Fermentation Profile and Probiotic-Related Characteristics of Bifidobacterium longum MC-42
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain Reactivation and Preparation of Starting Inoculum
2.2. Assessment of Inhibition of Pathogens
2.3. Antibiotic Resistance Assays
2.4. Search for Genes of Interest in Genome
2.5. Cholesterol-Removal Capacity Assay
2.6. Fermentations on Milk and Milk Supplemented with Growth-Promoting Additives
2.7. Enzymatic Activity Profile and Peptidase Assays
2.8. Proteolytic, Antioxidant, and Angiotensin-I-Converting Enzyme Inhibitory Activities
3. Results and Discussion
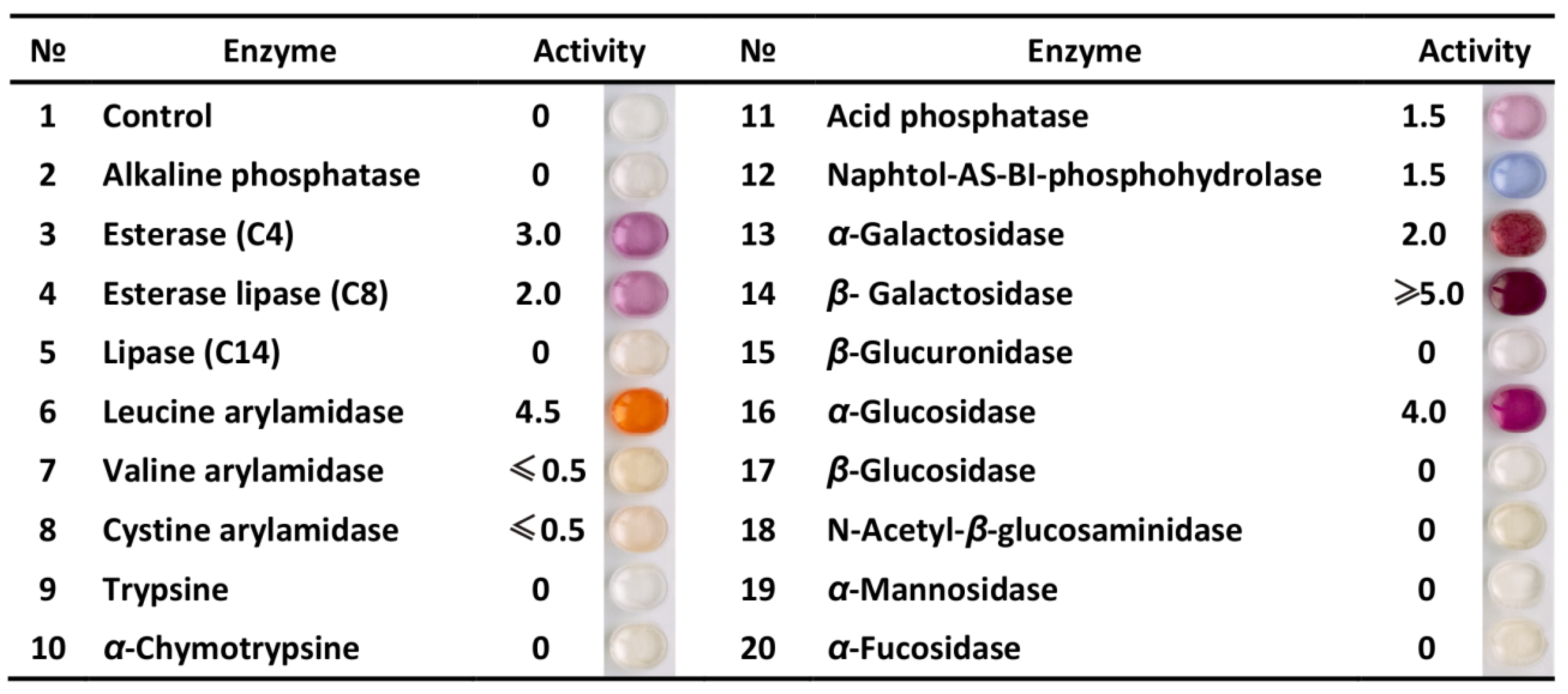
3.1. Profile of Enzymatic Activities
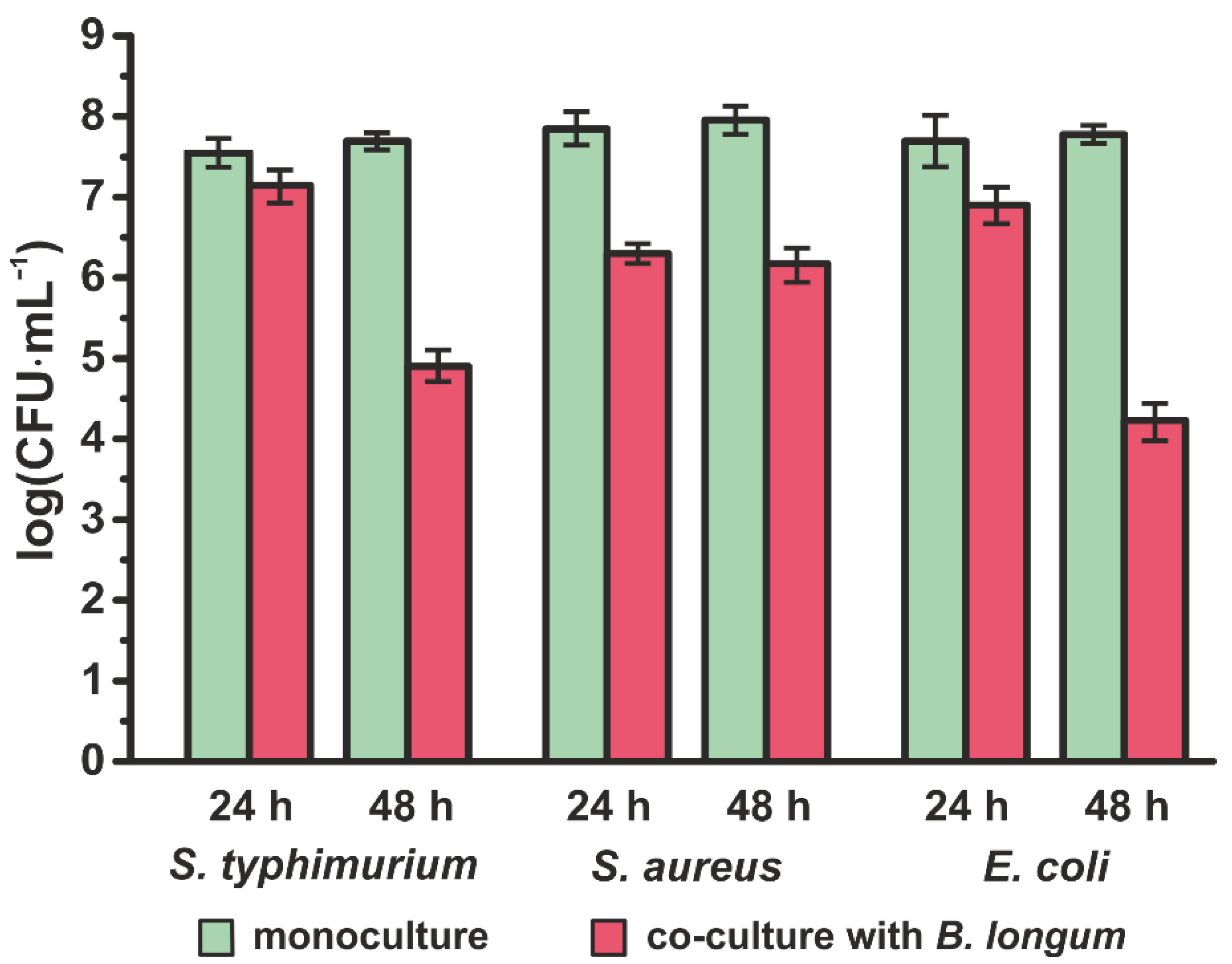
3.2. Inhibition of Pathogens
3.3. Resistance to Antibiotics
3.4. Cholesterol-Removal Capacity
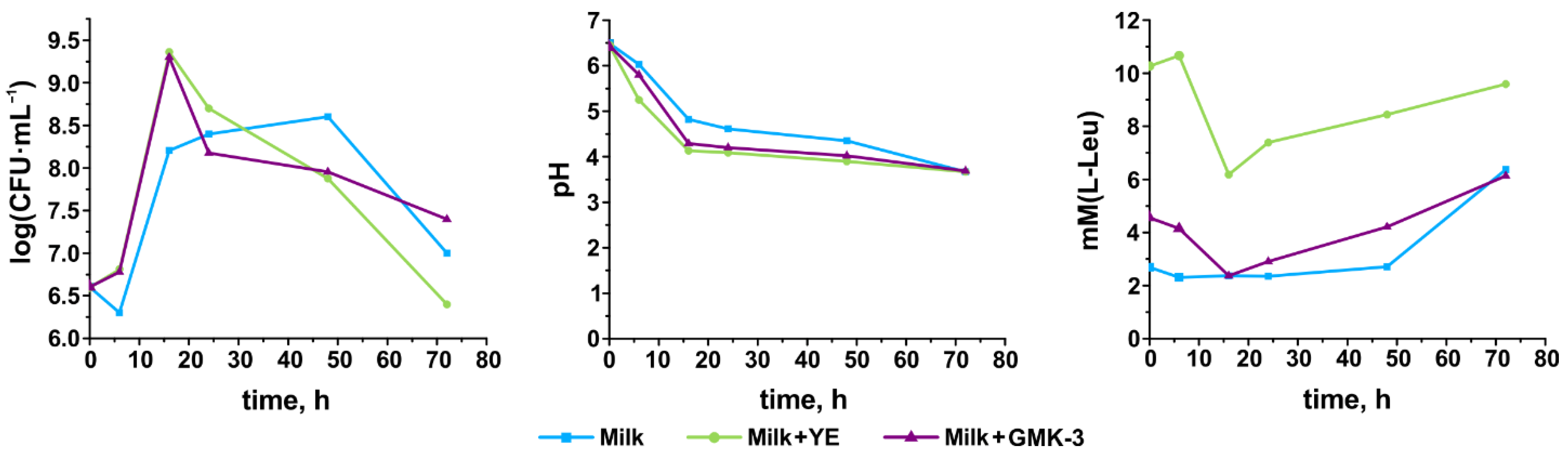
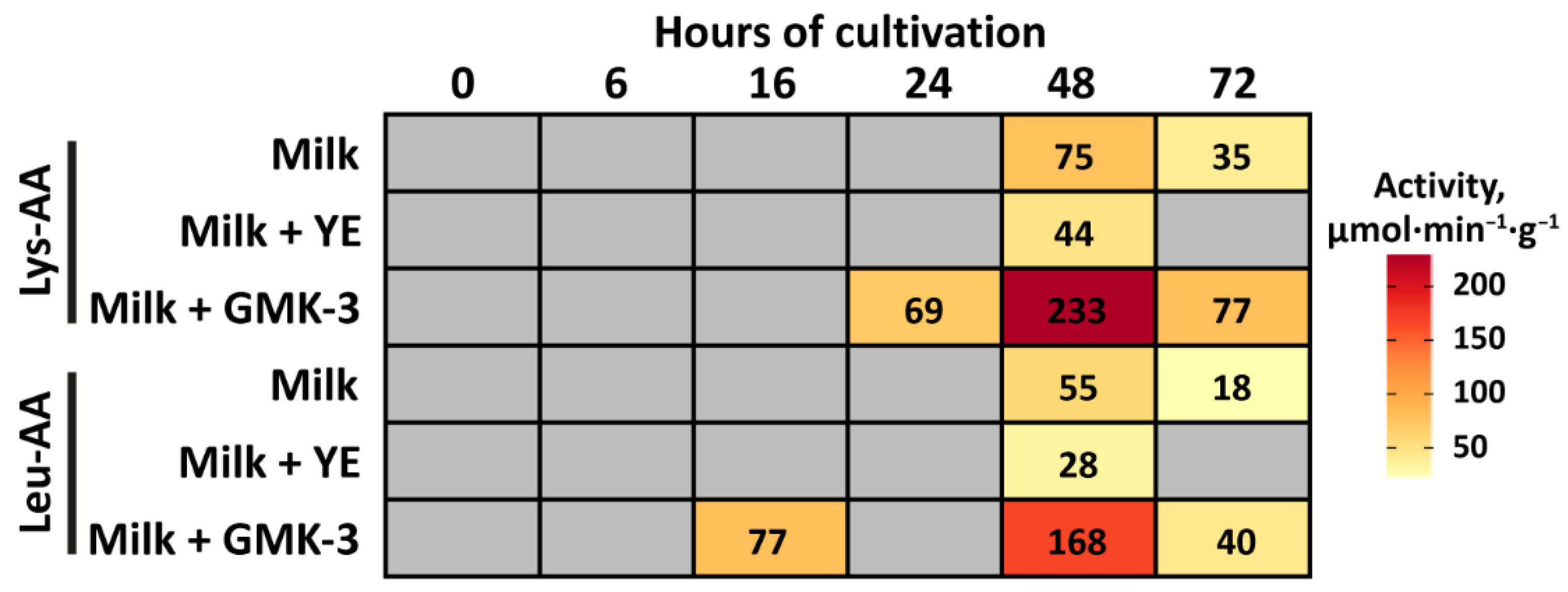
3.5. Growth Performance, Acidification Capability, and Degree of Proteolysis
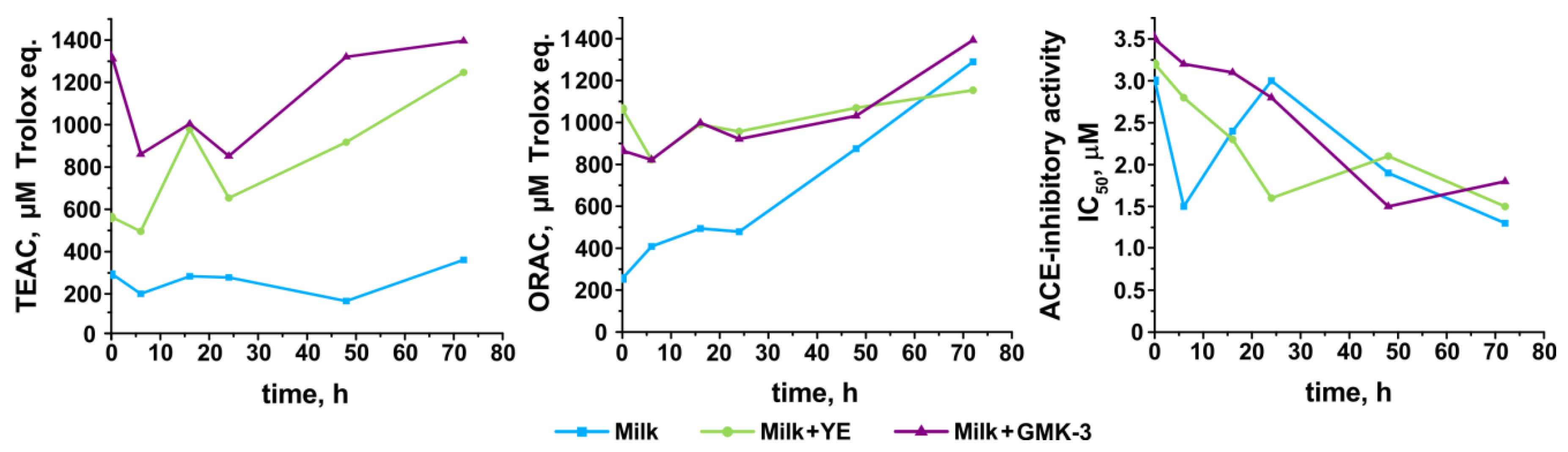
3.6. Development of Antioxidant and Antihypertensive Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saarela, M.; Mogensen, G.; Fondén, R.; Mättö, J.; Mattila-Sandholm, T. Probiotic bacteria: Safety, functional and technological properties. J. Biotechnol. 2000, 84, 197–215. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef] [PubMed]
- Ohland, C.L.; MacNaughton, W.K. Probiotic bacteria and intestinal epithelial barrier function. Am. J. Physiol. Liver Physiol. 2010, 298, G807–G819. [Google Scholar] [CrossRef] [Green Version]
- Begley, M.; Hill, C.; Gahan, C.G.M. Bile salt hydrolase activity in probiotics. Appl. Environ. Microbiol. 2006, 72, 1729–1738. [Google Scholar] [CrossRef] [Green Version]
- Maldonado Galdeano, C.; Cazorla, S.I.; Lemme Dumit, J.M.; Vélez, E.; Perdigón, G. Beneficial effects of probiotic consumption on the immune system. Ann. Nutr. Metab. 2019, 74, 115–124. [Google Scholar] [CrossRef]
- Wang, H.; Lee, I.-S.; Braun, C.; Enck, P. Effect of probiotics on central nervous system functions in animals and humans: A systematic review. J. Neurogastroenterol. Motil. 2016, 22, 589–605. [Google Scholar] [CrossRef]
- Korhonen, H. Milk-derived bioactive peptides: From science to applications. J. Funct. Foods 2009, 1, 177–187. [Google Scholar] [CrossRef]
- Lugli, G.A.; Milani, C.; Duranti, S.; Mancabelli, L.; Mangifesta, M.; Turroni, F.; Viappiani, A.; van Sinderen, D.; Ventura, M. Tracking the taxonomy of the genus Bifidobacterium based on a phylogenomic approach. Appl. Environ. Microbiol. 2017, 84, e02249-17. [Google Scholar] [CrossRef] [Green Version]
- Arboleya, S.; Watkins, C.; Stanton, C.; Ross, R.P. Gut bifidobacteria populations in human health and aging. Front. Microbiol. 2016, 7, 1204. [Google Scholar] [CrossRef] [Green Version]
- Picard, C.; Fioramonti, J.; Francois, A.; Robinson, T.; Neant, F.; Matuchansky, C. Review article: Bifidobacteria as probiotic agents—Physiological effects and clinical benefits. Aliment. Pharmacol. Ther. 2005, 22, 495–512. [Google Scholar] [CrossRef]
- Jungersen, M.; Wind, A.; Johansen, E.; Christensen, J.; Stuer-Lauridsen, B.; Eskesen, D. The science behind the probiotic strain Bifidobacterium animalis subsp. lactis BB-12®. Microorganisms 2014, 2, 92–110. [Google Scholar] [CrossRef]
- Ku, S.; Park, M.; Ji, G.; You, H. Review on Bifidobacterium bifidum BGN4: Functionality and nutraceutical applications as a probiotic microorganism. Int. J. Mol. Sci. 2016, 17, 1544. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.B.; Iwabuchi, N.; Xiao, J. Exploring the science behind Bifidobacterium breve M-16V in infant health. Nutrients 2019, 11, 1724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tupikin, A.E.; Kalmykova, A.I.; Kabilov, M.R. Draft genome sequence of the probiotic Bifidobacterium longum subsp. longum strain MC-42. Genome Announc. 2016, 4, e01411-16. [Google Scholar] [CrossRef] [Green Version]
- Naumova, N.; Alikina, T.; Tupikin, A.; Kalmykova, A.; Soldatova, G.; Vlassov, V.; Kabilov, M. Human gut microbiome response to short-term bifidobacterium-based probiotic treatment. Indian J. Microbiol. 2020, 60, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Coman, M.M.; Verdenelli, M.C.; Cecchini, C.; Silvi, S.; Orpianesi, C.; Boyko, N.; Cresci, A. In vitro evaluation of antimicrobial activity of Lactobacillus rhamnosus IMC 501®, Lactobacillus paracasei IMC 502® and SYNBIO® against pathogens. J. Appl. Microbiol. 2014, 117, 518–527. [Google Scholar] [CrossRef]
- Clark, K.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Sayers, E.W. GenBank. Nucleic Acids Res. 2016, 44, D67–D72. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, H.; Hashiba, H.; Kok, J.; Mierau, I. Bile salt hydrolase of Bifidobacterium longum—Biochemical and genetic characterization. Appl. Environ. Microbiol. 2000, 66, 2502–2512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanotti, I.; Turroni, F.; Piemontese, A.; Mancabelli, L.; Milani, C.; Viappiani, A.; Prevedini, G.; Sanchez, B.; Margolles, A.; Elviri, L.; et al. Evidence for cholesterol-lowering activity by Bifidobacterium bifidum PRL2010 through gut microbiota modulation. Appl. Microbiol. Biotechnol. 2015, 99, 6813–6829. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Exterkate, F.A. Location of peptidases outside and inside the membrane of Streptococcus cremoris. Appl. Environ. Microbiol. 1984, 47, 177–183. [Google Scholar] [CrossRef] [Green Version]
- Agarkova, E.Y.; Kruchinin, A.G.; Glazunova, O.A.; Fedorova, T.V. Whey protein hydrolysate and pumpkin pectin as nutraceutical and prebiotic components in a functional mousse with antihypertensive and bifidogenic properties. Nutrients 2019, 11, 2930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kruchinin, A.G.; Savinova, O.S.; Glazunova, O.A.; Moiseenko, K.V.; Agarkova, E.Y.; Fedorova, T.V. Hypotensive and hepatoprotective properties of the polysaccharide-stabilized foaming composition containing hydrolysate of whey proteins. Nutrients 2021, 13, 1031. [Google Scholar] [CrossRef] [PubMed]
- Torkova, A.A.; Ryazantseva, K.A.; Agarkova, E.Y.; Kruchinin, A.G.; Tsentalovich, M.Y.; Fedorova, T.V. Rational design of enzyme compositions for the production of functional hydrolysates of cow milk whey proteins. Appl. Biochem. Microbiol. 2017, 53, 669–679. [Google Scholar] [CrossRef]
- Indira, M.; Venkateswarulu, T.C.; Abraham Peele, K.; Nazneen Bobby, M.; Krupanidhi, S. Bioactive molecules of probiotic bacteria and their mechanism of action: A review. 3 Biotech 2019, 9, 306. [Google Scholar] [CrossRef]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef]
- Cockburn, D.W.; Koropatkin, N.M. Polysaccharide degradation by the intestinal microbiota and its influence on human health and disease. J. Mol. Biol. 2016, 428, 3230–3252. [Google Scholar] [CrossRef]
- Oak, S.J.; Jha, R. The effects of probiotics in lactose intolerance: A systematic review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1675–1683. [Google Scholar] [CrossRef]
- Nanno, M.; Morotomi, M.; Takayama, H.; Kuroshima, T.; Tanaka, R.; Mutai, M. Mutagenic activation of biliary metabolites of benzo(a)pyrene by β-glucuronidase-positive bacteria in human faeces. J. Med. Microbiol. 1986, 22, 351–355. [Google Scholar] [CrossRef] [Green Version]
- Davis, C.D.; Milner, J.A. Gastrointestinal microflora, food components and colon cancer prevention. J. Nutr. Biochem. 2009, 20, 743–752. [Google Scholar] [CrossRef] [Green Version]
- Arboleya, S.; Ruas-madiedo, P.; Margolles, A.; Solís, G.; Salminen, S.; Reyes-gavilán, C.G.D.L.; Gueimonde, M. Characterization and in vitro properties of potentially probiotic Bifidobacterium strains isolated from breast-milk. Int. J. Food Microbiol. 2011, 149, 28–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delgado, S.; O’Sullivan, E.; Fitzgerald, G.; Mayo, B. In vitro evaluation of the probiotic properties of human intestinal Bifidobacterium species and selection of new probiotic candidates. J. Appl. Microbiol. 2008, 104, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, M.L.; Roy, D.; Goulet, J. Growth of bifidobacteria and their enzyme profiles. J. Dairy Sci. 1990, 73, 299–307. [Google Scholar] [CrossRef]
- Martínez-Villaluenga, C.; Gómez, R. Characterization of bifidobacteria as starters in fermented milk containing raffinose family of oligosaccharides from lupin as prebiotic. Int. Dairy J. 2007, 17, 116–122. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Kim, J.S.; Kim, Y.G.; Jeong, Y.; Kim, J.E.; Paek, N.S.; Kang, C.H. Antioxidant and probiotic properties of lactobacilli and bifidobacteria of human origins. Biotechnol. Bioprocess Eng. 2020, 25, 421–430. [Google Scholar] [CrossRef]
- Kim, H.J.; Shin, S.I.; Lee, S.J.; Moon, T.W.; Lee, C.J. Screening and selection of Bifidobacterium strains isolated from human feces capable of utilizing resistant starch. J. Sci. Food Agric. 2018, 98, 5901–5907. [Google Scholar] [CrossRef]
- Corr, S.C.; Hill, C.; Gahan, C.G.M. Understanding the mechanisms by which probiotics inhibit gastrointestinal pathogens. Adv. Food Nutr. Res. 2009, 56, 1–15. [Google Scholar]
- Britton, R.A.; Versalovic, J. Probiotics and gastrointestinal infections. Interdiscip. Perspect. Infect. Dis. 2008, 2008, 1–10. [Google Scholar] [CrossRef]
- Isolauri, E.; Kirjavainen, P.V.; Salminen, S. Probiotics: A role in the treatment of intestinal infection and inflammation? Gut 2002, 50, iii54–iii59. [Google Scholar] [CrossRef]
- Cox, G.; Wright, G.D. Intrinsic antibiotic resistance: Mechanisms, origins, challenges and solutions. Int. J. Med. Microbiol. 2013, 303, 287–292. [Google Scholar] [CrossRef]
- Sharma, P.; Tomar, S.K.; Goswami, P.; Sangwan, V.; Singh, R. Antibiotic resistance among commercially available probiotics. Food Res. Int. 2014, 57, 176–195. [Google Scholar] [CrossRef]
- Álvarez-Cisneros, M.Y.; Ponce-Alquicira, E. Antibiotic resistance in lactic acid bacteria. In Antimicrobial Resistance—A Global Threat; IntechOpen: London, UK, 2019. [Google Scholar]
- Kumar, S.B.; Arnipalli, S.R.; Ziouzenkova, O. Antibiotics in food chain: The consequences for antibiotic resistance. Antibiotics 2020, 9, 688. [Google Scholar] [CrossRef]
- D’Aimmo, M.R.; Modesto, M.; Biavati, B. Antibiotic resistance of lactic acid bacteria and Bifidobacterium spp. isolated from dairy and pharmaceutical products. Int. J. Food Microbiol. 2007, 115, 35–42. [Google Scholar] [CrossRef]
- Moubareck, C.; Gavini, F.; Vaugien, L.; Butel, M.J.; Doucet-Populaire, F. Antimicrobial susceptibility of bifidobacteria. J. Antimicrob. Chemother. 2005, 55, 38–44. [Google Scholar] [CrossRef] [Green Version]
- Ammor, M.S.; Belén Flórez, A.; Mayo, B. Antibiotic resistance in non-enterococcal lactic acid bacteria and bifidobacteria. Food Microbiol. 2007, 24, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.C. Update on acquired tetracycline resistance genes. FEMS Microbiol. Lett. 2005, 245, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.; Kehrenberg, C.; Doublet, B.; Cloeckaert, A. Molecular basis of bacterial resistance to chloramphenicol and florfenicol. FEMS Microbiol. Rev. 2004, 28, 519–542. [Google Scholar] [CrossRef] [Green Version]
- Roberts, M.C. Update on macrolide-lincosamide-streptogramin, ketolide, and oxazolidinone resistance genes. FEMS Microbiol. Lett. 2008, 282, 147–159. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Nagpal, R.; Kumar, R.; Hemalatha, R.; Verma, V.; Kumar, A.; Chakraborty, C.; Singh, B.; Marotta, F.; Jain, S.; et al. Cholesterol-lowering probiotics as potential biotherapeutics for metabolic diseases. Exp. Diabetes Res. 2012, 2012, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Chand, D.; Avinash, V.S.; Yadav, Y.; Pundle, A.V.; Suresh, C.G.; Ramasamy, S. Molecular features of bile salt hydrolases and relevance in human health. Biochimica Biophysica Acta Gen. Subj. 2017, 1861, 2981–2991. [Google Scholar] [CrossRef] [PubMed]
- Kriaa, A.; Bourgin, M.; Mkaouar, H.; Jablaoui, A.; Akermi, N.; Soussou, S.; Maguin, E.; Rhimi, M. Microbial reduction of cholesterol to coprostanol: An old concept and new insights. Catalysts 2019, 9, 167. [Google Scholar] [CrossRef] [Green Version]
- Roy, D. Technological aspects related to the use of bifidobacteria in dairy products. Lait 2005, 85, 39–56. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Zhang, Y.; Li, H.; Liu, X. The potential of proteins, hydrolysates and peptides as growth factors for Lactobacillus and Bifidobacterium: Current research and future perspectives. Food Funct. 2020, 11, 1946–1957. [Google Scholar] [CrossRef]
- Poch, M.; Bezkorovainy, A. Growth-enhancing supplements for various species of the genus Bifidobacterium. J. Dairy Sci. 1988, 71, 3214–3221. [Google Scholar] [CrossRef]
- Shihata, A.; Shah, N.P. Proteolytic profiles of yogurt and probiotic bacteria. Int. Dairy J. 2000, 10, 401–408. [Google Scholar] [CrossRef]
- Meli, F.; Lazzi, C.; Neviani, E.; Gatti, M. Effect of protein hydrolizates on growth kinetics and aminopeptidase activities of some Bifidobacterium species. Anaerobe 2013, 22, 130–133. [Google Scholar] [CrossRef]
- Raveschot, C.; Cudennec, B.; Coutte, F.; Flahaut, C.; Fremont, M.; Drider, D.; Dhulster, P. Production of bioactive peptides by Lactobacillus species: From gene to application. Front. Microbiol. 2018, 9, 2354. [Google Scholar] [CrossRef] [Green Version]
- Udenigwe, C.C.; Aluko, R.E. Food protein-derived bioactive peptides: Production, processing, and potential health benefits. J. Food Sci. 2012, 77, R11–R24. [Google Scholar] [CrossRef]
- Zulueta, A.; Esteve, M.J.; Frígola, A. ORAC and TEAC assays comparison to measure the antioxidant capacity of food products. Food Chem. 2009, 114, 310–316. [Google Scholar] [CrossRef]
| Antibiotic | Amount, µg | Inhibition Zone Diameter, mm | Resistance Status |
|---|---|---|---|
| β-lactams (penams): | |||
| Ampicillin | 10 | 28 ± 0.5 | Susceptible |
| Amoxicillin | 20 | 28 ± 1 | Susceptible |
| Oxacillin | 1 | 12 ± 0.5 | Intermediate |
| Penicillin G | 10 | 28 ± 1 | Susceptible |
| Fosfomycins: | |||
| Fosfomycin | 200 | 23 ± 1 | Susceptible |
| Aminoglycosides: | |||
| Gentamicin | 120 | 10 ± 0.5 | Resistant |
| Kanamycin A | 30 | 11 ± 0.5 | Resistant |
| Neomycin | 30 | 7 ± 1 | Resistant |
| Tetracyclines: | |||
| Doxycycline | 30 | 32 ± 1 | Susceptible |
| Tetracycline | 30 | 28 ± 1 | Susceptible |
| Macrolides: | |||
| Azithromycin | 15 | 14 ± 1 | Intermediate |
| Lincosamides: | |||
| Lincomycin | 15 | 8 ± 1 | Resistant |
| Amphenicols: | |||
| Chloramphenicol | 30 | 26 ± 1 | Susceptible |
| Fluoroquinolones: | |||
| Levofloxacin | 5 | 14 ± 1 | Intermediate |
| Pefloxacin | 5 | 6 ± 1 | Resistant |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Begunova, A.V.; Rozhkova, I.V.; Glazunova, O.A.; Moiseenko, K.V.; Savinova, O.S.; Fedorova, T.V. Fermentation Profile and Probiotic-Related Characteristics of Bifidobacterium longum MC-42. Fermentation 2021, 7, 101. https://doi.org/10.3390/fermentation7030101
Begunova AV, Rozhkova IV, Glazunova OA, Moiseenko KV, Savinova OS, Fedorova TV. Fermentation Profile and Probiotic-Related Characteristics of Bifidobacterium longum MC-42. Fermentation. 2021; 7(3):101. https://doi.org/10.3390/fermentation7030101
Chicago/Turabian StyleBegunova, Anna V., Irina V. Rozhkova, Olga A. Glazunova, Konstantin V. Moiseenko, Olga S. Savinova, and Tatyana V. Fedorova. 2021. "Fermentation Profile and Probiotic-Related Characteristics of Bifidobacterium longum MC-42" Fermentation 7, no. 3: 101. https://doi.org/10.3390/fermentation7030101
APA StyleBegunova, A. V., Rozhkova, I. V., Glazunova, O. A., Moiseenko, K. V., Savinova, O. S., & Fedorova, T. V. (2021). Fermentation Profile and Probiotic-Related Characteristics of Bifidobacterium longum MC-42. Fermentation, 7(3), 101. https://doi.org/10.3390/fermentation7030101






