Influence of Cultivation pH on Composition, Diversity, and Metabolic Production in an In Vitro Human Intestinal Microbiota
Abstract
:1. Introduction
2. Materials and Methods
2.1. Donor Stool
2.2. In Vitro Cultivation System
2.3. Analysis of Cell Count
2.4. Analysis of Short-Chain Fatty Acids by High Performance Liquid Chromatography
2.5. Microbiota Profiling with 16S rRNA Sequencing
2.6. Statistical Analysis
3. Results and Discussion
3.1. Influence of Cultivation pH
3.1.1. Standardization of the Stable State
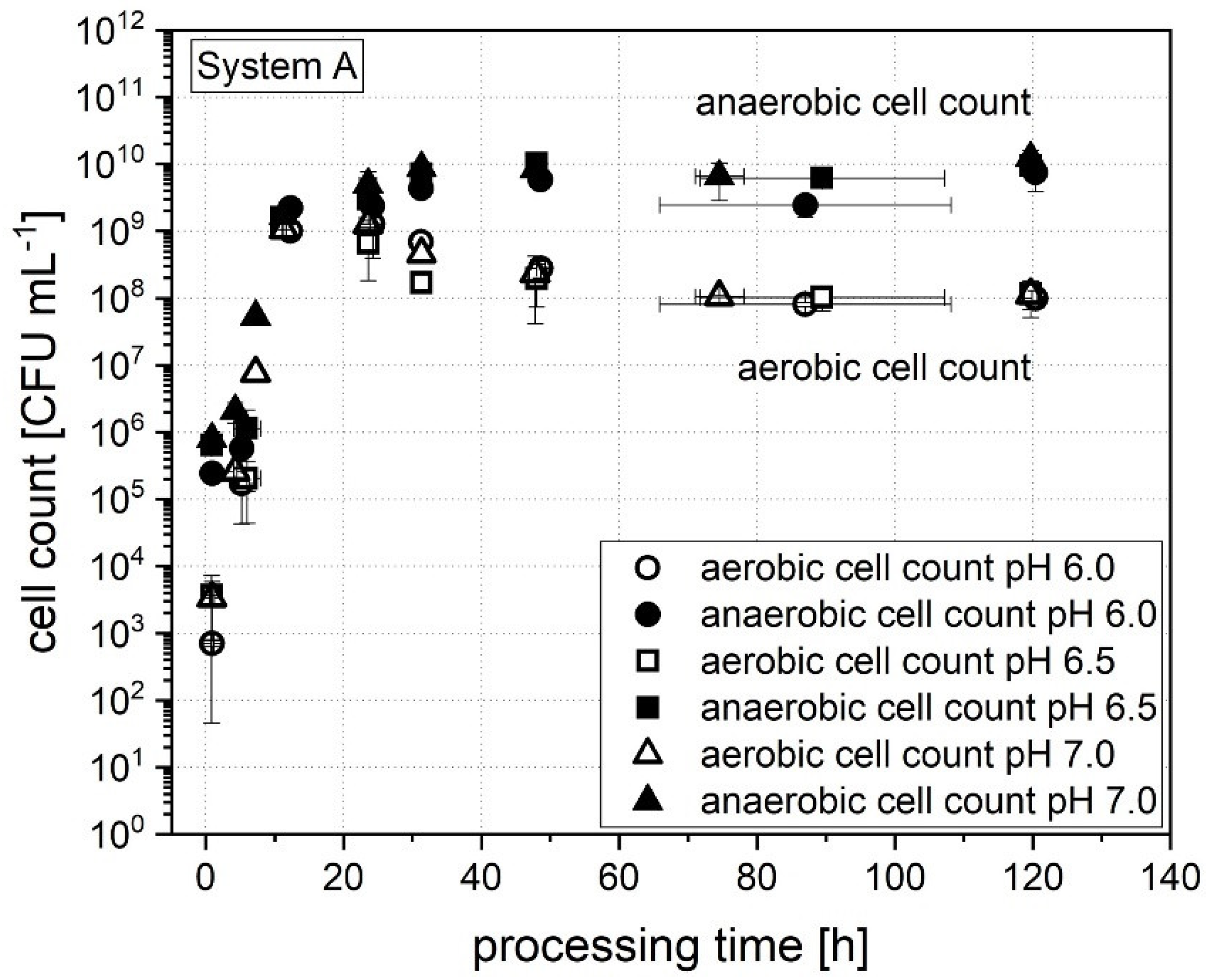
3.1.2. Cell Count
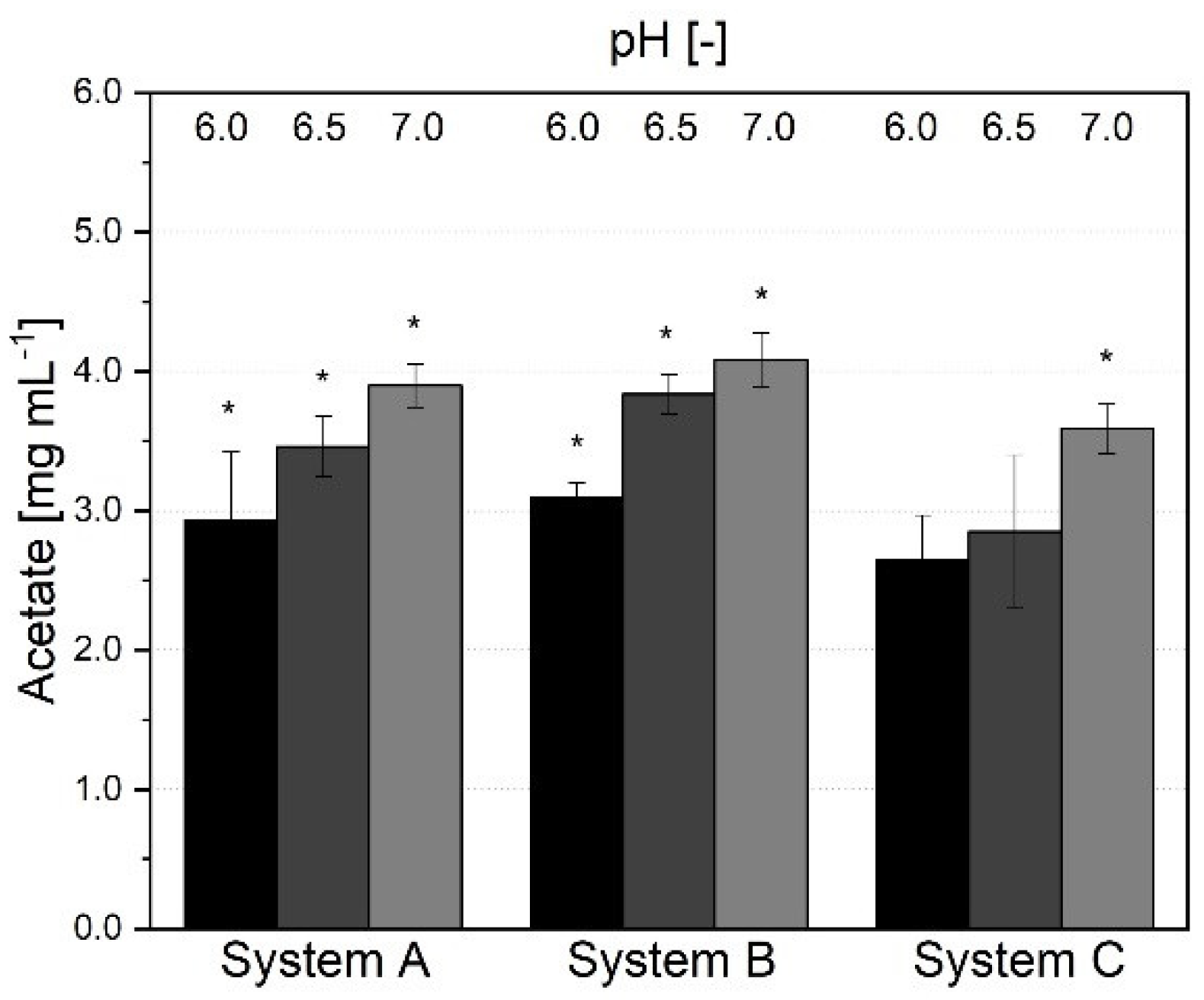
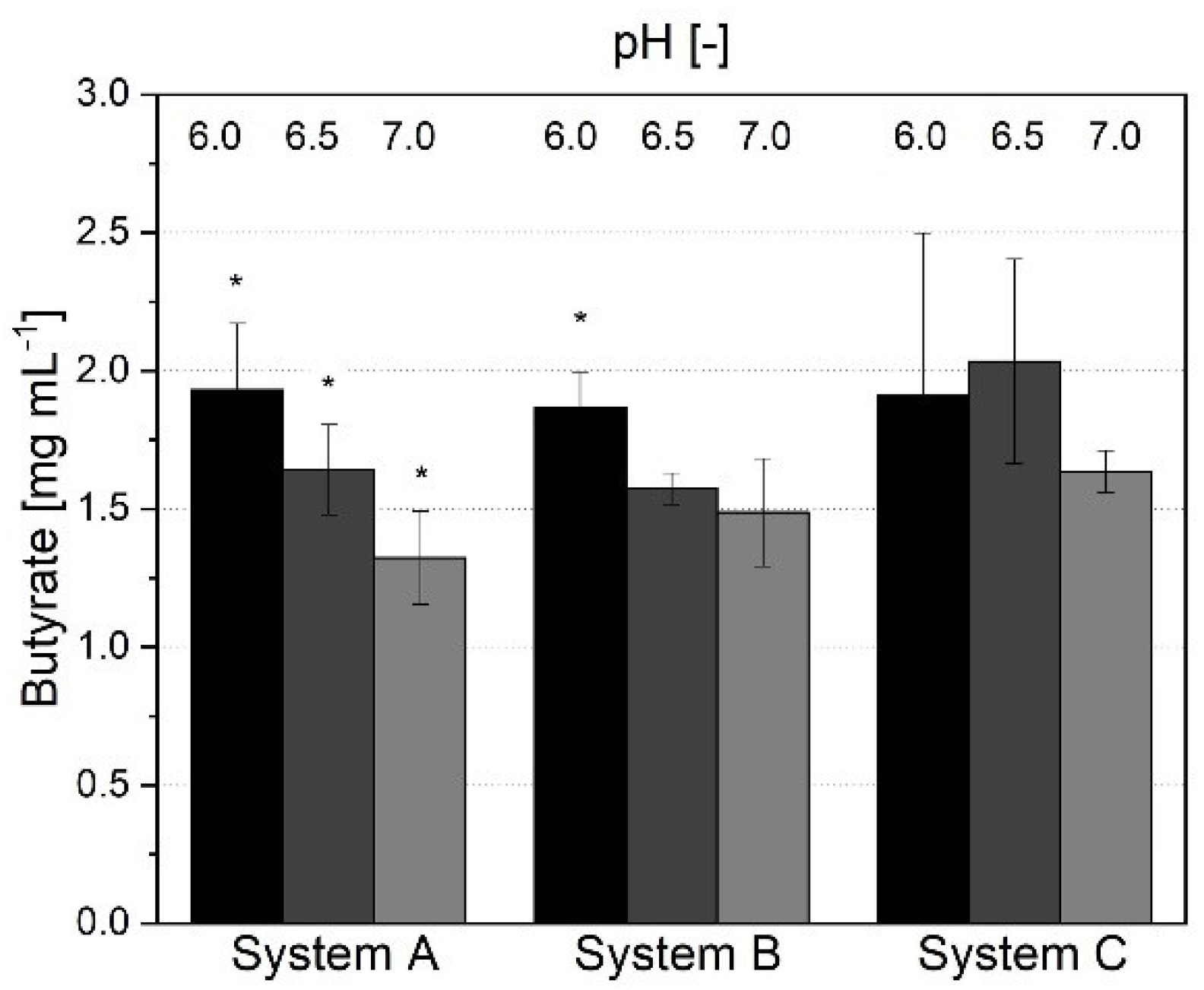
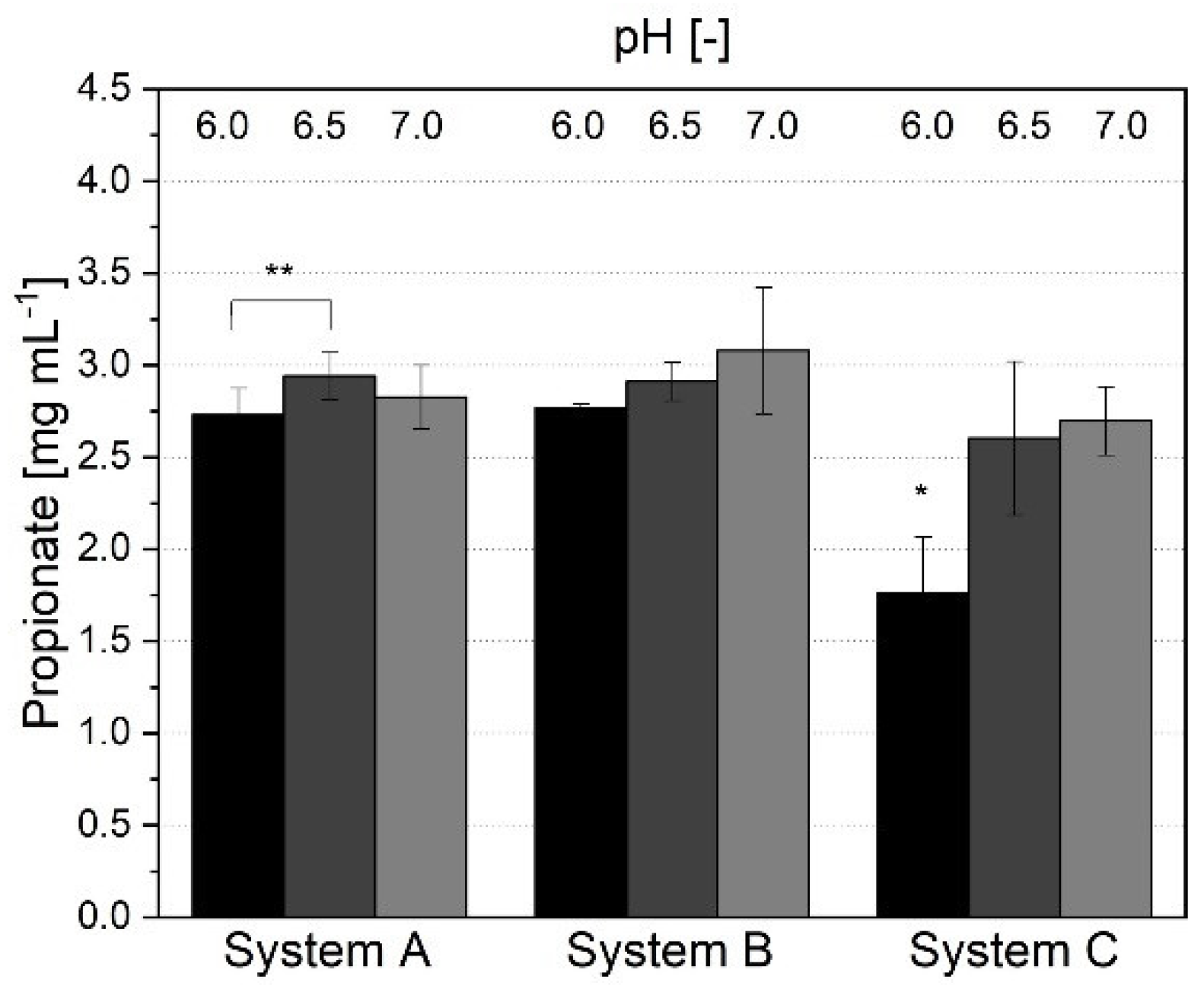
3.1.3. Short-Chain Fatty Acid Production and Metabolic Profile
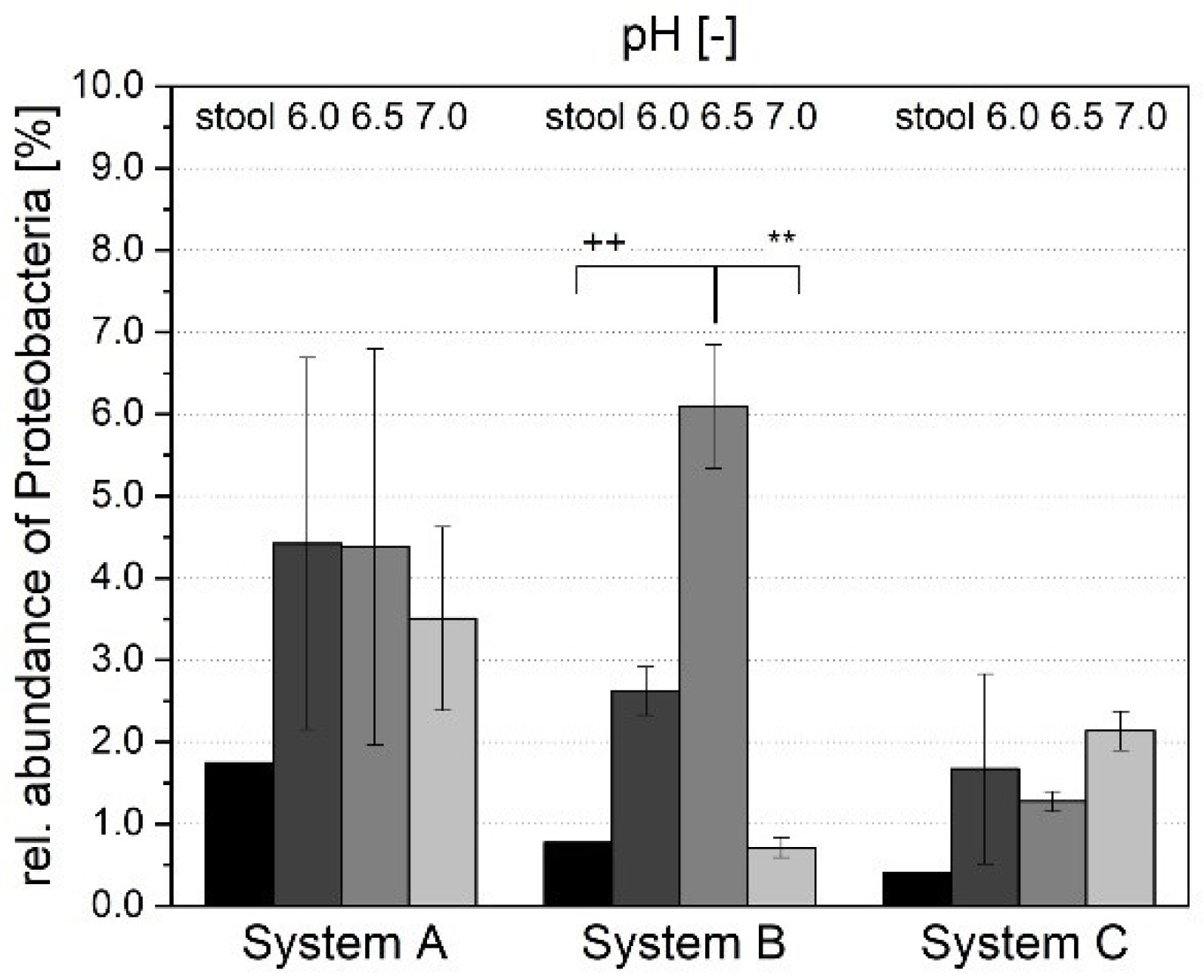
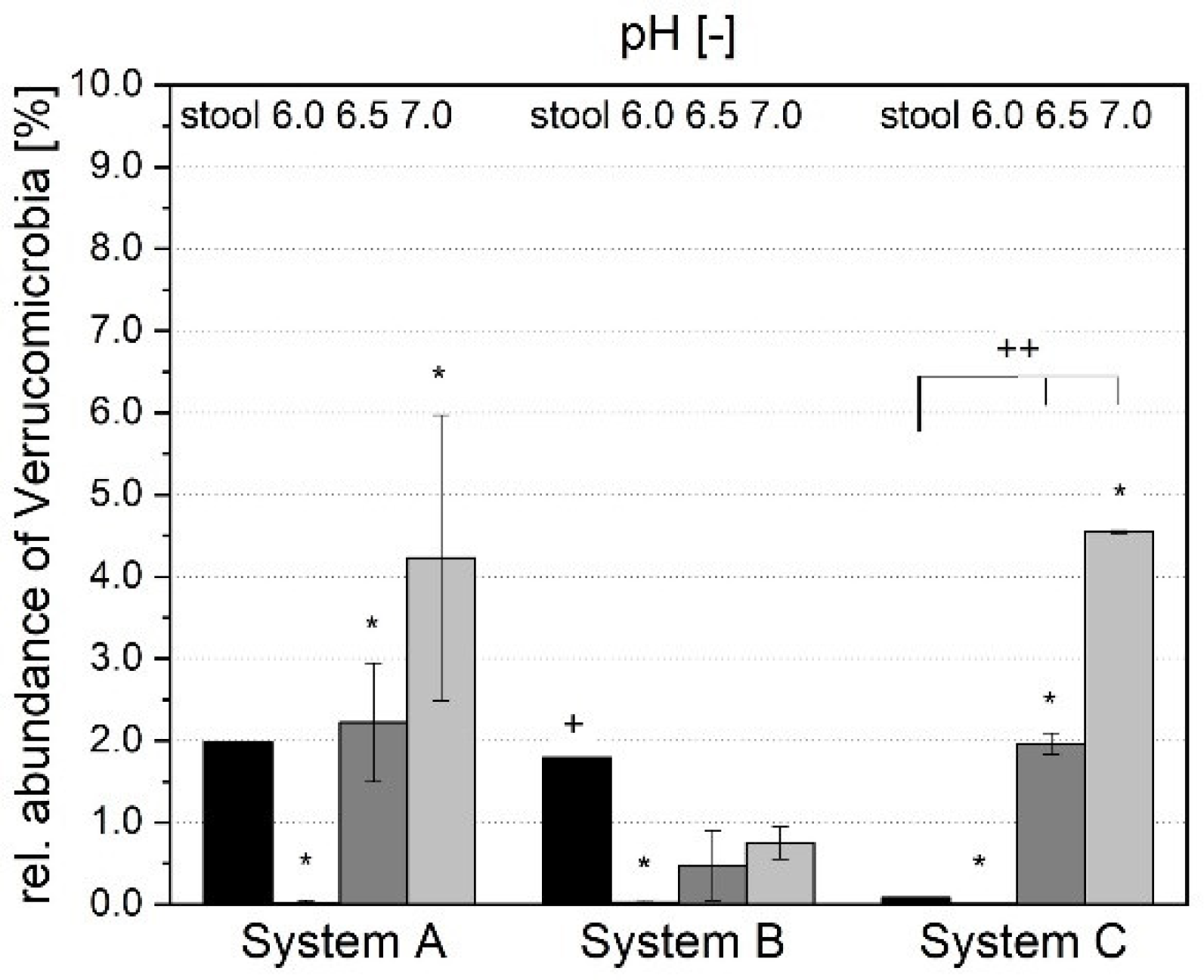
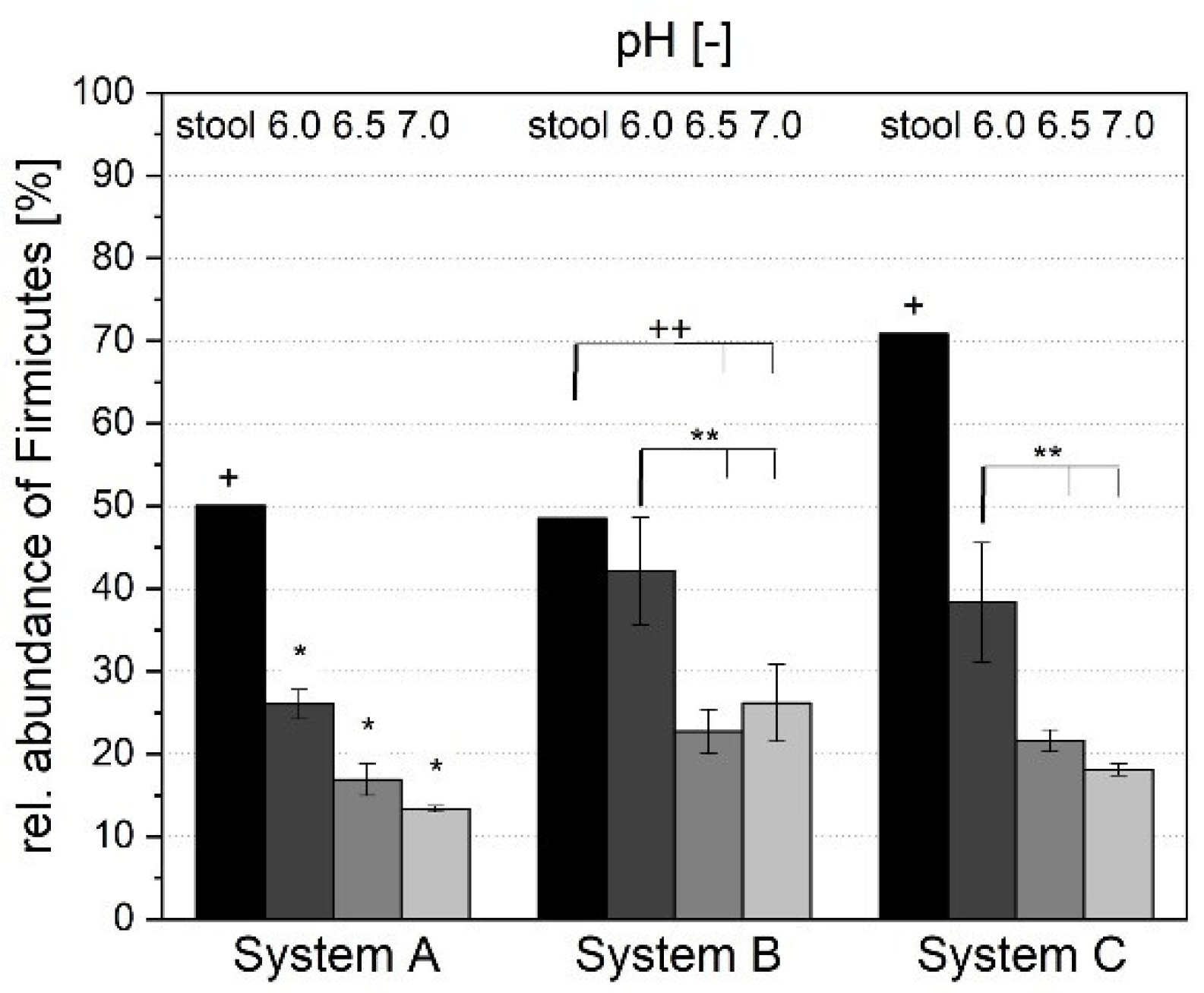
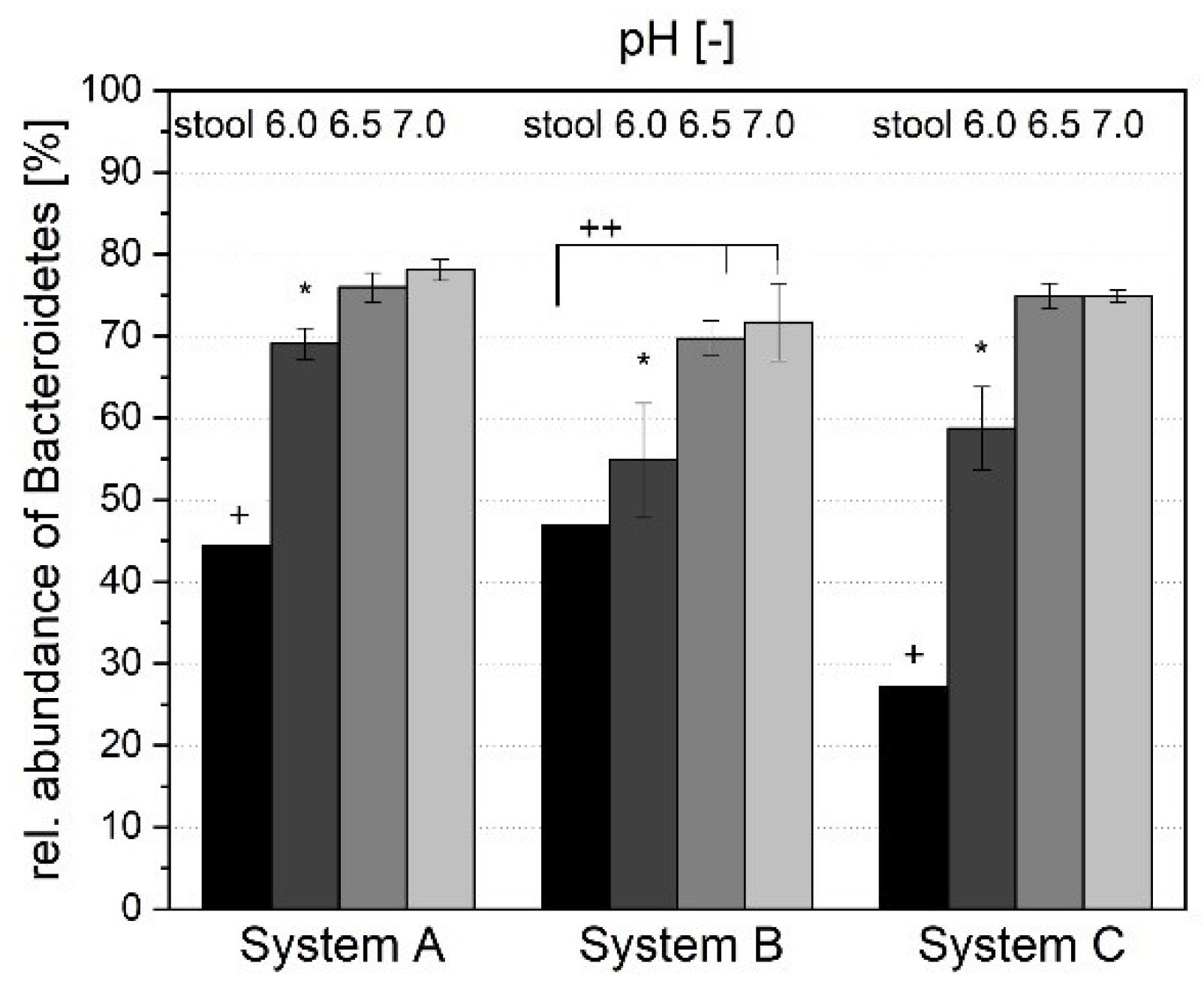
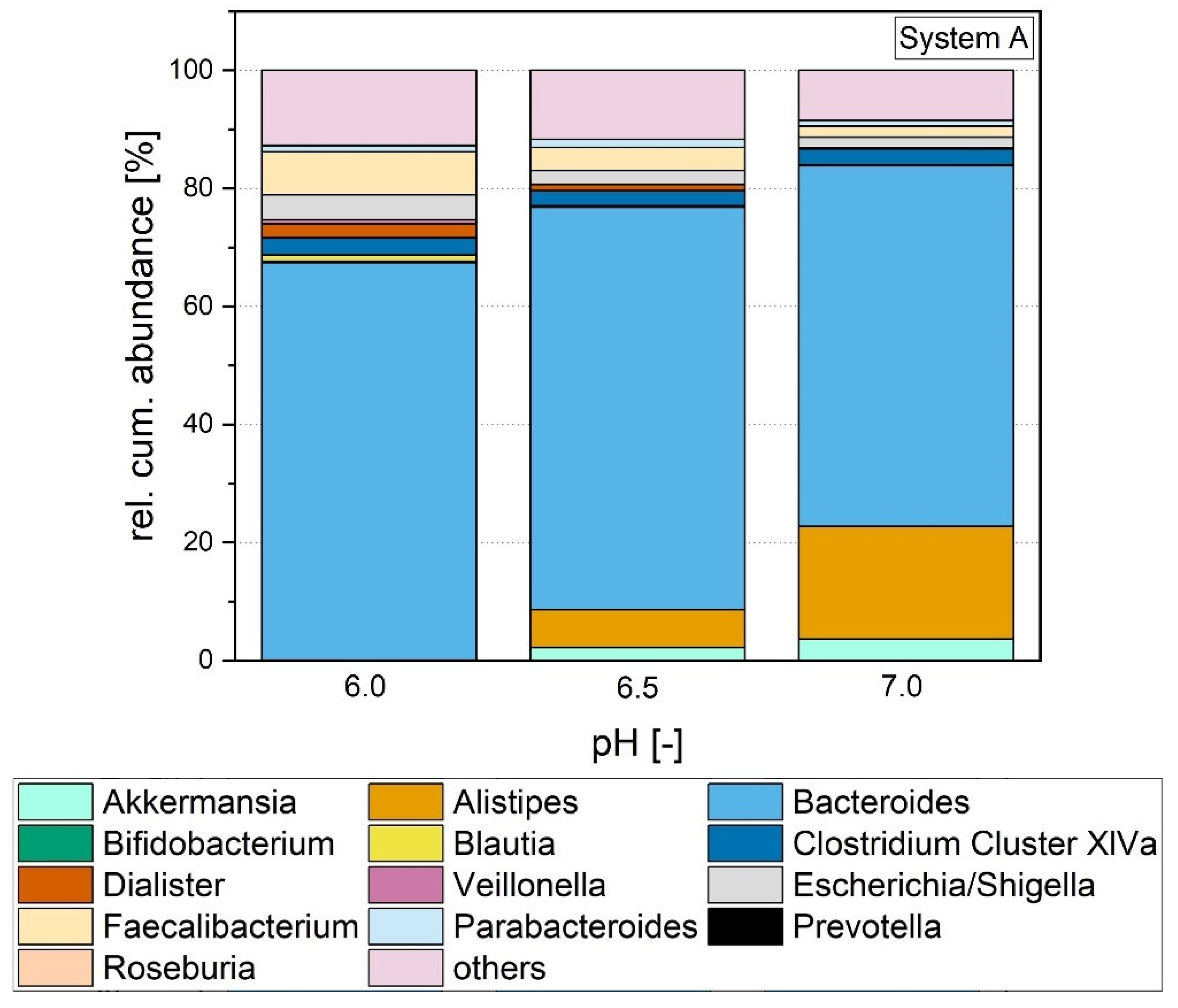
3.1.4. Microbial Composition
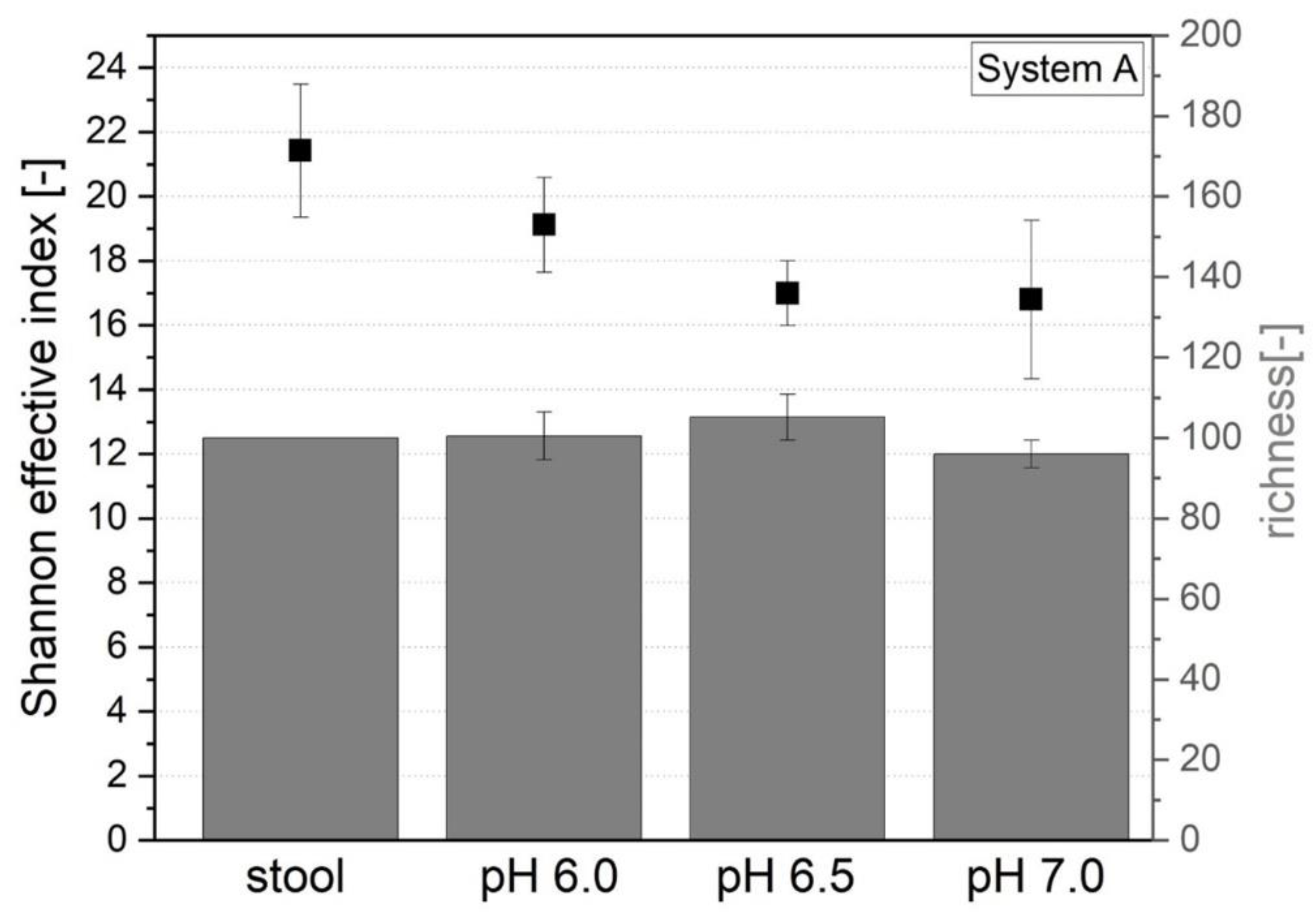
3.1.5. Microbial Richness and Diversity
3.2. Comparison with Original Donor Stool
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the Human Intestinal Microbial Flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gill, S.R.; Pop, M.; Deboy, R.T.; Eckburg, P.B.; Turnbaugh, P.J.; Samuel, B.S.; Gordon, J.I.; Relman, D.A.; Fraser-Liggett, C.M.; Nelson, K.E. Metagenomic Analysis of the Human Distal Gut Microbiome. Science 2006, 312, 1355–1359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dethlefsen, L.; Mcfall-Ngai, M.; Relman, D.A. An Ecological and Evolutionary Perspective on Human-Microbe Mutualism and Disease. Nature 2007, 449, 811–818. [Google Scholar] [CrossRef]
- Rios-Covian, D.; Salazar, N.; Gueimonde, M.; De Los Reyes-Gavilan, C.G. Shaping the Metabolism of Intestinal Bacteroides Population through Diet to Improve Human Health. Front. Microbiol. 2017, 8, 376. [Google Scholar] [CrossRef] [Green Version]
- Vadder, F.; De Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-Generated Metabolites Promote Metabolic Benefits Via Gut-Brain Neural Circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, R.; Grover, S.; Batish, V.K. Hypocholesterolaemic Effect of Dietary Inclusion of Two Putative Probiotic Bile Salt Hydrolase-Producing Lactobacillus Plantarum Strains in Sprague-Dawley Rats. Br. J. Nutr. 2011, 105, 561–573. [Google Scholar] [CrossRef] [Green Version]
- Holmes, E.; Li, J.V.; Athanasiou, T.; Ashrafian, H.; Nicholson, J.K. Understanding the Role of Gut Microbiome-Host Metabolic Signal Disruption in Health and Disease. Trends Microbiol. 2011, 19, 349–359. [Google Scholar] [CrossRef]
- Schreiber, S.; Nikolaus, S.; Rosenstiel, P. Microbiome and Nutrition. The Way to a Future Therapy for Chronic Inflammatory Bowel Diseases? Internist 2014, 55, 889–897. [Google Scholar] [CrossRef]
- Manichanh, C.; Rigottier-Gois, L.; Bonnaud, E.; Gloux, K.; Pelletier, E.; Frangeul, L.; Nalin, R.; Jarrin, C.; Chardon, P.; Marteau, P.; et al. Reduced Diversity of Faecal Microbiota in Crohn’s Disease Revealed by a Metagenomic Approach. Gut 2006, 55, 205–211. [Google Scholar] [CrossRef] [Green Version]
- Collado, M.C.; Calabuig, M.; Sanz, Y. Differences between the Fecal Microbiota of Coeliac Infants and Healthy Controls. Curr. Issues Intest. Microbiol. 2007, 8, 9–14. [Google Scholar]
- Larsen, N.; Vogensen, F.K.; Van Den Berg Frans, W.J.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sorensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut Microbiota in Human Adults with Type 2 Diabetes Differs From Non-Diabetic Adults. PLoS ONE 2010, 5, E9085. [Google Scholar] [CrossRef]
- Koliada, A.; Syzenko, G.; Moseiko, V.; Budovska, L.; Puchkov, K.; Perederiy, V.; Gavalko, Y.; Dorofeyev, A.; Romanenko, M.; Tkach, S.; et al. Association between Body Mass Index and Firmicutes/Bacteroidetes Ratio in an Adult Ukrainian Population. BMC Microbiol. 2017, 17, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity Alters Gut Microbial Ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [Green Version]
- Hill-Burns, E.M.; Debelius, J.W.; Morton, J.T.; Wissemann, W.T.; Lewis, M.R.; Wallen, Z.D.; Peddada, S.D.; Factor, S.A.; Molho, E.; Zabetian, C.P.; et al. Parkinson’s Disease and Parkinson’s Disease Medications Have Distinct Signatures of the Gut Microbiome. Mov. Disord. 2017, 32, 739–749. [Google Scholar] [CrossRef]
- Helmink, B.A.; Khan, M.A.W.; Hermann, A.; Gopalakrishnan, V.; Wargo, J.A. The Microbiome, Cancer, and Cancer Therapy. Nat. Med. 2019, 25, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Tremlett, H.; Bauer, K.C.; Appel-Cresswell, S.; Finlay, B.B.; Waubant, E. The Gut Microbiome in Human Neurological Disease: A Review. Ann. Neurol. 2017, 81, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Lagier, J.-C.; Million, M.; Hugon, P.; Armougom, F.; Raoult, D. Human Gut Microbiota: Repertoire and Variations. Front. Cell. Infect. Microbiol. 2012, 2, 136. [Google Scholar] [CrossRef] [Green Version]
- Zar, F.A.; Bakkanagari, S.R.; Moorthi, K.M.L.S.T.; Davis, M.B. A Comparison of Vancomycin and Metronidazole for the Treatment of Clostridium Difficile-Associated Diarrhea, Stratified by Disease Severity. Clin. Infect. Dis. 2007, 45, 302–307. [Google Scholar] [CrossRef]
- Aroniadis, O.C.; Brandt, L.J. Fecal Microbiota Transplantation: Past, Present and Future. Curr. Opin. Gastroenterol. 2013, 29, 79–84. [Google Scholar] [CrossRef]
- Gough, E.; Shaikh, H.; Manges, A.R. Systematic Review of Intestinal Microbiota Transplantation (Fecal Bacteriotherapy) for Recurrent Clostridium Difficile Infection. Clin. Infect. Dis. 2011, 53, 994–1002. [Google Scholar] [CrossRef] [Green Version]
- Terveer, E.M.; Van Beurden, Y.H.; Goorhuis, A.; Seegers, J.F.M.L.; Bauer, M.P.; Van Nood, E.; Dijkgraaf, M.G.W.; Mulder, C.J.J.; Vandenbroucke-Grauls, C.M.J.E.; Verspaget, H.W.; et al. How To: Establish and Run a Stool Bank. Clin. Microbiol. Infect. 2017, 23, 924–930. [Google Scholar] [CrossRef] [Green Version]
- Mennigen, R.; Bruewer, M. Effect of Probiotics on Intestinal Barrier Function. Ann. N. Y. Acad. Sci. 2009, 1165, 183–189. [Google Scholar] [CrossRef]
- Le Blay, G.; Rytka, J.; Zihler, A.; Lacroix, C. New In Vitro Colonic Fermentation Model for Salmonella Infection in the Child Gut. Fems Microbiol. Ecol. 2009, 67, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Meunier, V.; Bourrié, M.; Berger, Y.; Fabre, G. The Human Intestinal Epithelial Cell Line Caco-2; Pharmacological and Pharmacokinetic Applications. Cell Biol. Toxicol. 1995, 11, 187–194. [Google Scholar] [CrossRef]
- Hemarajata, P.; Versalovic, J. Effects of Probiotics on Gut Microbiota: Mechanisms of Intestinal Immunomodulation and Neuromodulation. Ther. Adv. Gastroenterol. 2013, 6, 39–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minekus, M.; Smeets-Peeters, M.; Bernalier, A.; Marol-Bonnin, S.; Havenaar, R.; Marteau, P.; Alric, M.; Fonty, G. A Computer-Controlled System to Simulate Conditions of the Large Intestine with Peristaltic Mixing, Water Absorption and Absorption of Fermentation Products. Appl. Microbiol. Biotechnol. 1999, 53, 108–114. [Google Scholar] [CrossRef]
- Molly, K.; Woestyne, M.V.; Smet, I.; De Verstraete, W. Validation of the Simulator of the Human Intestinal Microbial Ecosystem (Shime) Reactor Using Microorganism-Associated Activities. Microb. Ecol. Health Dis. 1994, 7, 191–200. [Google Scholar] [CrossRef]
- Mcdonald, J. Development of an In Vitro Fermentation Model to Culture the Human Distal Gut Microbiota. Ph.D. Thesis, University of Guelph, Guelph, ON, Canada, 2013. [Google Scholar]
- Bircher, L.; Schwab, C.; Geirnaert, A.; Lacroix, C. Cryopreservation of Artificial Gut Microbiota Produced with In Vitro Fermentation Technology. Microb. Biotechnol. 2018, 11, 163–175. [Google Scholar] [CrossRef]
- Walker, A.W.; Duncan, S.H.; Mcwilliam Leitch, E.C.; Child, M.W.; Flint, H.J. pH and Peptide Supply Can Radically Alter Bacterial Populations and Short-Chain Fatty Acid Ratios Within Microbial Communities from the Human Colon. Appl. Environ. Microbiol. 2005, 71, 3692–3700. [Google Scholar] [CrossRef] [Green Version]
- Duncan, S.H.; Louis, P.; Thomson, J.M.; Flint, H.J. The Role of pH in Determining the Species Composition of the Human Colonic Microbiota. Environ. Microbiol. 2009, 11, 2112–2122. [Google Scholar] [CrossRef]
- Duncan, S.H.; Scott, K.P.; Ramsay, A.G.; Harmsen, H.J.M.; Welling, G.W.; Stewart, C.S.; Flint, H.J. Effects of Alternative Dietary Substrates On Competition Between Human Colonic Bacteria in an Anaerobic Fermentor System. Appl. Environ. Microbiol. 2003, 69, 1136–1142. [Google Scholar] [CrossRef] [Green Version]
- Takagi, R.; Sasaki, K.; Sasaki, D.; Fukuda, I.; Tanaka, K.; Yoshida, K.-I.; Kondo, A.; Osawa, R. A Single-Batch Fermentation System to Simulate Human Colonic Microbiota for High-Throughput Evaluation of Prebiotics. PLoS ONE 2016, 11, E0160533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamora-Gasga, V.M.; Cárdenas-Castro, A.P.; Montalvo-González, E.; Loarca-Piña, M.G.F.; Pedro Alberto, V.-L.; Tovar, J.; Sáyago-Ayerdi, S.G. In Vitro Human Colonic Fermentation of Indigestible Fraction Isolated From Lunch Menus: Impact on the Gut Metabolites and Antioxidant Capacity. Int. J. Food Sci. Nutr. 2018, 69, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Campos-Vega, R.; Reynoso-Camacho, R.; Pedraza-Aboytes, G.; Acosta-Gallegos, J.A.; Guzman-Maldonado, S.H.; Paredes-Lopez, O.; Oomah, B.D.; Loarca-Piña, G. Chemical Composition and In Vitro Polysaccharide Fermentation of Different Beans (Phaseolus vulgaris L.). J. Food Sci. 2009, 74, T59–T65. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.T.; Macfarlane, S.; Gibson, G.R. Validation of a Three-Stage Compound Continuous Culture System for Investigating the Effect of Retention Time on the Ecology and Metabolism of Bacteria in the Human Colon. Microb. Ecol. 1998, 35, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.R.; Rhee, P.-L. How to Interpret a Functional or Motility Test-Colon Transit Study. J. Neurogastroenterol. Motil. 2012, 18, 94–99. [Google Scholar] [CrossRef]
- Haindl, R.; Engel, J.; Kulozik, U. Establishment of an In Vitro System of the Human Intestinal Microbiota: Effect of Cultivation Conditions and Influence of Three Donor Stool Samples. Microorganisms 2021, 9, 1049. [Google Scholar] [CrossRef]
- Kiviharju, K.; Leisola, M.; Eerikainen, T. Optimization of a Bifidobacterium Longum Production Process. J. Biotechnol. 2005, 117, 299–308. [Google Scholar] [CrossRef]
- Dalland, E.; Hofstad, T. Growth of Bacteroides Fragilis in Continuous Culture and in Batch Cultures at Controlled pH. Appl. Microbiol. 1974, 28, 856–860. [Google Scholar] [CrossRef]
- Baktash, A.; Terveer, E.M.; Zwittink, R.D.; Hornung, B.V.H.; Corver, J.; Kuijper, E.J.; Smits, W.K. Mechanistic Insights in the Success of Fecal Microbiota Transplants for the Treatment of Clostridium Difficile Infections. Front. Microbiol. 2018, 9, 1242. [Google Scholar] [CrossRef]
- Payne, A.N.; Zihler, A.; Chassard, C.; Lacroix, C. Advances and Perspectives in In Vitro Human Gut Fermentation Modeling. Trends Biotechnol. 2012, 30, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.F.; Pye, G.; Bramley, R.; Clark, A.G.; Dyson, T.J.; Hardcastle, J.D. Measurement of Gastrointestinal pH Profiles in Normal Ambulant Human Subjects. Gut 1988, 29, 1035–1041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reitmeier, S.; Kiessling, S.; Neuhaus, K.; Haller, D. Comparing Circadian Rhythmicity in the Human Gut Microbiome. Star Protoc. 2020, 1, 100148. [Google Scholar] [CrossRef]
- Lagkouvardos, I.; Joseph, D.; Kapfhammer, M.; Giritli, S.; Horn, M.; Haller, D.; Clavel, T. Imngs: A Comprehensive Open Resource of Processed 16s Rrna Microbial Profiles for Ecology and Diversity Studies. Sci. Rep. 2016, 6, 33721. [Google Scholar] [CrossRef]
- Reitmeier, S.; Hitch, T.C.A.; Fikas, N.; Hausmann, B.; Ramer-Tait, A.E.; Neuhaus, K.; Berry, D.; Haller, D.; Lagkouvardos, I.; Clavel, T. Handling of Spurious Sequences Affects the Outcome of High-Throughput 16s rRNA Gene Amplicon Profiling. ISME Commun. 2021, 1, 1–12. [Google Scholar] [CrossRef]
- Reitmeier, S.; Kiessling, S.; Clavel, T.; List, M.; Almeida, E.L.; Ghosh, T.S.; Neuhaus, K.; Grallert, H.; Linseisen, J.; Skurk, T.; et al. Arrhythmic Gut Microbiome Signatures Predict Risk of Type 2 Diabetes. Cell Host Microbe 2020, 28, 258–272. [Google Scholar] [CrossRef]
- Lagkouvardos, I.; Fischer, S.; Kumar, N.; Clavel, T. Rhea: A Transparent and Modular R Pipeline for Microbial Profiling Based On 16s Rrna Gene Amplicons. PeerJ 2017, 5, E2836. [Google Scholar] [CrossRef] [Green Version]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, E1002533. [Google Scholar] [CrossRef] [Green Version]
- Schwiertz, A.; Taras, D.; Schäfer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and Scfa in Lean and Overweight Healthy Subjects. Obesity 2010, 18, 190–195. [Google Scholar] [CrossRef]
- Macfarlane, G.T.; Gibson, G.R.; Cummings, J.H. Comparison of Fermentation Reactions in Different Regions of the Human Colon. J. Appl. Bacteriol. 1992, 72, 57–64. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut Microbiota Functions: Metabolism of Nutrients and Other Food Components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Schwan, W.R.; Lee, J.L.; Lenard, F.A.; Matthews, B.T.; Beck, M.T. Osmolarity and pH Growth Conditions Regulate Fim Gene Transcription and Type 1 Pilus Expression in Uropathogenic Escherichia Coli. Infect. Immun. 2002, 70, 1391–1402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Small, P.; Blankenhorn, D.; Welty, D.; Zinser, E.; Slonczewski, J.L. Acid and Base Resistance in Escherichia Coli and Shigella Flexneri: Role of Rpos and Growth pH. J. Bacteriol. 1994, 176, 1729–1737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Herreweghen, F.; Van Den Abbeele, P.; De Mulder, T.; De Weirdt, R.; Geirnaert, A.; Hernandez-Sanabria, E.; Vilchez-Vargas, R.; Jauregui, R.; Pieper, D.H.; Belzer, C.; et al. In Vitro Colonisation of the Distal Colon by Akkermansia Muciniphila Is Largely Mucin and pH Dependent. Benef. Microbes 2017, 8, 81–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheema, Z.M. The Role of Faecalibacterium Prausnitzii in Health and Disease. Catalyst 2019, 3, 11–17. [Google Scholar]
- Licht, T.R.; Hansen, M.; Bergström, A.; Poulsen, M.; Krath, B.N.; Markowski, J.; Dragsted, L.O.; Wilcks, A. Effects of Apples and Specific Apple Components on the Cecal Environment of Conventional Rats: Role of Apple Pectin. BMC Microbiol. 2010, 10, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barcenilla, A.; Pryde, S.E.; Martin, J.C.; Duncan, S.H.; Stewart, C.S.; Henderson, C.; Flint, H.J. Phylogenetic Relationships of Butyrate-Producing Bacteria from the Human Gut. Appl. Environ. Microbiol. 2000, 66, 1654–1661. [Google Scholar] [CrossRef] [Green Version]
- Louis, P.; Flint, H.J. Diversity, Metabolism and Microbial Ecology of Butyrate-Producing Bacteria from the Human Large Intestine. FEMS Microbiol. Lett. 2009, 294, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Zihler Berner, A.; Fuentes, S.; Dostal, A.; Payne, A.N.; Vazquez Gutierrez, P.; Chassard, C.; Grattepanche, F.; Vos, W.M.; De Lacroix, C. Novel Polyfermentor Intestinal Model (Polyferms) for Controlled Ecological Studies: Validation and Effect of pH. PLoS ONE 2013, 8, E77772. [Google Scholar] [CrossRef] [Green Version]
- Lopetuso, L.R.; Petito, V.; Graziani, C.; Schiavoni, E.; Paroni Sterbini, F.; Poscia, A.; Gaetani, E.; Franceschi, F.; Cammarota, G.; Sanguinetti, M.; et al. Gut Microbiota in Health, Diverticular Disease, Irritable Bowel Syndrome, and Inflammatory Bowel Diseases: Time for Microbial Marker of Gastrointestinal Disorders. Dig. Dis. 2018, 36, 56–65. [Google Scholar] [CrossRef]
- Tamanai-Shacoori, Z.; Smida, I.; Bousarghin, L.; Loreal, O.; Meuric, V.; Fong, S.B.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Roseburia Spp.: A Marker of Health? Future Microbiol. 2017, 12, 157–170. [Google Scholar] [CrossRef]
- Tojo, R.; Suárez, A.; Clemente, M.G.; Los Reyes-Gavilán, C.G.; De Margolles, A.; Gueimonde, M.; Ruas-Madiedo, P. Intestinal Microbiota in Health and Disease: Role of Bifidobacteria in Gut Homeostasis. World J. Gastroenterol. 2014, 20, 15163–15176. [Google Scholar] [CrossRef]
- Tamaki, H.; Nakase, H.; Inoue, S.; Kawanami, C.; Itani, T.; Ohana, M.; Kusaka, T.; Uose, S.; Hisatsune, H.; Tojo, M.; et al. Efficacy of Probiotic Treatment with Bifidobacterium Longum 536 for Induction of Remission in Active Ulcerative Colitis: A Randomized, Double-Blinded, Placebo-Controlled Multicenter Trial. Dig. Endosc. 2016, 28, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Miquel, S.; Martín, R.; Rossi, O.; Bermúdez-Humarán, L.G.; Chatel, J.M.; Sokol, H.; Thomas, M.; Wells, J.M.; Langella, P. Faecalibacterium Prausnitzii and Human Intestinal Health. Curr. Opin. Microbiol. 2013, 16, 255–261. [Google Scholar] [CrossRef]


| Donor A | Donor B | Donor C | ||
|---|---|---|---|---|
| Age | years | 28 | 25 | 27 |
| BMI | - | 23 | 21 | 21 |
| Cell count | Aerobic (105 CFU mL−1) | 0.7 ± 0.4 | 10 ± 9 | 9 ± 1 |
| Anaerobic (108 CFU mL−1) | 4 ± 3 | 2 ± 0.02 | 4 ± 0.6 | |
| Metabolic profile | Acetate (mg mL−1) | 3.17 ± 0.21 | 2.74 ± 0.07 | 2.59 ± 0.54 |
| Propionate (mg mL−1) | 2.00 ± 0.16 | 1.15 ± 0.04 | 1.02 ± 0.16 | |
| Butyrate (mg mL−1) | 1.48 ± 0.08 | 0.99 ± 0.03 | 2.14 ± 0.26 | |
| Isovalerate (mg mL−1) | 0.15 ± 0.02 | 0.24 ± 0.00 | 0.34 ± 0.04 | |
| Σ SCFAs (mg mL−1) | 6.80 ± 0.47 | 5.12 ± 0.14 | 6.09 ± 1.00 | |
| Microbial profile | Richness (-) | 100 | 123 | 122 |
| Shannon effective (-) | 21.42 | 38.43 | 46.34 | |
| Ratio Firmicutes:Bacteroidetes | 1.03 | 1.13 | 2.60 |
| Cultivation Parameter | Set Condition |
|---|---|
| Temperature | +37 °C |
| Stirring rate | 200 rpm |
| Aeration | 8 ccm forming gas (95% N2, 5% H2) |
| pH value | 6.0/6.5/7.0 regulated with 1.25 M NaOH and 0.5 M HCl |
| Medium inflow | 0.5 mL min−1 |
| Volume | 850 mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haindl, R.; Schick, S.; Kulozik, U. Influence of Cultivation pH on Composition, Diversity, and Metabolic Production in an In Vitro Human Intestinal Microbiota. Fermentation 2021, 7, 156. https://doi.org/10.3390/fermentation7030156
Haindl R, Schick S, Kulozik U. Influence of Cultivation pH on Composition, Diversity, and Metabolic Production in an In Vitro Human Intestinal Microbiota. Fermentation. 2021; 7(3):156. https://doi.org/10.3390/fermentation7030156
Chicago/Turabian StyleHaindl, Regina, Simon Schick, and Ulrich Kulozik. 2021. "Influence of Cultivation pH on Composition, Diversity, and Metabolic Production in an In Vitro Human Intestinal Microbiota" Fermentation 7, no. 3: 156. https://doi.org/10.3390/fermentation7030156
APA StyleHaindl, R., Schick, S., & Kulozik, U. (2021). Influence of Cultivation pH on Composition, Diversity, and Metabolic Production in an In Vitro Human Intestinal Microbiota. Fermentation, 7(3), 156. https://doi.org/10.3390/fermentation7030156






