Comparative Highly Efficient Production of β-glucan by Lasiodiplodia theobromae CCT 3966 and Its Multiscale Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Microorganism
2.2. Carbon Source: Sugarcane Bagasse Cellulosic Hydrolysate
2.3. Nitrogen Sources: Rice and Soybean Bran Extracts
2.4. Media and Cultivation Conditions for Lasiodiplodan Production
2.4.1. Lasiodiplodan and Fungal Biomass Quantification
2.4.2. Sugars Quantification
2.5. Central Composite Rotational Design (CCRD): Interplay of Carbon and Nitrogen Sources
2.6. Multiscale Characterization of Lasiodiplodan
2.6.1. Purity and Solubility Properties
2.6.2. Viscosity and Molecular Weight Analysis
2.6.3. Scanning Electron Microscopy (SEM)
2.6.4. Fourier Transform Infrared (FTIR) Spectroscopy
2.6.5. Differential Scanning Calorimetry (DSC)
2.6.6. X-ray Diffraction (XRD)
2.7. Statistical Analysis
3. Results and Discussion
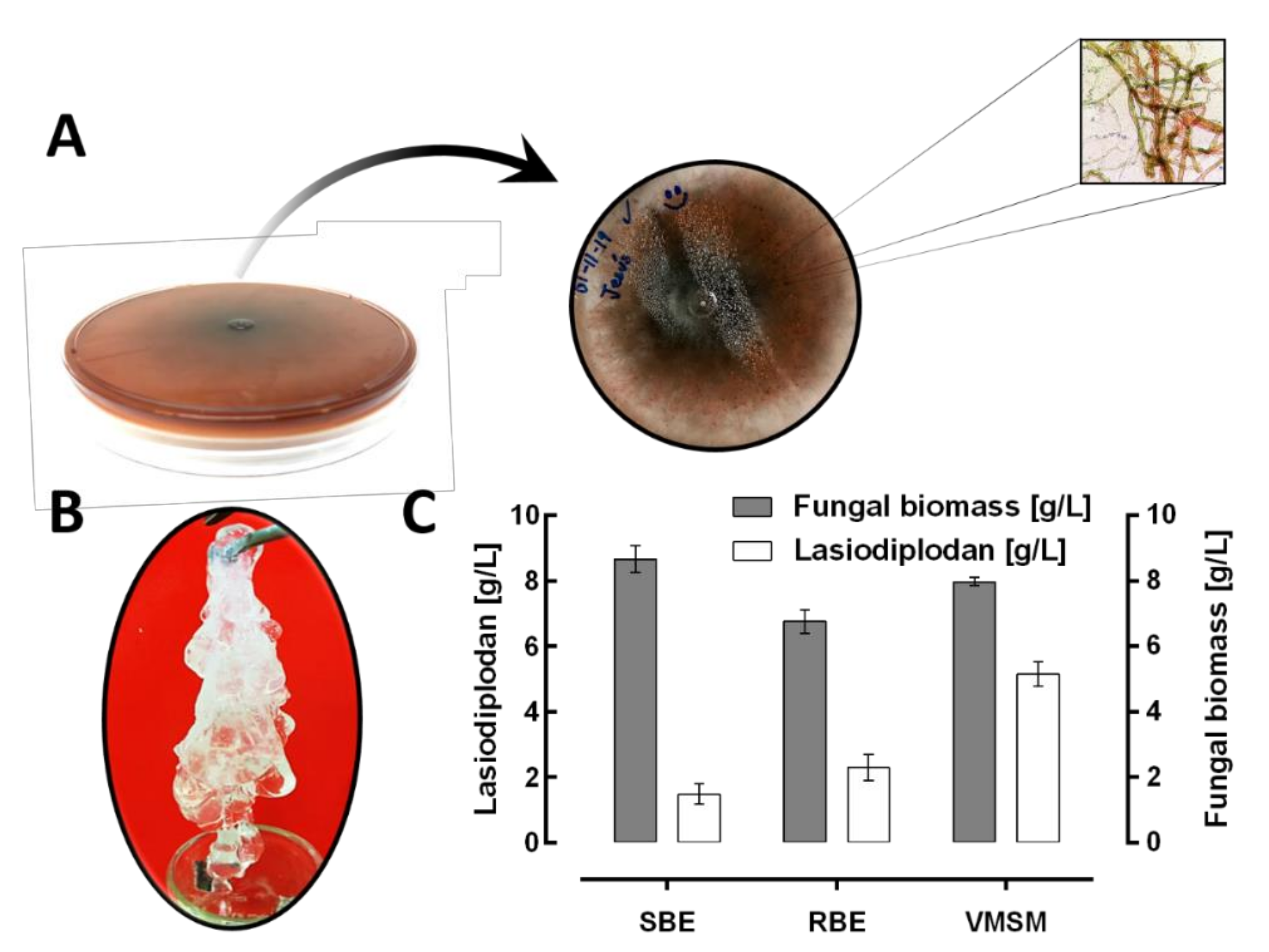
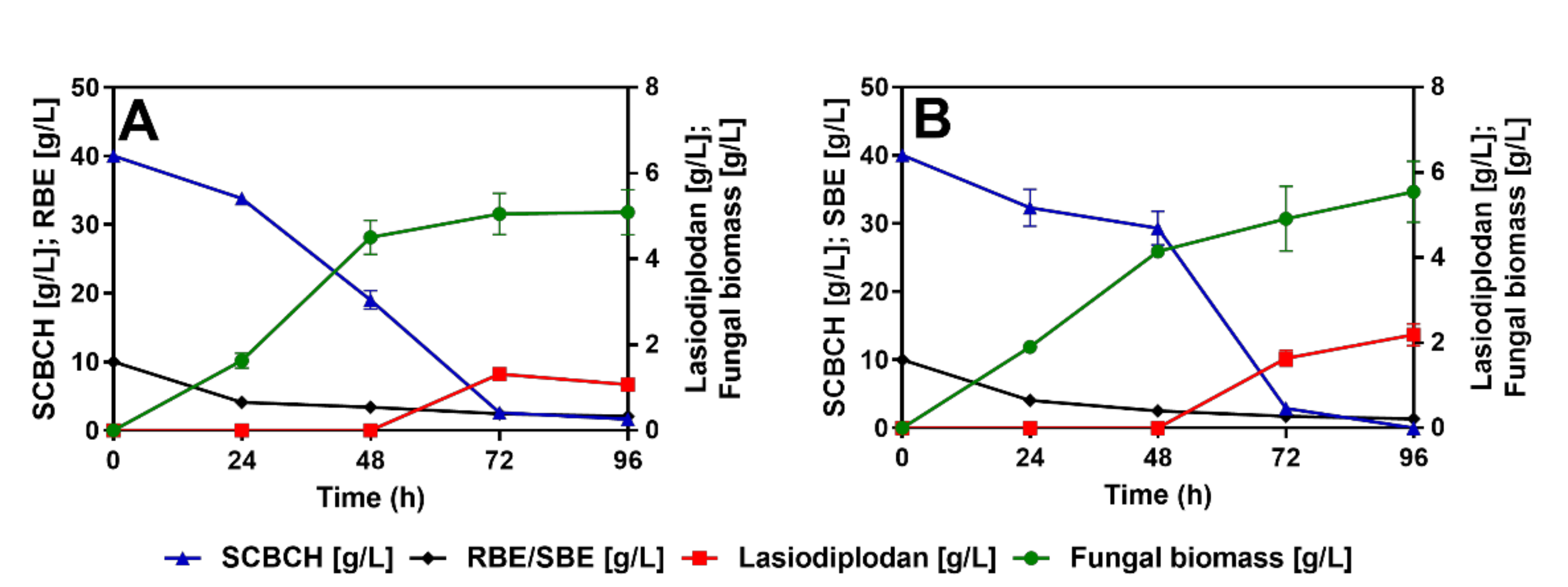
3.1. Importance of Alternative Nitrogen Sources on Lasiodiplodan Production
3.2. Impact of Lignocellulosic Carbon Source on Lasiodiplodan Production
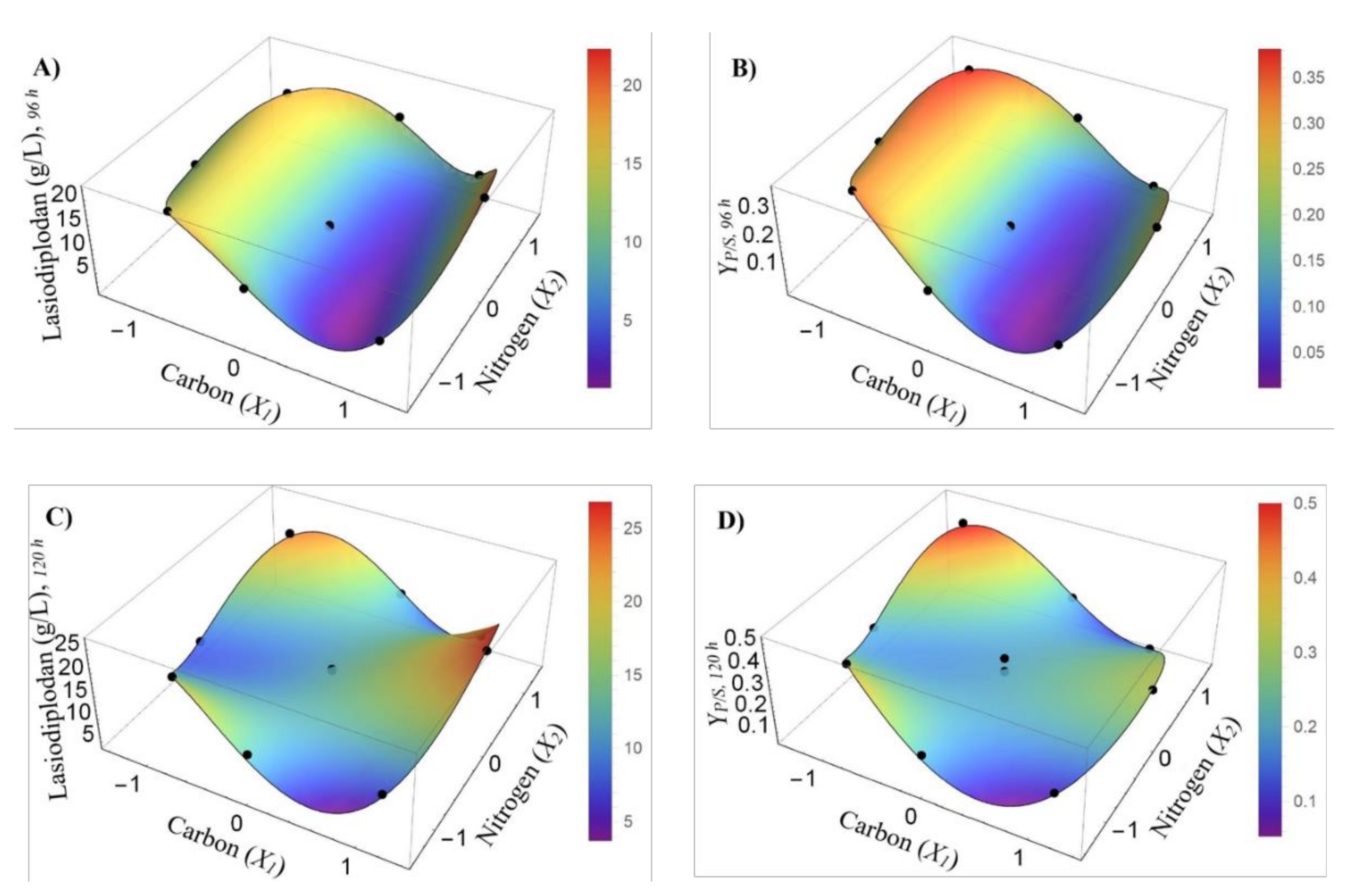
3.3. Carbon and Nitrogen Optimization Through CCRD and Genetic Programming
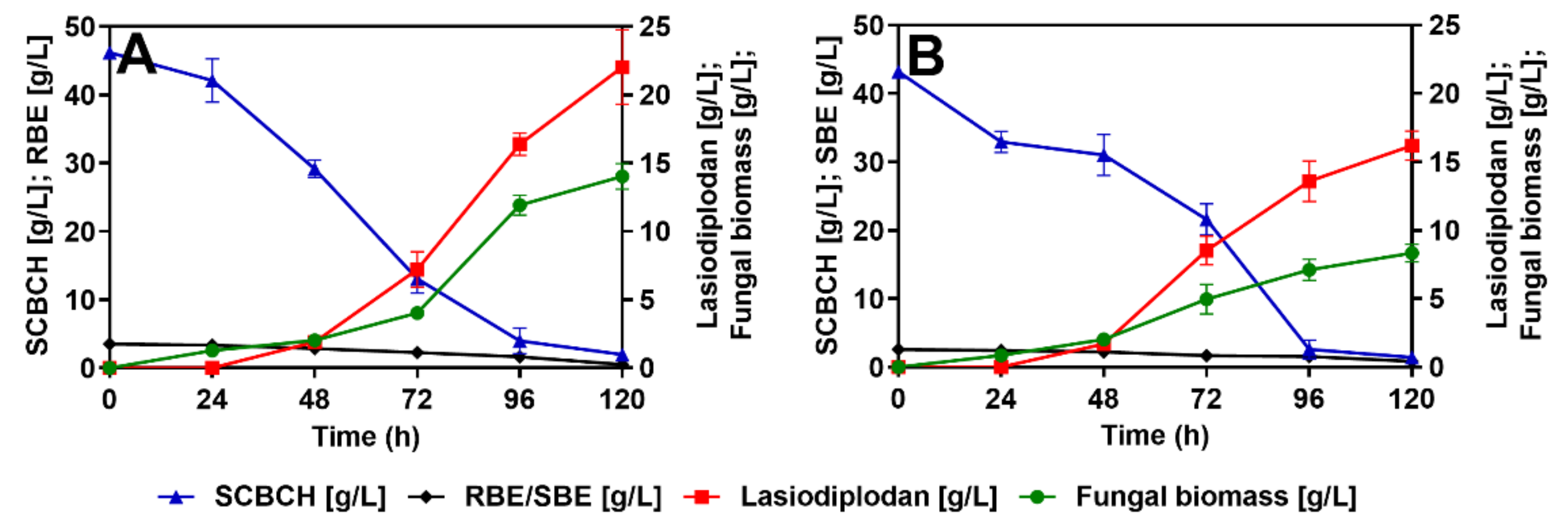
3.4. Lasiodiplodan Production Using the Optimal Carbon and Nitrogen Concentration
3.5. Multiscale Characterization of Lasiodiplodan from Sustainable Process
3.5.1. Physicochemical Properties Analysis
3.5.2. GPC, SEM, and Viscosity Analysis
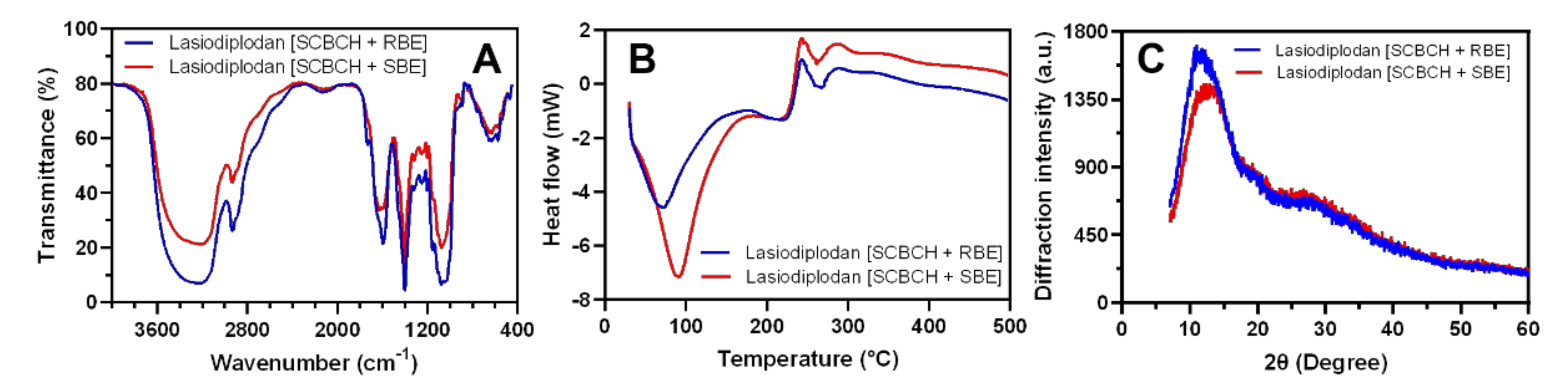
3.5.3. FTIR, DSC, and XRD Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Fang, X.-H.; Zou, M.-Y.; Chen, F.-Q.; Ni, H.; Nie, S.-P.; Yin, J.-Y. An overview on interactions between natural product-derived β-glucan and small-molecule compounds. Carbohydr. Polym. 2021, 261, 117850. [Google Scholar] [CrossRef]
- Han, B.; Baruah, K.; Cox, E.; Vanrompay, D.; Bossier, P. Structure-functional activity relationship of β-glucans from the per-spective of immunomodulation: A mini-review. Front. Immunol. 2020, 11, 658. [Google Scholar] [CrossRef] [Green Version]
- Zhu, F.; Du, B.; Xu, B. A critical review on production and industrial applications of beta-glucans. Food Hydrocoll. 2016, 52, 275–288. [Google Scholar] [CrossRef]
- Kaur, R.; Sharma, M.; Ji, D.; Xu, M.; Agyei, D. Structural Features, Modification, and Functionalities of Beta-Glucan. Fibers 2019, 8, 1. [Google Scholar] [CrossRef] [Green Version]
- Maheshwari, G.; Sowrirajan, S.; Joseph, B. Extraction and Isolation of β-Glucan from Grain Sources-A Review. J. Food Sci. 2017, 82, 1535–1545. [Google Scholar] [CrossRef] [Green Version]
- Bzducha-Wróbel, A.; Koczoń, P.; Błażejak, S.; Kozera, J.; Kieliszek, M. Valorization of Deproteinated Potato Juice Water into β-Glucan Preparation of C. utilis Origin: Comparative Study of Preparations Obtained by Two Isolation Methods. Waste Biomass Valoriz. 2020, 11, 3257–3271. [Google Scholar] [CrossRef] [Green Version]
- Żbikowska, A.; Kupiec, M.; Szymanska, I.; Osytek, K.; Kowalska, M.; Marciniak-Lukasiak, K.; Rutkowska, J. Microbial β-glucan Incorporated into Muffins: Impact on Quality of the Batter and Baked Products. Agriculture. 2020, 10, 126. [Google Scholar] [CrossRef] [Green Version]
- Acosta, S.B.P.; Marchioro, M.L.K.; Santos, V.A.Q.; Calegari, G.C.; Lafay, C.B.B.; Barbosa-Dekker, A.M.; Dekker, R.F.H.; Da Cunha, M.A.A. Valorization of Soybean Molasses as Fermentation Substrate for the Production of Microbial Exocellular β-Glucan. J. Polym. Environ. 2020, 28, 2149–2160. [Google Scholar] [CrossRef]
- Vasconcelos, A.F.D.; Dekker, R.F.; Barbosa, A.M.; Carbonero, E.R.; Silveira, J.L.; Glauser, B.; Pereira, M.S.; Silva, M.L.C. Sul-fonation and anticoagulant activity of fungal exocellular β-(1→6)-D-glucan (lasiodiplodan). Carbohydr. Polym. 2013, 92, 1908–1914. [Google Scholar] [CrossRef] [Green Version]
- da Cunha, M.A.A.; Turmina, J.A.; Ivanov, R.C.; Barroso, R.R.; Marques, P.T.; Fonseca, E.A.I.; Fortes, Z.B.; Dekker, R.F.H.; Khaper, N.; Barbosa, A.M. Lasiodiplodan, an exocellular (1→6)-β-d-glucan from Lasiodiplodia theobromae MMPI: Production on glucose, fermentation kinetics, rheology and anti-proliferative activity. J. Ind. Microbiol. Biotechnol. 2012, 39, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.; Crous, P.W.; Correia, A.; Phillips, A.J.L. Morphological and molecular data reveal cryptic speciation in Lasiodiplodia theobromae. Fungal Divers. 2008, 28, 1–13. [Google Scholar]
- Mahapatra, S.; Banerjee, D. Fungal Exopolysaccharide: Production, Composition and Applications. Microbiol. Insights 2013, 6, MBI S10957. [Google Scholar] [CrossRef] [Green Version]
- Theis, T.V.; Santos, V.A.Q.; Appelt, P.; Barbosa-Dekker, A.M.; Vetvicka, V.; Dekker, R.F.H.; Cunha, M.A.A. Fungal Exocellular (1-6)-β-d-glucan: Carboxymethylation, Characterization, and Antioxidant Activity. Int. J. Mol. Sci. 2019, 20, 2337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdeshahian, P.; Ascencio, J.J.; Philippini, R.R.; Antunes, F.A.F.; Dos Santos, J.C.; da Silva, S.S. Utilization of sugarcane straw for production of β-glucan biopolymer by Lasiodiplodia theobromae CCT 3966 in batch fermentation process. Bioresour. Technol. 2020, 314, 123716. [Google Scholar] [CrossRef] [PubMed]
- Philippini, R.R.; Martiniano, S.E.; Marcelino, P.R.F.; Chandel, A.K.; dos Santos, J.C.; Da Silva, S.S. Production of β-glucan exopolysaccharide lasiodiplodan by Lasiodiplodia theobromae CCT 3966 from corn bran acid hydrolysate. Appl. Microbiol. Biotechnol. 2021, 105, 2319–2332. [Google Scholar] [CrossRef] [PubMed]
- Chandel, A.K.; Garlapati, V.K.; Kumar, S.P.J.; Hans, M.; Singh, A.K. The role of renewable chemicals and biofuels in building a bioeconomy. Biofuels, Bioprod. Biorefining 2020, 14, 830–844. [Google Scholar] [CrossRef]
- Ascencio, J.J.; Chandel, A.K.; Philippini, R.R.; Da Silva, S.S. Comparative study of cellulosic sugars production from sugarcane bagasse after dilute nitric acid, dilute sodium hydroxide and sequential nitric acid-sodium hydroxide pretreatment. Biomass Convers. Biorefinery 2020, 10, 813–822. [Google Scholar] [CrossRef]
- Martiniano, S.E.; Philippini, R.R.; Chandel, A.K.; Rosa, C.A.; Pagnocca, F.C.; Da Silva, S.S. Evaluation of Rice Bran Extract as a Nitrogen Source for Improved Hemicellulosic Ethanol Production from Sugarcane Bagasse by New Xylose-Fermenting Yeast Strains Isolated from Brazilian Forests. Sugar Tech. 2013, 16, 1–8. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Vogel, H.J. A convenient growth medium for Neurospora (Medium N). Microb. Genet. Bull. 1956, 13, 42–43. [Google Scholar]
- Gomes, F.M.; Pereira, F.M.; Silva, A.F.; Silva, M.B. Multiple response optimization: Analysis of genetic programming for symbolic regression and assessment of desirability functions. Knowledge-Based Syst. 2019, 179, 21–33. [Google Scholar] [CrossRef]
- Vazquez-Rodriguez, A.; Vasto-Anzaldo, X.G.; Perez, D.B.; Garza, E.V.; Villanueva, H.C.; García-Rivas, G.; Garza-Cervantes, J.A.; Gómez-Lugo, J.J.; Gomez-Loredo, A.E.; Gonzalez, M.T.G.; et al. Microbial Competition of Rhodotorula mucilaginosa UANL-001L and E. coli increase biosynthesis of Non-Toxic Exopolysaccharide with Applications as a Wide-Spectrum Antimicrobial. Sci. Rep. 2018, 8, 798. [Google Scholar] [CrossRef] [Green Version]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Guerra, A.; Mendonça, R.; Ferraz, A. Molecular weight distribution of wood components extracted from Pinus taeda biotreated by Ceriporiopsis subvermispora. Enzym. Microb. Technol. 2003, 33, 12–18. [Google Scholar] [CrossRef]
- Gmoser, R.; Sintca, C.; Taherzadeh, M.; Lennartsson, P.R. Combining submerged and solid state fermentation to convert waste bread into protein and pigment using the edible filamentous fungus N. intermedia. Waste Manag. 2019, 97, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Karr-Lilienthal, L.K.; Grieshop, C.M.; Merchen, N.R.; Mahan, D.C.; Fahey, G.C. Chemical Composition and Protein Quality Comparisons of Soybeans and Soybean Meals from Five Leading Soybean-Producing Countries. J. Agric. Food Chem. 2004, 52, 6193–6199. [Google Scholar] [CrossRef]
- Wang, J.; Salem, D.R.; Sani, R.K. Synthesis of Biopolymers from a Geobacillus sp. WSUCF1 Using Unprocessed Corn Stover. ACS Sustain. Chem. Eng. 2020, 8, 9483–9496. [Google Scholar] [CrossRef]
- López, J.C.; Pérez, J.S.; Sevilla, J.M.F.; Fernandez, F.G.A.; Grima, E.M.; Chisti, Y. Production of lovastatin by Aspergillus terreus: Effects of the C:N ratio and the principal nutrients on growth and metabolite production. Enzym. Microb. Technol. 2003, 33, 270–277. [Google Scholar] [CrossRef] [Green Version]
- Hitzenhammer, E.; Büschl, C.; Sulyok, M.; Schuhmacher, R.; Kluger, B.; Wischnitzki, E.; Schmoll, M. YPR2 is a regulator of light modulated carbon and secondary metabolism in Trichoderma reesei. BMC Genom. 2019, 20, 211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Félix, C.; Libório, S.; Nunes, M.; Félix, R.; Duarte, A.S.; Alves, A.; Esteves, A.C. Lasiodiplodia theobromae as a producer of bio-technologically relevant enzymes. Int. J. Mol. Sci. 2018, 19, 29. [Google Scholar]
- Ko, Y.-S.; Kim, J.W.; Lee, J.A.; Han, T.; Kim, G.B.; Park, J.E.; Lee, S.Y. Tools and strategies of systems metabolic engineering for the development of microbial cell factories for chemical production. Chem. Soc. Rev. 2020, 49, 4615–4636. [Google Scholar] [CrossRef]
- Synytsya, A.; Novak, M. Structural analysis of glucans. Ann. Transl. Med. 2014, 2. [Google Scholar] [CrossRef]
- Johansson, L.; Virkki, L.; Maunu, S.L.; Lehto, M.; Ekholm, P.; Varo, P. Structural characterization of water soluble β-glucan of oat bran. Carbohydr. Polym. 2000, 42, 143–148. [Google Scholar] [CrossRef]
- Oliveira, K.S.; Di Bastiani, M.; Cordeiro, L.M.; Costa, M.F.; Toledo, K.A.; Iacomini, M.; Barbosa, A.M.; Dekker, R.F.H.; Nas-cimento, V.M.G. (1→6)-and (1→3)(1→6)-β-glucans from Lasiodiplodia theobromae MMBJ: Structural characterization and pro-inflammatory activity. Carbohydr. polym. 2015, 133, 539–546. [Google Scholar] [CrossRef]
- Luna, W.N.S.; Santos, V.A.; Teixeira, S.D.; Barbosa-Dekker, A.M.; Dekker, R.F.H.; Cunha, M.A.A. O-Acetylated (1→6)-β-D-Glucan (lasiodiplodan): Chemical derivatization, characterization and antioxidant activity. J. Pharm. Pharmacol. 2018, 6, 320–332. [Google Scholar]
- Kagimura, F.Y.; da Cunha, M.A.A.; Theis, T.V.; Malfatti, C.R.M.; Dekker, R.F.; Barbosa, A.M.; Teixeira, S.D.; Salome, K. Carboxymethylation of (1→6)-β-glucan (lasiodiplodan): Preparation, characterization and antioxidant evaluation. Carbohydr. Polym. 2015, 127, 390–399. [Google Scholar] [CrossRef] [Green Version]
- Uchiyama, H.; Dowaki, M.; Kadota, K.; Arima, H.; Sugiyama, K.; Tozuka, Y. Single-stranded β-1,3–1,6-glucan as a carrier for improved dissolution and membrane permeation of poorly water-soluble compounds. Carbohydr. Polym. 2020, 247, 116698. [Google Scholar] [CrossRef]


| Kinetic Parameter | SCBCH/RBE Medium 1 | SCBCH/SBE Medium 1 | Soybean Molasses 2 | Corn Bran Hydrolysate 3 | Glucose Medium 3 |
|---|---|---|---|---|---|
| P (g/L) | 22.03 | 16.20 | 1.06 | 5.73 | 5.25 |
| X (g/L) | 14.03 | 8.34 | 8.40 | 4.25 | 8.40 |
| YP/S (g/g) | 0.49 | 0.38 | 0.13 | 0.14 | 0.13 |
| YX/S (g/g) | 0.31 | 0.19 | 0.93 | 0.10 | 0.21 |
| YX/P (g/g) | 0.63 | 0.51 | 0.13 | 0.13 | 0.63 |
| QP (g/L.h) | 0.18 | 0.13 | 0.01 | 0.06 | 0.05 |
| QX (g/L.h) | 0.11 | 0.06 | 0.11 | 0.04 | 0.09 |
| QS (g/L.h) | 0.36 | 0.34 | 0.12 | 0.44 | 0.41 |
| YC (%) | 95.6 | 96.6 | 87.4 | 84.8 | 99.8 |
| Parameter | Lasiodiplodan 1 | Lasiodiplodan 2 | Glucose 3 |
|---|---|---|---|
| Total sugar (%, w/w) | 93.75 ± 0.01 | 80.11 ± 0.01 | 100.09 ± 0.05 |
| Glucose (%, w/w) | 94.20 ± 0.05 | 80.40 ± 0.04 | 100.12 ± 0.04 |
| Total protein (%, w/w) | 4.56 ± 0.01 | 15.25 ± 0.01 | ND |
| Solubility (%) | 16.81 ± 0.01 | 4.53 ± 0.03 | ND |
| Monosaccharide composition | Glucose | Glucose | Glucose |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ascencio, J.J.; Philippini, R.R.; Gomes, F.M.; Pereira, F.M.; da Silva, S.S.; Kumar, V.; Chandel, A.K. Comparative Highly Efficient Production of β-glucan by Lasiodiplodia theobromae CCT 3966 and Its Multiscale Characterization. Fermentation 2021, 7, 108. https://doi.org/10.3390/fermentation7030108
Ascencio JJ, Philippini RR, Gomes FM, Pereira FM, da Silva SS, Kumar V, Chandel AK. Comparative Highly Efficient Production of β-glucan by Lasiodiplodia theobromae CCT 3966 and Its Multiscale Characterization. Fermentation. 2021; 7(3):108. https://doi.org/10.3390/fermentation7030108
Chicago/Turabian StyleAscencio, Jesús J., Rafael R. Philippini, Fabricio M. Gomes, Félix M. Pereira, Silvio S. da Silva, Vinod Kumar, and Anuj K. Chandel. 2021. "Comparative Highly Efficient Production of β-glucan by Lasiodiplodia theobromae CCT 3966 and Its Multiscale Characterization" Fermentation 7, no. 3: 108. https://doi.org/10.3390/fermentation7030108
APA StyleAscencio, J. J., Philippini, R. R., Gomes, F. M., Pereira, F. M., da Silva, S. S., Kumar, V., & Chandel, A. K. (2021). Comparative Highly Efficient Production of β-glucan by Lasiodiplodia theobromae CCT 3966 and Its Multiscale Characterization. Fermentation, 7(3), 108. https://doi.org/10.3390/fermentation7030108








