Abstract
In recent years, interest in the biorefinery concept has emerged in the utilization of volatile fatty acids (VFAs) produced by acidogenic fermentation as precursors for various biotechnological processes. This has attracted substantial attention to VFA production from low-cost substrates such as organic waste and membrane based VFA recovery techniques to achieve cost-effective and environmentally friendly processes. However, there are few reviews which emphasize the acidogenic fermentation of organic waste into VFAs, and VFA recovery. Therefore, this article comprehensively summarizes VFA production, the factors affecting VFA production, and VFA recovery strategies using membrane-based techniques. Additionally, the outlook for future research on VFA production is discussed.
1. Introduction
The increase in global population size, economic growth, and urbanization development has led to massive generation of organic waste, often including sewage sludge (SS) from wastewater treatment plants, municipal solid waste, and animal manure (AM). This causes unsanitary conditions that affect human health and exacerbates environmental pollution worldwide. Disposal methods, such as landfill composting and combustion, are limited by the availability of disposal sites, greenhouse gas emissions, and high energy consumption [1]. Organic waste is rich in organic and inorganic nutrients, is highly accessible, cost-effective and has a high moisture content [2]. Thus, is it considered as a potential substrate for bioenergy and biochemical production by anaerobic digestion. There are four independent metabolic reactions conducted by a mixed microbial community in anaerobic digestion process: hydrolysis, acidogenesis, acetogenesis, and methanogenesis. Mixed microorganisms decompose organic matter into low-molecular-weight intermediates, such as volatile fatty acids (VFAs) and hydrogen in the acidogenesis stage, and methane in the methanogenesis stage [3]. Interest in these intermediate compounds, especially VFAs, has recently increased because they have been recognized to have more applications than methane [4]. Moreover, the market value of VFAs for 1 ton of biomass (USD 150) is estimated to be much higher than that of methane (USD 31) [5,6].
VFAs are low-molecular-weight carboxylic acids containing two to six carbon atoms. To date, VFAs are mostly produced from conventional chemical processes (such as methanol carbonylation, oxidation of propane, and oxidation of butyraldehyde) using petrochemicals as raw materials [7,8]. However, these processes are highly energy-intensive and use non-renewable sources, making them economically and environmentally less attractive. It has been reported that greenhouse gas (GHG) emissions from the production of acetic acids in the petrochemical industry released 3.3 t-CO2 eq/t from the cradle to the grave [7]. Therefore, VFA production by biological routes from organic waste is gaining attention because of its economic and environmental advantages. VFAs, including acetic acid, propionic acid, butyric acid, caproic acid, and valeric acid, are produced as the final products of acidogenic fermentation in the anaerobic digestion process. These compounds are reported to be the most critical precursors in the biorefinery concept. They can be directly or indirectly used as carbon sources for biological nutrient removal from wastewater [5] or as precursors to synthesize high-value-added products, such as bioplastics [9] and biodiesel [10].
Acidogenic fermentation employs an undefined mixed culture that is easy to handle under non-sterile conditions and can use a broad spectrum of substrates. However, it is necessary to be attentive to the process and operational parameters to maximize the VFA production. For example, the optimal organic loading rate (OLR) for acidogenic fermentation of organic waste is reported to be in the range of 2–15 g-volatile solid (VS)/L·day [3,8]. Hydraulic retention time (HRT) should be maintained for a short period to inhibit slow-growing methanogenic microorganisms [8]. In addition, the acidogenic fermentation of carbohydrate-rich substrates (e.g., food waste (FW) and agricultural residues) must be conducted under acidic and neutral pH ranges [11,12], whereas SS and AM prefer an alkaline pH range [4,5].
Although several studies have investigated the process and operational parameters of VFAs production, the process cannot be easily scaled up to the pilot-scale because of limitations in the recovery of the produced VFAs [7]. The challenges in VFA recovery include: (1) processing cost, which accounts for approximately 30–40% of the total production cost, (2) the mixture complexity of fermentation broth, and (3) VFAs with low concentration in the fermented stream [5,13]. Furthermore, the presence of suspended solids including cells and inorganic precipitates in real fermentation broth might hinder mass transfer coefficients of the VFA recovery step. Therefore, these fermented streams require a solid removal step to improve the efficiency of the recovery process [14]. Various techniques have been applied for VFA recovery, including liquid–liquid extraction [15], adsorption [16,17], membrane contactors [18], membrane reactors [19,20], electrodialysis [21,22] and membrane pervaporation [13]. Among them, membrane-based recovery appears to be a more promising technology than other techniques because of its economic and environmental potential [5,7,14].
This paper provides a comprehensive review of the production of VFAs from organic waste, factors influencing acidogenic fermentation, with the emphasis on the use of membrane based VFA recovery techniques to make acidogenic fermentation more economically feasible and environmentally benign. In addition, the advantages and disadvantages of membrane based VFA recovery techniques are summarized. Finally, potentially important ideas regarding the ongoing development of this technology are highlighted.
2. Types of Organic Waste
The organic waste is typically defined as waste composed of biomass rich in organic matter derived from renewable sources [23,24]. It includes mainly agricultural waste (e.g., AM, rice straw, wheat straw, corn stalk, fruit peels, and fallen leaves), industrial waste (e.g., palm oil mill effluent, SS, cheese way wastewater, rapeseed oil cakes), and municipal solid waste (e.g., FW, household waste, wastepaper). The biomass of the organic waste is abundant, cheap, and does not compete with food production. Among them, FW, SS, and AM are the three major organic waste products that have been studied for their potential as substrates to produce VFAs by acidogenic fermentation (Table 1). Some of the organic waste comprises a proportion of lignocellulosic materials that can hinder microbial degradation. Thus, an initial pretreatment can lose the recalcitrant structure and accelerate solubilization before acidogenic fermentation is required [25]. Additionally, the use of waste substrates has major drawbacks. Organic waste containing mixed microflora may also consist of VFA consuming bacteria, such as methanogenic bacteria [3] or other microorganisms that compete with VFA production in terms of nutrients and substrate uptake rate, such as lactic acid bacteria [26]. Methanogenic bacteria should be inhibited as much as possible. Several methods for inhibiting methanogenic activities have been developed, such as the addition of chemicals, physicochemical treatment, and the application of heat [25,27].

Table 1.
Various organic waste as the substrate for acidogenic fermentation.
FW is defined as fractions of food and inedible parts of food removed from the food supply chain [28]. There are four main types of FW related to edibility categorized by food type for bin digs. These include inedible parts of all types of food, edible foods of one food type, edible foods comprised of multiple food types, and unidentifiable foods [29]. Generally, the amount of FW generated is proportional to the level of local social and economic growth. The yield of FW is 0.26–0.32 kg/day per capita in Europe and North America, but only 0.02–0.03 kg/day in Africa and Southeast Asia [1]. FW has various characteristics because of the different food cultures in each region [30]. Normally, FW contains a high amount of biodegradable organic content, moisture, and trace elements. FW serves as a suitable substrate for acidogenic fermentation according to its chemical compositions. The major organic compounds in FW are carbohydrates (41–62%), proteins (15–25%), lipids (13–30%), and a considerable amount of nutrients. The carbon to nitrogen (C/N) ratio varies between 9 and 32, depending on the protein content of the FW [1]. The challenge of using FW as the feedstock for VFA production is to effectively separate FW from municipal solid waste because it contains many inert materials, such as plastic bags, eggshells, bones, aluminum cans, grit, and broken glass [8]. In this case, source separation or establishing a material recovery center to isolate the organic fraction of municipal solid waste may be a solution, but it would require a high level of public cooperation, which is not easy to achieve [31].
SS is waste generated in the activated sludge process, commonly used for wastewater treatment [32]. The disposal of SS has become a serious problem because of the large amount of SS produced annually. It has been reported that 7.8 and 13 million tons of dry sludge were generated in China in 2019 [33] and EU countries in 2020 [34], respectively. Additionally, the disposal of such massive amounts of SS is costly, accounting for up to 60% of the overall operating costs of wastewater treatment plants [35]. SS contains high levels of organic matter, making it an ideal renewable substrate for acidogenic fermentation. SS components, including microorganisms and extracellular polymeric substances (EPS), account for approximately 60% on a dry basis, followed by inorganic particles (e.g., silicates and metals), pathogens, and water (63–99%) [32]. SS has a VS to total solid (TS) ratio of 0.75, total chemical oxygen demand (tCOD) of 19.7 g-COD/L, total proteins of 8063 mg-COD/L, and total carbohydrates of 1524 mg-COD/L [36,37]. However, the soluble COD (sCOD) of SS is 10–100 times lower than its tCOD. Consequently, the rate of hydrolysis is impeded. Thus, a pretreatment step such as ultrasonication, hydrothermal and acid-alkaline pretreatment is needed to improve the production of VFAs from SS [31,38]. For example, hydrothermal pretreatment was found to significantly improve the biodegradability of SS in which the highest biodegradability of 56% was attained when SS was hydrothermally pretreated at 170 °C for 30 min. The biodegradability of hydrothermal pretreated SS was 36% higher than untreated SS [39].
AM is another organic waste that is widely used for VFA production. AM is typically livestock waste generated in large quantities in two forms, i.e., liquid manure (animal excrement mixed with water used for cleaning the concrete floor in an animal stall) and solid manure (a mixture of manure and urine of animals) [40,41]. Variation in the physicochemical properties of AM has revealed that the composition of manure is highly dependent on animal feed [42]. AM typically has a low C/N ratio (9–19), high buffering capacity, and high biodegradability [43,44]. It contains a VS/TS ratio ranging between 0.65 and 0.90 [40,43]. Compared to FW and SS, fewer studies have investigated the use of AM for VFA production because of the high level of ammonia (9.5–28 g/L), which is toxic to most microorganisms [19]. The remaining part of AM (mostly dietary fiber) is digested through the animal digestive tract. As a result, it is highly recalcitrant to enzymatic hydrolysis in acidogenic fermentation, which means that the pretreatment of AM is required [42]. A suitable pretreatment method for AM is highly dependent on its compositions. AM with high fiber contents and stone should be mechanically pretreated with, e.g., thermal and ultrasonic pretreatment prior to any other pretreatment. Alkaline pretreatment is appropriate for AM with high lignin contents [45]. For instance, Cristina et al., (2008) [46] reported that sCOD of swine manure was increased by 57% and 32% after applying alkaline (1 M NaOH) and thermal (170 °C for 30 min) pretreatment, respectively.
Production efficiency of VFAs is different and based on operational parameters (such as pH, temperature, and OLR), type of substrate, and microbial population. FW usually has a higher soluble organic content and digestibility than SS, resulting in higher VFA production. Co-digestion with various organic wastes results in higher VFA production. For instance, co-digestion of FW and SS produced the highest VFA yield of 0.87 g-COD/g-VS without pH control [47]. Most studies have focused on short-term VFA production operation with a maximum fermentation period of 30 days [47]. However, Agyeman et al. (2020) [48] investigated the production of VFAs in long-term operation mode by co-digestion of FW and SS and found that the operation time affected the type of dominant VFA, and the VFA production yield and rate. Overall, it is still unclear which types of waste biomass are the most suitable for VFA production because of different operational conditions and evaluation criteria in each study. Apart from the characteristics of waste biomass, the availability of waste should also be considered to ensure that waste is sufficient for acidogenic fermentation [31].
3. Acidogenic Fermentation
During acidogenic fermentation of organic waste, organic matter, such as carbohydrates, proteins, and lipids, are first hydrolyzed to their soluble form, which leads to an increase in the sCOD; this is the so-called hydrolysis step. Soluble organic matter (such as sugar amino acids and fatty acids) is rapidly fermented to pyruvate through glycolysis and finally to VFAs with hydrogen, carbon dioxide amount of alcohol as by-products in the acidogenic step [50]. The reactions take place in the digester, where the activity of methanogens is suppressed to prevent the utilization of VFAs to produce biogas. There are several methods for this, including changing the operational factors (e.g., shortening the reaction time, adjusting pH and food to microorganisms (F/M) ratio) to increase the activity of acid-fermentation bacteria [7,57], use of an inhibitor (e.g., carbon monoxide, 2-bromoethanesulfonate (BES), 2-mercaptoethanesulfonate (MES) to inhibit methanogen activity [49,58] and use of substrate pretreatment to inhibit the methanogens present [59].
The most common VFAs produced during acidogenic fermentation are acetic acid, propionic acid, butyric acid, and valeric acid. The properties of these acids are shown in Table 2. They are potential renewable carbon sources in a variety of biological processes, such as biopolymer formation, biological nutrient removal, and creation of bioenergy, and have a wide range of applications, such as food additives, pharmaceutical products, cosmetics, and chemical precursors [59,60]. In general, the distribution of fermentation products reflects prevailing metabolic pathways [61]. This could be explained by different metabolic pathways caused by the development of the microbial community when environmental conditions, such as pH and temperature, are different, which leads to different fermentation types [51,62]. Based on the main product produced during the fermentation process, acidogenic fermentation is generally classified as the acetate-ethanol, propionate, butyrate, mixed-acid, or lactate type. (Figure 1)

Table 2.
VFAs properties [63].
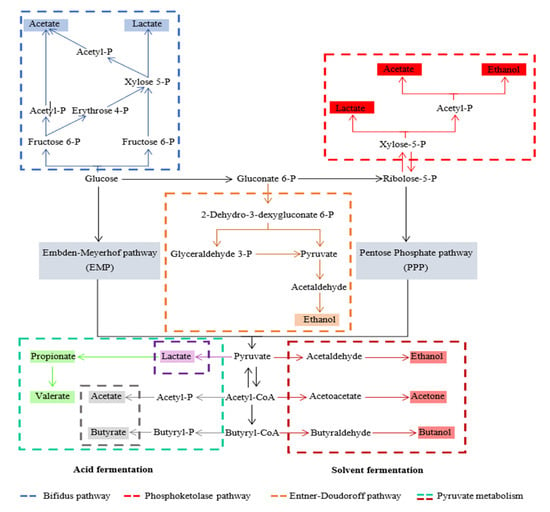
Figure 1.
Metabolic pathway of acidogenic fermentation, modified from [64].
Acetate can be produced from the acetyl-CoA pathway or the syntrophic oxidation of ethanol or long-chain fatty acids. Ethanol can be produced by converting pyruvate by pyruvate decarboxylation to acetaldehyde and its subsequent acetaldehyde reduction to ethanol. During ethanol production, acetone and butanol can be produced by some genera of Clostridia, such as Clostridium acetobutylicum, which is called acetone-butanol-ethanol (ABE) fermentation [65,66]. Propionate is produced by two distinct pathways. In pyruvate catalyzation, lactate is produced by lactate dehydrogenase, and lactate is then reduced to propionate by the action of propionate dehydrogenase and propionate production through the trans-carboxylate cycle. Butyrate is produced through the Embden–Meyerhof–Parnas (EMP) pathway that converts glucose to pyruvate before conversion to butyryl-CoA with acetoacetyl-CoA, 3-hydroxybutyryl-CoA, and crotonyl-CoA as intermediates, and then sequentially by the catalysis of thiolase, 3-hydroxybutyryl-CoA dehydrogenase, and butyryl-CoA dehydrogenase. Then, butyryl-CoA is converted into butyrate by phosphotransbutyrylase and butyrate-kinase enzymes [64,66]. For lactic acid fermentation, pyruvate produced from glycolysis is converted into lactate by lactate dehydrogenase. Lactate production can be classified into two types based on the products: homolactate fermentation (one mole of glucose is converted into two moles of lactate) and heterolactate fermentation (acetate or ethanol are produced as co-products in the system) through the phosphoketolase pathway and bifidus pathway [64,67]. Another type of metabolic pathway that can occur in acidogenic fermentation is mixed-acid fermentation. In mixed-acid fermentation, microbials use two or more different metabolic pathways to convert pyruvate into several fermentation products. There are no dominant VFAs in the fermentation products. Generally, acetic acid, propionic acid, and butyric acid are present in relatively high concentrations, while other products, such as lactate and ethanol are present in low concentrations [66].
4. Factors Affecting Acidogenic Fermentation
Because VFA production through acidogenic fermentation uses mixed microbial culture, various factors such as pH, temperature, inoculum, HRT, and OLR play a key role in VFA production yield and soluble metabolite distribution. These factors must be optimized to significantly improve the production efficiency and achieve selective production of specific VFAs while reducing the cost and saving energy consumed in the process.
4.1. pH
pH is one of the most important parameters affecting VFA production, yield and composition because it regulates the activities of different microbes involved in acidogenic fermentation. The pH level in the reactor affects the rate of hydrolysis, which is characterized by changes in sCOD [68]. Hydrolysis is well known to be the rate-limiting step of anaerobic digestion. Therefore, sCOD is very important because it shows the number of soluble substances that can be converted into VFAs. Wang et al. (2014) [69] observed that sCOD produced at pH 4 and uncontrolled pH was higher than that produced at pH 5 and pH 6 during VFA production from FW. However, most microorganisms cannot survive in extremely acidic (pH = 3) or alkaline (pH = 12) conditions; thus, it is necessary to maintain an optimal pH for inducing acidification depending on the type of wet waste and acids of interest [60]. Previous research reported that the highest VFA yield was achieved under slightly acid-neutral conditions (pH 5.5–7.0). Yin et al. (2021) [53] found that the highest VFA yield of 0.53 g-VFAs/g-VS was achieved at pH 7.7 and conditions with heat-shocked inoculum, substrate pretreatment, and a substrate to inoculum (S:I) ratio of 6 using chicken manure as the substrate. Similarly, Cavinato et al. (2017) [70] found that the production of VFAs from co-digestion of cow manure and maize silage was highest at pH between 5.5 and 6.5. In contrast, few studies have suggested that alkaline pH (pH 9–11) is effective in VFA production from waste biomass [53,71]. For example, the effect of pH on FW fermentation for VFA production was investigated by Khatami et al. (2021) [71]. They found that the highest VFA concentration from acidogenic fermentation of FW was obtained under alkaline pH conditions (pH 10). Another study conducted by Dahiya et al. (2015) [72] showed that the degradation efficiency of soluble proteins and fats under alkaline and neutral conditions was higher than that under acidic conditions and suppressed the growth of acid consumer bacteria. The highest VFAs production from anaerobic digestion of FW was obtained at pH 10 (6.3 g/L) followed by pH 9 (5.1 g/L), pH 6 (4.5 g/L), pH 5 (4.2 g/L), pH 7 (4.1 g/L), pH 8 (3.8 g/L), and pH 11 (3.5 g/L). According to the literature, alkaline pH conditions seem to be more favorable for improving VFA production from anaerobic digestion of sludge [73,74] while a pH range between 5.5 and 6.5 was suggested for VFA production using FW and other waste biomass.
pH not only plays a significant role in the synthesis of VFAs but also influences VFA composition. Acidogenic fermentation of VFAs using FW as a substrate was conducted by Jiang et al. (2013) [68]. The authors observed that the VFA composition in reactors with uncontrolled pH and at pH 5 was dominated by acetic acid, followed by butyric acid and propionic acid. When the pH was controlled at 6.0 or 7.0, butyric acid became the main product, followed by acetic acid, propionic acid, and valeric acid. Ye et al. (2018) [75] reported that a pH level of 6.0 was optimal for producing VFAs in which concentrations of butyric acid and acetic acid were dominant, whereas pH 8.0 increased propionic acid production. A previous study by Gameiro et al. (2016) [76] reported that the formation of propionic acid was favored at a pH higher than 6.5. However, the accumulation of VFAs will trigger a drop in pH. When the pH drops below the pKa value of the VFAs, most of the VFAs will be changed into the undissociated form. These undissociated acids can easily pass through the cell membrane and dissociate inside the microbial cell, causing imbalanced energy production in the cell, inhibiting cell activity [49]. Lu et al. (2020) [50] found that butyric acid was the main product under an acidic pH (pH 5.0 and uncontrolled pH), whereas acetic acid was the dominant product under neutral-alkaline pH (pH 7.0 and pH 11.0). The author further reported that the enzymatic activities of acetate kinase and butyrate kinase were slightly inhibited at pH 5.0 and 11.0, resulting in relatively low VFA production. Furthermore, Agyeman et al. (2020) [48] demonstrated that the composition of the VFAs obtained from the digestion of organic waste in a batch reactor with an initial pH of 5 (acetic acid, propionic acid, and caproic acid) differed from that obtained from a semi-continuous reactor with pH controlled at 5 (caproic acid, acetic acid, and butyric acid). Therefore, controlling the operational pH must be considered to obtain a high VFA production efficiency and selectively produce acid during long-term continuous operation.
4.2. Temperature
In addition to pH, temperature is a key parameter in the acidogenic fermentation of waste biomass because of its role in microbial growth, metabolic pathways, enzyme activity, and hydrolysis rate [74,77]. Additionally, the microbial population and rate of hydrolysis are highly affected by changes in operational temperature [70,76]. Jiang et al. (2013) [68] found that higher solubilization of FW was obtained at a thermophilic temperature. Furthermore, acidification decreased as temperature increased, which was in line with the results from He et al. (2012) [78], who found that the rate of hydrolysis was proportional to the increase in temperature because of a physicochemical effect at higher temperatures. However, an increase in VFA production is only possible when the optimum temperature for bacterial growth is met because many acidogens cannot survive at high temperatures. Additionally, VFA production from FW at psychrophilic temperatures was investigated by Komemoto et al. (2009) [79], who observed that the substrate consumption and sCOD released by the reactors at low temperatures (15 °C) were the lowest, while the maximum substrate consumption and solubilization rate was attained under mesophilic conditions (35 and 45 °C). From these findings, it can be concluded that the optimum temperature for VFA production in the mesophilic range is appropriate for substrate uptake by microorganisms. The rate of substrate solubilization can be accelerated by increasing the fermentation temperatures. However, at temperatures above 55 °C, microbial activity is inhibited.
The composition of VFAs is affected by temperature, although slightly lesser than pH [31]. Jiang et al. (2013) [68] reported that acetic acid and propionic acid were the most prevalent soluble products at 35 °C and 45 °C, accounting for approximately 70%. Butyric acid was the main product at 55 °C, accounting for 81%. Similar results were reported by Aguirre et al. (2017) [80], who found that a mixture of acetic acid and propionic acid was favored at 35 °C. When the temperature was further increased to 55 °C, butyric acid became the main product. Conversely, Hao and Wang (2015) [81] investigated the effect of fermentation temperature on VFA production from sludge by anaerobic fermentation. The authors reported that the percentages of acetic acid and valeric acid were higher at thermophilic temperatures (55 °C) than at mesophilic temperatures (35 °C), whereas propionic acid and butyric acid decreased, which is consistent with the results reported by Zhang et al. (2009) [82]. These contradictory results might be attributable to the initial inoculum seed and various physicochemical factors that influence microbial dynamics.
4.3. Inoculum
The type of inoculum can affect the VFA composition when a complex substrate is used. Most studies have performed acidogenic fermentation using anaerobic digestate/sludge as a seed inoculum because of the presence of acidogenic bacteria compared to other inoculums, such as aerobic sludge [83]. Furthermore, some studies have reported that the desired acid composition might be produced by selecting the inoculum type. Wang et al. (2014) [69] studied the effect of different mixed microbial cultures between anaerobic sludge and aerobic sludge on VFA production from FW at various pH values (4, 5, 6, and uncontrolled pH) in batch fermentation mode. They found that anaerobically activated sludge produced almost double the amount of VFAs (0.918 g-VFAs/g-VSS) compared to aerobically activated sludge (0.482 g-VFAs/g-VSS). In addition to the type of inoculum, VFA production is influenced by inoculum pretreatment, as illustrated by Blasco et al. (2020) [84]. They concluded that two inoculum pretreatments (thermal and freeze–thaw pretreatment) influenced the microbial community, resulting in a change in VFA composition and thermal pretreatment was the most effective inoculum pretreatment.
Acclimation of inoculum was favorable for achieving a higher VFA yield. Lukitawesa et al. (2020) [3] investigated the role of inoculum acclimatization by comparing the first reactor that was inoculated with the inoculum that had been fed with a high substrate loading for 14 days to the second reactor, which was inoculated with fresh inoculum. In comparison with fresh inoculum, acclimatization inoculum increased VFA production by approximately 35-fold. This implies that acclimatization of the inoculum can shift the microbial community to VFA production. In another study, Zhang et al. (2017) [85] reported that the acclimatization of inoculum could enrich Firmicutes (90%), followed by Bacteroides (12%) and Cloacimonetes (11%). The abundance of these phyla and their corresponding genera confirmed their preeminence in increasing the efficacy of acidogenic fermentation.
AM has been used as an inoculum for acidogenic fermentation. For instance, Tampio et al. (2019) [4] achieved a VFA production yield of 0.43 g-VFAs/g-VS added from acidogenic fermentation of FW with cow manure as an inoculum. Similar results were obtained by Jomnonkhaow et al. (2021) [19], who found that cow manure could be used as both substrate and inoculum to provide the maximum VFA production yield of 0.4 g-VFAs/g-VSadded. Furthermore, they found that cow manure has a high buffering capacity, leading to a very stable fermentation process, although the pH was not controlled throughout the process.
4.4. HRT
HRT can be described as the average time organic matter remains inside the reactor [56]. HRT is the inverse of the dilution rate (D), which is directly proportional to the maximum microbial growth rate (μmax). Fast-growing microbes can be actively sustained in bioreactors at high D or short HRTs [51]. In batch fermentation, the maximum VFA yield is typically achieved in 4–10 days [86,87,88]. Previous studies that conducted acidogenic fermentation in a continuous and semi-continuous mode showed that 3 days of HRT is required to achieve the maximum VFA yield, indicating that a short HRT can enhance acidogenic fermentation. Conversely, the highest VFA concentration (43.8 g-VFAs) was obtained at an HRT of 41 days when brewer’s spent grain (without any pretreatment) was used as substrate in long-term fed-batch acidogenic fermentation [89]. This suggests that a high HRT is advantageous for acidogenic fermentation of recalcitrant substrates because it allows sufficient contact time between microorganisms and the substrate. However, a very high HRT promotes the activity of slow-growing methanogens. An HRT of approximately 8–20 days is typically needed as a minimum HRT for methanogens. Thus, keeping HRT for a shorter period would drastically wash out methanogens [51,90]. Additionally, Strazzera et al. (2018) [74] revealed that acetic acid was the main product under a short HRT, whereas propionic acid was the predominant product under a higher HRT. This depended essentially on the substrates; some studies demonstrated that high molecular VFAs, such as valeric acid and caproic acid, were increased with an increase in the protein content of the substrate [48,56,91]. Previous studies found that the HRT varied based on the type of substrate and operating conditions. Moreover, it should be noted that a higher HRT requires a larger reactor, which is linked to higher functional costs [90].
4.5. OLR
OLR is the amount of organic matter fed per working volume of the reactor per day [8,77]. According to the literature, the recommended OLR for acidogenic fermentation ranges from 2 to 8 g-VS/L·day [77]. Additionally, the TS content increased with an increase in OLR, resulting in a higher organic content that could be converted into VFAs. However, a higher TS content beyond a certain level inhibited further degradation of organic matter, leading to a lower yield of VFAs [51,92]. It has been reported that increasing the OLR leads to a higher accumulation of VFAs and a subsequent decrease in pH, resulting in a low VFA production rate and yield [93]. Lukitawesa et al. (2020) [20] studied the effect of OLR on the acidogenic fermentation of pretreated citrus waste in a semi-continuous reactor. They observed that a maximum yield of VFAs of 0.84 g-VFAs/g-VS was obtained at an OLR of 4 g-VS/L·day. A further increase in OLR of up to 8 g-VS/L·day caused a sharp decrease in the yield of VFAs to 0.55 g-VFAs/g-VS. This indicates that an increase in OLR may cause the medium viscosity to increase, reducing heat and mass transfers, and consequently, the substrate conversion into VFAs [19,51]
Additionally, previous research has reported that the total ammonia nitrogen concentration gradually increases with an increase in OLR because of the biodegradation of nitrogenous organic matter by Stickland fermentation [48]. As long as the concentration of ammonia nitrogen does not exceed the inhibition limit according to the literature (above 3 g/L ammonia nitrogen), ammonia nitrogen can be neutralized with VFAs, allowing pH to be stabilized during fermentation [94].
4.6. S:I Ratio
In batch fermentation systems, the conversion rate and efficiency of organic waste into VFAs largely depend on the OLR, which affects the S:I ratio. According to the literature, an S:I ratio from 1:1 to 1:6 can lead to higher VFA yields for acidogenic fermentation of protein-rich and less degradable substrates, such as SS and pig manure. Holliger et al. (2016) [95] recommended that an S:I ratio of less than or equal to 1:1 should be applied to a less biodegradable substrate. Furthermore, it has been reported that increasing the S:I ratio can lead to the accumulation of VFAs and ammonium in the reactor [96]. However, Yin et al. (2021) [53] investigated the effect of the S:I ratio on VFA production from chick manure by acidogenic fermentation. They observed that the VFA yield increased with an increasing S:I ratio. The maximum VFA yield of 0.53 g-VFAs/g-VS was achieved at S:I ratio of 6:1. This might be attributed to an increase in organic matter and the fact that AM contains the preferred inoculum for VFA production and provides alkalinity to keep the system stable, which are all key factors of VFA production from chick manure. Conversely, previous studies using different kinds of substrates showed that the highest VFA production yield could be achieved by controlling the S:I ratio from 1:1 to 6:1 by using easily biodegradable materials, such as FW as the substrate [20,49]. Li et al. (2018) [93] observed that the maximum VFA production from the co-digestion of tomato residue, dairy manure, and corn stover was obtained in the experiment using an S:I ratio of 6:1, and VFA production decreased as the S:I ratio decreased from 6:1 to 4:1. Therefore, the appropriate S:I ratio depends not only on the type of substrate but also on the type of inoculum and the operational conditions.
4.7. Oxidation-Reduction Potential (ORP)
Several studies have reported the positive effects of low oxygen concentration (micro-oxygenation) in the bioreactor’s headspace on hydrolysis of acidogenic fermentation because of a significantly more diverse microbial community [20,79,97]. Compared to anaerobic systems, micro-oxygenation acidogenic fermentation contained a higher proportion of facultative acidogens, such as Firmicutes and Proteobacteria. According to the present studies, micro oxygenation conditions allow acidogenic reactors to metabolize various substrates, resulting in increased sCOD and VFA production [97,98]. In general, the oxygen content can be accurately determined by the ORP. ORP is a measure of intracellular metabolism regulated by electron transfer and redox balance [83]. The optimal ORP for acidogenic fermentation should be between −100 mV and −250 mV, whereas the completely anaerobic condition of ORP varied between −200 mV and −300 mV [99].
The effects of oxygen on acidogenic fermentation are evaluated. For example, the interaction effect of the addition of BES, S:I ratio and initial oxygen concentration on VFA production from FW was investigated by Lukitawesa et al. (2020) [3]. They found that VFAs produced in the reactors with low substrate loading (S:I 1:1), the addition of BES, and the presence of initial oxygen did not affect the VFA production yield. However, the presence of initial oxygen has affected VFA composition. The findings of Sawatdeenarunat et al. (2017) [98] showed that an oxygen concentration of 15 g/L, using anaerobic granular sludge as inoculum, yielded the highest VFA production yield of 107.25 mg/g-VSadded from Napier grass. The authors further reported that micro-aeration influenced VFA composition and inhibited methanogenesis, enhancing the VFA yield. Additionally, micro-aeration should be applied at the early stage of acidogenic fermentation to prevent the accumulation of lactic acid, which could lead to failure of the system [98]. The optimal aeration intensity is different because of the application of intermittent oxygen. Xu et al. (2014) [99] found that an optimal micro-aeration intensity of 258 L-air/kg-TS·day in a 3 L leach bed reactor could promote hydrolysis of the substrate, resulting in a 3-fold increase in VFAs produced. Similar findings were documented by Lim and Wang (2013) [100] and Johansan and Bakke (2006) [101], who observed that the optimal aeration intensity was 57 and 33 L-air/kg-TS·day, respectively. Therefore, a low oxygen concentration can enhance VFA production.
5. Membrane-Based VFA Recovery
Recovery of VFAs prevents the inhibition of microbes, leading to the stability and high production of VFAs by continuously removing VFAs from the reactor [14,19,21]. However, VFA recovery from fermented effluent is a challenging process because of the low concentration of VFAs, the complexity of the mixture, the physicochemical nature of the fermented effluent, and processing cost, which accounts for approximately about 30–40% of the total cost, all of which contribute to the VFA recovery processes [13]. VFA recovery can be broadly classified into non-membrane-based and membrane-based recovery. Previous studies have provided a complete explanation of the various strategies for recovery of VFAs by conventional non-membrane-based methods, including solvent extraction, distillation, chemical precipitation, adsorption, and ion exchange [7,14,102]. However, current researchers have focused on applying membrane technology to achieve effective VFA recovery strategies because it could reduce the number of recovery steps while shortening the residence time, resulting in a more economical process [14,103]. Table 3 summarizes the advantages and disadvantages of membrane-based techniques for VFA recovery from acidogenic effluents.

Table 3.
Membrane-based VFA recovery techniques.
5.1. Membrane Contactor
The membrane contactor uses a hydrophobic porous membrane to separate the two aqueous phases on opposite sides of the membrane, avoiding mixing (Figure 2) [14,106]. The commonly used polymers in membrane contactors, selected based on their properties, such as mechanical stability, chemical resistance, and hydrophilic or hydrophobic characteristics, are polypropylene (PP), polyvinylidene fluoride (PVDF), and polytetrafluoroethylene (PTFE) [107]. The transmembrane pressure is determined by the partial pressure or concentration difference between the two sides of the membrane. In most membrane contactor processes, the driving force is created by the pH gradient between the two sides of the membrane. For example, Aydin et al. (2018) [18] recovered VFAs from three types of fermented effluent, including fermentation broth of organic waste, landfill leachate, and chicken manure digestate using a PTFE membrane contactor. Because of the partial pressure gradient on the two sides of the PTFE membrane, uncharged VFA molecules can migrate from the feed side to react with the base solution on the permeate side of the membrane, resulting in the formation of dissociated VFAs with high solubility and non-volatility. Furthermore, the incorporation of amine-based extractants, such as trioctylamine (TOA) and tridodecylamine (TDDA) into the PTFE membrane pores resulted in higher flux transfer and higher VFA recovery [18]. The results showed that the acetic acid recoveries from the fermentation broth of organic waste and landfill leachate were higher than 45%, regardless of the initial acetic acid concentration. However, 80–95% recovery of propionic, butyric, valeric, and caproic acids from the fermentation broth of organic waste and landfill leachate was achieved using the PTFE-TOA membrane. Although the highest caproic acid concentration was observed in the chicken manure digestate, the lowest recovery of valeric and caproic acids was achieved. This could be caused by the high solids and ion content of the chicken manure digestate compared to the other two types of fermented effluent. These results were corroborated by the results of Angenent et al. (2016) [108], in which the VFA selectivity of the membrane contactor process could be improved by filling with a specific extractant. They found that a TOA-filled membrane was highly selective for valeric and caproic acids, moderately selective for butyric acid, and least selective for acetic acid.
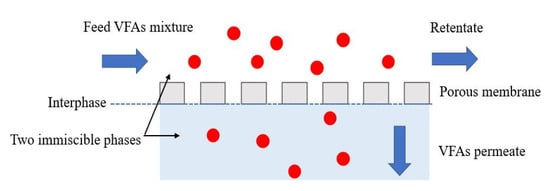
Figure 2.
Operating principle of VFA recovery by membrane contactor.
The main drawback of using extractants is their high cost. Thus, recent research has focused on applying silicone membranes filled with water as an extractant for VFA and alcohol recovery. The advantages of using water are its cost-effectiveness and recovery of VFAs in an undissociated form, which does not require the counter ion removal process. Additionally, fouling did not occur on the silicone surface, suggesting the feasibility of the membrane contactor for VFA recovery from a real fermenter [109].
5.2. Electrodialysis
Electrodialysis is a mature process applied in the early stages of industrial development on an industrial scale to produce drinking water. However, more recently, the use of electrodialysis for the recovery of VFAs has gained increased interest [110]. Electrodialysis is a membrane separation process used to transport ionic solutes from one solution to another through anion and cation-exchange membranes separated by a spacer gasket in an alternating pattern forming individual cells between two electrodes [7], as illustrated in Figure 3.
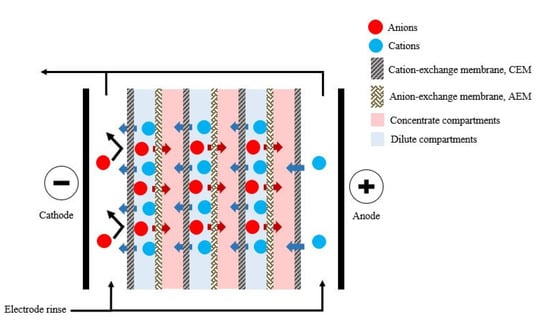
Figure 3.
Schematic representation of electrodialysis.
Because VFAs can exist in an ionized form in solutions with a pH greater than the pKa of each acid, this process can also be used for VFA recovery. Jones et al. (2015) [111] stated that conventional electrodialysis applied with 18 V of electrical potential could recover up to 99% of VFAs from a model solution containing 1.2 g/L of each VFA within 60 min electrodialysis operation. Jones et al. (2017) [112] used conventional electrodialysis under the same operating conditions as Jones et al. (2015) [111] to alleviate inhibitory VFAs from a continuously fed sucrose reactor to increase biohydrogen production yield. The fermentation broth was pretreated by centrifugation and filtration through a microfiltration membrane to separate the solid particles. The filtrate was pumped through the diluted chamber of the electrodialysis stack to recover VFAs. The continually fed sucrose reactor was operated for 6 days with VFA recovery every 24 h, and 23 g of acetic acid and 14 g of butyric acid were recovered. The removal of VFAs increased the biohydrogen production yield by 3.75-fold and increased the carbohydrate utilization rate from 12% to 15%. This is consistent with the findings of Hassan et al. (2017) [21], who have combined the production of dark hydrogen with electrodialysis. Tao et al. (2016) [113] used a microfiltration system combined with electrodialysis to recover VFAs from a VFA production bioreactor. The VFA-rich fermentation broth was transferred through the microfiltration system before feeding it into the electrodialysis stack to prevent solid particles from interfering with electrodialysis operation and to reducing the fouling tendency of the downstream process [102]. The authors also observed that, even though electrodialysis concentrated approximately 90% of the VFAs in the permeate, each acid behaved differently. The transport efficiency of small molecular weight acids was greater than that of larger molecular weight acids [113].
In addition to the microfiltration system, the PTFE membrane integration and electrodialysis were previously studied by Brown et al. (2020) [22] to overcome the challenge wherein electrodialysis does not selectively recover VFAs alone, leading to loss of nutrients from the fermentation broth and, therefore, a lower rate of microbial growth and VFA production. The process shows that up to 98% of the total VFA recovery was achieved with the PTFE and electrodialysis system, and the essential nutrients, such as ammonium phosphate, and nitrate, remained in the reactor.
5.3. Anaerobic Membrane Bioreactor
Anaerobic membrane technology is a hybrid system in which membranes are combined with an anaerobic bioreactor (Figure 4) [114]. Anaerobic membrane bioreactors have emerged as a promising alternative to VFA recovery technologies because of their low sludge generation and energy requirements. Moreover, it has the benefit of reducing the operating space and number of operational units [114]. Integrating a membrane recovery system with an acidogenic fermentation bioreactor would decrease cell washout from the bioreactor and the inhibition effect of the acids, thereby improving biodegradation [25].
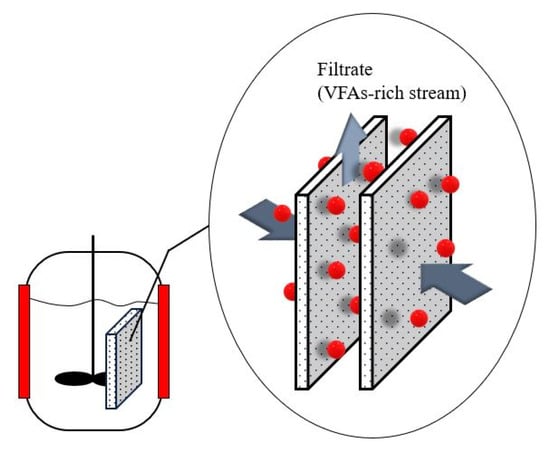
Figure 4.
Anaerobic membrane bioreactor.
In general, the efficiency and economics of membrane filtration are dependent on the membrane module design (tubular, plate and frame, rotary disk, or hollow fiber), pore size of the membrane (microfiltration, ultrafiltration, nanofiltration, or reverse osmosis), membrane material (organic, inorganic, metallic, hydrophobicity, or hydrophilicity), filtration mode (dead-end or cross-flow filtration), operating conditions (flux or hydraulic recirculation), and characteristics of sludge (biomass concentration, pH, EPS, or soluble microbial product) [115]. Based on published research, the most commonly used membranes for VFA recovery from organic waste fermentation broth are polyethersulfone (PES) [10,19,25,116], polyvinylidene fluoride (PVDF) [117,118,119]; however, chlorinated polyethylene (CPE) [120], polypropylene (PP) [20,121], and ceramic [122] microfiltration membranes have seldom been employed.
Long-term VFA production and recovery from acidogenic fermentation of cow manure were investigated by Jomnonkhaow et al. (2020) [19]. The authors observed that the maximum VFA yield of 0.41 g-VFAs/g-VS was obtained from heat-treated cow manure without pH control or inoculum addition. Furthermore, anaerobic membrane bioreactors can be operated at very high amounts of TSS ranging from 15 to 55 g-total suspended solid (TSS)/L by employing a routine reactor draining, backwashing, gas sparging, and chemical membrane cleaning to control the amount of TSS in the reactor. Consistent with the study by Parchami et al. (2020) [5], they observed that even though the anaerobic membrane bioreactor was operated at a maximum suspended solid loading of 32 g/L, minimal filtration flux deterioration was observed. Other studies have shown that anaerobic membrane bioreactors have the potential to operate at high organic loading with a complex medium for a long period [25,118].
5.4. Membrane Pervaporation
The membrane pervaporation process relies on diffusing different components in the feed mixture through a dense membrane [14,107]. The term pervaporation represents the combination of the “permeation” and “evaporation” processes [105]. Pervaporation membranes can also be broadly categorized into two groups based on the target molecule to be recovered: hydrophilic pervaporation membranes ensure rapid water transport, allowing for effective dehydration, whereas organophilic pervaporation membranes provide better transport of organic compounds, making them suitable for VFA recovery [106]. The hydrophilic membrane is made of polyvinyl alcohol (PVA) [123] and sulfonated polybenzimidazole (SPBI) [124]. In contrast, the most commonly used materials for the organophilic membrane are polydimethylsiloxane (PDMS) [125] polyether block amides (PEBA) [105], and hydrophobic zeolites [126]. The pervaporation process is also more suitable for separating VFAs from water (azeotropic mixtures) compared with other separation methods [15]. Additionally, membrane pervaporation has no adverse effects on microorganisms in the fermentation broth and can be directly integrated with an acidogenic bioreactor to continuously remove inhibitory products [13]. The driving force in membrane pervaporation is the chemical potential gradient across the membrane, which can be created by applying a vacuum or gas purge on the permeate side to keep the permeate vapor pressure lower than the partial pressure of the feed liquid [14,107]. The membrane controls the selective transport of species from the feed-liquid mixture to the vapor-permeate (Figure 5). Mass transport of volatile substances in the pervaporation process follows four consecutive steps: (1) a volatile substance dissolves in the feed side, (2) sorption into a permeable-selective membrane, (3) diffusion through the bulk membrane, and (4) evaporation of the vapor phase on the permeate side [13,106].
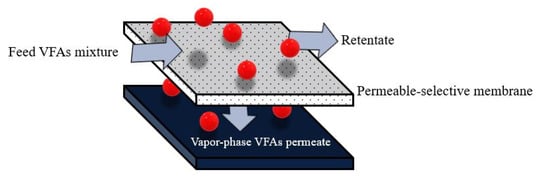
Figure 5.
Schematic diagram of membrane pervaporation.
Recent studies have enhanced the selectivity of VFA separation by increasing the hydrophobicity of membranes by the filling of various materials on the surface or inside the membrane. Choudhari et al. (2015) [105] attempted to recover butyric acid from anaerobic digestion effluent using a PEBA-graphene membrane composite via the pervaporation process. A comparison of the concentration of butyric acid in the feed (0.6%) with butyric acid in the permeate (11.4%) indicated that the PEBA-graphene composite could be used to concentrate butyric acid in anaerobic digestion effluent using the pervaporation technique. Furthermore, the authors reported that increasing graphene up to 0.75 wt% of the membrane matrix increased overall performance. Additionally, an increase in the feed pH decreased the recovery of butyric acid because of its higher solubility in water in its dissociated form.
6. Future Perspective
Over the last decade, organic waste has been considered the most promising source for anaerobic digestion processes. Previous research has focused on the production of hydrogen and methane from organic waste substrates. However, current research indicates that the highest profit from the anaerobic digestion process is VFAs produced during the acidogenic step. Compared to methane, VFA storage and transportation are simpler and safer. The market price of VFAs is tabulated in Table 4. The market size of VFAs was predicted to increase to 18,500 kTons in 2020 because of the economic growth [127]. Bastidas and Schmidt (2018) [128] confirmed this with a techno-economic analysis of acidogenic fermentation VFA production compared with anaerobic digestion methane production based on relevant data from the literature. They discovered that the production of VFAs by acidogenic fermentation of FW yielded a profit of 296 USD/ton-VS, which was better than the profit of 19 USD/ton-VS from anaerobic digestion, where the produced and upgraded methane was sold to a power grid. Consequently, acidogenic fermentation is more attractive than anaerobic digestion.

Table 4.
Market price of VFAs [129].
As a matter of fact that the concentration and distribution of VFAs are the result of acidogenic metabolic pathways, a good understanding of the VFA production pathways will provide guidelines for optimizing acidogenic product recovery and selective production of specific products. The influencing factors must be optimized to improve VFA production efficiency. Additionally, it is critical to consider the microbial community structure and its interactions with VFA metabolic production pathways.
VFAs need to be recovered to the maximum extent possible, while caution should be exercised to avoid incurring excessive costs that would make them economically unfeasible. Among the recovery strategies of VFAs, membrane-based VFA recovery seems to be the most promising strategy because it can alleviate the inhibition effects caused by VFA accumulation, reduce the number of operational units, and make the acidogenic fermentation process more stable, leading to higher VFA yields. However, some recovery processes, such as pressure-driven anaerobic membrane bioreactors and electrical-potential-driven electrodialysis, can be costly because of the high energy costs. The cost of the entire VFA production chain must be carefully considered. There are several possible ways to improve VFA recovery strategies, such as applying new methods, resolving membrane fouling, optimizing the VFA recovery process, membrane modification by applying alternative membrane materials, and large-scale experiments suggested for future studies. The overview of VFAs production and their recovery is illustrated in Figure 6.
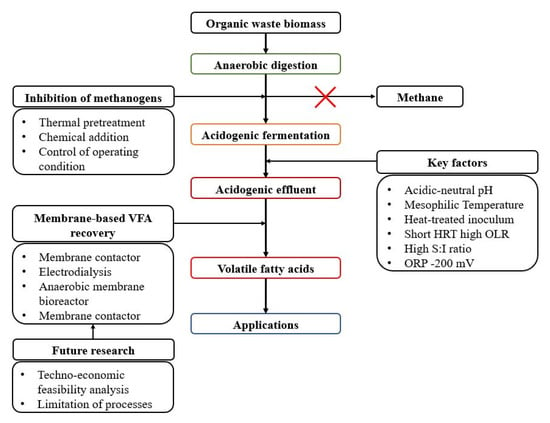
Figure 6.
Overview diagram of VFA production process.
7. Conclusions
Organic waste is abundant and rich in organic substances, making it a great resource for conversion into VFAs by acidogenic fermentation. The production of VFAs requires optimal conditions aiming to enhance VFAs’ production rate and yield. Acidogenic fermentation of organic waste favors an acidic-neutral pH range which stimulates VFAs’ production pathway. Moreover, operating factors such as temperatures and ORP should be maintained at a sufficient level for microbial growth. In addition, HRT and OLR are considered microbial growth-related factors that also play important roles in acidogenic fermentation. However, the characteristics of acidogenic effluent are complex making VFA recovery difficult, expensive and challenging. Membrane-based technology may progress the field of VFAs production and recovery. The economic point of view of VFA recovery from organic waste should be conducted to meet at the halfway point between economic benefits and environmental impacts. Moreover, the direct integration of membranes with bioreactors to recover VFAs is recommended for a thorough understanding of the limitations of the processes, which may promote industrial scale applicability.
Author Contributions
Wrote the manuscript with input from all authors, P.S.; edited the manuscript, S.S.; Conceptualization, supervised the project, edited the manuscript, A.R. All authors have read and agreed to the published version of the manuscript.
Funding
The present study is supported by TRF Senior Research Scholar (RTA628001). The scholarship for P.S. is provided by the Grant No. NRCT5-RGJ63003-059.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The author would like to thank the scholarship for PS from National Research Council of Thailand (NRCT) through the Royal Golden Jubilee Program (Grant NO. NRCT5-RGJ63003-059) and the financial support from Thailand Science Research and Innovation (TSRI) Senior Research Scholar (Grant No. RTA6280001), and Research and Graduate Studies, Khon Kaen University, Thailand.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, Q.; Li, H.; Feng, K.; Liu, J. Oriented fermentation of food waste towards high-value products: A review. Energies 2020, 13, 5638. [Google Scholar] [CrossRef]
- Sekoai, P.T.; Ghimire, A.; Ezeokoli, O.T.; Rao, S.; Ngan, W.Y.; Habimana, O.; Yao, Y.; Yang, P.; Yiu Fung, A.H.; Yoro, K.O.; et al. Valorization of volatile fatty acids from the dark fermentation waste streams-A promising pathway for a biorefinery concept. Renew. Sustain. Energy Rev. 2021, 143, 110971. [Google Scholar] [CrossRef]
- Lukitawesa; Patinvoh, R.J.; Millati, R.; Sárvári-Horváth, I.; Taherzadeh, M.J. Factors influencing volatile fatty acids production from food wastes via anaerobic digestion. Bioengineered 2020, 11, 39–52. [Google Scholar] [CrossRef] [Green Version]
- Tampio, E.A.; Blasco, L.; Vainio, M.M.; Kahala, M.M.; Rasi, S.E. Volatile fatty acids (VFAs) and methane from food waste and cow slurry: Comparison of biogas and VFA fermentation processes. Glob. Chang. Biol. Bioenergy 2019, 11, 72–84. [Google Scholar] [CrossRef]
- Parchami, M.; Wainaina, S.; Mahboubi, A.; I’Ons, D.; Taherzadeh, M.J. MBR-assisted VFAs production from excess sewage sludge and food waste slurry for sustainable wastewater treatment. Appl. Sci. 2020, 10, 2921. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Wang, W.; O.-Thong, S.; Yang, Z.; Zhang, S.; Liu, G.; Luo, G. Microbial insights of enhanced anaerobic conversion of syngas into volatile fatty acids by co-fermentation with carbohydrate-rich synthetic wastewater. Biotechnol. Biofuels 2020, 13. [Google Scholar] [CrossRef] [PubMed]
- Atasoy, M.; Owusu-Agyeman, I.; Plaza, E.; Cetecioglu, Z. Bio-based volatile fatty acid production and recovery from waste streams: Current status and future challenges. Bioresour. Technol. 2018, 268, 773–786. [Google Scholar] [CrossRef]
- Ramos-Suarez, M.; Zhang, Y.; Outram, V. Current perspectives on acidogenic fermentation to produce volatile fatty acids from waste. Rev. Environ. Sci. Biotechnol. 2021, 1–40. [Google Scholar] [CrossRef]
- Al Battashi, H.; Al-Kindi, S.; Gupta, V.K.; Sivakumar, N. Polyhydroxyalkanoate (PHA) production using volatile fatty acids derived from the anaerobic digestion of waste paper. J. Polym. Environ. 2021, 29, 250–259. [Google Scholar] [CrossRef]
- Patel, A.; Mahboubi, A.; Horváth, I.S.; Taherzadeh, M.J.; Rova, U.; Christakopoulos, P.; Matsakas, L. Volatile fatty acids (VFAs) generated by anaerobic digestion serve as feedstock for freshwater and marine oleaginous microorganisms to produce biodiesel and added-value compounds. Front. Microbiol. 2021, 12, 72. [Google Scholar] [CrossRef]
- Menzel, T.; Neubauer, P.; Stefan, J. Role of microbial hydrolysis in anaerobic digestion. Energies 2020, 13, 5555. [Google Scholar] [CrossRef]
- Castilla-Archilla, J.; Papirio, S.; Lens, P. Two step process for volatile fatty acid production from brewery spent grain: Hydrolysis and direct acidogenic fermentation using anaerobic granular sludge. Process. Biochem. 2021, 100, 272–283. [Google Scholar] [CrossRef]
- Yesil, H.; Taner, H.; Ugur Nigiz, F.; Hilmioglu, N.; Tugtas, A.E. Pervaporative separation of mixed volatile fatty acids: A study towards integrated VFA production and separation. Waste Biomass Valorization 2020, 11, 1737–1753. [Google Scholar] [CrossRef]
- Aghapour Aktij, S.; Zirehpour, A.; Mollahosseini, A.; Taherzadeh, M.J.; Tiraferri, A.; Rahimpour, A. Feasibility of membrane processes for the recovery and purification of bio-based volatile fatty acids: A comprehensive review. J. Ind. Eng. Chem. 2020, 81, 24–40. [Google Scholar] [CrossRef]
- Reyhanitash, E.; Fufachev, E.; van Munster, K.D.; van Beek, M.B.M.; Sprakel, L.M.J.; Edelijn, C.N.; Weckhuysen, B.M.; Kersten, S.R.A.; Bruijnincx, P.C.A.; Schuur, B. Recovery and conversion of acetic acid from a phosphonium phosphinate ionic liquid to enable valorization of fermented wastewater. Green Chem. 2019, 21, 2023–2034. [Google Scholar] [CrossRef] [Green Version]
- Eregowda, T.; Rene, E.R.; Rintala, J.; Lens, P.N.L. Volatile fatty acid adsorption on anion exchange resins: Kinetics and selective recovery of acetic acid. Sep. Sci. Technol. 2020, 55, 1449–1461. [Google Scholar] [CrossRef]
- Talebi, A.; Razali, Y.S.; Ismail, N.; Rafatullah, M.; Azan Tajarudin, H. Selective adsorption and recovery of volatile fatty acids from fermented landfill leachate by activated carbon process. Sci. Total Environ. 2020, 707, 134533. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Yesil, H.; Tugtas, A.E. Recovery of mixed volatile fatty acids from anaerobically fermented organic wastes by vapor permeation membrane contactors. Bioresour. Technol. 2018, 250, 548–555. [Google Scholar] [CrossRef]
- Jomnonkhaow, U.; Uwineza, C.; Mahboubi, A.; Wainaina, S.; Reungsang, A.; Taherzadeh, M.J. Membrane bioreactor-assisted volatile fatty acids production and in situ recovery from cow manure. Bioresour. Technol. 2021, 321, 124456. [Google Scholar] [CrossRef]
- Lukitawesa; Eryildiz, B.; Mahboubi, A.; Millati, R.; Taherzadeh, M.J. Semi-continuous production of volatile fatty acids from citrus waste using membrane bioreactors. Innov. Food Sci. Emerg. Technol. 2021, 67, 102545. [Google Scholar] [CrossRef]
- Hassan, G.K.; Massanet-Nicolau, J.; Dinsdale, R.; Jones, R.J.; Abo-Aly, M.M.; El-Gohary, F.A.; Guwy, A. A novel method for increasing biohydrogen production from food waste using electrodialysis. Int. J. Hydrogen Energy 2019, 44, 14715–14720. [Google Scholar] [CrossRef]
- Chalmers Brown, R.; Tuffou, R.; Massanet Nicolau, J.; Dinsdale, R.; Guwy, A. Overcoming nutrient loss during volatile fatty acid recovery from fermentation media by addition of electrodialysis to a polytetrafluoroethylene membrane stack. Bioresour. Technol. 2020, 301, 122543. [Google Scholar] [CrossRef] [PubMed]
- Argun, H.; Liz, P.G.; Karapinar, I. Biohydrogen production potential of different biomass sources. In Biohydrogen Production: Sustainability of Current Technology and Future Perspective; Singh, A., Rathore, D., Eds.; Springer: New Delhi, India, 2016; pp. 11–48. [Google Scholar] [CrossRef]
- Ghimire, A.; Frunzo, L.; Pirozzi, F.; Trably, E.; Escudie, R.; Lens, P.N.L.; Esposito, G. A review on dark fermentative biohydrogen production from organic biomass: Process parameters and use of by-products. Appl. Energy 2015, 144, 73–95. [Google Scholar] [CrossRef]
- Wainaina, S.; Lukitawesa; Kumar Awasthi, M.; Taherzadeh, M.J. Bioengineering of anaerobic digestion for volatile fatty acids, hydrogen or methane production: A critical review. Bioengineered 2019, 10, 437–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rombouts, J.L.; Kranendonk, E.M.M.; Regueira, A.; Weissbrodt, D.G.; Kleerebezem, R.; van Loosdrecht, M.C.M. Selecting for lactic acid producing and utilising bacteria in anaerobic enrichment cultures. Biotechnol. Bioeng. 2020, 117, 1281–1293. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Pang, Y.; Yang, Q.; Wang, D.; Li, X.; Lei, M.; Huang, Q. A critical review of volatile fatty acids produced from waste activated sludge: Enhanced strategies and its applications. Environ. Sci. Pollut. Res. 2019, 26, 13984–13998. [Google Scholar] [CrossRef]
- Gustavsson, J.; Bos-Brouwers, H.; Timmermans, T.; Hansen, O.J.; Møller, H.; Anderson, G.; O’connor, C.; Soethoudt, H.; Quested, T.; Easteal, S.; et al. FUSIONS Definitional Framework for Food Waste-Full Report. Project Report Fusions. 2014. Available online: https://www.eu-fusions.org/index.php/publications (accessed on 12 August 2021).
- Hoover Darby and Moreno Laura. Estimating Quantities and Types of Food Waste at the City Level. 2017. Available online: https://furtherwithfood.org/resources/estimating-quantities-types-food-waste-city-level/ (accessed on 12 August 2021).
- Ho, K.S.; Chu, L.M. Characterization of food waste from different sources in Hong Kong. J. Air Waste Manag. Assoc. 2019, 69, 277–288. [Google Scholar] [CrossRef]
- Lee, W.S.; Chua, A.S.M.; Yeoh, H.K.; Ngoh, G.C. A review of the production and applications of waste-derived volatile fatty acids. Chem. Eng. J. 2014, 235, 83–99. [Google Scholar] [CrossRef]
- Fang, W.; Zhang, X.; Zhang, P.; Wan, J.; Guo, H.; Ghasimi, D.S.M.; Morera, X.C.; Zhang, T. Overview of key operation factors and strategies for improving fermentative volatile fatty acid production and product regulation from sewage sludge. J. Environ. Sci. China 2020, 87, 93–111. [Google Scholar] [CrossRef]
- Wei, L.; Zhu, F.; Li, Q.; Xue, C.; Xia, X.; Yu, H.; Zhao, Q.; Jiang, L.; Bai, S. Development, current state and future trends of sludge management in China: Based on exploratory data and CO2-equivaient emissions analysis. Environ. Int. 2020, 144, 106093. [Google Scholar] [CrossRef]
- Kolosionis, A.; Kastanaki, E.; Veksha, A.; Wang, H.; He, C.; Lisak, G.; Giannis, A. The effects of washing techniques on thermal combustion properties of sewage sludge chars. Int. J. Environ. Res. 2021, 15, 285–297. [Google Scholar] [CrossRef]
- Fu, Q.; Wang, D.; Li, X.; Yang, Q.; Xu, Q.; Ni, B.J.; Wang, Q.; Liu, X. Towards hydrogen production from waste activated sludge: Principles, challenges and perspectives. Renew. Sustain. Energy Rev. 2021, 13, 110283. [Google Scholar] [CrossRef]
- Wei, W.; Zhang, Y.T.; Huang, Q.S.; Ni, B.J. Polyethylene terephthalate microplastics affect hydrogen production from alkaline anaerobic fermentation of waste activated sludge through altering viability and activity of anaerobic microorganisms. Water Res. 2019, 163. [Google Scholar] [CrossRef]
- Huang, X.; Dong, W.; Wang, H.; Feng, Y.; Sun, F.; Zhou, T. Sludge alkaline fermentation enhanced anaerobic- multistage anaerobic/oxic (A-MAO) process to treat low C/N municipal wastewater: Nutrients removal and microbial metabolic characteristics. Bioresour. Technol. 2020, 302, 122583. [Google Scholar] [CrossRef] [PubMed]
- Zhen, G.; Lu, X.; Kato, H.; Zhao, Y.; Li, Y.-Y. Overview of pretreatment strategies for enhancing sewage sludge disintegration and subsequent anaerobic digestion: Current advances, full-scale application and future perspectives. Renew. Sustain. Energy Rev. 2017, 69, 559–577. [Google Scholar] [CrossRef]
- Kakar, F.l.; El Sayed, A.; Purohit, N.; Elbeshbishy, E. Volatile Fatty Acids and Biomethane Recovery from Thickened Waste Activated Sludge: Hydrothermal pretreatment’s retention time impact. Processes 2020, 8, 1580. [Google Scholar] [CrossRef]
- Shen, J.; Zhao, C.; Liu, Y.; Zhang, R.; Liu, G.; Chen, C. Biogas production from anaerobic co-digestion of durian shell with chicken, dairy, and pig manures. Energy Convers. Manag. 2019, 198, 110535. [Google Scholar] [CrossRef]
- Huang, W.; Huang, W.; Yuan, T.; Zhao, Z.; Cai, W.; Zhang, Z.; Lei, Z.; Feng, C. Volatile fatty acids (VFAs) production from swine manure through short-term dry anaerobic digestion and its separation from nitrogen and phosphorus resources in the digestate. Water Res. 2016, 90, 344–353. [Google Scholar] [CrossRef] [Green Version]
- Ai, P.; Zhang, X.; Dinamarca, C.; Elsayed, M.; Yu, L.; Xi, J.; Mei, Z. Different effects of ozone and aqueous ammonia in a combined pretreatment method on rice straw and dairy manure fiber for enhancing biomethane production. Bioresour. Technol. 2019, 282, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wang, C.; Sun, Y.; Luo, L.; Wong, J.W.C. Assessing the stability of co-digesting sewage sludge with pig manure under different mixing ratios. Waste Manag. 2020, 114, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Sun, C.; Liu, R.; Yellezuome, D.; Zhu, X.; Bai, R.; Liu, M.; Sun, M. Anaerobic co-digestion of corn stover and chicken manure using continuous stirred tank reactor: The effect of biochar addition and urea pretreatment. Bioresour. Technol. 2021, 31, 124197. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, N.; Ismail, M.-G.; Tinia, I. Pretreatment of lignocellulosic biomass from animal manure as a means of enhancing biogas production. Eng. Life Sci. 2015, 15, 733–742. [Google Scholar] [CrossRef]
- Cristina, G.-F.; Cristina, L.-C.; Pedro, G.-E. Different pretreatments for increasing the anaerobic biodegradability in swine manure. Bioresour. Technol. 2008, 99, 8710–8714. [Google Scholar] [CrossRef]
- Wu, Q.L.; Guo, W.Q.; Zheng, H.S.; Luo, H.C.; Feng, X.C.; Yin, R.L.; Ren, N.Q. Enhancement of volatile fatty acid production by co-fermentation of food waste and excess sludge without pH control: The mechanism and microbial community analyses. Bioresour. Technol. 2016, 216, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Owusu-Agyeman, I.; Plaza, E.; Cetecioglu, Z. Production of volatile fatty acids through co-digestion of sewage sludge and external organic waste: Effect of substrate proportions and long-term operation. Waste Manag. 2020, 112, 30–39. [Google Scholar] [CrossRef]
- Eryildiz, B.; Lukitawesa; Taherzadeh, M.J. Effect of pH, substrate loading, oxygen, and methanogens inhibitors on volatile fatty acid (VFA) production from citrus waste by anaerobic digestion. Bioresour. Technol. 2020, 302, 122800. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, Q.; Wang, X.; Zhou, X.; Zhu, J. Effect of pH on volatile fatty acid production from anaerobic digestion of potato peel waste. Bioresour. Technol. 2020, 316, 123851. [Google Scholar] [CrossRef]
- Wainaina, S.; Parchami, M.; Mahboubi, A.; Horváth, I.S.; Taherzadeh, M.J. Food waste-derived volatile fatty acids platform using an immersed membrane bioreactor. Bioresour. Technol. 2019, 274, 329–334. [Google Scholar] [CrossRef]
- Shewa, W.A.; Hussain, A.; Chandra, R.; Lee, J.; Saha, S.; Lee, H.S. Valorization of food waste and economical treatment: Effect of inoculation methods. J. Clean. Prod. 2020, 261, 121170. [Google Scholar] [CrossRef]
- Yin, D.M.; Mahboubi, A.; Wainaina, S.; Qiao, W.; Taherzadeh, M.J. The effect of mono- and multiple fermentation parameters on volatile fatty acids (VFAs) production from chicken manure via anaerobic digestion. Bioresour. Technol. 2021, 330, 124992. [Google Scholar] [CrossRef]
- Xin, X.; She, Y.; Hong, J. Insights into microbial interaction profiles contributing to volatile fatty acids production via acidogenic fermentation of waste activated sludge assisted by calcium oxide pretreatment. Bioresour. Technol. 2021, 320, 124287. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Chen, Y.; Shen, N.; Wang, G.; Wan, J.; Huang, J. The influence of a stepwise pH increase on volatile fatty acids production and phosphorus release during Al-waste activated sludge fermentation. Bioresour. Technol. 2021, 320, 124276. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Gutiérrez, M.; Garcia-Aguirre, J.; Irizar, I.; Aymerich, E. From sewage sludge and agri-food waste to VFA: Individual acid production potential and up-scaling. Waste Manag. 2018, 77, 203–212. [Google Scholar] [CrossRef]
- Kuruti, K.; Nakkasunchi, S.; Begum, S.; Juntupally, S.; Arelli, V.; Anupoju, G.R. Rapid generation of volatile fatty acids (VFA) through anaerobic acidification of livestock organic waste at low hydraulic residence time (HRT). Bioresour. Technol. 2017, 238, 188–193. [Google Scholar] [CrossRef]
- Esquivel-Elizondo, S.; Miceli, J.; Torres, C.I.; Krajmalnik-Brown, R. Impact of carbon monoxide partial pressures on methanogenesis and medium chain fatty acids production during ethanol fermentation. Biotechnol. Bioeng. 2018, 115, 341–350. [Google Scholar] [CrossRef]
- Bhatt, A.H.; Ren, Z.; Tao, L. Value proposition of untapped wet wastes: Carboxylic acid production through anaerobic digestion. iScience 2020, 23, 101221. [Google Scholar] [CrossRef] [PubMed]
- Carvalheira, M.; Duque, A.F. From Food Waste to Volatile Fatty Acids towards a Circular Economy. In Fermentation-Processes, Benefits and Risks; Laranjo, M., Ed.; IntechOpen: London, UK, 2021; pp. 1–21. [Google Scholar] [CrossRef]
- Feng, L.; Chen, Y.; Zheng, X. Enhancement of waste activated sludge protein conversion and volatile fatty acids accumulation during waste activated sludge anaerobic fermentation by carbohydrate substrate addition: The effect of pH. Environ. Sci. Technol. 2009, 43, 4373–4380. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Loh, K.C.; Dai, Y.; Tong, Y.W. Acidogenic fermentation of food waste for production of volatile fatty acids: Bacterial community analysis and semi-continuous operation. Waste Manag. 2020, 109, 75–84. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 28 May 2021).
- Feng, K.; Li, H.; Zheng, C. Shifting product spectrum by pH adjustment during long-term continuous anaerobic fermentation of food waste. Bioresour. Technol. 2018, 270, 180–188. [Google Scholar] [CrossRef]
- Al-Shorgani, N.K.N.; Kalil, M.S.; Yusoff, W.M.W.; Hamid, A.A. Impact of pH and butyric acid on butanol production during batch fermentation using a new local isolate of Clostridium acetobutylicum YM1. Saudi J. Biol. Sci. 2018, 25, 339–348. [Google Scholar] [CrossRef]
- Zhou, M.; Yan, B.; Wong, J.W.C.; Zhang, Y. Enhanced volatile fatty acids production from anaerobic fermentation of food waste: A mini-review focusing on acidogenic metabolic pathways. Bioresour. Technol. 2018, 248, 68–78. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, H.; Zheng, M.; Wang, K. Lactic acid production from acidogenic fermentation of fruit and vegetable wastes. Bioresour. Technol. 2015, 191, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhang, Y.; Li, K.; Wang, Q.; Gong, C.; Li, M. Volatile fatty acids production from food waste: Effects of pH, temperature, and organic loading rate. Bioresour. Technol. 2013, 143, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yin, J.; Shen, D.; Li, N. Anaerobic digestion of food waste for volatile fatty acids (VFAs) production with different types of inoculum: Effect of pH. Bioresour. Technol. 2014, 161, 395–401. [Google Scholar] [CrossRef]
- Cavinato, C.; Da Ros, C.; Pavan, P.; Bolzonella, D. Influence of temperature and hydraulic retention on the production of volatile fatty acids during anaerobic fermentation of cow manure and maize silage. Bioresour. Technol. 2017, 223, 59–64. [Google Scholar] [CrossRef]
- Khatami, K.; Atasoy, M.; Ludtke, M.; Baresel, C.; Eyice, Ö.; Cetecioglu, Z. Bioconversion of food waste to volatile fatty acids: Impact of microbial community, pH and retention time. Chemosphere 2021, 275, 129981. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, S.; Sarkar, O.; Swamy, Y.V.; Venkata Mohan, S. Acidogenic fermentation of food waste for volatile fatty acid production with co-generation of biohydrogen. Bioresour. Technol. 2015, 182, 103–113. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Zhang, Y.; Pan, Y.R.; Li, L.; Liu, J.; Butler, D. Stepwise pH control to promote synergy of chemical and biological processes for augmenting short-chain fatty acid production from anaerobic sludge fermentation. Water Res. 2019, 155, 193–203. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Wang, D.; Liu, Y.; Ngo, H.H.; Guo, W.; Yang, Q.; Li, X. Novel stepwise pH control strategy to improve short chain fatty acid production from sludge anaerobic fermentation. Bioresour. Technol. 2018, 249, 431–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, M.; Liu, J.; Ma, C.; Li, Y.Y.; Zou, L.; Qian, G.; Xu, Z.P. Improving the stability and efficiency of anaerobic digestion of food waste using additives: A critical review. J. Clean. Prod. 2018, 192, 316–326. [Google Scholar] [CrossRef] [Green Version]
- Gameiro, T.; Lopes, M.; Marinho, R.; Vergine, P.; Nadais, H.; Capela, I. Hydrolytic-Acidogenic Fermentation of Organic Solid Waste for Volatile Fatty Acids Production at Different Solids Concentrations and Alkalinity Addition. Water. Air. Soil Pollut. 2016, 227, 1–16. [Google Scholar] [CrossRef]
- Strazzera, G.; Battista, F.; Garcia, N.H.; Frison, N.; Bolzonella, D. Volatile fatty acids production from food wastes for biorefinery platforms: A review. J. Environ. Manag. 2018, 226, 278–288. [Google Scholar] [CrossRef]
- He, M.; Sun, Y.; Zou, D.; Yuan, H.; Zhu, B.; Li, X.; Pang, Y. Influence of Temperature on Hydrolysis Acidification of Food Waste. Procedia Environ. Sci. 2012, 16, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Komemoto, K.; Lim, Y.G.; Nagao, N.; Onoue, Y.; Niwa, C.; Toda, T. Effect of temperature on VFA’s and biogas production in anaerobic solubilization of food waste. Waste Manag. 2009, 29, 2950–2955. [Google Scholar] [CrossRef]
- Garcia-Aguirre, J.; Aymerich, E.; de Goñi, J.G.-M.; Esteban-Gutiérrez, M. Selective VFA production potential from organic waste streams: Assessing temperature and pH influence. Bioresour. Technol. 2017, 244, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Wang, H. Volatile fatty acids productions by mesophilic and thermophilic sludge fermentation: Biological responses to fermentation temperature. Bioresour. Technol. 2015, 175, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Chen, Y.; Zhou, Q. Waste activated sludge hydrolysis and short-chain fatty acids accumulation under mesophilic and thermophilic conditions: Effect of pH. Water Res. 2009, 43, 3735–3742. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Yu, X.; Zhang, Y.; Shen, D.; Wang, M.; Long, Y.; Chen, T. Enhancement of acidogenic fermentation for volatile fatty acid production from food waste: Effect of redox potential and inoculum. Bioresour. Technol. 2016, 216, 996–1003. [Google Scholar] [CrossRef]
- Blasco, L.; Kahala, M.; Tampio, E.; Vainio, M.; Ervasti, S.; Rasi, S. Effect of inoculum pretreatment on the composition of microbial communities in anaerobic digesters producing volatile fatty acids. Microorganisms 2020, 8, 581. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Li, X.; Zhang, T.; Li, J.; Lai, S.; Chen, H.; Gao, P.; Xue, G. High-rate lactic acid production from food waste and waste activated sludge via interactive control of pH adjustment and fermentation temperature. Chem. Eng. J. 2017, 328, 197–206. [Google Scholar] [CrossRef]
- Bhargav, M.; Singh, K. Volatile fatty acid production using heat-treated potato starch: Impact of hydraulic retention time. J. Environ. Eng. 2020, 146, 04020111. [Google Scholar] [CrossRef]
- Atasoy, M.; Eyice, O.; Cetecioglu, Z. A comprehensive study of volatile fatty acids production from batch reactor to anaerobic sequencing batch reactor by using cheese processing wastewater. Bioresour. Technol. 2020, 311, 123529. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Iglesias, R.; Campanaro, S.; Treu, L.; Kennes, C.; Veiga, M.C. Valorization of sewage sludge for volatile fatty acids production and role of microbiome on acidogenic fermentation. Bioresour. Technol. 2019, 291, 121817. [Google Scholar] [CrossRef]
- Ribau Teixeira, M.; Guarda, E.C.; Freitas, E.B.; Galinha, C.F.; Duque, A.F.; Reis, M.A.M. Valorization of raw brewers’ spent grain through the production of volatile fatty acids. N. Biotechnol. 2020, 57, 4–10. [Google Scholar] [CrossRef]
- Worwag, M.; Kwarciak-Kozłowska, A. Volatile fatty acid (VFA) yield from sludge anaerobic fermentation through a biotechnological approach. In Industrial and Municipal Sludge: Emerging Concerns and Scope for Resource Recovery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 681–703. [Google Scholar] [CrossRef]
- Shen, D.; Yin, J.; Yu, X.; Wang, M.; Long, Y.; Shentu, J.; Chen, T. Acidogenic fermentation characteristics of different types of protein-rich substrates in food waste to produce volatile fatty acids. Bioresour. Technol. 2017, 227, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Jiang, J.; Yan, F.; Xu, Y.; Yang, M.; Gao, Y.; Aihemaiti, A.; Zou, Q. Optimization of simultaneous production of volatile fatty acids and bio-hydrogen from food waste using response surface methodology. RSC Adv. 2018, 8, 10457–10464. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, Y.; Yu, Z.; Lu, J.; Li, D.; Wang, G.; Li, Y.; Wu, Y.; Li, S.; Xu, F.; et al. Effect of inoculum and substrate/inoculum ratio on the performance and methanogenic archaeal community structure in solid state anaerobic co-digestion of tomato residues with dairy manure and corn stover. Waste Manag. 2018, 81, 117–127. [Google Scholar] [CrossRef]
- Magdalena, J.A.; Greses, S.; González-Fernández, C. Impact of organic loading rate in volatile fatty acids production and population dynamics using microalgae biomass as substrate. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Holliger, C.; Alves, M.; Andrade, D.; Angelidaki, I.; Astals, S.; Baier, U.; Bougrier, C.; Buffière, P.; Carballa, M.; De Wilde, V.; et al. Towards a standardization of biomethane potential tests. Water Sci. Technol. 2016, 74, 2515–2522. [Google Scholar] [CrossRef]
- Latifi, P.; Karrabi, M.; Danesh, S. Anaerobic co-digestion of poultry slaughterhouse wastes with sewage sludge in batch-mode bioreactors (effect of inoculum-substrate ratio and total solids). Renew. Sustain. Energy Rev. 2019, 107, 288–296. [Google Scholar] [CrossRef]
- Lim, J.W.; Chiam, J.A.; Wang, J.Y. Microbial community structure reveals how microaeration improves fermentation during anaerobic co-digestion of brown water and food waste. Bioresour. Technol. 2014, 171, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Sawatdeenarunat, C.; Sung, S.; Khanal, S.K. Enhanced volatile fatty acids production during anaerobic digestion of lignocellulosic biomass via micro-oxygenation. Bioresour. Technol. 2017, 237, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Selvam, A.; Wong, J.W.C. Optimization of micro-aeration intensity in acidogenic reactor of a two-phase anaerobic digester treating food waste. Waste Manag. 2014, 34, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.W.; Wang, J.Y. Enhanced hydrolysis and methane yield by applying microaeration pretreatment to the anaerobic co-digestion of brown water and food waste. Waste Manag. 2013, 33, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Johansen, J.E.; Bakke, R. Enhancing hydrolysis with microaeration. Water Sci. Technol. 2006, 53, 43–50. [Google Scholar] [CrossRef]
- Lü, F.; Wang, Z.; Zhang, H.; Shao, L.; He, P. Anaerobic digestion of organic waste: Recovery of value-added and inhibitory compounds from liquid fraction of digestate. Bioresour. Technol. 2021, 333, 125196. [Google Scholar] [CrossRef]
- Arslan, D.; Zhang, Y.; Steinbusch, K.J.J.; Diels, L.; Hamelers, H.V.M.; Buisman, C.J.N.; De Wever, H. In-situ carboxylate recovery and simultaneous pH control with tailor-configured bipolar membrane electrodialysis during continuous mixed culture fermentation. Sep. Purif. Technol. 2017, 175, 27–35. [Google Scholar] [CrossRef]
- Pan, X.R.; Li, W.W.; Huang, L.; Liu, H.Q.; Wang, Y.K.; Geng, Y.K.; Kwan-Sing Lam, P.; Yu, H.Q. Recovery of high-concentration volatile fatty acids from wastewater using an acidogenesis-electrodialysis integrated system. Bioresour. Technol. 2018, 260, 61–67. [Google Scholar] [CrossRef]
- Choudhari, S.K.; Cerrone, F.; Woods, T.; Joyce, K.; O’Flaherty, V.; O’Connor, K.; Babu, R. Pervaporation separation of butyric acid from aqueous and anaerobic digestion (AD) solutions using PEBA based composite membranes. J. Ind. Eng. Chem. 2015, 23, 163–170. [Google Scholar] [CrossRef]
- Zhu, X.; Leininger, A.; Jassby, D.; Tsesmetzis, N.; Ren, Z.J. Will membranes break barriers on volatile fatty acid recovery from anaerobic digestion? ACS EST Eng. 2021, 1, 141–153. [Google Scholar] [CrossRef]
- Basile, A.; Galiano, F.; Santoro, S.; Figoli, A. Pervaporation and membrane contactors. In Membrane Reactor Engineering: Applications for a Greener Process. Industry; Wiley: Hoboken, NJ, USA, 2016; pp. 280–312. [Google Scholar] [CrossRef]
- Angenent, L.T.; Richter, H.; Buckel, W.; Spirito, C.M.; Steinbusch, K.J.J.; Plugge, C.M.; Strik, D.P.B.T.B.; Grootscholten, T.I.M.; Buisman, C.J.N.; Hamelers, H.V.M. Chain elongation with reactor microbiomes: Open-culture biotechnology to produce biochemicals. Environ. Sci. Technol. 2016, 50, 2796–2810. [Google Scholar] [CrossRef] [PubMed]
- Ravishankar, H.; Dessì, P.; Trudu, S.; Asunis, F.; Lens, P.N.L. Silicone membrane contactor for selective volatile fatty acid and alcohol separation. Process. Saf. Environ. Prot. 2021, 148, 125–136. [Google Scholar] [CrossRef]
- Scoma, A.; Varela-Corredor, F.; Bertin, L.; Gostoli, C.; Bandini, S. Recovery of VFAs from anaerobic digestion of dephenolized olive mill wastewaters by electrodialysis. Sep. Purif. Technol. 2016, 159, 81–91. [Google Scholar] [CrossRef]
- Jones, R.J.; Massanet-Nicolau, J.; Guwy, A.; Premier, G.C.; Dinsdale, R.M.; Reilly, M. Removal and recovery of inhibitory volatile fatty acids from mixed acid fermentations by conventional electrodialysis. Bioresour. Technol. 2015, 189, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.J.; Massanet-Nicolau, J.; Mulder, M.J.J.; Premier, G.; Dinsdale, R.; Guwy, A. Increased biohydrogen yields, volatile fatty acid production and substrate utilisation rates via the electrodialysis of a continually fed sucrose fermenter. Bioresour. Technol. 2017, 229, 46–52. [Google Scholar] [CrossRef]
- Tao, B.; Passanha, P.; Kumi, P.; Wilson, V.; Jones, D.; Esteves, S. Recovery and concentration of thermally hydrolysed waste activated sludge derived volatile fatty acids and nutrients by microfiltration, electrodialysis and struvite precipitation for polyhydroxyalkanoates production. Chem. Eng. J. 2016, 295, 11–19. [Google Scholar] [CrossRef]
- Maaz, M.; Yasin, M.; Aslam, M.; Kumar, G.; Atabani, A.E.; Idrees, M.; Anjum, F.; Jamil, F.; Ahmad, R.; Khan, A.L.; et al. Anaerobic membrane bioreactors for wastewater treatment: Novel configurations, fouling control and energy considerations. Bioresour. Technol. 2019, 283, 358–372. [Google Scholar] [CrossRef]
- Dvořák, L.; Gómez, M.; Dolina, J.; Černín, A. Anaerobic membrane bioreactors—A mini review with emphasis on industrial wastewater treatment: Applications, limitations and perspectives. Desalin. Water Treat. 2016, 57, 19062–19076. [Google Scholar] [CrossRef] [Green Version]
- Wainaina, S.; Awasthi, M.K.; Horváth, I.S.; Taherzadeh, M.J. Anaerobic digestion of food waste to volatile fatty acids and hydrogen at high organic loading rates in immersed membrane bioreactors. Renew. Energy 2020, 152, 1140–1148. [Google Scholar] [CrossRef]
- Trad, Z.; Akimbomi, J.; Vial, C.; Larroche, C.; Taherzadeh, M.J.; Fontaine, J.P. Development of a submerged anaerobic membrane bioreactor for concurrent extraction of volatile fatty acids and biohydrogen production. Bioresour. Technol. 2015, 196, 290–300. [Google Scholar] [CrossRef]
- Khan, M.A.; Ngo, H.H.; Guo, W.; Liu, Y.; Nghiem, L.D.; Chang, S.W.; Nguyen, D.D.; Zhang, S.; Luo, G.; Jia, H. Optimization of hydraulic retention time and organic loading rate for volatile fatty acid production from low strength wastewater in an anaerobic membrane bioreactor. Bioresour. Technol. 2019, 271, 100–108. [Google Scholar] [CrossRef]
- Ding, Y.; Guo, Z.; Hou, X.; Mei, J.; Liang, Z.; Li, Z.; Zhang, C.; Jin, C. Performance analysis for the anaerobic membrane bioreactor combined with the forward osmosis membrane bioreactor: Process conditions optimization, wastewater treatment and sludge characteristics. Water 2020, 12, 2958. [Google Scholar] [CrossRef]
- Chen, R.; Jiang, H.; Li, Y.Y. Caffeine degradation by methanogenesis: Efficiency in anaerobic membrane bioreactor and analysis of kinetic behavior. Chem. Eng. J. 2018, 334, 444–452. [Google Scholar] [CrossRef]
- Akram, A.; Stuckey, D.C. Flux and performance improvement in a submerged anaerobic membrane bioreactor (SAMBR) using powdered activated carbon (PAC). Process. Biochem. 2008, 43, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Aslam, M.; McCarty, P.L.; Shin, C.; Bae, J.; Kim, J. Low energy single-staged anaerobic fluidized bed ceramic membrane bioreactor (AFCMBR) for wastewater treatment. Bioresour. Technol. 2017, 240, 33–41. [Google Scholar] [CrossRef]
- Cai, W.; Cheng, X.; Chen, X.; Li, J.; Pei, J. Poly(vinyl alcohol)-modified membranes by Ti3C2Tx for ethanol dehydration via pervaporation. ACS Omega 2020, 5, 6277–6287. [Google Scholar] [CrossRef] [Green Version]
- Shi, G.M.; Chen, H.; Jean, Y.C.; Chung, T.S. Sorption, swelling, and free volume of polybenzimidazole (PBI) and PBI/zeolitic imidazolate framework (ZIF-8) nano-composite membranes for pervaporation. Polymer 2013, 54, 774–783. [Google Scholar] [CrossRef]
- Chen, T.; Xu, F.; Zhang, W.; Zhou, J.; Dong, W.; Jiang, Y.; Lu, J.; Fang, Y.; Jiang, M.; Xin, F. High butanol production from glycerol by using Clostridium sp. strain CT7 integrated with membrane assisted pervaporation. Bioresour. Technol. 2019, 288, 121530. [Google Scholar] [CrossRef]
- Bowen, T.C.; Li, S.; Noble, R.D.; Falconer, J.L. Driving force for pervaporation through zeolite membranes. J. Memb. Sci. 2003, 225, 165–176. [Google Scholar] [CrossRef]
- Veluswamy, G.K.; Shah, K.; Ball, A.S.; Guwy, A.J.; Dinsdale, R.M. A techno-economic case for volatile fatty acid production for increased sustainability in the wastewater treatment industry. Environ. Sci. Water Res. Technol. 2021, 7, 927–941. [Google Scholar] [CrossRef]
- Bastidas-Oyanedel, J.-R.; Schmidt, J.E. Increasing profits in food waste biorefinery—A techno-economic analysis. Energies 2018, 11, 1551. [Google Scholar] [CrossRef] [Green Version]
- Production of High Value Chemicals from Low Value Biodegradable Materials an Introduction to Earth Energy Renewables. Available online: https://www.iowabio.org/documents/filelibrary/images/iba_events/pfg_2017/Earth_Energy_Renewables_501D04B83E4F3.pdf (accessed on 28 May 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).