Use of Corn-Steep Water Effluent as a Promising Substrate for Lactic Acid Production by Enterococcus faecium Strain WH51-1
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrate Collection
2.2. Seed and Fermentative Media
2.3. Isolation and Screening of Lactic Acid Producers
2.4. Characterization and Identification of Isolate WH51-1
2.5. Optimization of Fermentation Conditions
2.6. Analytical Methods
3. Results and Discussion
3.1. Characterization of CSW as a Promising Substrate
3.1.1. Physicochemical Characteristics of CSW
3.1.2. Inorganic Ions Content of CSW
3.1.3. Amino Acid Analysis of CSW
3.1.4. Analysis of Fat-Soluble and Water-Soluble Vitamins in CSW
3.1.5. Analysis of Non-Protein Nitrogenous Components of CSW
3.2. Isolation and Screening of the Most Potent Lactic Acid Producers
3.3. Effect of Inhibitors on LA Production by the Most Potent Isolates
3.4. Optimization of the Fermentation Conditions for LA Production
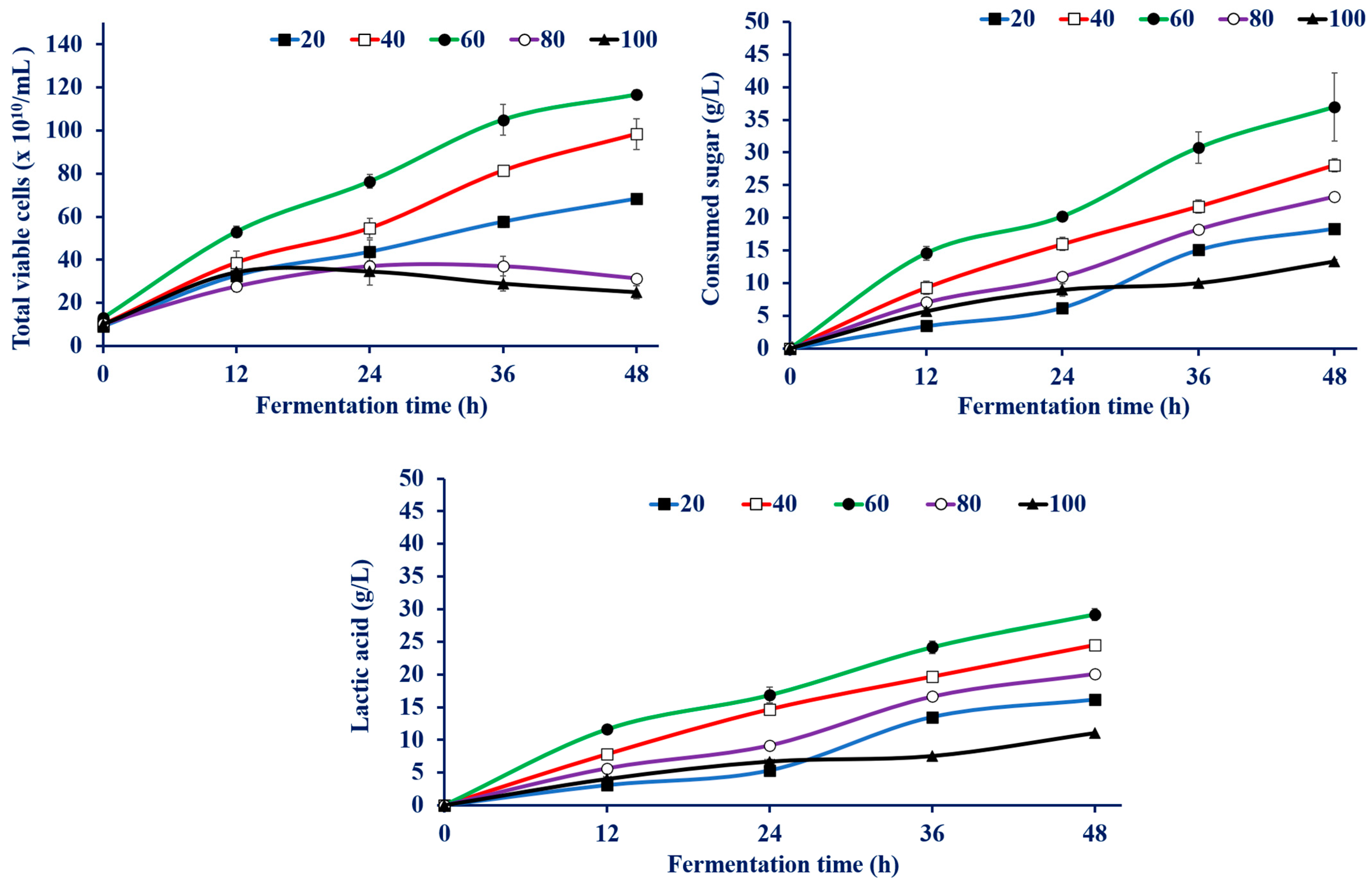
3.4.1. Effect of Sugar Concentration on LA Production by Enterococcus faecium WH51-1
3.4.2. Effect of Inoculum Size on LA Production by Enterococcus faecium WH51-1
3.4.3. Effect of Neutralizing Agents on LA Production by Enterococcus faecium WH51-1
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Din, N.A.S.; Lim, S.J.; Maskat, M.Y.; Mutalib, S.A.; Zaini, N.A.M. Lactic acid separation and recovery from fermentation broth by ion-exchange resin: A review. Bioresour. Bioprocess. 2021, 8, 31. [Google Scholar] [CrossRef]
- Gaber, M.A.; Abdel-Rahman, M.A.; Hassan, S.E.-D.; Azab, M.S. Efficient biorefinery process for lactic acid production from date wastes with alleviating substrate inhibition effect using thermo-alkaline repeated batch fermentation. Biomass Convers. Biorefinery 2021, 11, 1053–1066. [Google Scholar] [CrossRef]
- Vorawongsagul, S.; Pratumpong, P.; Pechyen, C. Preparation and foaming behavior of poly (lactic acid)/poly (butylene suc-cinate)/cellulose fiber composite for hot cups packaging application. Food Packag. Shelf Life 2021, 27, 100608. [Google Scholar] [CrossRef]
- Cubas Cano, E. Valorisation of Lignocellulosic Residues for Lactic Acid and Bioethanol Production in a Biorefinery Context. Ph.D. Thesis, Facultad de Ciencias Biológicas, Universidad Complutense de Madrid, Madrid, Spain, 2021. [Google Scholar]
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Recent advances in lactic acid production by microbial fermentation processes. Biotechnol. Adv. 2013, 31, 877–902. [Google Scholar] [CrossRef]
- Wang, Y.; Tashiro, Y.; Sonomoto, K. Fermentative production of lactic acid from renewable materials: Recent achievements, prospects, and limits. J. Biosci. Bioeng. 2015, 119, 10–18. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Hassan, S.E.D.; El-Din, M.N.; Azab, M.S.; El-Belely, E.F.; Alrefaey, H.M.A.; Elsakhawy, T. One-factor-at-a-time and response surface statistical designs for improved lactic acid production from beet molasses by Enterococcus hirae ds10. SN Appl. Sci. 2020, 2, 573. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Hu, J.-H.; Guo, C.; Liu, C.-Z. Enhanced laccase production by Trametes versicolor using corn steep liquor as both nitrogen source and inducer. Bioresour. Technol. 2014, 166, 602–605. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Xu, J.; Xia, J.; Lv, J.; Zhang, T.; Wu, Z.; Deng, Y.; He, J. Citric acid production by Yarrowia lipolytica SWJ-1b using corn steep liquor as a source of organic nitrogen and vitamins. Ind. Crop Prod. 2015, 78, 154–160. [Google Scholar] [CrossRef]
- Wang, D.; Eckhoff, S.R. Effect of Broken Corn Levels on Water Absorption and Steepwater Characteristics. Cereal Chem. J. 2000, 77, 525–528. [Google Scholar] [CrossRef]
- Singh, N.; Shevkani, K.; Kaur, A.; Thakur, S.; Parmar, N.; Virdi, A.S. Characteristics of starch obtained at different stages of purification during commercial wet milling of maize. Starch Stärke 2014, 66, 668–677. [Google Scholar] [CrossRef]
- Eneje, L.; Ogu, E.; Aloh, C.; Odibo, F.; Agu, R.; Palmer, G. Effect of steeping and germination time on malting performance of Nigerian white and yellow maize varieties. Process Biochem. 2004, 39, 1013–1016. [Google Scholar] [CrossRef]
- Brooker, D.B.; Bakker-Arkema, F.W.; Hall, C.W. Drying and Storage of Grains and Oilseeds; Springer Science & Business Media: Berlin, Germany, 1992. [Google Scholar]
- Hofer, A.; Hauer, S.; Kroll, P.; Fricke, J.; Herwig, C. In-depth characterization of the raw material corn steep liquor and its bioavailability in bioprocesses of Penicillium chrysogenum. Process Biochem. 2018, 70, 20–28. [Google Scholar]
- Li, X.; Xu, W.; Yang, J.; Zhao, H.; Pan, C.; Ding, X.; Zhang, Y. Effects of applying lactic acid bacteria to the fermentation on a mixture of corn steep liquor and air-dried rice straw. Anim. Nutr. 2016, 2, 229–233. [Google Scholar] [CrossRef]
- Bolobova, A.V.; Kondrashchenko, V.I. Use of yeast fermentation waste as a biomodifier of concrete (Review). Appl. Biochem. Microbiol. 2000, 36, 205–214. [Google Scholar]
- Pereira, F.B.; Guimarães, P.M.; Teixeira, J.; Domingues, L. Optimization of low-cost medium for very high gravity ethanol fermentations by Saccharomyces cerevisiae using statistical experimental designs. Bioresour. Technol. 2010, 101, 7856–7863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azizi-Shotorkhoft, A.; Sharifi, A.; Mirmohammadi, D.; Baluch-Gharaei, H.; Rezaei, J. Effects of feeding different levels of corn steep liquor on the performance of fattening lambs. J. Anim. Physiol. Anim. Nutr. 2015, 100, 109–117. [Google Scholar]
- Tauqir, N.A. Impact of Corn Steep Liquor and Enzose Mixture on Growth Performance of Chicks. Pak. J. Zoöl. 2021, 1–4. [Google Scholar]
- Babakhani, S.; Fahmi, A.; Katebi, H.; Ouria, A.; Majnouni-Toutakhane, A.; Ganbarov, K.; Kafil, H. Non-sterile corn steep liquor a novel, cost effective and powerful culture media for Sporosarcina pasteurii cultivation for sand improvement. J. Appl. Microbiol. 2021, 130, 1232–1244. [Google Scholar] [PubMed]
- Maddipati, P.; Atiyeh, H.K.; Bellmer, D.D.; Huhnke, R.L. Ethanol production from syngas by Clostridium strain P11 using corn steep liquor as a nutrient replacement to yeast extract. Bioresour. Technol. 2011, 102, 6494–6501. [Google Scholar] [CrossRef]
- Liu, B.; Yang, M.; Qi, B.; Chen, X.; Su, Z.; Wan, Y. Optimizing l-(+)-lactic acid production by thermophile Lactobacillus plantarum As.1.3 using alternative nitrogen sources with response surface method. Biochem. Eng. J. 2010, 52, 212–219. [Google Scholar] [CrossRef]
- Silveira, M.; Wisbeck, E.; Hoch, I.; Jonas, R. Production of glucose-fructose oxidoreductase and ethanol by Zymomonas mobilis ATCC 29191 in medium containing corn steep liquor as a source of vitamins. Appl. Microbiol. Biotechnol. 2001, 55, 442–445. [Google Scholar] [CrossRef] [PubMed]
- Saxena, J.; Tanner, R.S. Optimization of a corn steep medium for production of ethanol from synthesis gas fermentation by Clostridium ragsdalei. World J. Microbiol. Biotechnol. 2011, 28, 1553–1561. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Tashiro, Y.; Zendo, T.; Sonomoto, K. Improved lactic acid productivity by an open repeated batch fermentation system using Enterococcus mundtii QU 25. RSC Adv. 2013, 3, 8437–8445. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Sonomoto, K. Opportunities to overcome the current limitations and challenges for efficient microbial production of optically pure lactic acid. J. Biotechnol. 2016, 236, 176–192. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, M.A.; Tashiro, Y.; Zendo, T.; Sakai, K.; Sonomoto, K. Enterococcus faecium QU 50: A novel thermophilic lactic acid bacterium for high-yield l-lactic acid production from xylose. FEMS Microbiol. Lett. 2014, 362, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Miller, D.; Bryant, J.E.; Madsen, E.L.; Ghiorse, W.C. Evaluation and optimization of DNA extraction and purification proce-dures for soil and sediment samples. Appl. Environ. Microbiol. 1999, 65, 4715–4724. [Google Scholar] [CrossRef] [Green Version]
- Lane, D. 16S/23S rRNA sequencing. In Nucleic Acid Techniques in Bacterial Systematics; Stackebrandt, E., Goodfellow, M., Eds.; John Wiley & Sons: New York, NY, USA, 1991; pp. 115–175. [Google Scholar]
- Association, A.P.H. Standard Methods for the Examination of Water and Wastewater; Clesceri, L.S., Greenberg, A.E., Eaton, A.D., Eds.; American Public Health Association: Washington, DC, USA, 1912; Volume 2. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Herbert, D. Chapter III Chemical analysis of microbial cells. VII-E Determination of nucleic acids in micro-organisms. In Methods in Microbiology; Academic Press: Cambridge, MA, USA, 1971; Part B; Volume 5, pp. 324–328. [Google Scholar]
- Barker, S.; Summerson, W.H. The colorimetric determination of lactic acid in biological material. J. Biol. Chem. 1941, 138, 535–554. [Google Scholar] [CrossRef]
- Hull, S.R.; Peters, E.; Cox, C.; Montgomery, R. Composition of corn steep water during experimental steeping. J. Agric. Food Chem. 1996, 44, 3521–3527. [Google Scholar] [CrossRef]
- Watson, S.A. Measurement and maintenance of quality. In Corn: Chemistry and Technology; Watson, S.A., Ramstad, P.E., Eds.; American Association of Cereal Chemists: St. Paul, MN, USA, 1987; pp. 125–183. [Google Scholar]
- Yasri, N.G.; Yaghmour, A.; Gunasekaran, S. Effective removal of organics from corn wet milling steepwater effluent by electrochemical oxidation and adsorption on 3-D granulated graphite electrode. J. Environ. Chem. Eng. 2015, 3, 930–937. [Google Scholar] [CrossRef]
- Whistler, R.L.; BeMiller, J.N.; Paschall, E.F. Starch: Chemistry and Technology; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Winston Liggett, R.; Koffler, H. Corn steep liquor in microbiology. Bacteriol. Rev. 1948, 12, 297. [Google Scholar] [CrossRef]
- Xiao, X.; Hou, Y.; Liu, Y.; Liu, Y.; Zhao, H.; Dong, L.; Du, J.; Wang, Y.; Bai, G.; Luo, G. Classification and analysis of corn steep liquor by UPLC/Q-TOF MS and HPLC. Talanta 2013, 107, 344–348. [Google Scholar] [CrossRef]
- Tammam, J.; Williams, A.; Noble, J.; Lloyd, D. Amino acid fermentation in non-starter Lactobacillus spp. isolated from Cheddar cheese. Lett. Appl. Microbiol. 2000, 30, 370–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kieronczyk, A.; Skeie, S.; Langsrud, T.; Le Bars, D.; Yvon, M. The nature of aroma compounds produced in a cheese model by glutamate dehydrogenase positive Lactobacillus INF15D depends on its relative aminotransferase activities towards the different amino acids. Int. Dairy J. 2004, 14, 227–235. [Google Scholar] [CrossRef]
- Williams, A.G.; Noble, J.; Banks, J.M. Catabolism of amino acids by lactic acid bacteria isolated from Cheddar cheese. Int. Dairy J. 2001, 11, 203–215. [Google Scholar] [CrossRef]
- Liu, S.-Q.; Holland, R.; Crow, V.L. The potential of dairy lactic acid bacteria to metabolise amino acids via non-transaminating reactions and endogenous transamination. Int. J. Food Microbiol. 2003, 86, 257–269. [Google Scholar] [CrossRef]
- Qiao, Y.; Liu, G.; Leng, C.; Zhang, Y.; Lv, X.; Chen, H.; Sun, J.; Feng, Z. Metabolic profiles of cysteine, methionine, glutamate, glutamine, arginine, aspartate, asparagine, alanine and glutathione in Streptococcus thermophilus during pH-controlled batch fermentations. Sci. Rep. 2018, 8, 12441. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J.; Seeras, A.; Sanchez-Maldonado, A.F.; Zhang, C.; Su, M.S.-W.; Gänzle, M.G. Glutamine, glutamate, and arginine-based acid resistance in Lactobacillus reuteri. Food Microbiol. 2014, 42, 172–180. [Google Scholar] [CrossRef]
- Loy, D.D.; Wright, K. Nutritional properties and feeding value of corn and its by-products. In Corn: Chemistry and Technology; White, P.J., Johnson, L.A., Eds.; American Association of Cereal Chemists: St. Paul, MN, USA, 1987. [Google Scholar]
- Limauro, D.; Falciatore, A.; Basso, A.L.; Forlani, G.; De Felice, M. Proline biosynthesis in Streptococcus thermophilus: Characterization of the proBA operon and its products. Microbiolology 1996, 142, 3275–3282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, S.; Zhou, J.; Liu, L.; Chen, J. Proline enhances Torulopsis glabrata growth during hyperosmotic stress. Biotechnol. Bioprocess Eng. 2010, 15, 285–292. [Google Scholar] [CrossRef]
- Tian, X.; Wang, Y.; Chu, J.; Zhuang, Y.; Zhang, S. Enhanced L-lactic acid production in Lactobacillus paracasei by exogenous proline addition based on compar-ative metabolite profiling analysis. Appl. Microbiol. Biotechnol. 2016, 100, 2301–2310. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Zhu, Y.; Mao, S.; Li, Y. Proteomic analyses to reveal the protective role of glutathione in resistance of Lactococcus lactis to osmotic stress. Appl. Environ. Microbiol. 2010, 76, 3177–3186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, S.W.; El-Nezami, H.; Shah, N.P. Effects of supplementation of citrulline and Lactobacillus helveticus ASCC 511 on intestinal epithelial cell integrity. J. Funct. Foods 2020, 64, 103571. [Google Scholar] [CrossRef]
- Litchfield, J. Lactic Acid, Microbially Produced. In Encyclopedia of Microbiology, 3rd ed.; Schaechter, M., Ed.; Academic Press: Cambridge, MA, USA, 2009; pp. 362–372. [Google Scholar]
- Reimundo, P.; Menendez, A.; Mendez, J.; Perez-Pascual, D.; Navais, R.; Gomez, E.; Brana, A.; Guijarro, J. dltA gene mutation in the teichoic acids alanylation system of Lactococcus garvieae results in diminished proliferation in its natural host. Vet. Microbiol. 2010, 143, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Chou, J.; Wang, T.; Zhao, H.; Zhang, B. Pantothenic Acid, Vitamin C, and Biotin Play Important Roles in the Growth of Lactobacillus helveticus. Front. Microbiol. 2018, 9, 1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jørgensen, H.; Nielsen, J.; Villadsen, J.; Møllgaard, H. Metabolic flux distributions in Penicillium chrysogenum during fed-batch cultivations. Biotechnol. Bioeng. 1995, 46, 117–131. [Google Scholar] [CrossRef]
- Hofer, A.; Herwig, C. Quantitative determination of nine water-soluble vitamins in the complex matrix of corn steep liquor for raw material quality assessment. J. Chem. Technol. Biotechnol. 2017, 92, 2106–2113. [Google Scholar] [CrossRef]
- Carr, J.G. The vitamin requirements of lactic acid bacteria from ciders. Antonie van Leeuwenhoek J. Microbiol. Serol. 1958, 24, 63–68. [Google Scholar] [CrossRef]
- Tanner, F.W., Jr.; Pfeiffer, S.E.; Van Lanen, J.M. Vitamin and protein content of residues from the production of penicillin by sub-merged fermentation. Arch. Biochem. 1945, 8, 29–36. [Google Scholar]
- Choi, J.-D.-R.; Jang, Y.-S.; Cho, J.-H.; Seung, D.; Lee, S.Y.; Papoutsakis, E.T.; Bennett, G.N.; Song, H. Characterization and evaluation of corn steep liquid in acetone-butanol-ethanol production by Clostridium acetobutylicum. Biotechnol. Bioprocess Eng. 2013, 18, 266–271. [Google Scholar] [CrossRef]
- Daniels, R.S. Corn Steep Liquor as a Biostimulant Composition. Google Patents PCT/US2010/058315, 30 November 2010. [Google Scholar]
- Cook, A.M.; Denger, K. Dissimilation of the C2 sulfonates. Arch. Microbiol. 2002, 179, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, A.M.; Denger, K. Metabolism of taurine in microorganisms. Taurine 2006, 6, 3–13. [Google Scholar]
- Denger, K.; Ruff, J.; Schleheck, D.; Cook, A.M. Rhodococcus opacus expresses the xsc gene to utilize taurine as a carbon source or as a nitrogen source but not as a sulfur source. Microbiology 2004, 150, 1859–1867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clifford, E.L.; Varela, M.M.; De Corte, D.; Bode, A.; Ortiz, V.; Herndl, G.J.; Sintes, E. Taurine Is a Major Carbon and Energy Source for Marine Prokaryotes in the North Atlantic Ocean off the Iberian Peninsula. Microb. Ecol. 2019, 78, 299–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christianson, D.D.; Cavins, J.F.; Wall, J.S. Steep Liquor Constituents, Identification and Determination of Nonprotein Nitrogenous Substances in Corn Steep Liquor. J. Agric. Food Chem. 1965, 13, 277–280. [Google Scholar] [CrossRef]
- Curis, E.; Nicolis, I.; Moinard, C.; Osowska, S.; Zerrouk, N.; Bénazeth, S.; Cynober, L. Almost all about citrulline in mammals. Amino Acids 2005, 29, 177–205. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.; Lee, J.-H. Characterization of Arginine Catabolism by Lactic Acid Bacteria Isolated from Kimchi. Molecules 2018, 23, 3049. [Google Scholar] [CrossRef] [Green Version]
- He, G.; Wu, C.; Huang, J.; Zhou, R. Metabolic response of Tetragenococcus halophilus under salt stress. Biotechnol. Bioprocess Eng. 2017, 22, 366–375. [Google Scholar] [CrossRef]
- Cusumano, Z.T.; Caparon, M.G. Citrulline Protects Streptococcus pyogenes from Acid Stress Using the Arginine Deiminase Pathway and the F1Fo-ATPase. J. Bacteriol. 2015, 197, 1288–1296. [Google Scholar] [CrossRef] [Green Version]
- Held, C.; Sadowski, G. Compatible solutes: Thermodynamic properties relevant for effective protection against osmotic stress. Fluid Phase Equilibria 2016, 407, 224–235. [Google Scholar] [CrossRef]
- Zhou, A.; Baidoo, E.; He, Z.; Mukhopadhyay, A.; Baumohl, J.K.; Benke, P.; Joachimiak, M.P.; Xie, M.; Song, R.; Arkin, A.P.; et al. Characterization of NaCl tolerance in Desulfovibrio vulgaris Hildenborough through experimental evolution. ISME J. 2013, 7, 1790–1802. [Google Scholar] [CrossRef] [Green Version]
- de Carvalho, C.C.C.R.; Marques, M.P.C.; Hachicho, N.; Heipieper, H.J. Rapid adaptation of Rhodococcus erythropolis cells to salt stress by synthesizing polyunsaturated fatty acids. Appl. Microbiol. Biotechnol. 2014, 98, 5599–5606. [Google Scholar] [CrossRef]
- Guillot, A.; Obis, D.; Mistou, M.-Y. Fatty acid membrane composition and activation of glycine-betaine transport in Lac-tococcus lactis subjected to osmotic stress. Int. J. Food Microbiol. 2000, 55, 47–51. [Google Scholar] [CrossRef]
- Li, Q.; Singh, V.; Gonzalez de Mejia, E.; Somavat, P. Effect of sulfur dioxide and lactic acid in steeping water on the extraction of anthocyanins and bioactives from purple corn pericarp. Cereal Chem. J. 2019, 96, 575–589. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Hassan, S.E.-D.; Azab, M.S.; Mahin, A.-A.; Gaber, M.A. High Improvement in Lactic Acid Productivity by New Alkaliphilic Bacterium Using Repeated Batch Fermentation Integrated with Increased Substrate Concentration. BioMed Res. Int. 2019, 2019, 7212870. [Google Scholar] [CrossRef] [Green Version]
- Kadam, S.R.; Patil, S.S.; Bastawde, K.B.; Khire, J.M.; Gokhale, D.V. Strain improvement of Lactobacillus delbrueckii NCIM 2365 for lactic acid production. Process Biochem. 2006, 41, 120–126. [Google Scholar] [CrossRef]
- Michelson, T.; Kask, K.; Jõgi, E.; Talpsep, E.; Suitso, I.; Nurk, A. L (+)-Lactic acid producer Bacillus coagulans SIM-7 DSM 14043 and its comparison with Lactobacillus delbrueckii ssp. lactis DSM 20073. Enzym. Microb. Technol. 2006, 39, 861–867. [Google Scholar] [CrossRef]
- Mohan, D.; Sarswat, A.; Ok, Y.S.; Pittman, C.U. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent—A critical review. Bioresour. Technol. 2014, 160, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.-K.; Wee, Y.-J.; Choi, G.-W. A novel lactic acid bacterium for the production of high purity l-lactic acid, Lactobacillus paracasei subsp. paracasei CHB2121. J. Biosci. Bioeng. 2012, 114, 155–159. [Google Scholar] [CrossRef]
- Ge, X.-Y.; Yuan, J.; Qin, H.; Zhang, W.-G. Improvement of l-lactic acid production by osmotic-tolerant mutant of Lactobacillus casei at high temperature. Appl. Microbiol. Biotechnol. 2010, 89, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Vishnu, C.; Seenayya, G.; Reddy, G. Direct fermentation of various pure and crude starchy substrates to L(+) lactic acid using Lactobacillus amylophilus GV6. World J. Microbiol. Biotechnol. 2002, 18, 429–433. [Google Scholar] [CrossRef]
- Panesar, P.S.; Kennedy, J.F.; Knill, C.J.; Kosseva, M. Production of L(+) lactic acid using Lactobacillus casei from whey. Braz. Arch. Biol. Technol. 2010, 53, 219–226. [Google Scholar] [CrossRef]
- Rivas, B.; Torrado, A.; Torre, P.; Converti, A.; Domínguez, J.M. Submerged Citric Acid Fermentation on Orange Peel Autohydrolysate. J. Agric. Food Chem. 2008, 56, 2380–2387. [Google Scholar] [CrossRef]
- Bernardo, M.P.; Coelho, L.F.; Sass, D.C.; Contiero, J. L-(+)-Lactic acid production by Lactobacillus rhamnosus B103 from dairy industry waste. Braz. J. Microbiol. 2016, 47, 640–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.; Lin, Y.; Zhang, Z.; Xiang, T.; Mei, Y.; Zhao, S.; Liang, Y.; Peng, N. High-titer lactic acid production by Lactobacillus pentosus FL0421 from corn stover using fed-batch simultaneous saccharification and fermentation. Bioresour. Technol. 2016, 214, 74–80. [Google Scholar] [CrossRef] [PubMed]
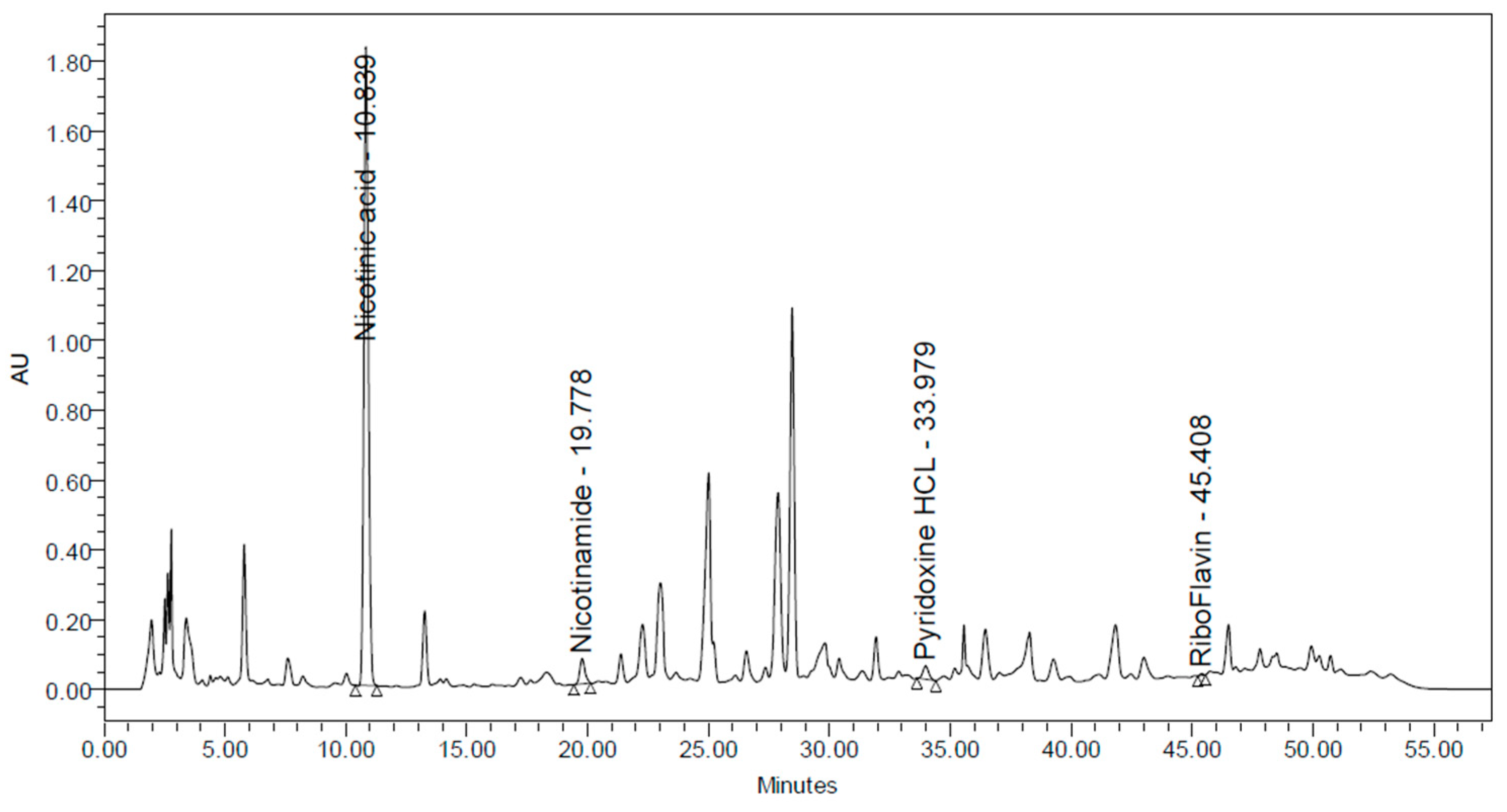


| Parameters | Results |
|---|---|
| Form | Liquid |
| Appearance | Viscous |
| Color | Deep brown |
| Odor | Rottenness |
| pH | 4.5 ± 0.2 |
| Temperature (°C) | 39.0 ± 1.1 |
| Conductivity (µs/cm) | 11,400 ± 0.42 |
| TDS (mg/L) | 6452 ± 1.2 |
| Dissolved oxygen (mg/L) | 0.890 ± 0.04 |
| COD (mg/L) | 78.0 ± 3.4 |
| BOD5 (g/L) | 23.0 ± 1.4 |
| Total Carbohydrates (g/L) | 250.4 ± 1.3 |
| Lactic acid (g/L) | 3.41 ± 0.87 |
| Total Protein (g/L) | 11.3 ± 1.4 |
| Total water-soluble vitamins (mg in 100 mL) | 145.5 |
| Total fat-soluble vitamins (mg in 100 mL) | 0.0 |
| Total amino acid (g/L) | 10.7 |
| Total non-protein nitrogenous components (μg/mL) | 34.8 |
| Inorganic Ions content (mg/kg of CSW) | |
| Aluminum | 3.48 |
| Boron | 3.85 |
| Barium | 0.374 |
| Calcium | 132.6 |
| Cadmium | <0.0006 |
| Cobalt | <0.001 |
| Chromium | 0.235 |
| Copper | 0.221 |
| Iron | 2.84 |
| Magnesium | 392.65 |
| Manganese | 3.734 |
| Molybdenum | 0.2205 |
| Nickel | 0.4315 |
| Lead | 0.6125 |
| Vanadium | <0.01 |
| Zinc | 29.21 |
| Phosphorus | 4515.5 |
| NO. | Amino Acid | Retention Time (min) | Concentration (g/L) |
|---|---|---|---|
| 1 | Aspartic acid | 8.40 | 0.766 |
| 2 | Threonine | 10.6 | 0.430 |
| 3 | Serine | 11.4 | 0.520 |
| 4 | Glutamic acid | 13.2 | 1.656 |
| 5 | Proline | 15.4 | 1.071 |
| 6 | Glycine | 19.4 | 0.580 |
| 7 | Alanine | 20.9 | 0.878 |
| 8 | Cystine | 22.0 | 0.180 |
| 9 | Valine | 22.9 | 0.556 |
| 10 | Methionine | 25.0 | 0.203 |
| 11 | Isoleucine | 27.2 | 0.303 |
| 12 | Leucine | 28.6 | 0.910 |
| 13 | Tyrosine | 31.0 | 0.210 |
| 14 | Phenylalanine | 32.3 | 0.331 |
| 15 | Histidine | 35.0 | 0.431 |
| 16 | Lysine | 39.3 | 0.527 |
| 17 | Arginine | 42.7 | 0.726 |
| Compound | Retention Time (min) | Concentration (μg/mL) |
|---|---|---|
| Ethanolamine | 4.8 | 5.12 |
| Ornithine | 6.0 | 6.33 |
| Citrulline | 8.0 | 7.41 |
| Taurine | 9.0 | 13.6 |
| Ɣ-Aminobutyric acid | 10.0 | 2.36 |
| Bacterial Isolate | Sodium Metabisulfate | Sodium Chloride | Sodium Acetate | Formic Acid | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inhibitor Conc. (g/L) | Consumed Sugar (g/L) | LA Conc. (g/L) a | YLA (g/g) b | Inhibitor Conc. (%) | Consumed Sugar (g/L) | LA Conc. (g/L) a | YLA (g/g) b | Inhibitor Conc. (g/L) | Consumed Sugar (g/L) | LA Conc. (g/L) a | YLA (g/g) b | Inhibitor Conc. (g/L) | Consumed Sugar (g/L) | LA Conc. (g/L) a | YLA (g/g) b | |
| SSD 16-1 | 1 | 15.09 | 13.12 | 0.87 | 2.5 | 14.09 | 12.12 | 0.86 | 5 | 14.84 | 12.14 | 0.82 | 2.5 | 14.41 | 11.21 | 0.78 |
| 2 | 15.98 | 9.78 | 0.61 | 5 | 13.98 | 8.15 | 0.58 | 10 | 13.89 | 8.45 | 0.61 | 5 | 12.79 | 7.98 | 0.62 | |
| 4 | 6.01 | 4.65 | 0.77 | 7.5 | 5.81 | 4.22 | 0.73 | 15 | 8.33 | 5.02 | 0.60 | 7.5 | 9.22 | 4.92 | 0.53 | |
| 8 | 3.55 | 0.89 | 0.25 | 10 | 4.95 | 1.34 | 0.27 | 20 | 2.09 | 0.55 | 0.26 | 10 | 1.29 | 0.32 | 0.25 | |
| WH 51-1 | 1 | 17.69 | 15.89 | 0.90 | 2.5 | 16.59 | 14.29 | 0.86 | 5 | 10.99 | 9.56 | 0.87 | 2.5 | 15.99 | 11.56 | 0.72 |
| 2 | 16.2 | 12.45 | 0.77 | 5 | 15.2 | 11.25 | 0.74 | 10 | 11.23 | 6.85 | 0.61 | 5 | 11.03 | 7.45 | 0.68 | |
| 4 | 6.25 | 4.85 | 0.78 | 7.5 | 5.25 | 3.85 | 0.73 | 15 | 6.81 | 3.85 | 0.57 | 7.5 | 6.51 | 4.05 | 0.62 | |
| 8 | 3.89 | 1.21 | 0.31 | 10 | 3.89 | 1.21 | 0.31 | 20 | 3.97 | 1.41 | 0.36 | 10 | 3.89 | 0.99 | 0.25 | |
| CSW Conc. (g/L) | Total Viable Cell (×1010) | Consumed Sugar (g/L) | LA Conc. (g/L) a | YLA (g/g) b | PLA (g/L/h) c | Max PLA (g/L/h) d at the Indicated Time |
|---|---|---|---|---|---|---|
| 20 | 68.3 ± 2.08 | 18.3 ± 0.37 | 16.1 ± 0.55 | 0.88 ± 0.02 | 0.33 ± 0.01 | 0.68 ± 0.06 (36 h) |
| 40 | 89.3 ± 7.02 | 28.0 ± 0.41 | 24.5 ± 0.11 | 0.87 ± 0.01 | 0.51 ± 0.01 | 0.65 ± 0.01(12 h) |
| 60 | 116.6 ± 2.08 | 36.9 ± 5.22 | 29.1 ± 0.87 | 0.80 ± 0.13 | 0.60 ± 0.01 | 0.96 ± 0.04 (12 h) |
| 80 | 31.3 ± 1.52 | 23.2 ± 0.37 | 20.1 ± 0.2 | 0.86 ± 0.01 | 0.41 ± 0.01 | 0.62 ± 0.02 (36 h) |
| 100 | 25.0 ± 3.0 | 13.2 ± 0.28 | 11.0 ± 0.1 | 0.82 ± 0.01 | 0.22 ± 0.01 | 0.33 ± 0.02 (12 h) |
| Inocula Sizes (v/v, %) | Total Viable Cell (×1010) | Consumed Sugar (g/L) | LA Conc. (g/L) a | YLA (g/g) b | PLA (g/L/h) c | Max PLA (g/L/h) d at the Indicated Time |
|---|---|---|---|---|---|---|
| 2.5 | 78.0 ± 3.60 | 25.4 ± 1.19 | 23.0 ± 0.15 | 0.90 ± 0.03 | 0.47 ± 0.01 | 0.83 ± 0.16 (36 h) |
| 5 | 98.0 ± 7.0 | 29.5 ± 0.56 | 26.8 ± 0.9 | 0.90 ± 0.01 | 0.55 ± 0.01 | 0.82 ± 0.12 (36 h) |
| 7.5 | 117.0 ± 2.64 | 36.6 ± 0.40 | 29.1 ± 0.87 | 0.79 ± 0.02 | 0.60 ± 0.01 | 0.96 ± 0.04 (12 h) |
| 10 | 121.3 ± 2.88 | 37.4 ± 1.01 | 32.8 ± 1.01 | 0.87 ± 0.04 | 0.68 ± 0.02 | 1.11 ± 0.07 (12 h) |
| 12.5 | 86.0 ± 6.24 | 26.4 ± 0.62 | 23.4 ± 1.34 | 0.88 ± 0.03 | 0.48 ± 0.02 | 0.59 ± 0.01 (12 h) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selim, M.T.; Salem, S.S.; Fouda, A.; El-Gamal, M.S.; Abdel-Rahman, M.A. Use of Corn-Steep Water Effluent as a Promising Substrate for Lactic Acid Production by Enterococcus faecium Strain WH51-1. Fermentation 2021, 7, 111. https://doi.org/10.3390/fermentation7030111
Selim MT, Salem SS, Fouda A, El-Gamal MS, Abdel-Rahman MA. Use of Corn-Steep Water Effluent as a Promising Substrate for Lactic Acid Production by Enterococcus faecium Strain WH51-1. Fermentation. 2021; 7(3):111. https://doi.org/10.3390/fermentation7030111
Chicago/Turabian StyleSelim, Mohamed T., Salem S. Salem, Amr Fouda, Mamdouh S. El-Gamal, and Mohamed Ali Abdel-Rahman. 2021. "Use of Corn-Steep Water Effluent as a Promising Substrate for Lactic Acid Production by Enterococcus faecium Strain WH51-1" Fermentation 7, no. 3: 111. https://doi.org/10.3390/fermentation7030111
APA StyleSelim, M. T., Salem, S. S., Fouda, A., El-Gamal, M. S., & Abdel-Rahman, M. A. (2021). Use of Corn-Steep Water Effluent as a Promising Substrate for Lactic Acid Production by Enterococcus faecium Strain WH51-1. Fermentation, 7(3), 111. https://doi.org/10.3390/fermentation7030111








