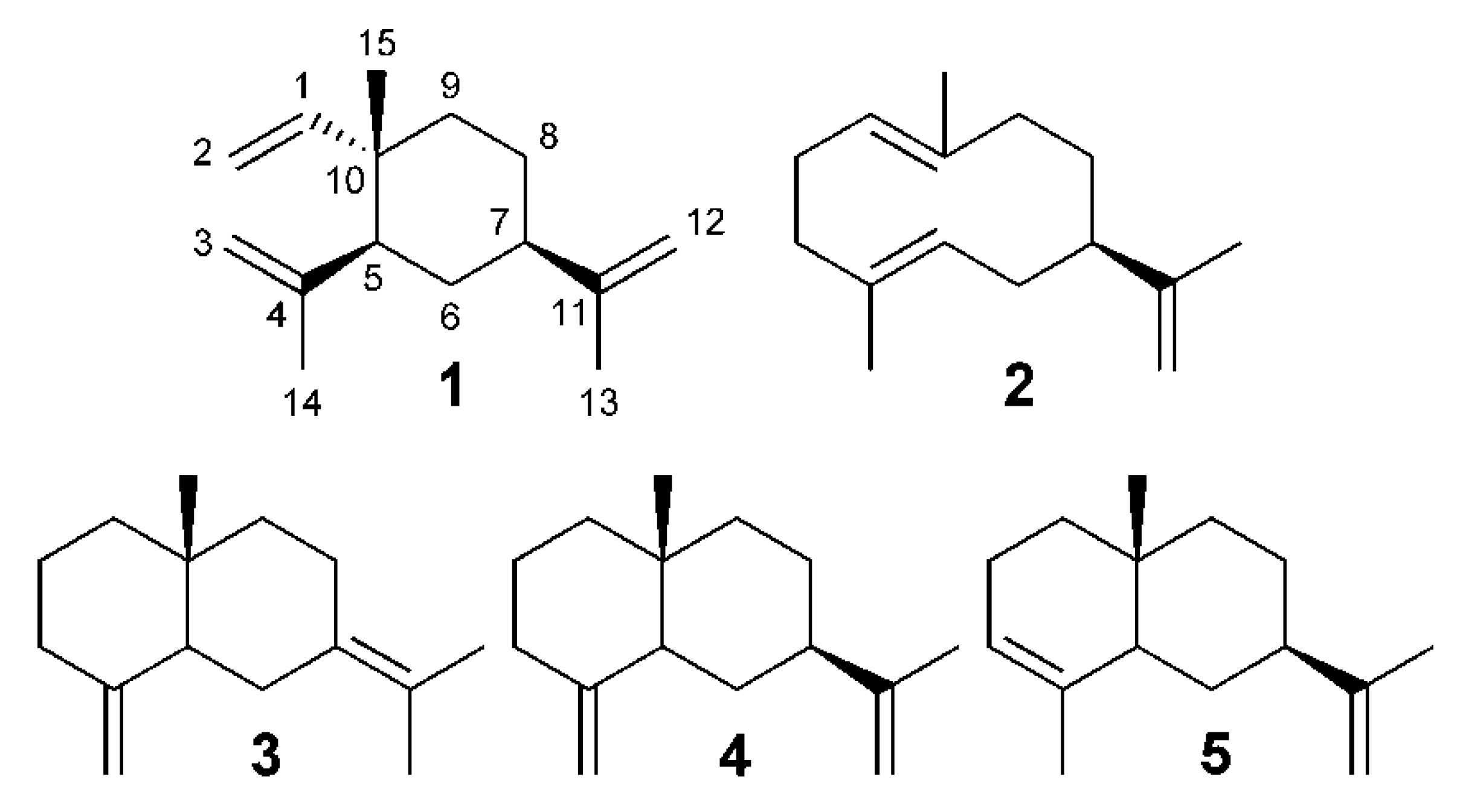
3.4. Identification of Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, G.; Li, X.; Huang, F.; Zhao, J.; Ding, H.; Cunningham, C.; Coad, J.E.; Flynn, D.C.; Reed, E.; Li, Q.Q. Antitumor effect of beta-elemene in non-small-cell lung cancer cells is mediated via induction of cell cycle arrest and apoptotic cell death. Cell Mol. Life Sci. 2005, 62, 881–893. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, H.D.; Yao, Y.F.; Zhong, S.L.; Zhao, J.H.; Tang, J.H. beta-Elemene reverses chemoresistance of breast cancer cells by reducing resistance transmission via exosomes. Cell Physiol. Biochem. 2015, 36, 2274–2286. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.Q.; Ding, X.; Jia, Y.C.; Huang, C.X.; Wang, Y.Z.; Xu, Y.H. Anti-tumor effect of beta-elemene in glioblastoma cells depends on p38 MAPK activation. Cancer Lett. 2008, 264, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Mei, X.L.; Yao, H.; Zhang, T.; Zhang, T.; Lu, N.; Liu, Y.S.; Xu, W.Y.; Wan, C.Y. beta-elemene enhances anticancer and anti-metastatic effects of osteosarcoma of ligustrazine in vitro and in vivo. Oncol. Lett. 2018, 15, 3957–3964. [Google Scholar] [PubMed] [Green Version]
- Zhai, B.T.; Zeng, Y.Y.; Zeng, Z.W.; Zhang, N.N.; Li, C.X.; Zeng, Y.J.; You, Y.; Wang, S.L.; Chen, X.B.; Sui, X.B.; et al. Drug delivery systems for elemene, its main active ingredient beta-elemene, and its derivatives in cancer therapy. Int. J. Nanomed. 2018, 13, 6279–6296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, G.N.; Ashby, C.R., Jr.; Zhang, Y.K.; Chen, Z.S.; Guo, H. The reversal of antineoplastic drug resistance in cancer cells by beta-elemene. Chin. J. Cancer 2015, 34, 488–495. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.S.; Xie, T.; Lin, J.; Fan, H.Z.; Huang-Fu, H.J.; Ni, L.F.; Yan, H.F. An investigation of the ability of elemene to pass through the blood-brain barrier and its effect on brain carcinomas. J. Pharm. Pharmacol. 2009, 61, 1653–1656. [Google Scholar] [CrossRef]
- Tan, W.; Lu, J.; Huang, M.; Li, Y.; Chen, M.; Wu, G.; Gong, J.; Zhong, Z.; Xu, Z.; Dang, Y.; et al. Anti-cancer natural products isolated from chinese medicinal herbs. Chin. Med. 2011, 6, 27. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.C.; Wang, R.F.; Wang, T.Y.; Ding, Q.L.; Khalil, A.; Xu, S.T.; Lin, A.J.; Yao, H.Q.; Xie, W.J.; Zhu, Z.Y.; et al. Antioxidant properties of novel dinners derived from natural beta-elemene through inhibiting H2O2-induced apoptosis. ACS Med. Chem. Lett. 2017, 8, 443–448. [Google Scholar] [CrossRef]
- Govindarajan, M.; Benelli, G. alpha-Humulene and beta-elemene from Syzygium zeylanicum (Myrtaceae) essential oil: Highly effective and eco-friendly larvicides against Anopheles subpictus, Aedes albopictus, and Culex tritaeniorhynchus (Diptera: Culicidae). Parasitol. Res. 2016, 115, 2771–2778. [Google Scholar] [CrossRef]
- Hong, L.L.; Zeng, Y.M.; Yang, D.Y. Inhibitory effect of beta-elemene on human airway granulation tissue in vivo and in vitro. Respiration 2016, 92, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.G.; Li, N.; Zhang, Y.; Fan, D.F.; Yang, C.; Li, H.; Guo, D.Z.; Pan, S.Y. Beneficial effect of beta-elemene alone and in combination with hyperbaric oxygen in traumatic brain injury by inflammatory pathway. Transl. Neurosci. 2018, 9, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.B.; Wang, C.Y.; Cui, Z.J.; Guo, P.F.; Meng, Q.N.; Shi, X.; Gao, Y.; Yang, G.Y.; Han, Z.F. beta-Elemene induces apoptosis of human rheumatoid arthritis fibroblast-like synoviocytes via reactive oxygen species-dependent activation of p38 mitogen-activated protein kinase. Pharmacol. Rep. 2016, 68, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, L.; Dai, Z.; Wang, D.; Zhang, L.; Guo, J.; Liu, Y. A Recombinant Bacterium and Its Uses. China Patent CN201711064197.5, 27 August 2018. [Google Scholar]
- de Kraker, J.W.; Franssen, M.C.R.; de Groot, A.; Konig, W.A.; Bouwmeester, H.J. (+)-Germacrene A biosynthesis—The committed step in the biosynthesis of bitter sesquiterpene lactones in chicory. Plant Physiol. 1998, 117, 1381–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adio, A.M. Germacrenes A–E and related compounds: Thermal, photochemical and acid induced transannular cyclizations. Tetrahedron 2009, 65, 1533–1552. [Google Scholar] [CrossRef]
- Toledo, A.G.; de Souza, J.G.D.; da Silva, J.P.B.; Favreto, W.A.J.; da Costa, W.F.; Pinto, F.G.D. Chemical composition, antimicrobial and antioxidant activity of the essential oil of leaves of Eugenia involucrata dc. Biosci. J. 2020, 36, 568–577. [Google Scholar] [CrossRef] [Green Version]
- Saric-Krsmanovic, M.; Umiljendic, J.G.; Radivojevic, L.; Rajkovic, M.; Santric, L.; Durovic-Pejcev, R. Chemical composition of Ambrosia trifida essential oil and phytotoxic effect on other plants. Chem. Biodivers. 2020, 17, e1900508. [Google Scholar] [CrossRef]
- Mehdizadeh, L.; Taheri, P.; Pirbalouti, A.G.; Moghaddam, M. Phytotoxicity and antifungal properties of the essential oil from the Juniperus polycarpos var. turcomanica (B. Fedsch.) RP Adams leaves. Physiol. Mol. Biol. Plants 2020, 26, 759–771. [Google Scholar] [CrossRef]
- Ali, I.; Li, J.; Cui, L.; Zhao, H.; He, Q.; Wang, D. Efficient extraction and purification of benzo[c]phenanthridine alkaloids from Macleaya cordata (Willd) R. Br. by combination of ultrahigh pressure extraction and pH-zone-refining counter-current chromatography with anti-breast cancer activity in vitro. Phytochem. Anal. PCA 2021, 32, 423–432. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, L.; Yu, J.; Cui, L.; Ali, I.; Song, X.; Park, J.H.; Wang, D.; Wang, X. Flavonoid epimers from custard apple leaves, a rapid screening and separation by HSCCC and their antioxidant and hypoglycaemic activities evaluation. Sci. Rep. 2020, 10, 8819. [Google Scholar] [CrossRef]
- Ali, I.; Mu, Y.; Atif, M.; Hussain, H.; Li, J.; Li, D.; Shabbir, M.; Bankeu, J.J.K.; Cui, L.; Sajjad, S.; et al. Separation and anti-inflammatory evaluation of phytochemical constituents from Pleurospermum candollei (Apiaceae) by high-speed countercurrent chromatography with continuous sample load. J. Sep. Sci. 2021, 44, 2663–2673. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Chen, G.; Tong, S.; Feng, Y.; Sheng, L.; Lou, J. Preparative isolation and purification of germacrone and curdione from the essential oil of the rhizomes of Curcuma wenyujin by high-speed counter-current chromatography. J. Chromatogr. A 2005, 1070, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Ali, I.; Hussain, H.; Hussain, M.; Wang, X.B.; Song, X.; Luo, G.; Zhang, Z.; Wang, Z.; Wang, D. Extraction and purification of cis/trans asarone from Acorus tatarinowii Schott: Accelerated solvent extraction and silver ion coordination high-speed counter-current chromatography. J. Chromatogr. A 2021, 1643, 462080. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y. Golden rules and pitfalls in selecting optimum conditions for high-speed counter-current chromatography. J. Chromatogr. A 2005, 1065, 145–168. [Google Scholar] [CrossRef] [PubMed]
- Pallenberg, A.J.; Marschner, T.M.; Barnhart, D.M. Phenanthroline complexes of the d10 metals nickel(0), zinc(II) and silver(I)—comparison to copper(I) species. Polyhedron 1997, 16, 2711–2719. [Google Scholar] [CrossRef]
- Wen, Y.; Wang, J.; Chen, X.; Le, Z.; Chen, Y.; Zheng, W. Application of silver ion in the separation of macrolide antibiotic components by high-speed counter-current chromatography. J. Chromatogr. A 2009, 1216, 4668–4672. [Google Scholar] [CrossRef] [PubMed]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange—Correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Dolg, M. Chapter 14—Relativistic Effective Core Potentials. In Theoretical and Computational Chemistry; Schwerdtfeger, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2002; Volume 11, pp. 793–862. [Google Scholar]
- Liang, L.F.; Guo, J.W.; Fan, T.; Yao, L.G.; Guo, Y.W. Terpenes from south China sea soft coral Sarcophyton trocheliophrum. Chin. Tradit. Herb. Drugs 2016, 47, 4331–4335. [Google Scholar]
- Adio, A.M.; Paul, C.; Tesso, H.; Kloth, P.; Konig, W.A. Absolute configuration of helminthogermacrene. Tetrahedron Asymmetr. 2004, 15, 1631–1635. [Google Scholar] [CrossRef]
- Wang, C.-C.; Kuoh, C.-S.; Wu, T.-S. Constituents of Persea japonica. J. Nat. Prod. 1996, 59, 409–411. [Google Scholar] [CrossRef]
- Williams, H.J.; Sattler, I.; Moyna, G.; Ian Scott, A.; Bell, A.A.; Bradleigh Vinson, S. Diversity in cyclic sesquiterpene production by Gossypium hirsutum. Phytochemistry 1995, 40, 1633–1636. [Google Scholar] [CrossRef]

| n-Hexane:Ethyl Acetate:Methanol:Water | KD Value | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | α12 | α23 | |
| 5:5:5:5 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 1.00 | 1.00 |
| 8:2:8:2 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 1.00 | 1.00 |
| 5:5:8:2 | 0.07 | 0.07 | 0.04 | 0.06 | 0.05 | 1.00 | 1.00 |
| 9:1:9:1 | 0.05 | 0.05 | 0.05 | 0.06 | 0.05 | 1.03 | 0.99 |
| 10:0:9.5:0.5 | 0.11 | 0.11 | 0.08 | 0.09 | 0.09 | 1.00 | 1.00 |
| 9:1:9:1 1 mol/L [Ag+] | 0.68 | 0.87 | 0.06 | 0.13 | 0.05 | 1.51 | 1.27 |
| 9:1:9:1 2 mol/L [Ag+] | 0.77 | 1.07 | 0.05 | 0.10 | 0.05 | 1.44 | 1.39 |
| 9:1:9:1 3 mol/L [Ag+] | 1.82 | 2.86 | 0.11 | 0.27 | 0.14 | 1.55 | 1.57 |
| 10:0:9.5:0.5 1 mol/L [Ag+] | 0.42 | 0.45 | 0.14 | 0.22 | 0.16 | 1.47 | 1.06 |
| 10:0:9.5:0.5 2 mol/L [Ag+] | 0.77 | 0.91 | 0.14 | 0.26 | 0.15 | 1.51 | 1.19 |
| 10:0:9.5:0.5 3 mol/L [Ag+] | 1.33 | 1.74 | 0.16 | 0.38 | 0.18 | 1.50 | 1.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Song, X.; Ali, I.; Hussain, M.; Mehmood, A.; Siyo, B.; Gao, Q.; Cui, L.; Aziz, S.; Hussain, H.; et al. Silver Ion-Complexation High-Speed Countercurrent Chromatography Coupled with Prep-HPLC for Separation of Sesquiterpenoids from Germacrene A Fermentation Broth. Fermentation 2021, 7, 230. https://doi.org/10.3390/fermentation7040230
Zhao H, Song X, Ali I, Hussain M, Mehmood A, Siyo B, Gao Q, Cui L, Aziz S, Hussain H, et al. Silver Ion-Complexation High-Speed Countercurrent Chromatography Coupled with Prep-HPLC for Separation of Sesquiterpenoids from Germacrene A Fermentation Broth. Fermentation. 2021; 7(4):230. https://doi.org/10.3390/fermentation7040230
Chicago/Turabian StyleZhao, Huanzhu, Xiangyun Song, Iftikhar Ali, Manzoor Hussain, Andleeb Mehmood, Baraa Siyo, Qianshan Gao, Li Cui, Shahid Aziz, Hidayat Hussain, and et al. 2021. "Silver Ion-Complexation High-Speed Countercurrent Chromatography Coupled with Prep-HPLC for Separation of Sesquiterpenoids from Germacrene A Fermentation Broth" Fermentation 7, no. 4: 230. https://doi.org/10.3390/fermentation7040230
APA StyleZhao, H., Song, X., Ali, I., Hussain, M., Mehmood, A., Siyo, B., Gao, Q., Cui, L., Aziz, S., Hussain, H., Ma, W., Qin, D., & Wang, D. (2021). Silver Ion-Complexation High-Speed Countercurrent Chromatography Coupled with Prep-HPLC for Separation of Sesquiterpenoids from Germacrene A Fermentation Broth. Fermentation, 7(4), 230. https://doi.org/10.3390/fermentation7040230







