Abstract
Lentinula edodes 3565 and Ganoderma lucidum 9621 were compared for their ability to produce lignocellulolytic enzymes in submerged (SM) and surface liquid (SL) fermentation of hydrolysed colza straw lignin waste that remained after the production of furfural and bioethanol (CS lignin). Application of cultivated mushrooms to dispose of pretreated colza straw agricultural waste is an approach to decrease the quantity of residual lignin while simultaneously obtaining active substances, e.g., the ligninolytic enzyme complex from mycelium. The effect of adding CS lignin to culture media on the yield of L. edodes and G. lucidum mycelium and extracellular laccase activity was studied. It was revealed that the mycelial growth of G. lucidum on solid media was significantly improved by adding CS lignin. Laccase activity during SL cultivation of L. edodes on medium with CS lignin gradually increased over the experiment starting on day 21 and peaked at 520 U/mL on day 28. G. lucidum expressed the maximum laccase activity, 540 U/mL, during the first 14 days of mycelium SM cultivation. Extracellular laccase activity was enhanced about 35- to 40-fold at cultivation of L. edodes and about 10- to 15-fold in the case of G. lucidum by supplementing liquid culture media with CS lignin.
1. Introduction
The increasing expansion of agro-industrial activity has led to the accumulation of a large quantity of lignocellulosic residues from wood (e.g., sawdust), agricultural (e.g., CS), and various industrial wastes all over the world. Lignocellulose consists of three major components—cellulose, hemicellulose, and lignin—and is a renewable organic material and the major structural component of all plants.
There are several approaches towards the disposal of industrial and agricultural lignocellulose-containing waste or residues. For example, they can be used as raw material for the production of second-generation biofuel [1,2]. Colza straw can be used as the source of cellulose and hemicellulose in the production of furfural and bioethanol [3,4]. This process generates the most recalcitrant lignin residues that must undergo further biodegradation to make the production of furfural and bioethanol or lipids as a waste-less process. The lignin waste may be used to improve the growth of medicinal mushrooms and to produce laccase-containing enzymes complexes [5,6].
White-rot fungi (WRF) are the most efficient organisms among the known lignin degraders. WRF depolymerise lignin with an extracellular enzyme complex formed by phenol oxidases (i.e., laccases and peroxidases), manganese peroxidases, lignin peroxidases, and other versatile peroxidases [7,8,9,10]. Genomic sequences of G. lucidum and L. edodes revealed more than 20 genes that potentially encode lignin oxidising enzymes in each fungus [11,12]. Unlike G. lucidum, the genome of L. edodes does not have a lignin peroxidase gene. Meanwhile, L. edodes possesses a more versatile spectrum of non-specific peroxidases [12]. The use of fungi in the processing and recycling of some difficult-to-degrade technogenic wastes has been examined [13,14,15]. Bioconversion of lignin with lignin-degrading fungi has significant advantages over other alternative strategies because it offers the potential to produce both valuable mycelial biomass and biologically active intermediates. Furthermore, white-rot fungi have been used as a potent resource for lignocellulose degrading enzymes for many years [16,17]. Studies that have been carried out during the last years have shown that Basidiomycetes produce many biologically active substances: proteins, lipids, polysaccharides, organic acids, enzymes, and vitamins, among others. These compounds are effective and less toxic than chemotherapeutical drugs for many clinical indications.
The production of mycelium is considered to be a more promising approach in modern biotechnology than the process of obtaining fruiting bodies. Growing mushroom mycelia in liquid culture on a defined nutrient medium has long been a simple and fast alternative method to produce fungal biomass [18]. Submerged cultivation (SM) has been widely used in the production of mycelia and bioactive compounds [19,20,21]. This method allows for the synthesis of the target product, and standardisation of the conditions for its production and its quality indicators. It has been established that biologically active substances extracted from mycelial biomass and cultivation media are not inferior in their spectrum of action to compounds extracted from mushroom fruiting bodies [22,23,24]. SM of mushrooms enhances the growing process by inducing the synthesis of target metabolites, increasing the productivity of strains, and reducing the duration of the process. The synthesis of desired components can be controlled by changing the cultivation conditions.
Many studies have reported that WRF L. edodes and G. lucidum are capable of producing lignin-degrading enzymes while growing on a variety of lignocellulosic materials [25,26,27,28,29,30,31,32]. Pharmaceutical and biotechnological potential of both basidiomycetes has been studied. Biological degradation of lignocellulosic compounds is a complex process influenced by a number of variables: culture conditions, fungal strains, induction, regulation, and the mechanism of various enzyme activities [33].
The utilisation of CS lignin, which remains after furfural and bioethanol production, is an approach to obtain ligninolytic enzyme complexes while simultaneously decreasing the quantity of residual lignin waste. This research aimed to study and evaluate the production of lignin-degrading enzymes by L. edodes strain 3565 and G. lucidum strain 9621 under different cultivation conditions on CS lignin supplemented substrates to evaluate the prospects of agricultural waste bioconversion into low-cost enzyme preparations. The conditions for increasing mycelium biomass and enzyme yields were explored by changing the incubation settings.
2. Materials and Methods
2.1. Reagents
All reagents used were purchased from Sigma-Aldrich (St. Louis, MO, USA) and Thermo Fisher Scientific (Waltham, MA, USA) unless otherwise stated. All reagents were of analytical reagent grade.
2.2. Fungal Strains and Culture Conditions
The white-rot basidiomycete strains Lentinula edodes DSM 3565 (Berk.) Pegler and Ganoderma lucidum DSM 9621 (Curtis) P. Karst were purchased from the DSMZ Collection Center (Germany). Stock cultures were maintained on malt extract agar slants and stored at 4 °C. Mycelial discs from the peripheral region of actively growing culture from Malt Extract Broth (MEB, Merck, Darmstadt, Germany) agar plates were used as the inoculum. The control culture medium consisted of 10 g/L MEB. The initial pH of the culture medium was adjusted to 5.0–6.0 before sterilisation.
Cultivation of this mushrooms was performed in liquid media (28 days) or on solid nutrient medium (16 days) at 22 °C for L. edodes and 28 °C for G. lucidum. The diameter of the mycelium extension was measured on agar medium every 3–4 days over 14 days. For cultivation, the flasks were inoculated with two mycelium discs (d = 0.8 cm) from the MEB agar plates. Submerged cultivation of basidial mushrooms was carried out in 250 mL Erlenmeyer flasks with 75 mL of the MEB medium on a rotary shaker (140 rpm) or in static conditions without agitation (surface liquid cultivation, SL). The effects of culture pH ranging from pH 5.0 to 7.0 on biomass and laccase production were examined in shake flask cultures in preliminary experiments. The optimum pH for biomass production was 6.0, while that for laccase production was pH 5.0.
2.3. Substrate for Mycelium
Liquid and solid nutrient media were supplemented with colza straw (CS) lignin that remained after chemical and biotechnological production of furfural and bioethanol [1,2]. The first hydrothermal pre-treatment of colza straw was performed in a pilot scale reactor system using Al2SO4 as a catalyst at the temperature range of 170 °C during 60 min. The steam pressure at this hydrothermal process corresponded to 7.2 bar. The lignocellulose-containing residue of colza straw that remained after this first pre-treatment and obtaining of furfural was subjected to drying. Then, this residue was hydrolysed using commercial preparations of cellulase—Accellerase 1500 DuPont (Genencor, The Netherlands). The enzymatic hydrolysis was performed at a temperature of 50 °C during 72 h [4]. Glucose-containing supernatant was used for the obtaining of ethanol. The remaining lignin-containing residue was used in our study. Chemical analysis showed that lignin content in colza straw was 17.5 ± 0.19% [4]. After hydrothermal and enzymatic treatments total lignin amount has not changed considerably—it has decreased by 1–2 ± 0.02%. The substrate was crushed before it was added to a cultivation medium. CS lignin for mushroom cultivation was added to control MEB medium in the amounts of 0.5–2% (w/v). Preliminary experiments suggested a concentration of 2% CS lignin as the optimum for laccase production. Therefore, for enzyme production, 2% CS lignin added to the MEB culture media and sterilised at 121 °C for 15 min.
2.4. Determination of Dry Weight
After 28 days of incubation, mycelium biomass was separated from the medium by centrifugation and washed with sterile distilled water. Then, it was dried at 45 °C 48 h, and the weight of the biomass was determined. Qualitative mycelium biomass evaluation was done by measuring the diametric growth (mm) of mycelium on agar medium. The supernatants were collected and stored for the measurement of pH, laccase, and peroxidases.
2.5. Enzyme Assays
The supernatants were separated from the mycelia and substrates by centrifugation at 6000 rpm for 15 min. The supernatants were analysed for extracellular enzyme activity periodically every seven days. The data presented in this study were the mean values of triplicate measurements. After sampling, the filtrates were frozen (−20 °C) and lyophilised in the Alpha 1–2 LD plus vacuum freeze dryer (Christ, Osterode am Hanz, Germany).
Screening of laccase was carried out by spotting 20 mM 2, 2-azino-bis-[3-ethyltiazoline-6-sulfonate] (ABTS) on agar plates after removing the mycelium from the surface. The green colour indicates a positive result [34]. The activity of extracellular enzymes was determined using an Ultrospec 3100 pro UV/vis spectrophotometer (Amersham Biosciences, Amersham, UK). Samples were collected, and the supernatants were assayed for laccase and peroxidase. Laccase activity was determined by monitoring the change in absorbance at 420 nm (extinction coefficient = 36,000 M−1 cm−1) related to the rate of oxidation of 1 mM ABTS in 100 mM sodium acetate buffer (pH 5.0). One unit was defined as the amount of enzyme that leads to the oxidation of 1 µmol/min of ABTS per mL of medium filtrate [35].
2.6. Zymogram Analysis
Native PAGE was performed by the method of Laemmli [36] using a 10% polyacrylamide gel in non-denaturing conditions.
Aliquots of crude extract (2 mL) were frozen at −20 °C before freeze-drying. The dry pellets were then concentrated 10 times by solubilisation them in water and used for the native PAGE. Ten micrograms of total protein per gel lane were loaded. For zymography analysis, non-reducing loading buffer was used, and the sample was not boiled before loading. Tris-glycine running buffer (pH 8.3) was used. The native PAGE was run in a Mini Protean (Bio-Rad Laboratories, Hercules, CA, USA) gel system for about 90–120 min at 100 V. After electrophoresis, the gel was fixed for 10 min in a solution containing 10% v/v acetic acid and 40% v/v methanol, then soaked in 25 mM succinate buffer (pH 4.5) containing 2.7 mg/mL ABTS for laccase activity [34]. Zymography for peroxidase activity was performed with 50 mM N,N,N′,N′-tetramethylphenylenediamine TMPD in 50 mM Tris-Cl buffer (pH 7) containing 13 mM H2O2 [37].
2.7. Statistical Analysis
The average values of three independent experiments (each in triplicate) were used to calculate the results. Standard deviation of the mean was determined using statistical functions in Microsoft Excel. Results were expressed as the mean ± SD. The value p < 0.05 was accepted as the level of statistical significance.
3. Results and Discussion
Lentinula and Ganoderma, white-rot basidiomycetes widely distributed worldwide, can be cultivated on various substrates using different cultivation models, and secrete lignin-modifying enzymes. The effect of CS lignin substrate through the optimisation of different process parameters was studied. The values of cultivation variables that affect mycelial yields and enzymes activity (temperature, medium pH, agitation, and incubation period) were selected based on initial screening data. Due to the variable composition of the pretreated straw samples used, the mycelial growth abilities were evaluated in a control medium to develop a more reliable cultivation methodology. The optimal conditions for biomass and laccase production were significantly different.
3.1. Mycelial Growth on Pretreated Colza Straw
At the first stage of our experiments, we checked the effect of CS lignin as a medium-enriching additive on L. edodes and G. lucidum substrate colonisation rate. The diameters of mycelium were measured (mm, n = 4) after three and seven days of incubation on solid media.
It appeared that mycelium of G. lucidum grew slowly with a diameter of 44 mm. In contrast, mycelium of L. edodes grew rapidly with diameters greater than 70 mm after 10 days on the control medium. CS lignin additions significantly improved the mycelial growth of G. lucidum on solid media during the first seven days of cultivation (Figure 1a,b). At the same time, it was revealed that the mycelial diameters of L. edodes were not noticeably different in either culture media.
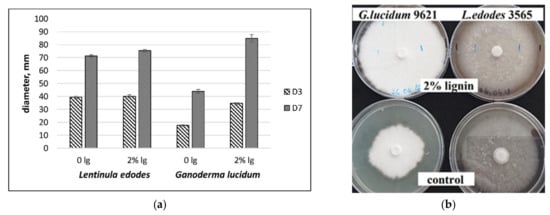
Figure 1.
Diameter of mycelia grown on MEB agar and MEB + 2% CS lignin (lg). Experiments were performed in triplicate. (a) D3, D7–days of cultivation. (b) L. edodes and G. lucidum mycelial growth on solid media, cultivation period—10 days.
The mycelial biomass yield of the mushrooms in the liquid cultures was larger under agitation than during static cultivation conditions (Figure 2). Mycelial biomass was determined by dry weight measurements. Biomass production for L. edodes ranged from 45.0 ± 8.9 (SL cultivation) to 69.3 ± 0.1 mg (SM cultivation) on 100 mL of control medium. The biomass production of G. lucidum ranged from 63.1 ± 8.3 (SL cultivation) to 245.0 ± 13.1 mg (SM cultivation) on 100 mL of control medium (Figure 2), and was significantly affected (p < 0.01) by the SM culture conditions: agitation at 140 rpm enhanced the final mycelium yield.
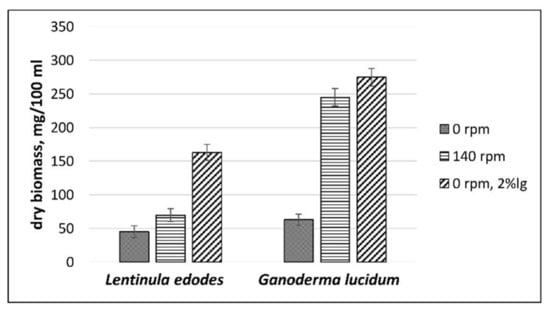
Figure 2.
Effect of agitation at 140 rpm and the addition of CS lignin on the production of mycelial biomass.
L. edodes and G. lucidum revealed a significantly higher yield (p < 0.01) when grown on medium with CS lignin. Surface liquid cultivation without agitation of both mushrooms resulted in 3- to 5-fold higher mycelial biomass yield when supplemented with CS lignin (Figure 2).
Using SL cultivation under stationary conditions, mycelium formed a film on the surface of the medium, and an increase in the viscosity of the liquid medium was observed. During submerged cultivation mycelial pellets or clumps are formed. When mycelium growth occurs in pellet form, the yield of biologically active metabolites depends on the pellet size [21]. Pellet sizes and morphologies vary under different cultivation conditions [38]. L. edodes L54 grows very poorly in agitated submerged conditions [35]. The agitation at low speed would prevent rapid aggregation of the mycelia and improve nutrient availability. Fraga et al. [39] demonstrated that reducing mycelium aggregation at different stages of fermentation by various means was beneficial to the rapid growth of mycelia of G. lucidum in submerged cultures. In our study, the size of mycelial pellets ranged from 2 to 5 mm during cultivation in MEB medium with agitation at 140 rpm and ranged from 5 to 14 mm under static conditions.
The addition of CS lignin changed the colour of the cultivation media to dark brown and opaque, which in turn made it impossible to accurately quantify the pellet size and mycelium biomass during SM fermentation. It is possible that lignin particles hindered the aggregation of mycelium and improved the growth of mushrooms in submerged culture.
3.2. Cultivation in SM with Agitation and SL Media for Laccase Production
Two commercial strains of L. edodes and G. lucidum mushrooms were evaluated for their ligninolytic enzyme profiles during cultivation with CS lignin supplemented media. The effect of the CS lignin supplement on extracellular laccase production was studied in MEB broth cultures. L. edodes cultures were incubated at 23 ± 2 °C, and G. lucidum cultures were incubated at 28 ± 2 °C. The supernatant was used as a crude enzyme solution. The supernatants received after mycelial biomass separation were analysed for pH and extracellular enzyme activity after 7, 14, 21, and 28 days of growth. The effects of agitation and the concentration of the CS lignin supplement on laccase enzyme production were evaluated.
The effect of pretreated CS lignin on laccase activity was evaluated in submerged culture medium with CS lignin added at various concentrations: 0.5%, 1.0%, 1.5%, and 2.0% (w/v; Figure 3). It became clear that adding CS lignin increased laccase activity in submerged cultures.
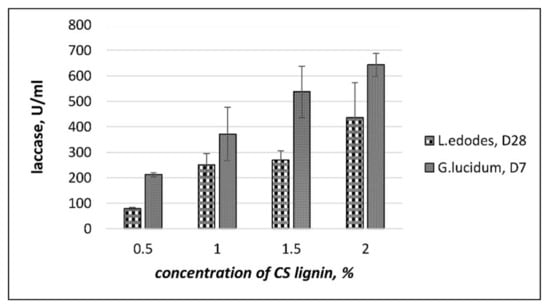
Figure 3.
The effect of CS lignin on the accumulation of laccase activity during SM cultivation with agitation. D7 and D28—days of incubation. Values are means with standard errors (vertical bars).
Under CS lignin-free conditions, L. edodes and G. lucidum produced only a small amount of laccase but did not produce peroxidases. The laccase activity in culture liquids of G. lucidum was greater than that for L. edodes (Figure 4).
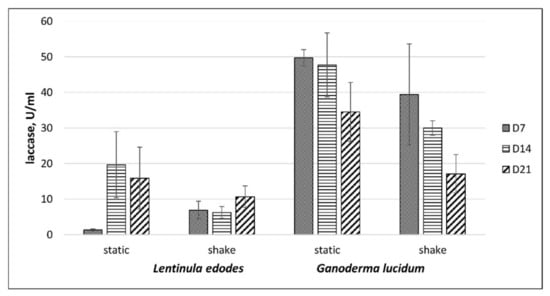
Figure 4.
Laccase activity of L. edodes and G. lucidum grown in control MEB liquid media under shaken and static conditions. D7 and D21—period of incubation (days).
Higher laccase activities of L. edodes were observed when these mushrooms were cultivated in medium containing CS lignin under static culture conditions than when shaken. Laccase activities of L. edodes gradually increased over the experiment starting at 21 days with a peak at 520 U/mL at day 28 in static conditions with CS lignin (Figure 5).
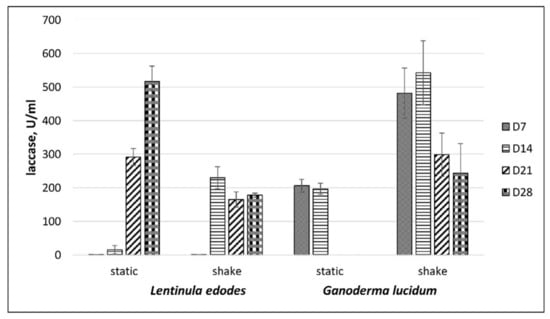
Figure 5.
Laccase activity of L. edodes and G. lucidum depending on CS lignin addition in media under shaken and static conditions. D7 and D28—period of incubation (days).
As shown in Figure 5, fermentation under agitation at 140 rpm resulted in a significant increase in laccase activity of G. lucidum when compared to static fermentation. The maximum laccase activities were detected during the first 14 days of G. lucidum cultivation with agitation. In the case of G. lucidum, laccase activity was seen with a peak of approximately 540 U/mL at day 14 with a decrease at day 21. Thus, the presence of CS lignin in the cultivation media significantly increased laccase activity in submerged cultures. As can be seen from Figure 4 and Figure 5, extracellular laccase activity, measured in the supernatants after cultivation with CS lignin additive, was 35- to 40-fold higher for L. edodes and 10- to 15- fold higher for G. lucidum than in supernatants without lignin.
3.3. Native PAGE
The literature reviews clearly show that the method of cultivation and presence of lignocellulosic wastes affect the synthesis of ligninolytic enzymes in a fungal-specific manner. The conditions of mycelial growth can increase or decrease the production of some of the enzymes and/or of their isoenzymes [10]. Isoenzymes can differ considerably in biochemical properties; therefore, their production expand the potential applications of ligninolytic enzymes complexes. In order to study the set of secreted ligninolytic isozymes, we performed zymogram analysis of L. edodes and G. lucidum culture supernatants.
The culture supernatants were harvested and concentrated by lyophilisation. Chromogenic substrates for laccases and peroxidases, ABTS and TMPD, respectively, were used to detect the activity of extracellular enzymes after PAGE.
Zymogram analysis using ABTS as a substrate revealed one green zone of Lac activity in concentrated extracellular culture liquids from L. edodes (Figure 6). This implies that the effect of the cultural conditions on laccase activity was not due to the expression of new isozymes. Peroxidase activity was not detected in the supernatants of L. edodes grown (28 days) under liquid culture conditions.
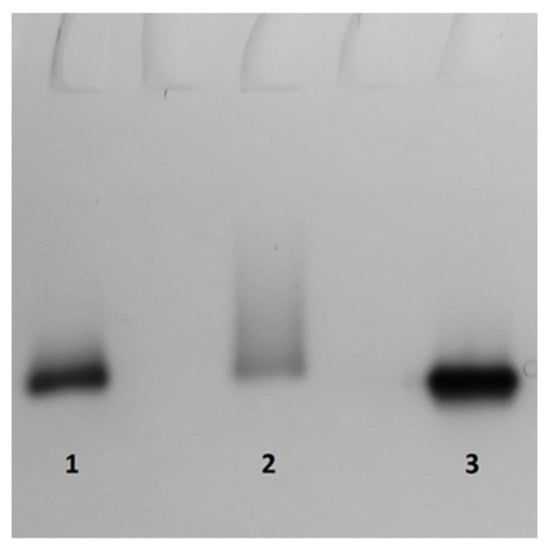
Figure 6.
Zymogram of laccase from the culture medium of L. edodes at 28 days of cultivation. The gel was stained with ABTS as substrate of laccase. Lanes: 1—SL cultivation without agitation; 2—SM cultivation with agitation with 2% CS lignin supplement; and 3—SL cultivation without agitation with 2% CS lignin supplement.
Isoforms of extracellular Lac from G. lucidum varied in number, mobility, and intensity of the electrophoretic bands with different growth conditions. In the control medium with agitation, there were visible three Lac isoforms: one Lac isoform with considerably lower electrophoretic mobility, and two weak bands with higher electrophoretic mobility (Figure 7a, lane 2); in the CS lignin-enriched medium there were two bands of similar intensity (Figure 7a, lane 3). Native PAGE electrophoresis of the crude extracellular proteins of G. lucidum confirmed the presence of an active peroxidase (Figure 7b). Two bands with peroxidase activity were obtained by culturing on the control medium with agitation at 140 rpm, and one isozyme was resolved in the CS lignin-enriched medium. These results indicate that the mycelial growth conditions of G. lucidum have a certain effect on the profile of extracellular laccase and peroxidase isoforms. The addition of CS lignin changed the colour of the cultivation media to dark brown due to the products of straw degradation. The standard methods previously adopted for assaying manganese peroxidase (MnP) and lignin peroxidase (LiP) activity in colourless fungal cultures were not suitable for coloured samples [40]. In this study, spectrophotometric assays that were used to quantify peroxidases in liquid cultures of L. edodes and G. lucidum failed to detect LiP and MnP activities. However, Native-PAGE results suggested the presence of peroxidases in G. lucidum liquid cultures after staining with TMPD (Figure 7b).
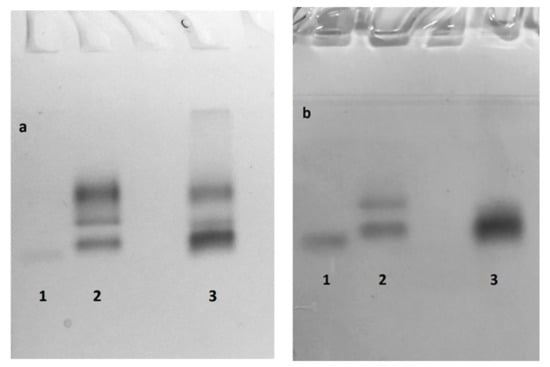
Figure 7.
Zymogram of ligninolytic enzymes from G. lucidum culture medium at 14 days of cultivation. The gel was stained: with ABTS (a), and with TMPD (b). Lanes: 1—SL cultivation without agitation; 2—SM cultivation with agitation with 2% CS lignin supplement; and 3—SL cultivation without agitation with 2% CS lignin supplement.
Summarizing the results of this study, it is necessary to mention once more that it is a part of a big complex research aimed at the development of completely new approach for the complete conversion of cellulose-containing agricultural residues into the various value-added products and compounds. At the first stage of this process using specific hydrothermal treatment, we obtained furfural from hemicellulose without usual irreversible damage of remaining lignocellulose. At the second stage, application of enzymatic hydrolysis led to the obtaining of glucose that was used for further microbial production of bioethanol. At the third stage, we used the remaining lignin-containing residue for the cultivation of medicinal mushrooms Lentinula edodes and Ganoderma lucidum. Therefore, the main purpose of this study was to show that lignin-containing residue after enzymatic hydrolysis of lignocellulose does not have any inhibitory effects and can be successfully used for cultivation of medicinal mushrooms.
Some studies have previously evaluated the role of WRF in bioremediation of wastes. Laccases and peroxidases have been reported in the secretomes of several white-rot basidiomycetes, including species of Ganoderma and Lentinula [35,41,42,43,44,45]. Laccases are commercially important enzymes due to their ability to degrade phenolic and non-phenolic lignin along with recalcitrant pollutants. It was shown that the addition of lignocelluloses residues to culture media could influence the increase in laccase activity of white-rot fungi by several-fold [10,30,46,47]. Additionally, our experiments showed that laccase production increased under submerged fermentation with L. edodes 3565 after the addition of pretreated wheat straw [5]. At the same time, we must note that in previous experiments, where all of the results were obtained with the addition of lignocellulosic residues, we used lignin waste obtained after furfural and bioethanol production from colza straw.
White-rot fungi produce multiple extracellular isoforms of laccase [48]. Variation in culture conditions can affect the production of distinct isozymes. Cavalazzi et al. [49] studied the effect of some compounds as laccase inducers in L. edodes cultures grown under stationary conditions and detected only one band in all treatments. G. lucidum secreted multiple laccase isozymes under diverse growth conditions [8,46]. Ko et al. [50] characterised three laccase isozymes of G. lucidum in liquid culture. Manavalan et al. [32] used zymography and confirmed four isoforms of laccase in G. lucidum, which was grown on sugarcane bagasse as a lignocellulose substrate.
4. Conclusions
Our study suggests that CS lignin, remaining after the chemical and biotechnological production of furfural and bioethanol from colza (rapeseed) straw, may be a suitable substrate for the production of mycelial biomass and laccases by L. edodes and G. lucidum mushrooms. We found that the mycelial growth of G. lucidum on solid media was significantly improved by adding CS lignin. SL cultivation of both mushrooms on media with supplemented CS lignin resulted in a 3- to 5-fold higher mycelial biomass yield in comparison to the control media that contained malt extract. Our study demonstrated that adding CS lignin to the cultivation medium increased laccase activity. Higher laccase activity in L. edodes liquid cultures was observed under static conditions, whereas for G. lucidum there was a preference for agitation to yield high laccase activity. Our results showed that CS lignin plays an important role not only at the level of soluble secreted ligninolytic enzymes of G. lucidum, but also in their isoforms profile.
Author Contributions
Conceptualization, A.R. and I.M.; investigation, G.M., V.B., E.A. and D.Z.; writing—original draft preparation, G.M.; writing—review and editing, A.R. and I.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the European Regional Development Fund project Nr.1.1.1.1/16/A/113.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rapoport, A.; Vedernikov, N.; Kruma, I.; Puke, M.; Borovikova, D.; Rozenfelde, L.; Khroustalyova, G.; Matyuskova, N. Waste-less bioethanol and other valuable substances production from hardwood. WIT Trans. Eng. Sci. 2014, 88, 311–317. [Google Scholar] [CrossRef]
- Brandenburg, J.; Poppele, I.; Blomqvist, J.; Puke, M.; Pickova, J.; Sandgren, M.; Rapoport, A.; Vedernikovs, N.; Passoth, V. Bioethanol and lipid production from the enzymatic hydrolysate of wheat straw after furfural extraction. Appl. Microbiol. Biotechnol. 2018, 102, 6269–6277. [Google Scholar] [CrossRef] [PubMed]
- López-Linares, J.C.; Ballesteros, I.; Tourán, J.; Cara, C.; Castro, E.; Ballesteros, M.; Romero, I. Optimization of uncatalyzed steam explosion pretreatment of rapeseed straw for biofuel production. Bioresour. Technol. 2015, 190, 97–105. [Google Scholar] [CrossRef]
- Rozenfelde, L.; Puke, M.; Vedernikovs, N.; Scherbaka, R.; Rapoport, A. Catalytic treatment of rapeseed straw for enhanced production of furfural and glucose for bioethanol production. Process. Biochem. 2021, 102, 102–107. [Google Scholar] [CrossRef]
- Matjuskova, N.; Okmane, L.; Zala, D.; Rozenfelde, L.; Puke, M.; Kruma, I.; Vedernikovs, N.; Rapoport, A. Effect of lignin-containing media on growth of medicinal mushroom Lentinula edodes. Proceeding Latv. Acad. Sci. 2017, 71, 38–42. [Google Scholar] [CrossRef]
- Liao, Y.; Koelewijn, S.-F.; Van den Bossche, G.; Van Aelst, J.; Van den Bosch, S.; Renders, T.; Navare, K.; Nicolaï, T.; Van Aelst, K.; Maesen, M.; et al. A sustainable wood biorefinery for low–carbon footprint chemicals production. Science 2020, 367, 1385–1390. [Google Scholar] [CrossRef] [PubMed]
- Leatham, G.F. Extracellular enzymes produced by the cultivated mushroom Lentinus edodes during degradation of a lignocellulosic medium. Appl. Environ. Microbiol. 1985, 50, 859–867. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, T.M.; Merritt, C.S.; Reddy, C.A. Lignin-modifying enzymes of the white rot basidiomycete Ganoderma lucidum. Appl. Environ. Microbiol. 1999, 65, 5307–5313. [Google Scholar] [CrossRef]
- Saeki, N.; Takeda, H.; Tanesaka, E.; Yoshida, M. Induction of manganese peroxidase and laccase by Lentinula edodes under liquid culture conditions and their isozyme detection by enzymatic staining on native-PAGE. Mycoscience 2011, 52, 132–136. [Google Scholar] [CrossRef]
- Martani, F.; Beltrametti, F.; Porro, D.; Branduardi, P.; Lotti, M. The importance of fermentative conditions for the biotechnological production of lignin modifying enzymes from white-rot fungi. FEMS Microbiol. Lett. 2017, 364, 1–18. [Google Scholar] [CrossRef]
- Chen, S.; Xu, J.; Liu, C.; Zhu, Y.; Nelson, D.R.; Zhou, S.; Li, C.; Wang, L.; Guo, X.; Sun, Y.; et al. Genome sequence of the model medicinal mushroom Ganoderma lucidum. Nat. Commun. 2012, 3, 1–9. [Google Scholar] [CrossRef]
- Chen, L.; Gong, Y.; Cai, Y.; Liu, W.; Zhou, Y.; Xiao, Y.; Xu, Z.; Liu, Y.; Lei, X.; Wang, G.; et al. Genome sequence of the edible cultivated mushroom Lentinula edodes (Shiitake) reveals insights into lignocellulose degradation. PLoS ONE 2016, 11, e0160336. [Google Scholar] [CrossRef]
- Hatakka, A. Lignin—Modifying enzymes from selected white—Rot fungi—Production and role in lignin degradation. FEMS Microbiol Rev. 1994, 13, 125–135. [Google Scholar] [CrossRef]
- Tripathi, M.; Mishra, A.; Misra, A.; Vaithiyanathan, S.; Prasad, R.; Jakhmola, R. Selection of white-rot basidiomycetes for bioconversion of mustard (Brassica compestris) straw under solid-state fermentation into energy substrate for rumen microorganism. Lett. Appl. Microbiol. 2008, 46, 364–370. [Google Scholar] [CrossRef]
- Dashtban, M.; Schraft, H.; Qin, W. Fungal Bioconversion of Lignocellulosic Residues; Opportunities & Perspectives. Int. J. Biol. Sci. 2009, 5, 578–595. [Google Scholar] [CrossRef]
- Winquist, E.; Moilanen, U.; Mettälä, A.; Leisola, M.; Hatakka, A. Production of lignin modifying enzymes on industrial waste material by solid-state cultivation of fungi. Biochem. Eng. J. 2008, 42, 128–132. [Google Scholar] [CrossRef]
- Masran, R.; Zanirun, Z.; Bahrin, E.K.; Ibrahim, M.F.; Lai Yee, P.; Abd-Aziz, S. Harnessing the potential of ligninolytic enzymes for lignocellulosic biomass pretreatment. Appl. Microbiol. Biotechnol. 2016, 100, 5231–5246. [Google Scholar] [CrossRef]
- Zhong, J.J.; Tang, Y.-J. Submerged Cultivation of Medicinal Mushrooms for Production of Valuable Bioactive Metabolites. Adv. Biochem. Eng. Biotechnol. 2004, 87, 25–59. [Google Scholar] [CrossRef]
- Yang, F.C.; Liau, C.B. Effects of cultivating conditions on the mycelial growth of Ganoderma lucidum in submerged flask cultures. Bioprocess. Eng. 1998, 19, 233–236. [Google Scholar] [CrossRef]
- Berović, M.; Habijanić, J.; Zore, I.; Wraber, B.; Hodžar, D.; Boh, B.; Pohleven, F. Submerged cultivation of Ganoderma lucidum biomass and immunostimulatory effects of fungal polysaccharides. J. Biotechnol. 2003, 103, 77–86. [Google Scholar] [CrossRef]
- Elisashvili, V. Submerged cultivation of medicinal mushrooms: Bioprocesses and products (review). Int. J. Med. Mushrooms 2012, 14, 211–239. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Chen, Y.; Yu, H.; Barseghyan, G.S.; Asatiani, M.D.; Wasser, S.P.; Mau, J.L. Comparative Study of Contents of Several Bioactive Components in Fruiting Bodies and Mycelia of Culinary-Medicinal Mushrooms. Int. J. Med. Mushrooms 2013, 15, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Grienke, U.; Kaserer, T.; Pfluger, F.; Mair, C.E.; Langer, T.; Schuster, D.; Rollinger, J.M. Accessing biological actions of Ganoderma secondary metabolites by in silico profiling. Phytochemistry 2015, 114, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Kała, K.; Kryczyk-Poprawa, A.; Rzewińska, A.; Muszyńska, B. Fruiting bodies of selected edible mushrooms as a potential source of lovastatin. Eur. Food Res. Technol. 2020, 246, 713–722. [Google Scholar] [CrossRef]
- Hofrichter, M.; Vares, T.; Kalsi, M.; Galkin, S.; Scheibner, K.; Fritsche, W.; Hatakka, A. Production of manganese peroxidase and organic acids and mineralization of 14C-labelled lignin (14C-DHP) during solid-state fermentation of wheat straw with the white rot fungus Nematoloma frowardii. Appl. Environ. Microbiol. 1999, 65, 1864–1870. [Google Scholar] [CrossRef]
- Mata, G.; Delpech, P.; Savoie, J.M. Selection of strains of Lentinula edodes and Lentinula boryana adapted for efficient mycelial growth on wheat straw. Rev. Iberoam. Micol. 2001, 18, 118–122. [Google Scholar]
- Nagai, M.; Sato, T.; Watanabe, H.; Saito, K.; Kawata, M.; Enei, H. Purification and characterization of an extracellular laccase from the edible mushuroom Lentinula edodes, and decolorization of chemically different dyes. Appl. Microbiol. Biotechnol. 2002, 60, 327–335. [Google Scholar] [CrossRef]
- Gaitán-Hernández, R.; Esqueda, M.; Gutiérrez, A.; Sánchez, A.; Beltrán-García, M.; Mata, G. Bioconversion of agrowastes by Lentinula edodes: The high potential of viticulture residues. Appl. Microbiol. Biotechnol. 2006, 71, 432–439. [Google Scholar] [CrossRef]
- Sánchez, C. Lignocellulosic residues: Biodegradation and bioconversion by fungi. Biotechnol. Adv. 2009, 27, 185–194. [Google Scholar] [CrossRef]
- Asgher, M.; Bhatti, H.N.; Ashraf, M.; Legge, R.L. Recent developments in biodegradation of industrial pollutants by white rot fungi and their enzyme system. Biodegradation 2008, 19, 771–783. [Google Scholar] [CrossRef]
- Sharma, R.; Arora, D. Changes in biochemical constituents of paddy straw during degradation by white rot fungi and its impact on in vitro digestibility. J. Appl. Microbiol. 2010, 109, 679–686. [Google Scholar] [CrossRef]
- Manavalan, T.; Manavalan, A.; Thangavelu, K.P.; Heese, K. Secretome analysis of Ganoderma lucidum cultivated in sugarcane bagasse. J. Proteom. 2012, 77, 298–309. [Google Scholar] [CrossRef]
- Wan, C.; Li, Y. Fungal pretreatment of lignocellulosic biomass. Biotechnol. Adv. 2012, 30, 1447–1457. [Google Scholar] [CrossRef]
- Niku-Paavola, M.; Raaska, L.; Itavaara, M. Detection of white-rot fungi by a non-toxic stain. Mycol. Res. 1990, 94, 27–31. [Google Scholar] [CrossRef]
- Buswell, J.A.; Cai, Y.; Chang, S.T. Effect of nutrient nitrogen and manganese on manganese peroxidase and laccase production by Lentinula (Lentinus) edodes. FEMS Microbiol. Lett. 1995, 128, 81–88. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Butler, M.J.; Lachance, M.A. The use of N,N,N’,N’-tetramethylphenylenediamine to detect peroxidase activity on polyacrylamide electrophoresis gels. Anal. Biochem. 1987, 162, 443–445. [Google Scholar] [CrossRef]
- Yang, F.C.; Yang, M.-J.; Cheng, C.H. A novel method to enhance the mycelia production of Ganoderma lucidum in submerged cultures by polymer additives and agitation strategies. J. Taiwan Inst. Chem. Eng. 2009, 40, 148–154. [Google Scholar] [CrossRef]
- Fraga, I.; Coutinho, J.; Bezerra, R.M.; Dias, A.; Marques, G.; Nunes, F. Influence of culture medium growth variables on Ganoderma lucidum exopolysaccharides structural features. Carbohydr. Polym. 2014, 111, 936–946. [Google Scholar] [CrossRef]
- Vares, T.; Kalsi, M.; Hatakka, A. Lignin peroxidases, manganese peroxidases, and other ligninolytic enzymes produced by Phlebia radiata during solid-state fermentation of wheat straw. Appl. Environ. Microbiol. 1995, 61, 3515–3520. [Google Scholar] [CrossRef]
- Makkar, R.S.; Tsuneda, A.; Tokuyasu, K.; Mori, Y. Lentinula edodes produces a multicomponent protein complex containing manganese (II)-dependent peroxidase, laccase and beta-glucosidase. FEMS Microbiol Lett. 2001, 200, 175–179. [Google Scholar] [CrossRef]
- De Souza Silva, C.M.M.; De Melo, I.S.; De Oliveira, P.R. Ligninolytic enzyme production by Ganoderma spp. Enzym. Microb. Technol. 2005, 37, 324–329. [Google Scholar] [CrossRef]
- Sitarz, A.; Mikkelsen, J.; Højrup, P.; Meyer, A. Identification of a laccase from Ganoderma lucidum CBS 229.93 having potential for enhancing cellulase catalyzed lignocellulose degradation. Enzym. Microb. Technol. 2013, 53, 378–385. [Google Scholar] [CrossRef]
- Elisashvili, V.; Kachlishvili, E.; Asatiani, M. Shiitake medicinal mushroom, Lentinus edodes (Higher Basidiomycetes) productivity and lignocellulolytic enzyme profiles during wheat straw and tree leaf bioconversion. Int. J. Med. Mushrooms 2015, 17, 77–86. [Google Scholar] [CrossRef]
- Cai, Y.; Gong, Y.; Liu, W.; Hu, Y.; Chen, L.; Yan, L.; Zhou, Y.; Bian, Y. Comparative secretomic analysis of lignocellulose degradation by Lentinula edodes grown on microcrystalline cellulose, lignosulfonate and glucose. J. Proteom. 2017, 163, 92–101. [Google Scholar] [CrossRef]
- Stajić, M.; Kukavica, B.; Vukojević, J.; Simonić, J.; Veljović-Jovanović, S.; Duletić-Laušević, S. Wheat straw conversion by enzymatic system of Ganoderma lucidum. Bioresources 2010, 5, 2362–2373. [Google Scholar] [CrossRef]
- Hariharan, S.; Nambisan, P. Optimization of Lignin Peroxidase, Manganese Peroxidase, and Lac Production from Ganoderma lucidum Under Solid State Fermentation of Pineapple Leaf. Bioresources 2013, 8, 250–271. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, D.; Sharma, K.K.; Arora, S.; Singh, A.K.; Gill, S.S.; Singhal, B. Gel-Based Purification and Biochemical Study of Laccase Isozymes from Ganoderma sp. and Its Role in Enhanced Cotton Callogenesis. Front. Microbiol. 2017, 8, 674. [Google Scholar] [CrossRef]
- Cavallazzi, J.; Oliveira, M.; Kasuya, M. Screening of inducers for laccase production by Lentinula edodes in liquid medium. Braz. J. Microbiol. 2005, 36, 383–387. [Google Scholar] [CrossRef]
- Ko, E.M.; Leem, Y.E.; Choi, H.T. Purification and characterization of laccase isozymes from the white-rot basidiomycete Ganoderma lucidum. Appl. Microbiol. Biotechnol. 2001, 57, 98–102. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).