Utilization of Whey for Red Pigment Production by Monascus purpureus in Submerged Fermentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms and Culture Media
2.2. Preparation of Inoculum and Fermentation Medium
2.3. The Effect of Whey Type on Pigment Production
2.4. Optimization of Fermentation Parameters
2.5. Analytical Methods
2.6. Cost Analysis
3. Results and Discussion
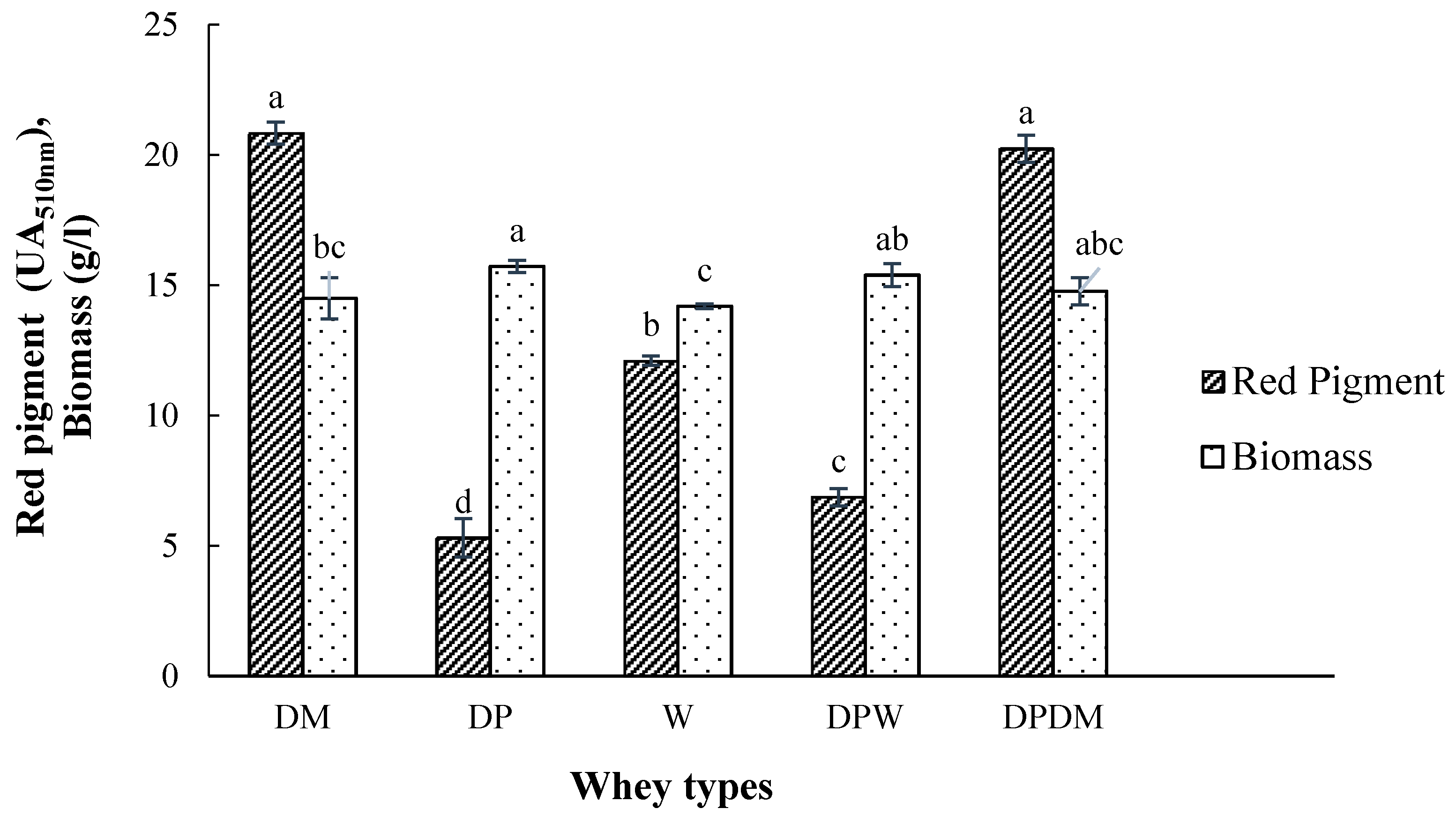
3.1. The Effect of Whey Type as Fermentation Substrate on Pigment Production
3.2. Effects of Different Fermentation Methods on Pigment Production
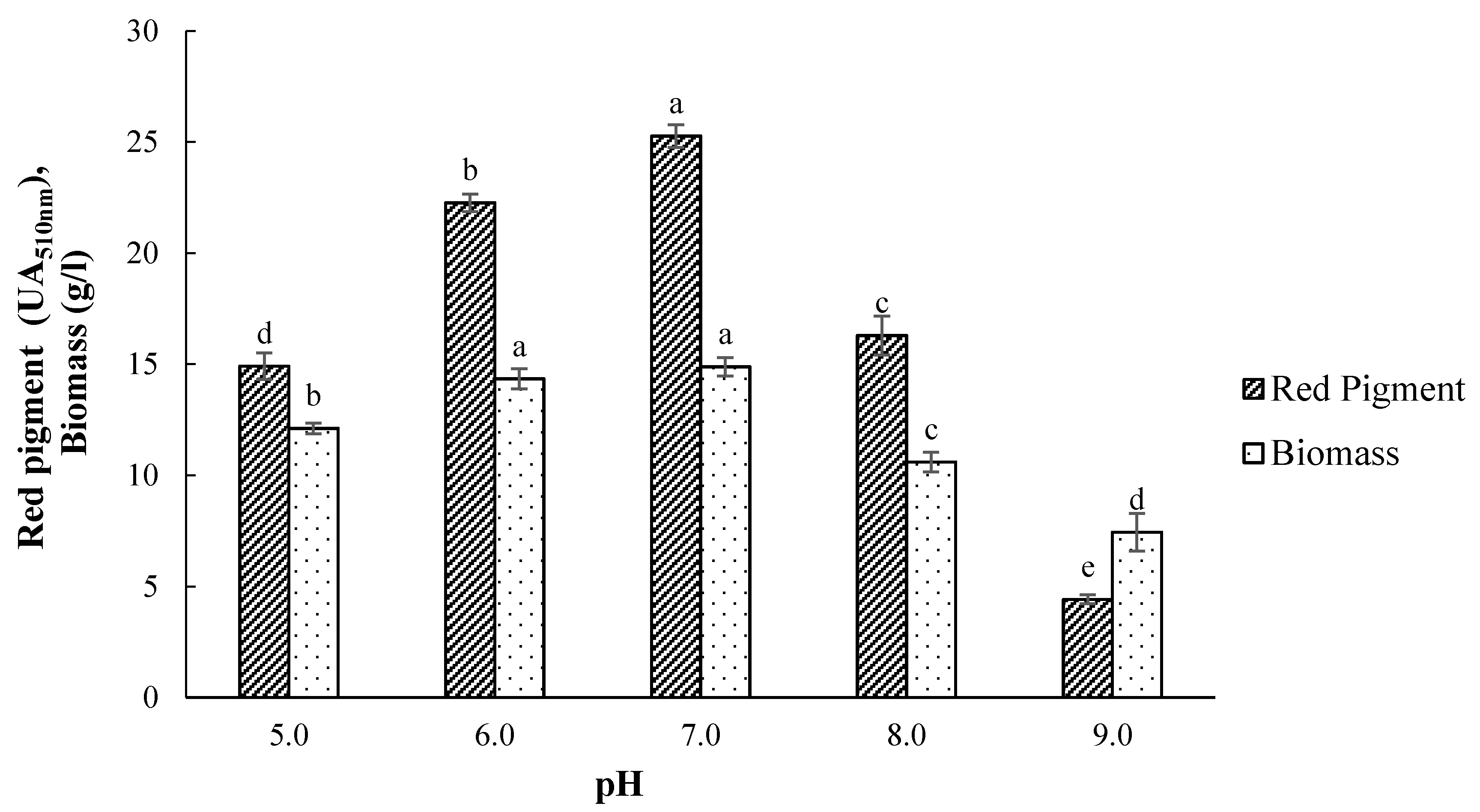
3.3. Effect of Initial pH on Pigment Production
3.4. Effect of Initial Lactose Concentration on Pigment Production
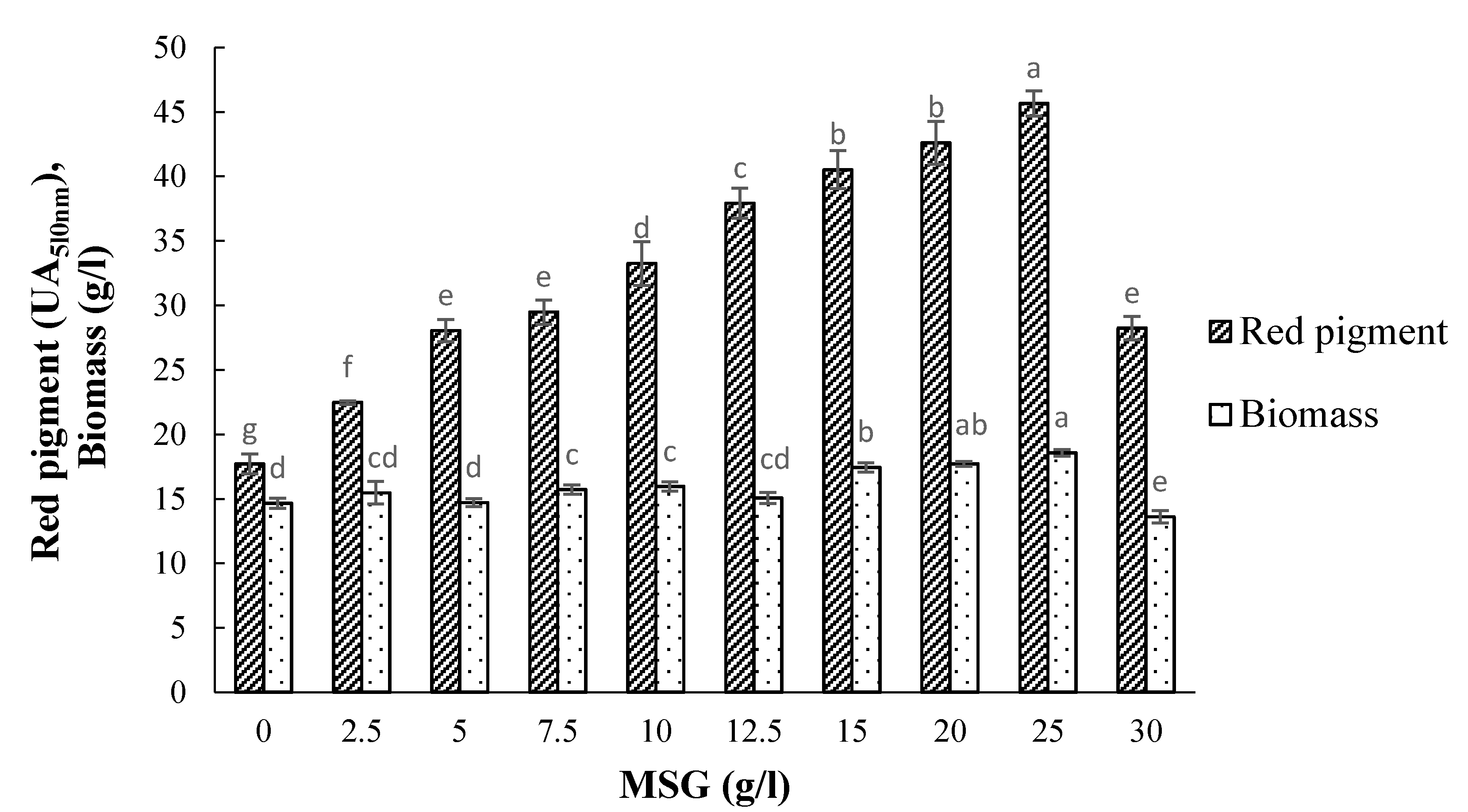
3.5. Effect of Monosodium Glutamate (MSG) Concentration on Pigment Production
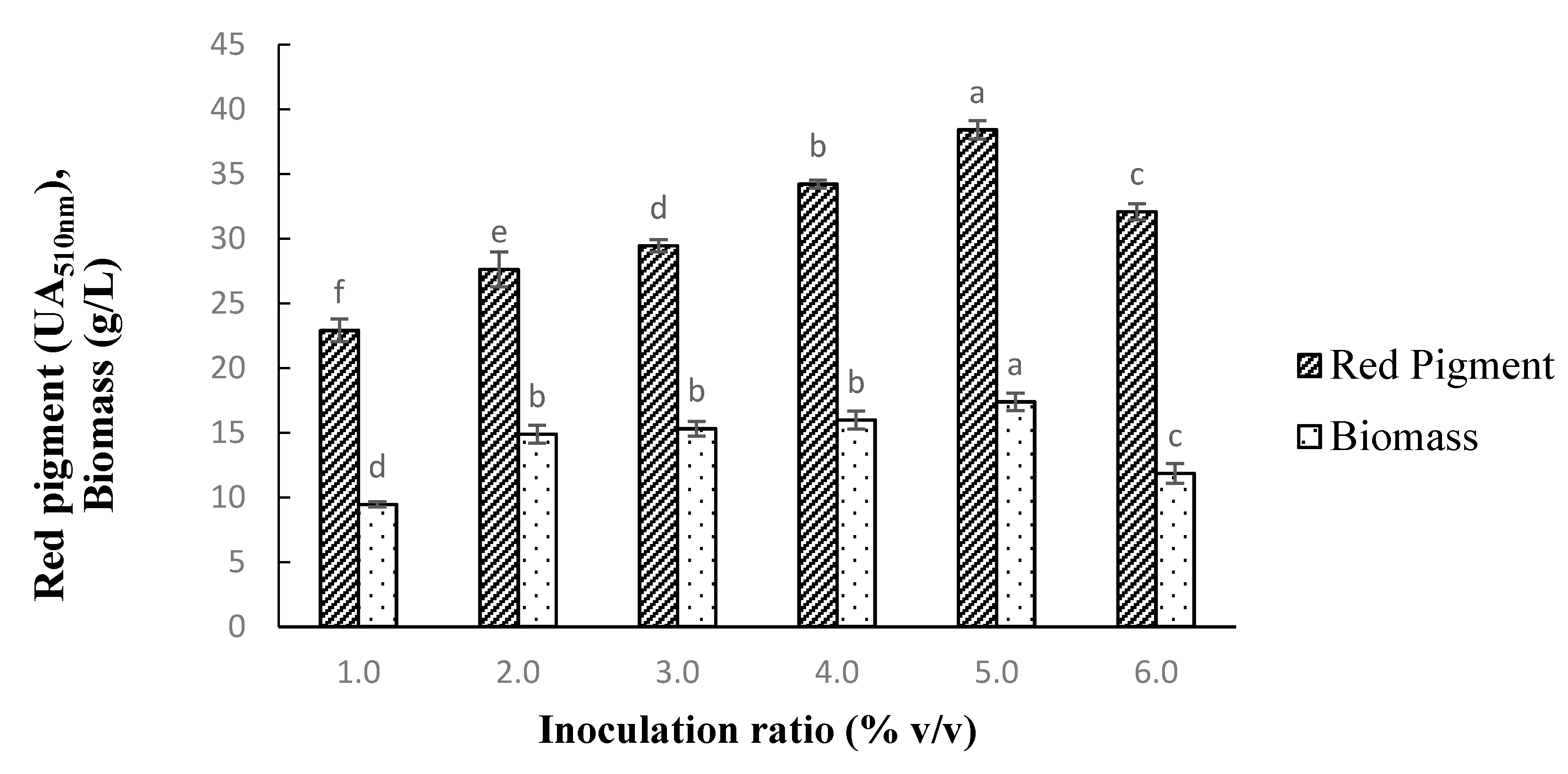
3.6. Effect of Inoculation Ratio on Pigment Production
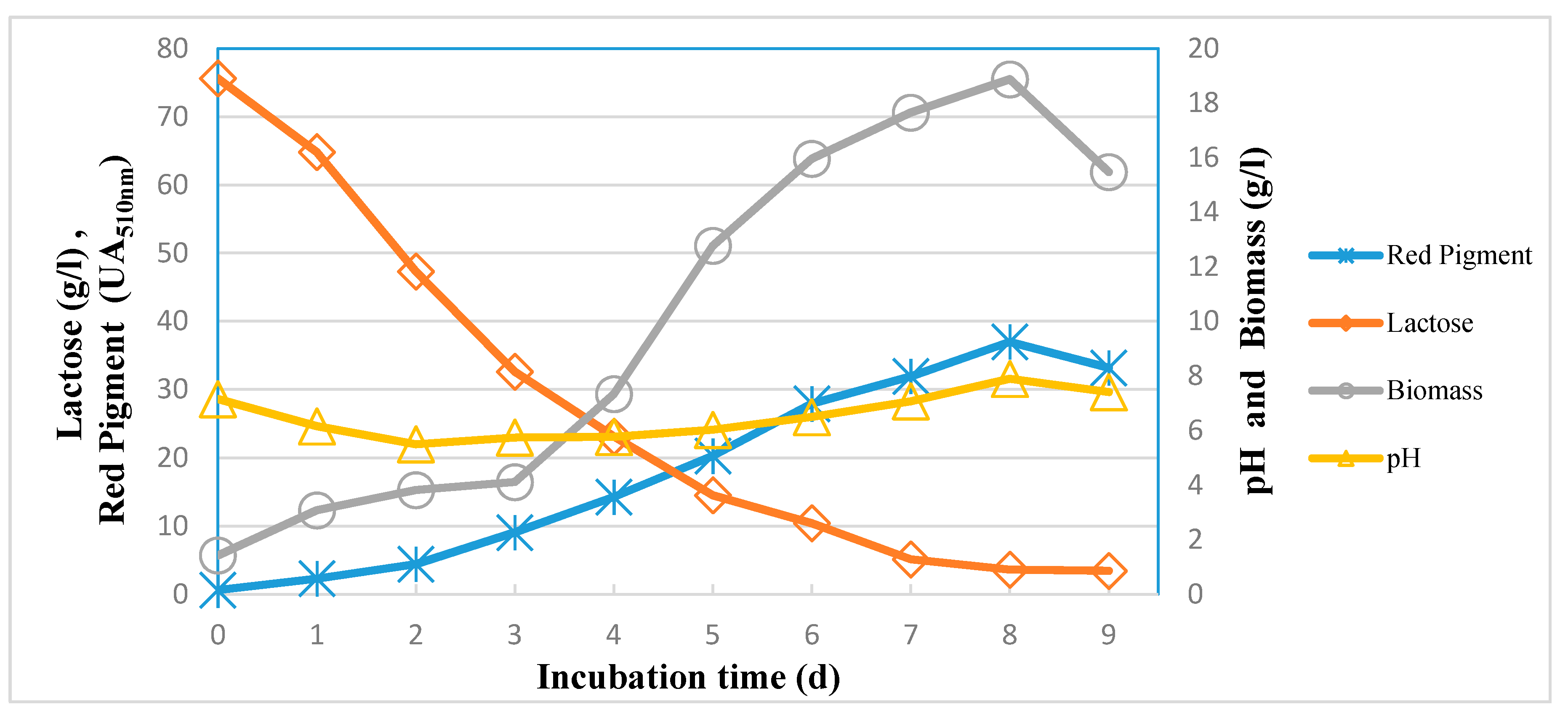
3.7. Kinetics of Red Pigment Production by M. purpureus
3.8. Assesment of Production Cost of Pigment Production
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| W | Whey powder |
| DM | Demineralized whey powder |
| DP | Deproteinized whey powder |
| DPDM | Treated deproteinized demineralized whey powder |
| DPW | Treated deproteinized raw whey powder |
| MSG | Monosodium glutamate |
| PHSF | Prehydrolysis and separate fermentation |
References
- Sharmila, G.; Nidhi, B.; Muthukumaran, C. Sequential statistical optimization of red pigment production by Monascus pur-pureus (MTCC 369) using potato powder. Ind. Crops Prod. 2013, 44, 158–164. [Google Scholar] [CrossRef]
- Nimnoi, P.; Pongsilp, N.; Lumyong, S. Utilization of agro-industrial products for increasing red pigment production of Monascus purpureus (AHK12). Chiang Mai J. Sci. 2015, 42, 331–338. [Google Scholar]
- Aberoumand, A. A review article on edible pigments properties and sources as natural biocolorants in foodstuff and food industry. World J. Dairy Food Sci. 2013, 6, 71–78. [Google Scholar]
- Panesar, R.; Kaur, S.; Panesar, P.S. Production of microbial pigments utilizing agro-industrial waste: A review. Curr. Opin. Food Sci. 2015, 1, 70–76. [Google Scholar] [CrossRef]
- Domínguez-Espinosa, R.M.; Webb, C. Submerged fermentation in wheat substrates for production of Monascus pigments. World J. Microbiol. Biotechnol. 2003, 19, 329–336. [Google Scholar] [CrossRef]
- Dufossé, L. Microbial production of food grade pigments. Food Technol. Biotechnol. 2006, 44, 313–321. [Google Scholar]
- Kumura, H.; Ohtsuyama, T.; Matsusaki, Y.; Taitoh, M.; Koyanagi, H.; Kobayashi, K.; Hayakawa, T.; Wakamatsu, J.; Ishizuka, S. Application of red pigment producing edible fungi for development of a novel type of functional cheese. J. Food Process. Preserv. 2018, 42, e13707. [Google Scholar] [CrossRef]
- Agboyibor, C.; Kong, W.-B.; Chen, D.; Zhang, A.-M.; Niu, S.-Q. Monascus pigments production, composition, bioactivity and its application: A review. Biocatal. Agric. Biotechnol. 2018, 16, 433–447. [Google Scholar] [CrossRef]
- Silbir, S.; Goksungur, Y. Natural red pigment production by Monascus purpureus in submerged fermentation systems using a food industry waste: Brewer’s spent grain. Foods 2019, 8, 161. [Google Scholar] [CrossRef]
- Silveira, S.T.; Daroit, D.J.; Brandelli, A. Pigment production by Monascus purpureus in grape waste using factorial design. LWT Food Sci. Technol. 2008, 41, 170–174. [Google Scholar] [CrossRef]
- Feng, Y.; Shao, Y.; Chen, F. Monascus pigments. Appl. Microbiol. Biotechnol. 2012, 96, 1421–1440. [Google Scholar] [CrossRef] [PubMed]
- Vendruscolo, F.; Müller, B.L.; Moritz, D.E.; Oliveira, D.; Schmidell, W.; Ninow, J.L. Thermal stability of natural pigments pro-duced by Monascus ruber in submerged fermentation. Biocatal. Agric. Biotechnol. 2013, 2, 278–284. [Google Scholar] [CrossRef]
- Günerhan, Ü.; Us, E.; Dumlu, L.; Yılmaz, V.; Carrère, H.; Perendeci, A. Impacts of chemical-assisted thermal pretreatments on methane production from fruit and vegetable harvesting wastes: Process optimization. Molecules 2020, 25, 500. [Google Scholar] [CrossRef]
- Liu, J.; Luo, Y.; Guo, T.; Tang, C.; Chai, X.; Zhao, W.; Bai, J.; Lin, Q. Cost-effective pigment production by Monascus purpureus using rice straw hydrolysate as substrate in submerged fermentation. J. Biosci. Bioeng. 2020, 129, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Atalay, P.; Sargın, S.; Goksungur, Y. Utilization of residual beer for red pigment production by Monascus purpureus in submerged fermantation. Fresenius Environ. Bull. 2020, 29, 1025–1034. [Google Scholar]
- Kantifedaki, A.; Kachrimanidou, V.; Mallouchos, A.; Papanikolaou, S.; Koutinas, A. Orange processing waste valorisation for the production of bio-based pigments using the fungal strains Monascus purpureus and Penicillium purpurogenum. J. Clean. Prod. 2018, 185, 882–890. [Google Scholar] [CrossRef]
- Orak, T.; Caglar, O.; Ortucu, S.; Ozkan, H.; Taskin, M. Chicken feather peptone: A new alternative nitrogen source for pigment production by Monascus purpureus. J. Biotechnol. 2018, 271, 56–62. [Google Scholar] [CrossRef]
- Silveira, S.T.; Daroit, D.J.; Sant’Anna, V.; Brandelli, A. Stability Modeling of red pigments produced by Monascus purpureus in submerged cultivations with Sugarcane Bagasse. Food Bioprocess Technol. 2011, 6, 1007–1014. [Google Scholar] [CrossRef]
- Hilares, R.T.; de Souza, R.A.; Marcelino, P.F.; da Silva, S.S.; Dragone, G.; Mussatto, S.I.; Santos, J.C. Sugarcane bagasse hy-drolysate as a potential feedstock for red pigment production by Monascus ruber. Food Chem. 2018, 245, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Haque, A.; Kachrimanidou, V.; Koutinas, A.; Lin, C.S.K. Valorization of bakery waste for biocolorant and enzyme production by Monascus purpureus. J. Biotechnol. 2016, 231, 55–64. [Google Scholar] [CrossRef]
- Singh, N.; Goel, G.; Singh, N.; Pathak, B.K.; Kaushik, D. Modeling the red pigment production by Monascus purpureus MTCC 369 by Artificial Neural Network using rice water based medium. Food Biosci. 2015, 11, 17–22. [Google Scholar] [CrossRef]
- Srivastav, P.; Yadav, V.K.; Govindasamy, S.; Chandrasekaran, M. Red pigment production by Monascus purpureus using sweet potato-based medium in submerged fermentation. Nutrafoods 2015, 14, 159–167. [Google Scholar] [CrossRef]
- Velmurugan, P.; Hur, H.; Balachandar, V.; Kamala-Kannan, S.; Lee, K.-J.; Lee, S.-M.; Chae, J.-C.; Shea, P.J.; Oh, B.-T. Monascus pigment production by solid-state fermentation with corn cob substrate. J. Biosci. Bioeng. 2011, 112, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Yin, Z.; Hu, X. Corncob hydrolysate, an efficient substrate for Monascus pigment production through submerged fermentation. Biotechnol. Appl. Biochem. 2014, 61, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Hamano, P.S.; Kilikian, B.V. Production of red pigments by Monascus ruber in culture media containing corn steep liquor. Braz. J. Chem. Eng. 2006, 23, 443–449. [Google Scholar] [CrossRef]
- Babitha, S.; Soccol, C.R.; Pandey, A. Solid-state fermentation for the production of Monascus pigments from jackfruit seed. Bioresour. Technol. 2007, 98, 1554–1560. [Google Scholar] [CrossRef]
- Hamdi, M.; Blanc, P.J.; Goma, G. Effect of aeration conditions on the production of red pigments by Monascus purpureus growth on prickly pear juice. Process. Biochem. 1996, 31, 543–547. [Google Scholar] [CrossRef]
- Güven, G.; Perendeci, A.; Özdemir, K.; Tanyolaç, A. Specific energy consumption in electrochemical treatment of food industry wastewaters. J. Chem. Technol. Biotechnol. 2012, 87, 513–522. [Google Scholar] [CrossRef]
- Hausjell, J.; Miltner, M.; Herzig, C.; Limbeck, A.; Saracevic, Z.; Saracevic, E.; Weissensteiner, J.; Molitor, C.; Halbwirth, H.; Spadiut, O.; et al. Valorisation of cheese whey as substrate and inducer for recombinant protein production in E. coli HMS174(DE3). Bioresour. Technol. Rep. 2019, 8, 100340. [Google Scholar] [CrossRef]
- Nadais, M.H.; Capela, M.I.; Arroja, L.M.; Hung, Y.T. Anaerobic treatment of milk processing wastewater. In Environmental Bioengineering; Humana Press: Totowa, NJ, USA, 2010; Volume 11, pp. 555–618. [Google Scholar]
- Britz, J.T.; van Schalwyk, C.; Hung, Y.T. Treatment of dairy processing wastewaters. In Waste Treatment in the Food Processing Industry; Wang, L.K., Hung, Y.T., Lo, H.H., Yapijakis, C., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 1–25. [Google Scholar]
- Karadag, D.; Köroğlu, O.E.; Ozkaya, B.; Cakmakci, M. A review on anaerobic biofilm reactors for the treatment of dairy industry wastewater. Process. Biochem. 2015, 50, 262–271. [Google Scholar] [CrossRef]
- Yadav, J.S.; Yan, S.; Pilli, S.; Kumar, L.; Tyagi, R.D.; Surampalli, R.Y. Cheese whey: A potential resource to transform into bio-protein, functional/nutritional proteins and bioactive peptides. Biotechnol. Adv. 2015, 33, 756–774. [Google Scholar] [CrossRef]
- Smithers, G.W. Whey-ing up the options—Yesterday, today and tomorrow. Int. Dairy J. 2015, 48, 2–14. [Google Scholar]
- Prazeres, A.R.; Carvalho, F.; Rivas, J. Cheese whey management: A review. J. Environ. Manag. 2012, 110, 48–68. [Google Scholar] [CrossRef]
- Das, M.; Raychaudhuri, A.; Ghosh, S.K. Supply chain of bioethanol production from whey: A review. Procedia Environ. Sci. 2016, 35, 833–846. [Google Scholar] [CrossRef]
- Şilbir, S. Bira Atığından Monascus Renk Pigmentleri Üretimi ve Stabilitesinin Belirlenmesi. Ph.D. Thesis, Ege University, Bornova/İzmir, Turkey, 2019; p. 590713. (In Turkish). [Google Scholar]
- Roukas, T.; Mantzouridou, F.; Boumpa, T.; Vafiadou, A.; Goksungur, Y. Production of β-carotene from beet molasses and deproteinized whey by Blakeslea trispora. Food Biotechnol. 2003, 17, 203–215. [Google Scholar] [CrossRef]
- Mukherjee, G.; Singh, S.K. Purification and characterization of a new red pigment from Monascus purpureus in submerged fermentation. Process. Biochem. 2011, 46, 188–192. [Google Scholar] [CrossRef]
- Shi, K.; Song, D.; Chen, G.; Pistolozzi, M.; Wu, Z.; Quan, L. Controlling composition and color characteristics of Monascus pigments by pH and nitrogen sources in submerged fermentation. J. Biosci. Bioeng. 2015, 120, 145–154. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Kjeldahl, J.G.C. En ny Methode til Kvaelstofvestemmelse i organiske Stoffer. Z. Anal. Chem. 1883, 22, 366–382. (In German) [Google Scholar] [CrossRef]
- Cataldi, T.R.I.; Angelotti, M.; D’Erchia, L.; Altieri, G.; Di Renzo, G.C. Ion-exchange chromatographic analysis of soluble cations, anions and sugars in milk whey. Eur. Food Res. Technol. 2003, 216, 75–82. [Google Scholar] [CrossRef]
- Duke, M.; Vasiljevic, T. Whey demineralization with membrane operations. In Encyclopedia of Membranes; Droli, E., Giorno, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Da Costa, J.P.V.; Vendruscolo, F. Production of red pigments by Monascus ruber CCT 3802 using lactose as a substrate. Biocatal. Agric. Biotechnol. 2017, 11, 50–55. [Google Scholar] [CrossRef]
- Lopes, F.C.; Tichota, D.M.; Pereira, J.Q.; Segalin, J.; Rios, A.D.O.; Brandelli, A. Pigment production by filamentous fungi on agro-industrial byproducts: An eco-friendly alternative. Appl. Biochem. Biotechnol. 2013, 171, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Pisareva, E.; Kujumdzieva, A. Taxonomic investigation and growth characteristics of citrinin free Monascus pilosus C1 strain. Biotechnol. Biotechnol. Equip. 2006, 20, 88–96. [Google Scholar] [CrossRef]
- Prajapati, V.S.; Soni, N.; Trivedi, U.B.; Patel, K.C. An enhancement of red pigment production by submerged culture of Monascus purpureus MTCC 410 employing statistical methodology. Biocatal. Agric. Biotechnol. 2014, 3, 140–145. [Google Scholar] [CrossRef]
- Kang, B.; Zhang, X.; Wu, Z.; Qi, H.; Wang, Z. Effect of pH and nonionic surfactant on profile of intracellular and extracellular Monascus pigments. Process. Biochem. 2013, 48, 759–767. [Google Scholar] [CrossRef]
- Orozco, S.F.B.; Kilikian, B.V. Effect of pH on citrinin and red pigments production by Monascus purpureus CCT3802. World J. Microbiol. Biotechnol. 2007, 24, 263–268. [Google Scholar] [CrossRef]
- Lee, B.-K.; Park, N.-H.; Piao, H.Y.; Chung, W.-J. Production of red pigments by Monascus purpureus in submerged culture. Biotechnol. Bioprocess Eng. 2001, 6, 341–346. [Google Scholar] [CrossRef]
- Chen, M.-H.; Johns, M.R. Effect of carbon source on ethanol and pigment production by Monascus purpureus. Enzym. Microb. Technol. 1994, 16, 584–590. [Google Scholar] [CrossRef]
- Liu, J.; Guo, T.; Luo, Y.; Chai, X.; Wu, J.; Zhao, W.; Jiao, P.; Luo, F.; Lin, Q. Enhancement of Monascus pigment productivity via a simultaneous fermentation process and separation system using immobilized-cell fermentation. Bioresour. Technol. 2019, 272, 552–560. [Google Scholar] [CrossRef]
- Wong, H.C.; Lin, Y.C.; Koehler, P.E. Regulation of growth and pigmentation of Monascus purpureus by carbon and nitrogen concentrations. Mycologia 1981, 73, 649–654. [Google Scholar] [CrossRef]
- Pastrana, L.; Blanc, P.; Santerre, A.; Loret, M.; Goma, G. Production of red pigments by Monascus ruber in synthetic media with a strictly controlled nitrogen source. Process. Biochem. 1995, 30, 333–341. [Google Scholar] [CrossRef]
- Meinicke, R.M.; Vendruscolo, F.; Moritz, D.E.; de Oliveira, D.; Schmidell, W.; Samohyl, R.W.; Ninow, J.L. Potential use of glycerol as substrate for the production of red pigments by Monascus ruber in submerged fermentation. Biocatal. Agric. Biotechnol. 2012, 1, 238–242. [Google Scholar] [CrossRef]
- Zhang, X.-W.; Wang, J.-H.; Chen, M.-H.; Wang, C.-L. Effect of nitrogen sources on production and photostability of Monascus pigments in liquid fermentation. IERI Procedia 2013, 5, 344–350. [Google Scholar] [CrossRef]
- Perendeci, N.A.; Gökgöl, S.; Orhon, D. Impact of alkaline H2O2 pretreatment on methane generation potential of greenhouse crop waste under anaerobic conditions. Molecules 2018, 23, 1794. [Google Scholar] [CrossRef]


| Agricultural Waste Material | Pigment Yield (mg/g Dry Substrate) | Waste Material (g Dry Substrate/g Pigment) | Cost ($/kg) | References |
|---|---|---|---|---|
| Soy bean residues (Supp. with 6% glucose) | 1.65 | 606.06 | [2] | |
| Coconut residues (Supp. with 6% glucose) | 5.65 | 176.99 | [2] | |
| Bagasse (Supp. with 6% glucose) | 7.5 | 133.33 | [2] | |
| Corn meal (Supp. with 6% glucose) | 20.86 | 47.94 | [2] | |
| Corn meal (Supp. with 6% glucose + 1% whey) | 47.42 | 21.09 | [2] | |
| Demineralized whey (6% glucose equivalent) | 133.3 | 7.50 | 14.92 | This Study |
| Glucose (6%) | 166.67 | 6.00 | 14.84 | Theoretical |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehri, D.; Perendeci, N.A.; Goksungur, Y. Utilization of Whey for Red Pigment Production by Monascus purpureus in Submerged Fermentation. Fermentation 2021, 7, 75. https://doi.org/10.3390/fermentation7020075
Mehri D, Perendeci NA, Goksungur Y. Utilization of Whey for Red Pigment Production by Monascus purpureus in Submerged Fermentation. Fermentation. 2021; 7(2):75. https://doi.org/10.3390/fermentation7020075
Chicago/Turabian StyleMehri, Dilara, N. Altinay Perendeci, and Yekta Goksungur. 2021. "Utilization of Whey for Red Pigment Production by Monascus purpureus in Submerged Fermentation" Fermentation 7, no. 2: 75. https://doi.org/10.3390/fermentation7020075
APA StyleMehri, D., Perendeci, N. A., & Goksungur, Y. (2021). Utilization of Whey for Red Pigment Production by Monascus purpureus in Submerged Fermentation. Fermentation, 7(2), 75. https://doi.org/10.3390/fermentation7020075






