Bioethanol Production via Herbaceous and Agricultural Biomass Gasification Integrated with Syngas Fermentation
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Safarian, S.; Khodaparast, P.; Kateb, M. Modeling and Technical–Economic Optimization of Electricity Supply Network by Three Photovoltaic Systems. J. Sol. Energy Eng. 2014, 136, 024501. [Google Scholar] [CrossRef]
- Safarian, S.; Unnthorsson, R.; Richter, C. Techno–Economic Analysis of Power Production by Using Waste Biomass Gasification. J. Power Energy Eng. 2020, 8, 1. [Google Scholar] [CrossRef]
- Begum, S.; Rasul, M.; Akbar, D.A. Numerical Investigation of Municipal Solid Waste Gasification Using Aspen Plus. Procedia Eng. 2014, 90, 710–717. [Google Scholar] [CrossRef] [Green Version]
- Zeng, J.; Xiao, R.; Zhang, H.; Wang, Y.; Zeng, D.; Ma, Z. Chemical Looping Pyrolysis–Gasification of Biomass for High H2/CO Syngas Production. Fuel Process. Technol. 2017, 168, 116–122. [Google Scholar] [CrossRef]
- Luo, H.; Lin, W.; Song, W.; Li, S.; Dam-Johansen, K.; Wu, H. Three Dimensional Full–Loop CFD Simulation of Hydrodynamics in a Pilot–Scale Dual Fluidized Bed System for Biomass Gasification. Fuel Process. Technol. 2019, 195, 106146. [Google Scholar] [CrossRef]
- Kumar, B.; Bhardwaj, N.; Agrawal, K.; Chaturvedi, V.; Verma, P. Current Perspective on Pretreatment Technologies using Lignocellulosic Biomass: An Emerging Biorefinery Concept. Fuel Process. Technol. 2020, 199, 106244. [Google Scholar] [CrossRef]
- Safarian, S.; Unnthorsson, R.; Richter, C. Hydrogen Production via Biomass Gasification: Simulation and Performance Analysis under Different Gasifying Agents. Biofuels 2021, 1–10. [Google Scholar] [CrossRef]
- Amaducci, S.; Facciotto, G.; Bergante, S.; Perego, A.; Serra, P.; Ferrarini, A.; Chimento, C. Biomass Production and Energy Balance of Herbaceous and Woody Crops on Marginal Soils in the Po Valley. Gcb Bioenergy 2017, 9, 31–45. [Google Scholar] [CrossRef] [Green Version]
- Shankar Tumuluru, J.; Sokhansanj, S.; Hess, J.R.; Wright, C.T.; Boardman, R.D. A Review on Biomass Torrefaction Process and Product Properties for Energy Applications. Ind. Biotechnol. 2011, 7, 384–401. [Google Scholar] [CrossRef] [Green Version]
- Talebnia, F.; Karakashev, D.; Angelidaki, I. Production of Bioethanol from Wheat Straw: An Overview on Pretreatment, Hydrolysis and Fermentation. Bioresour. Technol. 2010, 101, 4744–4753. [Google Scholar] [CrossRef]
- Safarian, S.; Sattari, S.; Unnthorsson, R.; Hamidzadeh, Z. Prioritization of Bioethanol Production Systems from Agricultural and Waste Agricultural Biomass Using Multi–criteria Decision Making. Biophys. Econ. Resour. Qual. 2019, 4, 4. [Google Scholar] [CrossRef]
- Safarian, S.; Unnthorsson, R. An Assessment of The Sustainability of Lignocellulosic Bioethanol Production from Wastes in Iceland. Energies 2018, 11, 1493. [Google Scholar] [CrossRef] [Green Version]
- Hirschnitz-Garbers, M.; Gosens, J. Producing Bio–Ethanol from Residues and Wastes: A Technology with Enormous Potential in need of Further Research and Development. Policy Brief 2015, 5, 1–5. [Google Scholar]
- Michailos, S.; Parker, D.; Webb, C. Design, Sustainability Analysis and Multiobjective Optimisation of Ethanol Production via Syngas Fermentation. Waste Biomass Valorization 2017, 10, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Liguori, R.; Soccol, C.R.; Porto de Souza Vandenberghe, L.; Woiciechowski, A.L.; Faraco, V. Second Generation Ethanol Production from Brewers’ Spent Grain. Energies 2015, 8, 2575–2586. [Google Scholar] [CrossRef] [Green Version]
- Mu, D.; Seager, T.; Rao, P.S.; Zhao, F. Comparative Life Cycle Assessment of Lignocellulosic Ethanol Production: Biochemical Versus Thermochemical Conversion. Environ. Manag. 2010, 46, 565–578. [Google Scholar] [CrossRef]
- Safarian, S.; Unnthorsson, R.; Richter, C. Simulation and Performance Analysis of Integrated Gasification–Syngas Fermentation Plant for Lignocellulosic Ethanol Production. Fermentation 2020, 6, 68. [Google Scholar] [CrossRef]
- Pardo-Planas, O.; Atiyeh, H.K.; Phillips, J.R.; Aichele, C.P.; Mohammad, S. Process. Simulation of Ethanol Production from Biomass Gasification and Syngas Fermentation. Bioresour. Technol. 2017, 245, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Safarian, S.; Unnthorsson, R.; Richter, C. Techno–Economic and Environmental Assessment of Power Supply Chain by using Waste Biomass Gasification in Iceland. Biophys. Econ. Sustain. 2020, 5, 7. [Google Scholar] [CrossRef]
- Safarian, S.; Ebrahimi Saryazdi, S.M.; Unnthorsson, R.; Richter, C. Gasification of Woody Biomasses and Forestry Residues: Simulation, Performance Analysis, and Environmental Impact. Fermentation 2021, 7, 61. [Google Scholar] [CrossRef]
- Phillips, J.R.; Huhnke, R.L.; Atiyeh, H.K. Syngas Fermentation: A Microbial Conversion Process of Gaseous Substrates to Various Products. Fermentation 2017, 3, 28. [Google Scholar] [CrossRef] [Green Version]
- de Medeiros, E.M.; Posada, J.A.; Noorman, H.; Filho, R.M. Dynamic Modeling of Syngas Fermentation in a Continuous Stirred–Tank Reactor: Multi–response Parameter Estimation and Process Optimization. Biotechnol. Bioeng. 2019, 116, 2473–2487. [Google Scholar] [CrossRef]
- Munasinghe, P.C.; Khanal, S.K. Biomass–Derived Syngas Fermentation into Biofuels: Opportunities and Challenges. Bioresour. Technol. 2010, 101, 5013–5022. [Google Scholar] [CrossRef]
- Sun, X.; Atiyeh, H.K.; Huhnke, R.L.; Tanner, R.S. Syngas Fermentation Process Development for Production of Biofuels and Chemicals: A Review. Bioresour. Technol. Rep. 2019, 7, 100279. [Google Scholar] [CrossRef]
- Safarian, S.; Unnthorsson, R.; Richter, C. Performance Analysis of Power Generation by Wood and Woody Biomass Gasification in a Downdraft Gasifier. J. Appl. Power Eng. 2021, 10, 80–88. [Google Scholar]
- Safarianbana, S.; Unnthorsson, R.; Richter, C. Development of A New Stoichiometric Equilibrium–based Model for Wood Chips and Mixed Paper Wastes Gasification by ASPEN Plus. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition, Portland, OR, USA, 11 November 2019. [Google Scholar]
- Safarian, S.; Unnthorsson, R.; Richter, C. Performance Analysis and Environmental Assessment of Small–Scale Waste Biomass Gasification Integrated CHP in Iceland. Energy 2020, 197, 117268. [Google Scholar] [CrossRef]
- Safarian, S.; Unnthorsson, R.; Richter, C. Simulation of Small–Scale Waste Biomass Gasification Integrated Power Production: A Comparative Performance Analysis for Timber and Wood Waste. Int. J. Appl. Power Eng. 2020, 9, 147–152. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An Overview of The Chemical Composition of Biomass. Fuel 2010, 89, 913–933. [Google Scholar] [CrossRef]
- Demirbas, A. Combustion characteristics of different biomass fuels. Prog. Energy Combust. Sci. 2004, 30, 219–230. [Google Scholar] [CrossRef]
- Scurlock, J.M.; Dayton, D.C.; Hames, B. Bamboo: An Overlooked Biomass Resource? Biomass Bioenergy 2000, 19, 229–244. [Google Scholar] [CrossRef] [Green Version]
- Risnes, H.; Fjellerup, J.; Henriksen, U.; Moilanen, A.; Norby, P.; Papadakis, K.; Posselt, D.; Sørensen, L. Calcium Addition in Straw Gasification. Fuel 2003, 82, 641–651. [Google Scholar] [CrossRef]
- Thy, P.; Lesher, C.; Jenkins, B. Experimental Determination of High–Temperature Elemental Losses from Biomass Slag. Fuel 2000, 79, 693–700. [Google Scholar] [CrossRef]
- Moilanen, A. Thermogravimetric Characterisations of Biomass and Waste for Gasification Processes. VTT 2006, 607, 1–103. [Google Scholar]
- Theis, M.; Skrifvars, B.-J.; Hupa, M.; Tran, H. Fouling Tendency of Ash Resulting from Burning Mixtures of Biofuels. Part. 1: Deposition Rates. Fuel 2006, 85, 1125–1130. [Google Scholar] [CrossRef]
- Thy, P.; Jenkins, B.; Grundvig, S.; Shiraki, R.; Lesher, C. High. Temperature Elemental Losses and Mineralogical Changes in Common Biomass Ashes. Fuel 2006, 85, 783–795. [Google Scholar] [CrossRef]
- Werther, J.; Saenger, M.; Hartge, E.-U.; Ogada, T.; Siagi, Z. Combustion of Agricultural Residues. Prog. Energy Combust. Sci. 2000, 26, 1–27. [Google Scholar] [CrossRef]
- Miles, T.; Baxter, L.; Bryers, R.; Jenkins, B.; Oden, L. Alkali Deposits Found in Biomass Power Plants: A Preliminary Investigation of Their Extent and Nature. Alikali Depos. Found Biomass Power Plants 1995. [Google Scholar] [CrossRef] [Green Version]
- Masiá, A.T.; Buhre, B.; Gupta, R.; Wall, T. Characterising Ash of Biomass and Waste. Fuel Process. Technol. 2007, 88, 1071–1081. [Google Scholar] [CrossRef]
- Nutalapati, D.; Gupta, R.; Moghtaderi, B.; Wall, T. Assessing Slagging and Fouling During Biomass Combustion: A Thermodynamic Approach Allowing for Alkali/Ash Reactions. Fuel Process. Technol. 2007, 88, 1044–1052. [Google Scholar] [CrossRef]
- Safarian, S.; Ebrahimi Saryazdi, S.M.; Unnthorsson, R.; Richter, C. Artificial Neural Network Modeling of Bioethanol Production Via Syngas Fermentation. Biophys. Econ. Sustain. 2021, 6, 1. [Google Scholar] [CrossRef]
- Safarian, S.; Ebrahimi Saryazdi, S.M.; Unnthorsson, R.; Richter, C. Dataset of Biomass Characteristics and Net Output Power from Downdraft Biomass Gasifier Integrated Power Production Unit. Data Brief 2020, 33, 106390. [Google Scholar] [CrossRef]
- Safarian, S.; Ebrahimi Saryazdi, S.M.; Unnthorsson, R.; Richter, C. Modeling of Hydrogen Production by Applying Biomass Gasification: Artificial Neural Network Modeling Approach. Fermentation 2021, 7, 71. [Google Scholar] [CrossRef]
- Safarian, S.; Ebrahimi Saryazdi, S.M.; Unnthorsson, R.; Richter, C. Artificial Neural Network Integrated with Thermodynamic Equilibrium Modeling of Downdraft Biomass Gasification–Power Production Plant. Energy 2020, 213, 118800. [Google Scholar] [CrossRef]
- Damartzis, T.; Michailos, S.; Zabaniotou, A. Energetic Assessment of a Combined Heat and Power Integrated Biomass Gasification–Internal Combustion Engine System by using Aspen Plus®. Fuel Process. Technol. 2012, 95, 37–44. [Google Scholar] [CrossRef]
- Tauqir, W.; Zubair, M.; Nazir, H. Parametric Analysis of a Steady State Equilibrium–Based Biomass Gasification Model for Syngas and Biochar Production and Heat Generation. Energy Convers. Manag. 2019, 199, 111954. [Google Scholar] [CrossRef]
- Safarian, S.; Unnthorsson, R.; Richter, C. The Equivalence of Stoichiometric and Non–Stoichiometric Methods for Modeling Gasification and Other Reaction Equilibria. Renew. Sustain. Energy Rev. 2020, 131, 109982. [Google Scholar] [CrossRef]
- Safarianbana, S. Simulation of a Small Scale Biowaste Gasification System for Energy Production. PhD Dissertation, University of Iceland, Reykjavík, Iceland, 2021. [Google Scholar]
- Ray, R.C.; Ramachandran, S. Bioethanol Production from Food Crops: Sustainable Sources, Interventions, and Challenges; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Teo, W.K.; Ruthven, D.M. Adsorption of Water from Aqueous Ethanol using 3–. ANG. Molecular Sieves. Ind. Eng. Chem. Process. Des. Dev. 1986, 25, 17–21. [Google Scholar] [CrossRef]

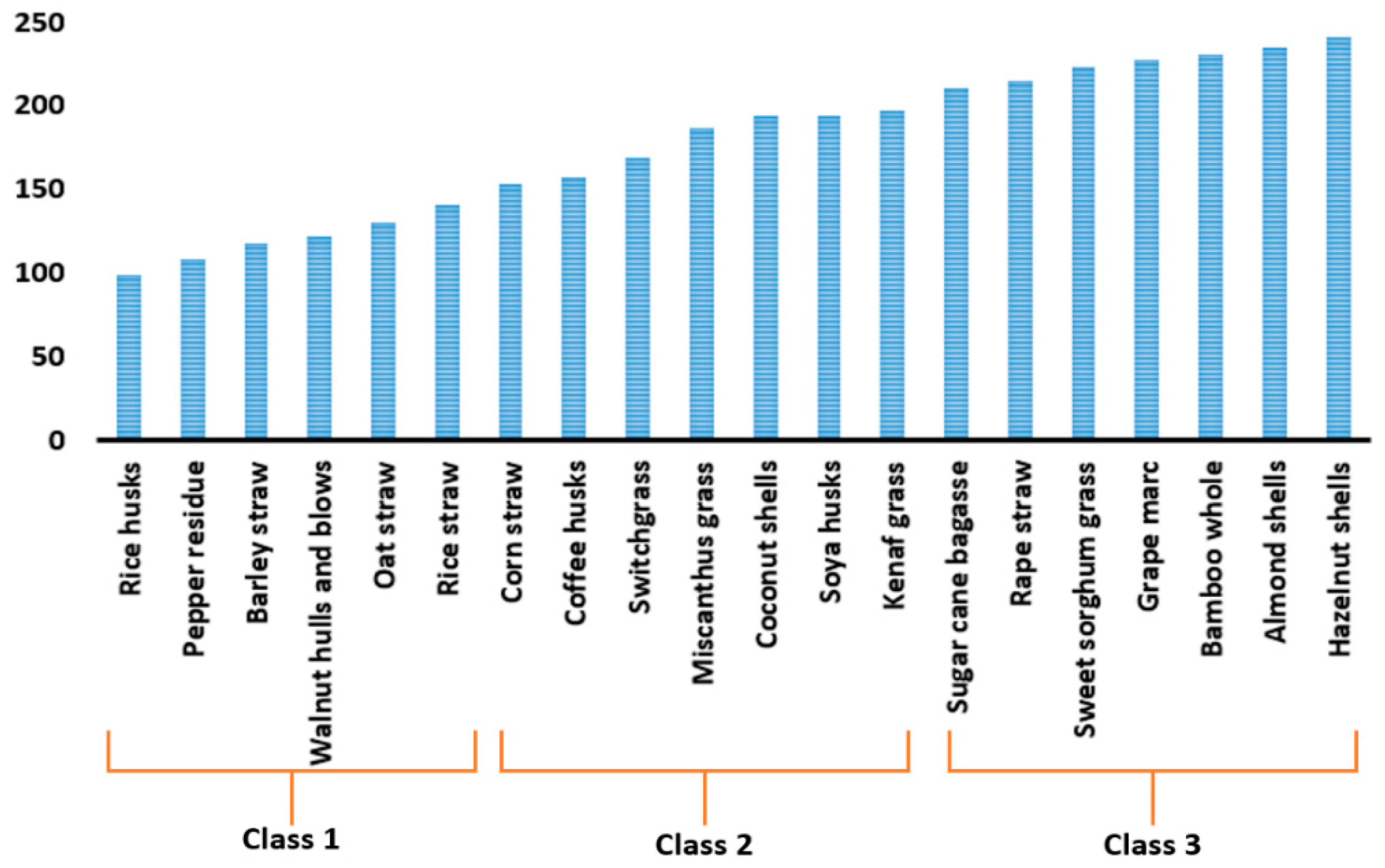
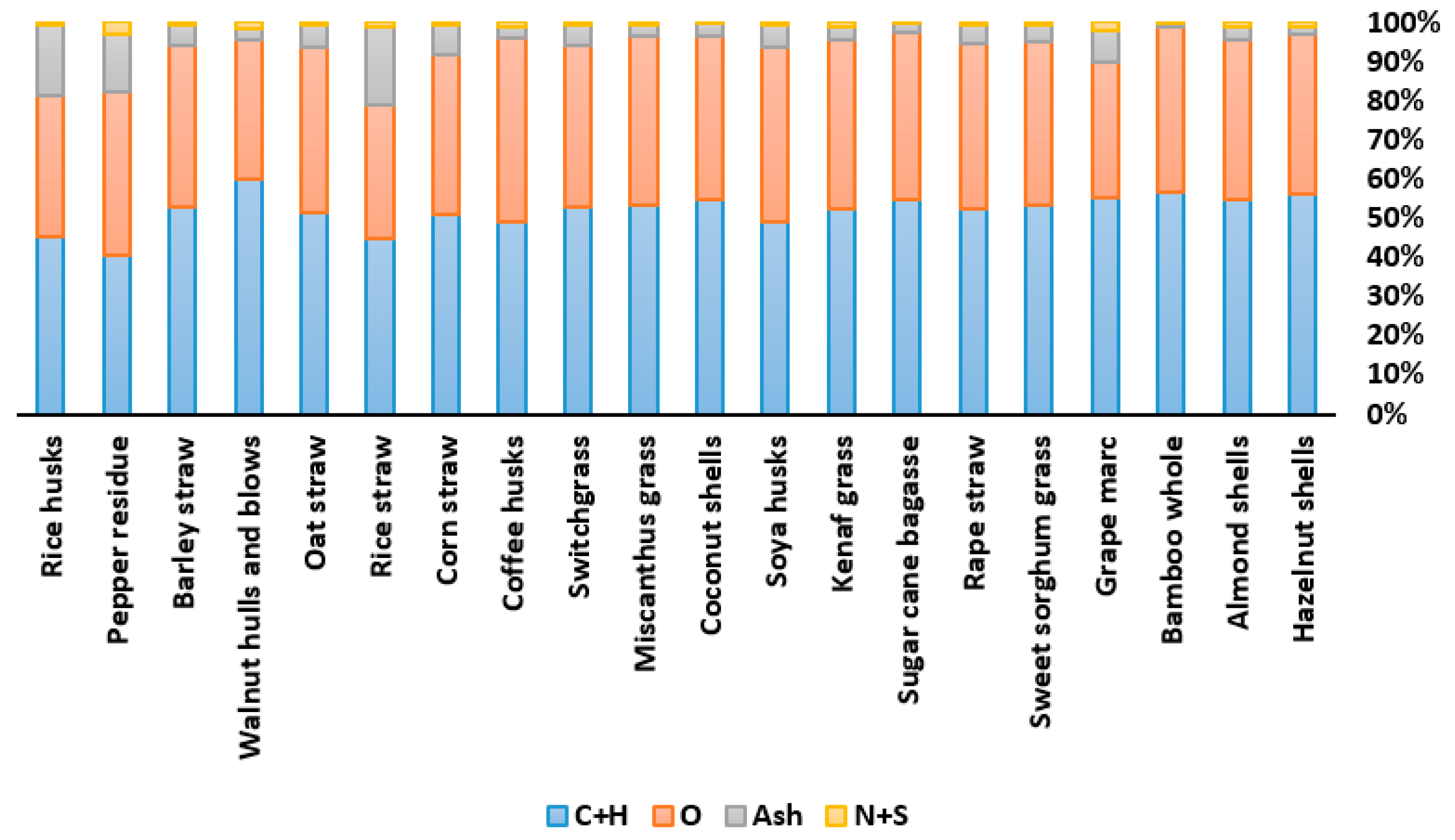
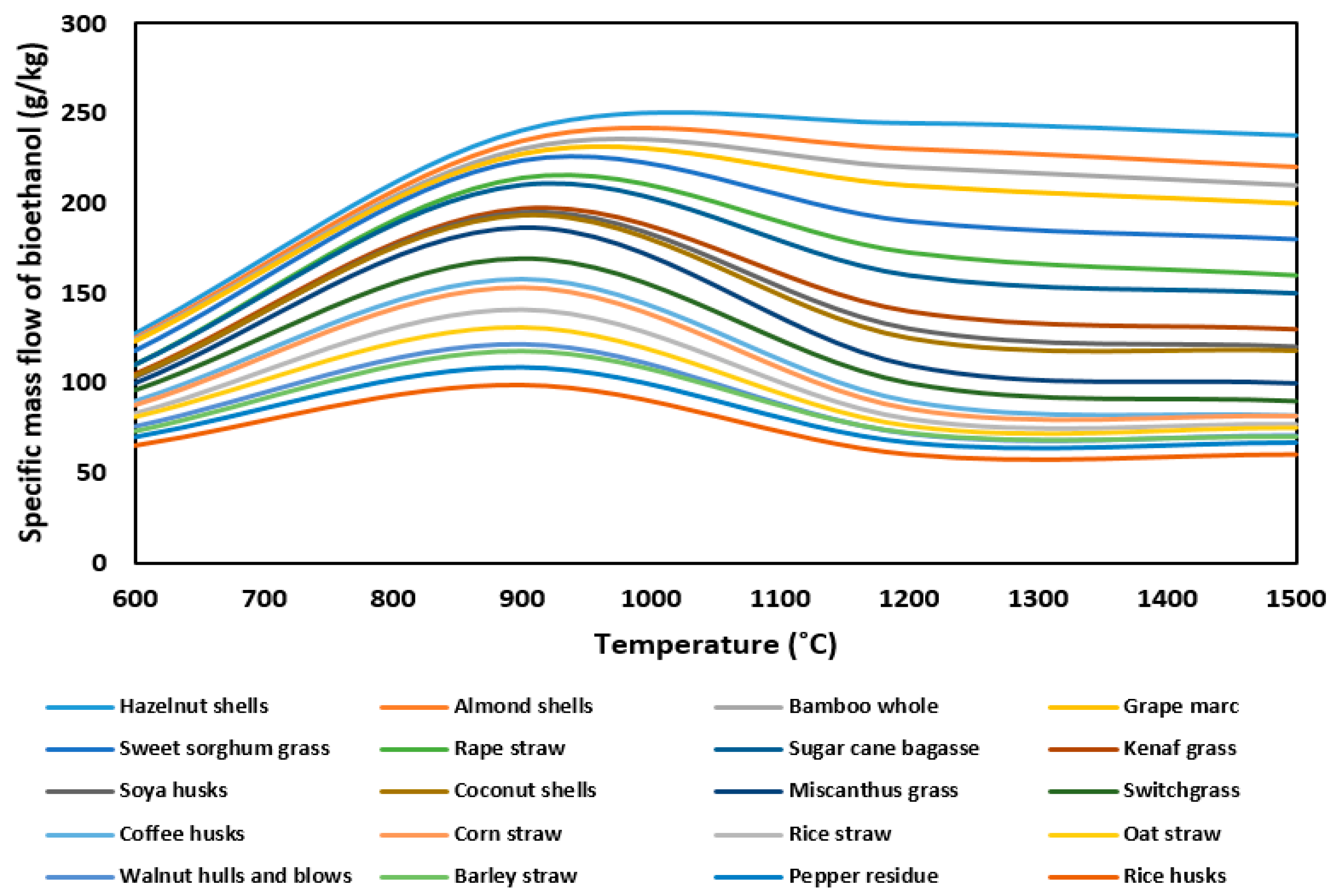

| Proximate Analysis (wt%) | Elemental Analysis (wt%—Dry Basis) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | VM | FC | A | C | O | H | N | S | ||
| 1 | Bamboo whole | 13 | 81.6 | 17.5 | 0.9 | 51.53 | 42.1 | 5.054 | 0.396 | 0 |
| 2 | Kenaf grass | 7.5 | 79.4 | 17 | 3.6 | 46.66 | 42.9 | 5.784 | 0.964 | 0.0964 |
| 3 | Miscanthus grass | 11.4 | 81.2 | 15.8 | 3 | 47.72 | 42.9 | 5.82 | 0.388 | 0.194 |
| 4 | Sweet sorghum grass | 7 | 77.2 | 18.1 | 4.7 | 47.36 | 41.6 | 5.813 | 0.381 | 0.0953 |
| 5 | Switchgrass | 11.9 | 80.4 | 14.5 | 5.1 | 47.17 | 41.2 | 5.789 | 0.664 | 0.0949 |
| 6 | Barley straw | 11.5 | 76.2 | 18.5 | 5.3 | 46.78 | 41.3 | 5.871 | 0.663 | 0.0947 |
| 7 | Corn straw | 7.4 | 73.1 | 19.2 | 7.7 | 44.95 | 40.7 | 5.907 | 0.646 | 0.0923 |
| 8 | Oat straw | 8.2 | 80.5 | 13.6 | 5.9 | 45.92 | 42 | 5.646 | 0.471 | 0.0941 |
| 9 | Rape straw | 8.7 | 77.4 | 17.9 | 4.7 | 46.22 | 42.4 | 6.099 | 0.477 | 0.0953 |
| 10 | Rice straw | 7.6 | 64.3 | 15.6 | 20.1 | 40.03 | 34.4 | 4.554 | 0.799 | 0.1598 |
| 11 | Almond shells | 7.2 | 74.9 | 21.8 | 3.3 | 48.64 | 41.1 | 5.995 | 0.967 | 0 |
| 12 | Coconut shells | 4.4 | 73.8 | 23 | 3.2 | 49.46 | 41.7 | 5.421 | 0.097 | 0.0968 |
| 13 | Coffee husks | 10.8 | 76.5 | 20.7 | 2.8 | 44.13 | 46.9 | 4.763 | 1.069 | 0.2916 |
| 14 | Rice husks | 10.6 | 62.8 | 19.2 | 18 | 40.43 | 35.8 | 5.002 | 0.656 | 0.082 |
| 15 | Soya husks | 6.3 | 74.3 | 20.3 | 5.4 | 42.95 | 44.4 | 6.338 | 0.851 | 0.0946 |
| 16 | Sugar cane bagasse | 10.4 | 85.5 | 12.4 | 2.1 | 48.75 | 43 | 5.874 | 0.196 | 0.0979 |
| 17 | Walnut hulls and blows | 47.9 | 79.6 | 17.5 | 2.9 | 53.5 | 35.4 | 6.506 | 1.554 | 0.0971 |
| 18 | Pepper residue | 9.7 | 64.8 | 27 | 8.2 | 41.95 | 43.2 | 2.938 | 3.121 | 0.5508 |
| 19 | Grape marc | 10 | 65.8 | 26.4 | 7.8 | 49.79 | 34.5 | 5.624 | 2.213 | 0.0922 |
| 20 | Hazelnut shells | 7.2 | 77.1 | 21.4 | 1.5 | 50.73 | 41 | 5.418 | 1.379 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safarian, S.; Unnthorsson, R.; Richter, C. Bioethanol Production via Herbaceous and Agricultural Biomass Gasification Integrated with Syngas Fermentation. Fermentation 2021, 7, 139. https://doi.org/10.3390/fermentation7030139
Safarian S, Unnthorsson R, Richter C. Bioethanol Production via Herbaceous and Agricultural Biomass Gasification Integrated with Syngas Fermentation. Fermentation. 2021; 7(3):139. https://doi.org/10.3390/fermentation7030139
Chicago/Turabian StyleSafarian, Sahar, Runar Unnthorsson, and Christiaan Richter. 2021. "Bioethanol Production via Herbaceous and Agricultural Biomass Gasification Integrated with Syngas Fermentation" Fermentation 7, no. 3: 139. https://doi.org/10.3390/fermentation7030139
APA StyleSafarian, S., Unnthorsson, R., & Richter, C. (2021). Bioethanol Production via Herbaceous and Agricultural Biomass Gasification Integrated with Syngas Fermentation. Fermentation, 7(3), 139. https://doi.org/10.3390/fermentation7030139







