In Vitro Fermentation Characteristics and Methane Mitigation Responded to Flavonoid Extract Levels from Alternanthera sissoo and Dietary Ratios
Abstract
1. Introduction
2. Material and Methods
2.1. Experiment 1: Preliminary Study
2.1.1. Location and Plantation
2.1.2. Yield Collection and Chemical Analysis
2.1.3. In Vitro Degradability
Animal Ethics
Sample Processing
Ruminal Fluid Donors and Preparation of Inoculum
2.2. Experiment 2: In Vitro Gas Production Technique (Main Experiment)
2.2.1. Flavonoid Extract
2.2.2. Study Design and In Vitro Fermentation
2.2.3. In Vitro Gas Production Sampling and Analysis
3. Statistics
4. Results
4.1. Experiment 1: Preliminary Study
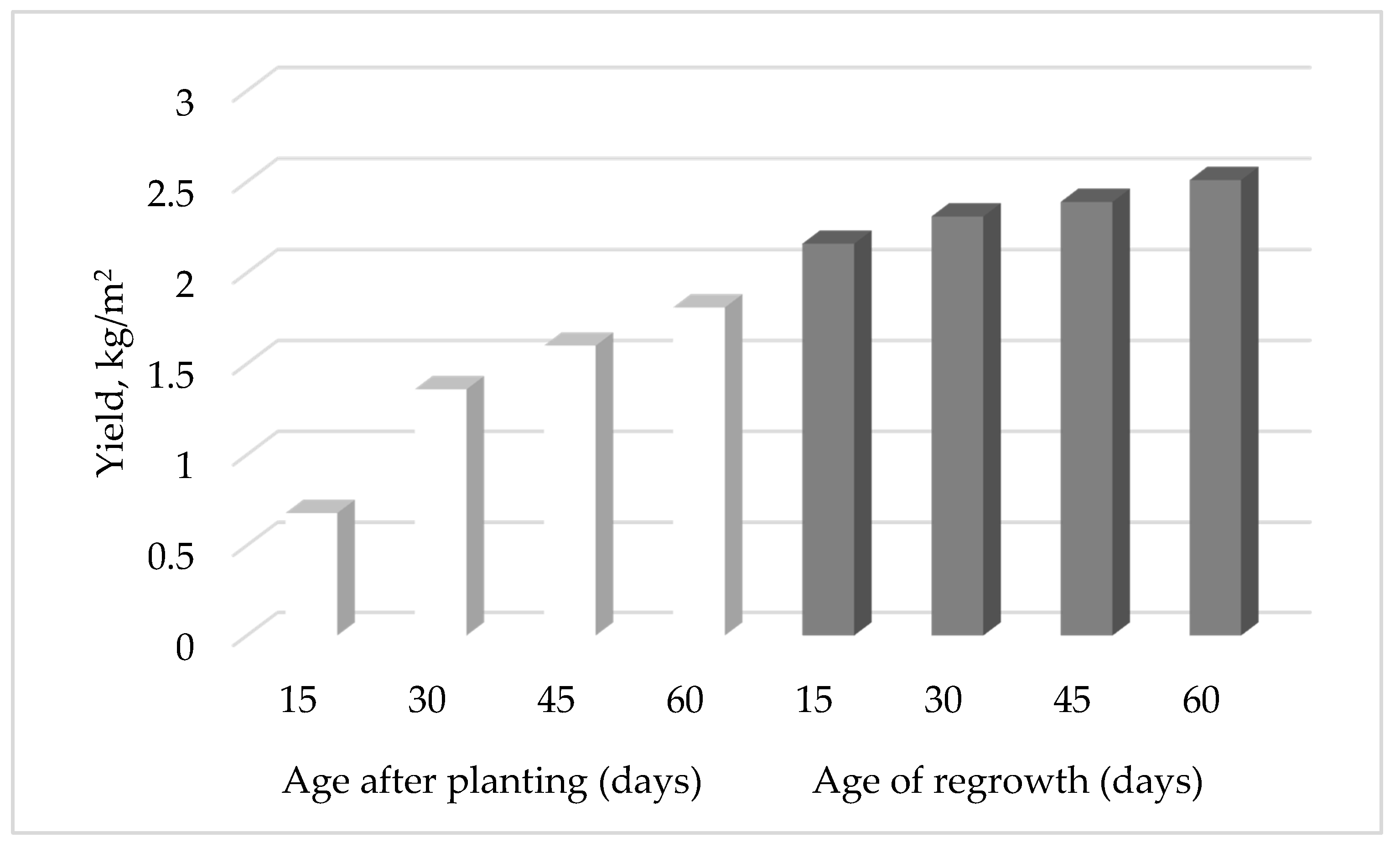
4.1.1. Production of Brazilian Spinach
4.1.2. Chemical Composition and Digestibility of Brazilian Spinach
4.2. Experiment 2: In Vitro Gas Technique (Main Experiment)
4.2.1. Gas Production Kinetics and In Vitro True Dry Matter Digestibility (DMDvt)
4.2.2. Rumen Fermentation, Volatile Fatty Acids (VFAs) and Protozoal Population
5. Discussion
5.1. Brazilian Spinach Yield
5.2. Chemical Composition and Digestibility of Brazilian Spinach
5.3. Gas Production Kinetics and In Vitro Dry Matter Degradability (DMDvt)
5.4. Rumen Fermentation, VFAs and Protozoal Population
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beauchemin, K.A.; Ungerfeld, E.; Eckard, R.J.; Wang, M. Review: Fifty years of research on rumen methanogenesis: Lessons learned and future challenges for mitigation. Animal 2020, 14, s2–s16. [Google Scholar] [CrossRef]
- Halmemies-Beauchet-Filleau, A.; Rinne, M.; Lamminen, M.; Mapato, C.; Ampapon, T.; Wanapat, M.; Vanhatalo, A. Review: Alternative and novel feeds for ruminants: Nutritive value, product quality and environmental aspects. Animal 2018, 12, s295–s309. [Google Scholar] [CrossRef]
- Steinfeld, H.; Gerber, P.; Wassenaar, T.D.; Castel, V.; Rosales, M.; Rosales, M.; de Haan, C. Livestock’s Long Shadow: Environmental Issues and Options; Food and Agriculture Organization: Rome, Italy, 2006; p. 377. [Google Scholar]
- Unnawong, N.; Cherdthong, A.; So, S. Influence of supplementing Sesbania grandiflora pod meal at two dietary crude protein levels on feed intake, fermentation characteristics, and methane mitigation in Thai purebred beef cattle. Vet. Sci. 2021, 8, 35. [Google Scholar] [CrossRef]
- Unnawong, N.; Cherdthong, A.; So, S. Crude saponin extract from Sesbania grandiflora (L.) Pers pod meal could modulate ruminal fermentation, and protein utilization, as well as mitigate methane production. Trop. Anim. Health Prod. 2021, 53, 1–9. [Google Scholar] [CrossRef]
- Hristov, A.N.; Ott, T.; Tricarico, J.; Rotz, A.; Waghorn, G.; Adesogan, A.; Dijkstra, J.; Montes, F.; Oh, J.; Kebreab, E.; et al. Mitigation of methane and nitrous oxide emissions from animal operations: III. A review of animal management mitigation options1. J. Anim. Sci. 2013, 91, 5095–5113. [Google Scholar] [CrossRef]
- Ku-Vera, J.C.; Jiménez-Ocampo, R.; Valencia-Salazar, S.S.; Montoya-Flores, M.D.; Molina-Botero, I.C.; Arango, J.; Gómez-Bravo, C.A.; Aguilar-Pérez, C.F.; Solorio-Sánchez, F.J. Role of secondary plant metabolites on enteric methane mitigation in ruminants. Front. Vet. Sci. 2020, 7, 584. [Google Scholar] [CrossRef] [PubMed]
- Manh, N.S.; Wanapat, M.; Uriyapongson, S.; Khejornsart, P.; Chanthakhoun, V. Effect of eucalyptus (Camaldulensis) leaf meal powder on rumen fermentation characteristics in cattle fed on rice straw. Afr. J. Agric. Res. 2012, 7, 2142–2148. [Google Scholar]
- Olagaray, K.E.; Bradford, B.J. Plant flavonoids to improve productivity of ruminants—A review. Anim. Feed Sci. Technol. 2019, 251, 21–36. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, W.; Tang, F.; Chen, X.; Ren, L. Antibacterial activities of flavonoids: Structure-activity relationship and mecha-nism. Curr. Med. Chem. 2015, 22, 132–149. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Wanapat, M.; Pakdee, P.; Pilajun, R.; Cherdthong, A. Effects of energy level and Leucaena leucocephala leaf meal as a protein source on rumen fermentation efficiency and digestibility in swamp buffalo. Anim. Feed Sci. Technol. 2012, 174, 131–139. [Google Scholar] [CrossRef]
- Oskoueian, E.; Abdullah, N.; Oskoueian, A. Effects of flavonoids on rumen fermentation activity, methane production, and microbial population. BioMed Res. Int. 2013, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sinz, S.; Kunz, C.; Liesegang, A.; Braun, U.; Marquardt, S.; Soliva, C.R.; Kreuzer, M. In vitro bioactivity of various pure fla-vonoids in ruminal fermentation, with special reference to methane formation. Czech J. Anim. Sci. 2018, 63, 293–304. [Google Scholar]
- Stoldt, A.-K.; Derno, M.; Daş, G.; Weitzel, J.M.; Wolffram, S.; Metges, C.C. Effects of rutin and buckwheat seeds on energy metabolism and methane production in dairy cows. J. Dairy Sci. 2016, 99, 2161–2168. [Google Scholar] [CrossRef] [PubMed]
- Tiveron, A.P.; Melo, P.S.; Bergamaschi, K.B.; Vieira, T.M.; Regitano-d’Arce, M.A.; Alencar, S.M. Antioxidant activity of Bra-zilian vegetables and its relation with phenolic composition. Int. J. Mol. Sci. 2012, 13, 8943–8957. [Google Scholar] [CrossRef] [PubMed]
- Manurung, H.; Kustiawan, W.; Kusuma, I.W.; Marjenah, M. Total flavonoid content and antioxidant activity of tabat Barito (Ficus deltoidea Jack) on different plant organs and ages. J. Med. Plants 2017, 5, 120–125. [Google Scholar]
- AOAC. Official Methods of Analysis, 16th ed.; AOAC: International—Association of Official Analytical Chemists: Gaithersburg, MD, USA, 1995. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Menke, K.H.; Steingass, H. Estimation of the energetic feed value obtained from chemical analysis and gas production using rumen fluid. Anim. Res. Devel. 1988, 28, 7–55. [Google Scholar]
- Tilley, J.M.A.; Terry, D.R. A two-stage technique for the in vitro digestion of forage crops. Grass Forage Sci. 1963, 18, 104–111. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 19th ed.; AOAC: International—Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Bohm, B.A.; Koupai-Abyazani, M.R. Flavonoids and condensed tannins from leaves of Hawaiian Vaccinium reticulatum and V. calycinum (Ericaceae). Pac. Sci. 1994, 48, 458–463. [Google Scholar]
- Ørskov, E.R.; McDonald, I. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J. Agric. Sci. 1979, 92, 499–503. [Google Scholar] [CrossRef]
- So, S.; Cherdthong, A.; Wanapat, M. Growth performances, nutrient digestibility, ruminal fermentation and energy partition of Thai native steers fed exclusive rice straw and fermented sugarcane bagasse with Lactobacillus, cellulase and molasses. J. Anim. Physiol. Anim. Nutr. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Galyean, M. Laboratory Procedures in Animal Nutrition Research; New Mexico State University: Las Cruces, NM, USA, 1989. [Google Scholar]
- Sittijunda, S.; Reungsang, A.; Sompong, O.T. Biohydrogen production from dual digestion pretreatment of poultry slaugh-terhouse sludge by anaerobic self-fermentation. Int. J. Hydrog. Energy 2010, 35, 13427–13434. [Google Scholar] [CrossRef]
- SAS (Statistical Analysis System). User’s Guide: Statistic, Version 9, 4th ed.; SAS Inst. Inc.: Cary, NC, USA, 2013. [Google Scholar]
- Crichton, N. Information point: Tukey multiple comparison test. J. Clin. Nurs. 1999, 8, 299–304. [Google Scholar]
- Martin, F.W.; Ruberté, R.M. Edible Leaves of the Tropics, 3rd ed.; Agency for International Development, Department of State: Washington, DC, USA; Agricultural Research Service, U.S. Department of Agriculture: Washington, DC, USA, 1975; p. 235. [Google Scholar]
- Lee, S.K.; Kader, A.A. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol. Technol. 2000, 20, 207–220. [Google Scholar] [CrossRef]
- Chiesa, A. Factors determining postharvest quality of leafy vegetables. In Proceedings of the International Conference on Quality in Chains: An Integrated View on Fruit and Vegetable Quality, Wageningen, The Netherlands, 6–9 July 2003; Tijskens, L.M.M., Vollebregt, H.M., Eds.; ISHS Acta Horticulturae: Leuven, Belgium, 2003. [Google Scholar]
- Baranga, D. Changes in chemical composition of food parts in the diet of Colobus monkeys. Ecology 1983, 64, 668–673. [Google Scholar] [CrossRef]
- Oloyede, F.M.; Oloyede, F.A.; Obuotor, E.M. Effect of plant maturity on the antioxidant profile of Amaranthus cruentus L. and Celosia argentea L. Bull. Env. Pharmacol. Life Sci. 2013, 2, 18–21. [Google Scholar]
- Mehta, D.; Belemkar, S. Pharmacological activity of Spinacia oleracea linn. Asian J. Pharm. Res. Devel. 2014, 2, 32–35. [Google Scholar]
- Bergquist, S.Å.; Gertsson, U.E.; Olsson, M.E. Influence of growth stage and postharvest storage on ascorbic acid and carotenoid content and visual quality of baby spinach (Spinacia oleracea L.). J. Sci. Food Agric. 2005, 86, 346–355. [Google Scholar] [CrossRef]
- Fallovo, C.; Rouphael, Y.; Rea, E.; Battistelli, A.; Colla, G. Nutrient solution concentration and growing season affect yield and quality of Lactuca sativa L. var. acephala in floating raft culture. J. Sci. Food Agric. 2009, 89, 1682–1689. [Google Scholar] [CrossRef]
- Fico, G.; Bilia, A.R.; Morelli, I.; Tomè, F. Flavonoid distribution in Pyracantha coccinea plants at different growth phases. Biochem. Syst. Ecol. 2000, 28, 673–678. [Google Scholar] [CrossRef]
- Lucier, G.; Allshouse, J.E.; Lin, B.H. Factors Affecting Spinach Consumption in the United States; US Department of Agriculture, Economic Research Service: Washington, DC, USA, 2004; p. 15. [Google Scholar]
- Bergquist, S.Å.; Gertsson, U.E.; Knuthsen, P.; Olsson, M.E. Flavonoids in Baby Spinach (Spinacia oleracea L.): Changes during plant growth and storage. J. Agric. Food Chem. 2005, 53, 9459–9464. [Google Scholar] [CrossRef]
- Khan, S.H.; Khan, A.G.; Sarwar, M.; Azim, A. Effect of maturity on production efficiency, nutritive value and in situ nutrients digestibility of three cereal fodders. Int. J. Agric. Res. 2007, 2, 900–906. [Google Scholar]
- Insoongnern, H.; Wachirapakorn, C. Effect of roughage to concentrate ratio and mineral salt block supplementation to optimize rumen fermentation in vitro gas technique. Khon Kaen Agri. J. 2017, 45, 48–52. [Google Scholar]
- Phesatcha, K.; Phesatcha, B.; Wanapat, M.; Cherdthong, A. Roughage to concentrate ratio and saccharomyces cerevisiae inclusion could modulate feed digestion and in vitro ruminal fermentation. Vet. Sci. 2020, 7, 151. [Google Scholar] [CrossRef] [PubMed]
- Nagadi, S.A. In vitro gas production, methane emission and rumen fermentation characteristics with increasing roughage to concentrate ratios. J. King Abdulaziz Univ. Meteorol. Environ. Arid. Land Agric. Sci. 2019, 28, 27–36. [Google Scholar] [CrossRef]
- So, S.; Wanapat, M.; Cherdthong, A. Effect of sugarcane bagasse as industrial by-products treated with Lactobacillus casei TH14, cellulase and molasses on feed utilization, ruminal ecology and milk production of mid-lactating Holstein Friesian cows. J. Sci. Food Agric. 2021. [Google Scholar] [CrossRef] [PubMed]
- Ogata, T.; Makino, H.; Ishizuka, N.; Iwamoto, E.; Masaki, T.; Kizaki, K.; Kim, Y.-H.; Sato, S. Long-term high-grain diet alters ruminal pH, fermentation, and epithelial transcriptomes, leading to restored mitochondrial oxidative phosphorylation in Japanese Black cattle. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Wanapat, M. Manipulation of cassava cultivation and utilization to improve protein to energy biomass for livestock feeding in the tropics. Asian Australas. J. Anim. Sci. 2003, 16, 463–472. [Google Scholar] [CrossRef]
- Cherdthong, A.; Wanapat, M.; Kongmun, P.; Pilajun, R.; Khejornsar, P. Rumen fermentation, microbial protein synthesis and cellulolytic bacterial population of swamp buffaloes as affected by roughage to concentrate ratio. J. Anim. Vet. Adv. 2010, 9, 1667–1675. [Google Scholar] [CrossRef]
- Aziz, H. Effect of roughage to concentrate ratio on digestibility in small ruminants. J. Anim. Poult. Prod. 2014, 5, 673–696. [Google Scholar] [CrossRef][Green Version]
- Dehority, B.A.; Orpin, C.G. Development of, and natural fluctuation in, rumen microbial population. In The Rumen Microbial Ecosystem; Hobson, P.N., Ed.; Elsevier Sci. Publ.: New York, NY, USA, 1988; pp. 151–183. [Google Scholar]
- Euge’ne, M.; Archimed, H.; Sauvant, D. Quantitiative meta-analysis on effects of defaunation of the rumen on growth, intake and digestion in ruminants. Livest. Prod. Sci. 2004, 85, 81–97. [Google Scholar] [CrossRef]
- Aziz, H.A.; Nassar, M.S.; Abd Badway, H.S.; Elrahaman, M.A. Rumen fermentations and rumen ciliate protozoa of goat kids fed diets with different concentrate: Roughage ratio. Egypt. J. Nutr. Feed. 2018, 21, 667–683. [Google Scholar] [CrossRef]
- Nijveldt, R.J.; van Nood, E.; van Hoorn, D.E.; Boelens, P.G.; van Norren, K.; van Leeuwen, P.A. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Kalantar, M. The importance of flavonoids in ruminant nutrition. Arch. Anim. Husb. Dairy Sci. 2018, 1, 1–4. [Google Scholar]
- Bergman, E.N.; Bergman, E.N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 1990, 70, 567–590. [Google Scholar] [CrossRef] [PubMed]
- Aluwong, T.; Kobo, P.I.; Abdullahi, A. Volatile fatty acids production in ruminants and the role of monocarboxylate trans-porters: A review. Afr. J. Biotechnol. 2010, 9, 6229–6232. [Google Scholar]
- Dieho, K.; Dijkstra, J.; Schonewille, J.T.; Bannink, A. Changes in ruminal volatile fatty acid production and absorption rate during the dry period and early lactation as affected by rate of increase of concentrate allowance. J. Dairy Sci. 2016, 99, 5370–5384. [Google Scholar] [CrossRef]
- So, S.; Cherdthong, A.; Wanapat, M. Improving sugarcane bagasse quality as ruminant feed with Lactobacillus, cellulase, and molasses. J. Anim. Sci. Technol. 2020, 62, 648–658. [Google Scholar] [CrossRef]
- So, S.; Cherdthong, A.; Wanapat, M.; Uriyapongson, S. Fermented sugarcane bagasse with Lactobacillus combined with cellulase and molasses promotes in vitro gas kinetics, degradability, and ruminal fermentation patterns compared to rice straw. Anim. Biotechnol. 2020, 1–12. [Google Scholar] [CrossRef]
- Saini, J.K.; Hundal, J.S.; Wadhwa, M.; Bakshi, M.P.S. Effect of roughage to concentrate ratio in the diet on the rumen envi-ronment and nutrient utilization in goat and sheep. Indian J. Anim. Nutr. 2012, 4, 333–338. [Google Scholar]
- McSweeney, C.S.; Palmer, B.; McNeill, D.M.; Krause, D.O. Microbial interactions with tannins: Nutritional consequences for ruminants. Anim. Feed Sci. Technol. 2001, 91, 83–93. [Google Scholar] [CrossRef]
- Tassone, S.; Fortina, R.; Peiretti, P.G. In vitro techniques using the daisyii incubator for the assessment of digestibility: A review. Animals. 2020, 10, 775. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Morales, E.; Rossi, G.; Cattin, M.; Jones, E.; Braganca, R.; Newbold, C.J. The effect of an isoflavonid-rich liquorice extract on fermentation, methanogenesis and the microbiome in the rumen simulation technique. FEMS Microbiol. Ecol. 2018, 94, fiy009. [Google Scholar] [CrossRef]
- Kim, E.T.; Guan, L.L.; Lee, S.J.; Lee, S.M.; Lee, S.S.; Lee, I.D.; Lee, S.K.; Lee, S.S. Effects of flavonoid-rich plant extracts on in vitro ruminal methanogenesis, microbial populations and fermentation characteristics. Asian-Australas. J. Anim. Sci. 2015, 28, 530–537. [Google Scholar] [CrossRef] [PubMed]
| Items | Concentrate | Rice Straw |
|---|---|---|
| Ingredients (g/kg DM) | ||
| Cassava chip | 530 | |
| Soybean meal | 165 | |
| Rice bran | 120 | |
| Palm kernel meal | 136 | |
| Urea | 10 | |
| Premix | 10 | |
| Molasses | 14 | |
| Sulfur | 5 | |
| Salt | 10 | |
| Chemical composition | ||
| Dry matter (DM), g/kg | 870.7 | 926 |
| Crude protein, g/kg DM | 143.8 | 29 |
| Neutral detergent fiber, g/kg DM | 216.1 | 716 |
| Acid detergent fiber, g/kg DM | 121.4 | 558 |
| Ash, g/kg DM | 49.7 | 133 |
| Items | Leaf | SEM | p-Value | Leaf + Leaf-Stalk | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 15 | 30 | 45 | 60 | 15 | 30 | 45 | 60 | |||||
| Chemical Composition (g/kg) | ||||||||||||
| DM | 118.2 b | 126.1 a | 128.2 a | 129.9 a | 1.14 | 0.004 | 148.5 d | 153.2 c | 159.9 b | 168.9 a | 0.14 | 0.001 |
| OM | 973.0 b | 973.6 b | 976.7 a | 976.9 a | 0.03 | 0.001 | 958.8 | 958.6 | 957.4 | 955.3 | 0.10 | 0.092 |
| CP | 256.4 a | 233.8 b | 214.7 c | 212.0 c | 0.17 | 0.000 | 196.2 a | 170.1 b | 163.0 b | 149.6 c | 0.33 | 0.001 |
| NDF | 343.2 b | 303.4 a | 406.1 a | 417.0 a | 0.71 | 0.002 | 477.5 b | 481.9 b | 485.7 b | 497.3 a | 0.35 | 0.019 |
| ADF | 173.8 c | 192.7 b | 200.6 b | 210.9 a | 0.33 | 0.002 | 207.1 | 209.9 | 212.1 | 212.9 | 0.68 | 0.202 |
| TF (g/kg) | 82.16 a | 72.21 b | 50.73 c | 49.57 c | 0.48 | 0.000 | 78.14 a | 71.34 b | 50.04 c | 45.52 d | 1.06 | 0.000 |
| Items (g/kg) | Leaf | SEM | p-Value | Leaf and Leaf + Leaf-Stalk | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 15 | 30 | 45 | 60 | 15 | 30 | 45 | 60 | |||||
| 12 h | ||||||||||||
| DM | 559.4 | 556.0 | 551.4 | 542.1 | 0.70 | 0.220 | 468.5 a | 406.4 b | 366.0 c | 346.6 d | 0.37 | 0.000 |
| OM | 871.2 a | 863.9 b | 857.0 c | 839.2 a | 0.76 | 0.000 | 862.2 a | 844.1 b | 841.9 b | 839.9 b | 0.15 | 0.002 |
| NDF | 398.1 a | 389.3 a | 364.9 b | 358.7 b | 0.43 | 0.002 | 341.6 a | 312.6 b | 262.6 c | 234.0 d | 0.07 | 0.000 |
| ADF | 229.0 a | 218.1 b | 207.6 c | 186.0 d | 0.32 | 0.001 | 177.1 a | 134.7 b | 130.1 c | 129.2 c | 0.04 | 0.000 |
| 24 h | ||||||||||||
| DM | 629.4 a | 615.8 b | 611.0 c | 602.8 d | 0.08 | 0.000 | 606.0 a | 600.0 b | 574.6 c | 542.7 d | 0.07 | 0.000 |
| OM | 934.6 a | 927.6 b | 912.7 c | 900.6 d | 0.07 | 0.000 | 906.0 a | 899.4 b | 881.7 c | 879.2 d | 0.07 | 0.000 |
| NDF | 458.8 a | 457.2 a | 449.3 b | 438.9 c | 0.07 | 0.000 | 423.1 a | 396.9 b | 385.5 bc | 374.7 c | 0.71 | 0.009 |
| ADF | 259.3 a | 257.4 a | 246.6 b | 238.8 c | 0.07 | 0.000 | 247.0 a | 224.8 b | 212.2 c | 207.0 d | 0.07 | 0.000 |
| Treatments | R:C | FE (mg) | a (mL) | B (mL) | c (mL) | a + b (mL) | Gas Production at 96 h (mL/0.5 g Substrate) | DMDvt (%) | |
|---|---|---|---|---|---|---|---|---|---|
| 12 h | 24 h | ||||||||
| T1 | 50:50 | 0 | −6.17 | 109.17 | 0.039 | 103.03 | 93.1 | 51.72 | 63.65 |
| T2 | 10 | −3.1 | 127.8 | 0.02 | 124.7 | 99.5 | 51.58 | 65.2 | |
| T3 | 20 | −1.6 | 116.9 | 0.027 | 115.43 | 102.7 | 51.13 | 67.9 | |
| T4 | 30 | −2.3 | 110.13 | 0.024 | 107.93 | 88.9 | 46.93 | 68.52 | |
| T5 | 40 | −1.33 | 107.43 | 0.078 | 106.03 | 101.7 | 57.23 | 68.9 | |
| T6 | 40:60 | 0 | −1.3 | 117.53 | 0.019 | 116.4 | 105.4 | 60 | 62.98 |
| T7 | 10 | −4.23 | 121.07 | 0.018 | 116.83 | 94.4 | 62.33 | 72.25 | |
| T8 | 20 | −5.53 | 111.93 | 0.032 | 108.4 | 100.9 | 61.22 | 70.48 | |
| T9 | 30 | −4.2 | 130.6 | 0.019 | 126.4 | 101.5 | 65.05 | 72.02 | |
| T10 | 40 | −2 | 107.7 | 0.018 | 105.7 | 82.4 | 64.77 | 71.6 | |
| T11 | 30:70 | 0 | −1.25 | 126 | 0.038 | 124.75 | 113.1 | 68.8 | 78.3 |
| T12 | 10 | −2.07 | 119.53 | 0.022 | 117.7 | 98.7 | 67.28 | 79.37 | |
| T13 | 20 | −3.4 | 112.75 | 0.021 | 119.3 | 95.6 | 70.2 | 86.17 | |
| T14 | 30 | −3.57 | 100.5 | 0.03 | 97 | 88.7 | 74.57 | 84.88 | |
| T15 | 40 | −3.1 | 113.73 | 0.028 | 110.63 | 97.3 | 70.6 | 74.8 | |
| SEM | 3.098 | 17.87 | 0.01 | 15.9 | 14.18 | 12.46 | 18 | ||
| R:C | 50:50 | −1.29 | 114.31 | 0.029 | 111.41 | 97.2 | 51.72 b | 66.84 b | |
| 40:60 | −2.88 | 112.17 | 0.027 | 114.55 | 94.51 | 62.68 ab | 69.86 b | ||
| 30:70 | −2.89 | 111.71 | 0.028 | 110.41 | 98.71 | 68.83 a | 80.73 a | ||
| FE (mg) | 0 | −1 | 113.12 | 0.034 a | 112.13 | 103.87 | 60.18 | 68.31 | |
| 10 | −3.04 | 122.80 | 0.020 b | 119.73 | 97.54 | 60.40 | 72.28 | ||
| 20 | −2.43 | 113.64 | 0.028 ab | 111.2 | 99.73 | 60.85 | 74.84 | ||
| 30 | −3.36 | 113.78 | 0.024 ab | 110.43 | 93.07 | 59.75 | 75.14 | ||
| 40 | −1.93 | 100.29 | 0.032 a | 107.13 | 89.81 | 64.20 | 71.80 | ||
| Orthogonal polynomial | Lin | 0.510 | 0.075 | 0.985 | 0.259 | 0.621 | 0.651 | 0.678 | |
| Quad | 0.172 | 0.107 | 0.030 | 0.485 | 0.620 | 0.722 | 0.548 | ||
| Cub | 0.925 | 0.784 | 0.359 | 0.424 | 0.264 | 0.746 | 0.924 | ||
| R:C × FE | 0.061 | 0.234 | 0.832 | 0.533 | 0.153 | 0.999 | 0.999 | ||
| Treatments | R:C | FE (mg) | pH | NH3-N (mg/dL) | Protozoa (×105 Cells/mL) |
|---|---|---|---|---|---|
| T1 | 50:50 | 0 | 6.85 | 10.82 | 3.50 |
| T2 | 10 | 6.87 | 17.03 | 3.25 | |
| T3 | 20 | 6.91 | 10.63 | 3.00 | |
| T4 | 30 | 6.94 | 17.13 | 1.25 | |
| T5 | 40 | 6.91 | 17.92 | 1.50 | |
| T6 | 40:60 | 0 | 6.61 | 20.02 | 3.50 |
| T7 | 10 | 6.66 | 18.43 | 2.75 | |
| T8 | 20 | 6.60 | 20.63 | 3.25 | |
| T9 | 30 | 6.64 | 16.52 | 2.75 | |
| T10 | 40 | 6.65 | 21.42 | 2.00 | |
| T11 | 30:70 | 0 | 6.49 | 22.92 | 5.50 |
| T12 | 10 | 6.40 | 22.22 | 4.52 | |
| T13 | 20 | 6.50 | 24.32 | 3.50 | |
| T14 | 30 | 6.41 | 22.22 | 4.00 | |
| T15 | 40 | 6.60 | 21.82 | 1.87 | |
| SEM | 0.013 | 0.255 | 0.052 | ||
| R:C | 50:50 | 6.90 a | 14.71 b | 2.50 b | |
| 40:60 | 6.63 b | 19.41 b | 2.85 b | ||
| 30:70 | 6.48 b | 22.70 a | 3.88 a | ||
| FE (mg) | 0 | 6.65 | 17.92 | 4.17 a | |
| 10 | 6.65 | 19.23 | 3.51 ab | ||
| 20 | 6.67 | 18.53 | 3.25 ab | ||
| 30 | 6.66 | 18.62 | 2.67 bc | ||
| 40 | 6.72 | 20.39 | 1.79 b | ||
| Orthogonal polynomial | Lin | 0.556 | 0.394 | 0.000 | |
| Quad | 0.773 | 0.773 | 0.534 | ||
| Cub | 0.895 | 0.467 | 0.502 | ||
| R:C × FE | 0.995 | 0.437 | 0.299 | ||
| Treatments | R:C | FE (mg) | C2:C3 Ratio | Molar Proportions of VFA (mmol/L) | Total VFA (mmol/L) | CH4 (mmol/L) | ||
|---|---|---|---|---|---|---|---|---|
| C2 | C3 | C4 | ||||||
| T1 | 50:50 | 0 | 4.14 a | 74.75 | 18.06 | 10.43 | 94.85 | 24.96 |
| T2 | 10 | 3.92 a | 72.44 | 18.46 | 10.62 | 94.53 | 21.99 | |
| T3 | 20 | 3.08 bc | 72.59 | 24.24 | 10.71 | 102.12 | 20.93 | |
| T4 | 30 | 3.17 bcd | 72.65 | 24.48 | 12.12 | 107.31 | 20.30 | |
| T5 | 40 | 3.86 a | 72.28 | 20.16 | 10.56 | 99.09 | 19.59 | |
| T6 | 40:60 | 0 | 3.21 b | 69.97 | 21.79 | 12.51 | 104.28 | 19.42 |
| T7 | 10 | 2.87 bcde | 68.36 | 23.86 | 11.41 | 103.62 | 16.30 | |
| T8 | 20 | 3.25 bc | 68.18 | 21.57 | 11.01 | 102.76 | 11.55 | |
| T9 | 30 | 2.84 bcde | 66.24 | 24.52 | 10.3 | 104.55 | 10.74 | |
| T10 | 40 | 2.83 bcde | 63.48 | 25.62 | 12.02 | 110.13 | 10.04 | |
| T11 | 30:70 | 0 | 2.8 bcde | 66.35 | 23.73 | 11.52 | 109.99 | 16.29 |
| T12 | 10 | 2.65 de | 65.45 | 24.73 | 11.71 | 108.88 | 13.25 | |
| T13 | 20 | 2.43 e | 65.34 | 27.66 | 11.84 | 114.08 | 12.55 | |
| T14 | 30 | 2.66 cde | 65.22 | 26.58 | 12.59 | 116.81 | 11.91 | |
| T15 | 40 | 2.49 e | 64.87 | 27.5 | 12.61 | 117.89 | 10.82 | |
| SEM | 0.25 | 0.100 | 1.93 | 1.076 | 4.25 | 0.156 | ||
| R:C | 50:50 | 3.64 a | 72.94 a | 21.22 c | 10.89 b | 99.61 c | 21.55 a | |
| 40:60 | 3.02 b | 67.25 b | 23.47 b | 11.45 ab | 104.87 b | 13.61 b | ||
| 30:70 | 2.61 c | 65.45 c | 26.24 a | 12.06 a | 113.53 a | 12.97 b | ||
| FE (mg) | 0 | 3.41 a | 70.36 ab | 20.59 b | 11.49 | 103.04 b | 20.22 a | |
| 10 | 3.15 ab | 68.75 ab | 22.87 ab | 11.25 | 101.44 b | 17.18 b | ||
| 20 | 2.94 b | 68.70 ab | 24.49 a | 11.19 | 106.38 ab | 15.01 bc | ||
| 30 | 2.89 b | 68.04 b | 25.19 a | 11.67 | 109.56 a | 14.32 bc | ||
| 40 | 3.06 b | 66.88 b | 24.43 a | 11.74 | 109.04 a | 13.48 c | ||
| Orthogonal polynomial | Lin | 0.010 | 0.001 | 0.002 | 0.523 | 0.003 | 0.000 | |
| Quad | 0.019 | 0.904 | 0.120 | 0.493 | 0.932 | 0.121 | ||
| Cub | 0.595 | 0.308 | 0.265 | 0.670 | 0.091 | 0.743 | ||
| R:C × FE | 0.042 | 0.356 | 0.122 | 0.398 | 0.483 | 0.785 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sommai, S.; Cherdthong, A.; Suntara, C.; So, S.; Wanapat, M.; Polyorach, S. In Vitro Fermentation Characteristics and Methane Mitigation Responded to Flavonoid Extract Levels from Alternanthera sissoo and Dietary Ratios. Fermentation 2021, 7, 109. https://doi.org/10.3390/fermentation7030109
Sommai S, Cherdthong A, Suntara C, So S, Wanapat M, Polyorach S. In Vitro Fermentation Characteristics and Methane Mitigation Responded to Flavonoid Extract Levels from Alternanthera sissoo and Dietary Ratios. Fermentation. 2021; 7(3):109. https://doi.org/10.3390/fermentation7030109
Chicago/Turabian StyleSommai, Sukruthai, Anusorn Cherdthong, Chanon Suntara, Sarong So, Metha Wanapat, and Sineenart Polyorach. 2021. "In Vitro Fermentation Characteristics and Methane Mitigation Responded to Flavonoid Extract Levels from Alternanthera sissoo and Dietary Ratios" Fermentation 7, no. 3: 109. https://doi.org/10.3390/fermentation7030109
APA StyleSommai, S., Cherdthong, A., Suntara, C., So, S., Wanapat, M., & Polyorach, S. (2021). In Vitro Fermentation Characteristics and Methane Mitigation Responded to Flavonoid Extract Levels from Alternanthera sissoo and Dietary Ratios. Fermentation, 7(3), 109. https://doi.org/10.3390/fermentation7030109








