Enzymatic Esterification as Potential Strategy to Enhance the Sorbic Acid Behavior as Food and Beverage Preservative
Abstract
1. Introduction
2. Materials and Methods
2.1. Enzyme and Chemicals
2.2. Synthesis of Glycerol Ester of Sorbic Acid
2.3. Thin Layer Chromatography (TLC)
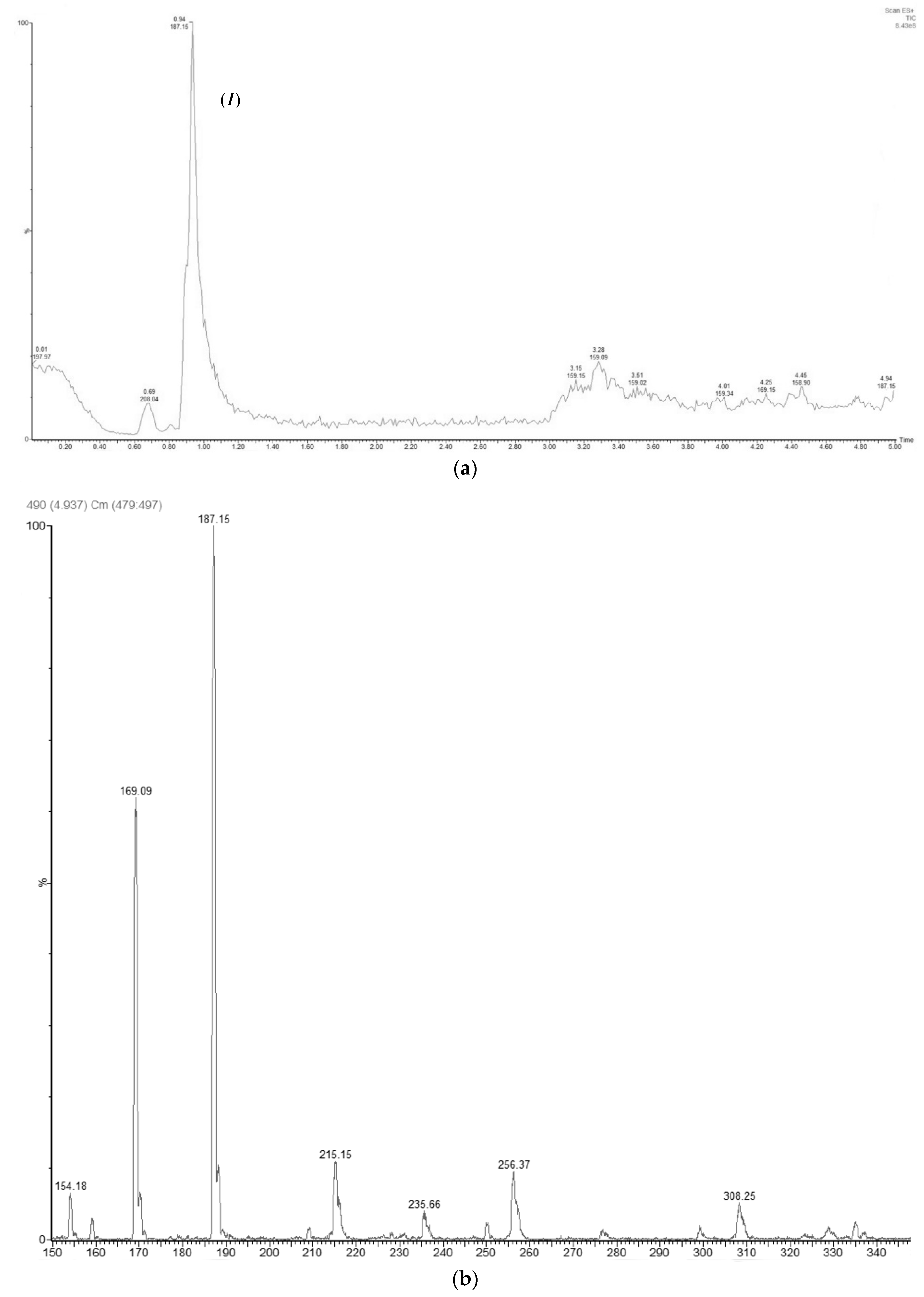
2.4. Purification and Spectroscopic Characterization of Glycerol Ester of Sorbic Acid
2.5. Analytical uHPLC-MS Method
2.6. Evaluation of Antibacterial and Antifungal Activity
3. Results and Discussion
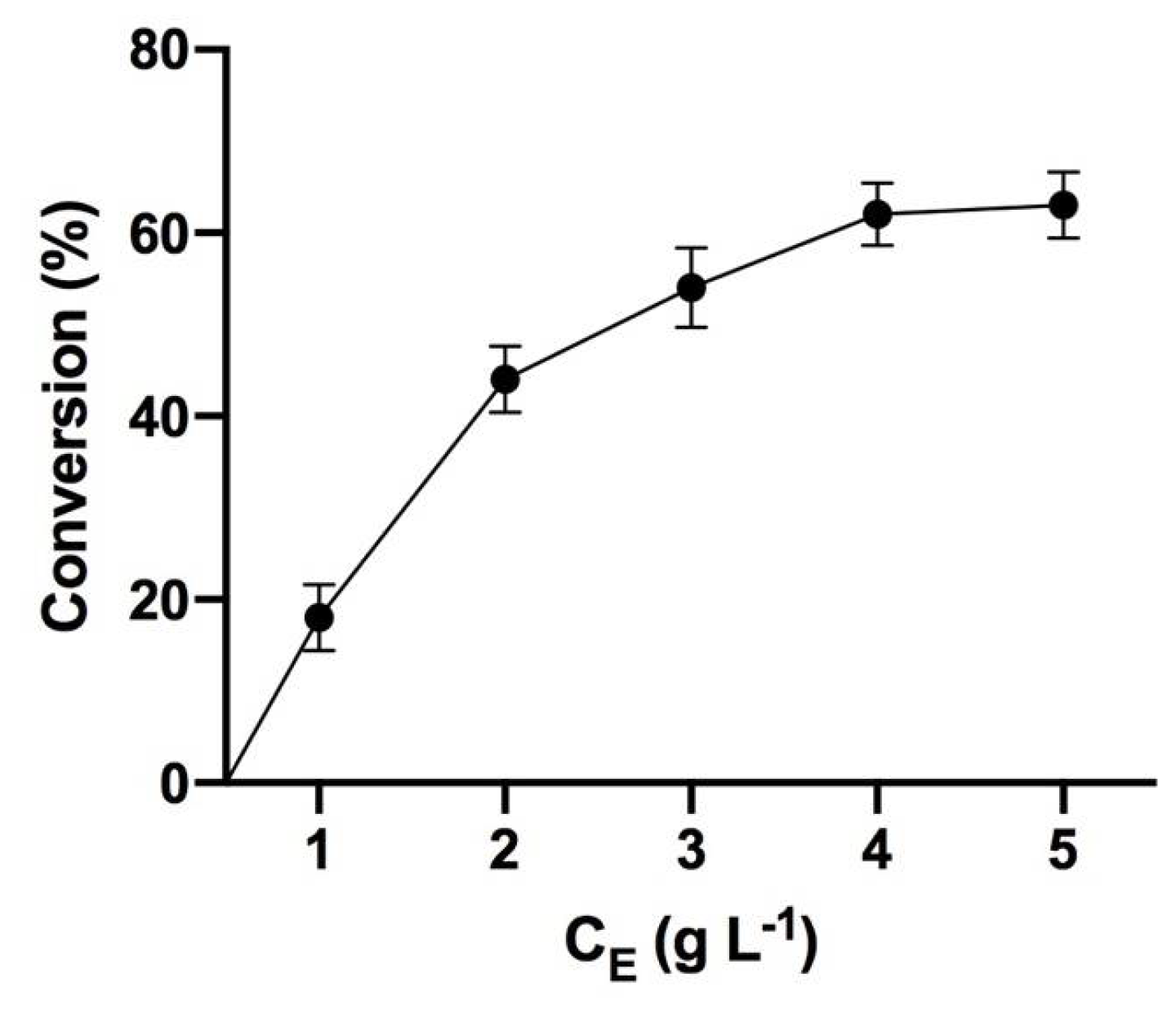
3.1. Sorbic Acid Monoglyceride Production
3.2. Antimicrobial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sofos, J.N. Sorbate Food Preservatives; CRC Press: Boca Raton, FL, USA, 1989; 248p. [Google Scholar]
- Liewen, M.B.; Marth, E.H. Growth and Inhibition of Microorganisms in the Presence of Sorbic Acid: A Review. J. Food Prot. 1985, 48, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Naidu, A.S. Natural Food Antimicrobial Systems. Nat. Food Antimicrob. Syst. 2000. [Google Scholar] [CrossRef]
- Harrison, R. Food Preservatives. Springer Sci. Bus. Media 1906, 168, 4338. [Google Scholar] [CrossRef]
- Sofos, J.N.; Pierson, M.D.; Blocher, J.C.; Busta, F.F. Mode of Action of Sorbic Acid on Bacterial Cells and Spores. Int. J. Food Microbiol. 1986, 3, 1–17. [Google Scholar] [CrossRef]
- York, G.K.; Vaughn, R.H. Mechanisms in the Inhibition of Microorganisms by Sorbic Acid. J. Bacteriol. 1964, 88, 411–417. [Google Scholar] [CrossRef]
- Smoot, L.A.; Pierson, M.D. Mechanisms of Sorbate Inhibition of Bacillus cereus T and Clostridium botulinum 62A Spore Germination. Appl. Environ. Microbiol. 1981, 42, 477–483. [Google Scholar] [CrossRef]
- Costilow, R.; Ferguson, W.; Ray, S. Sorbic Acid as a Selective Agent in Cucumber Fermentations. Appl. Microbiol. 1955, 6, 341–345. [Google Scholar] [CrossRef]
- Sayeed, S.A.; Sankaran, R. Action of Sorbic Acid on Staphylococcus Metabolism: A Microcalorimetric Investigation. Indian J. Exp. Biol. 1991, 29, 628–630. [Google Scholar]
- Kinderlerer, P.V. Fungal Metabolites of Sorbic Acid. Food Addit. Contam. 1990, 7, 657–669. [Google Scholar] [CrossRef]
- Lide, D.R.; Baysinger, G. Physical Constants of Organic Compounds. CRC Handb. Chem. Phys. 2019, 313–576. [Google Scholar] [CrossRef]
- Hoag, S.W.; Hussain, A.S. The Impact of Formulation on Bioavailability. J. Nutr. 2001, 131, 1389–1391. [Google Scholar] [CrossRef] [PubMed]
- Guzman, J.D. Natural Cinnamic Acids, Synthetic Derivatives and Hybrids with Antimicrobial Activity. Molecules 2014, 19, 19292–19349. [Google Scholar] [CrossRef] [PubMed]
- Kabara, J.J. Antimicrobial Agents Derived from Fatty Acids. J. Am. Oil Chem. Soc. 1984, 61, 397–403. [Google Scholar] [CrossRef]
- Jahangiri, A.; Møller, A.H.; Danielsen, M.; Madsen, B.; Joernsgaard, B.; Vaerbak, S.; Adlercreutz, P.; Dalsgaard, T.K. Hydrophilization of Bixin by Lipase-Catalyzed Transesterification with Sorbitol. Food Chem. 2018, 268, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Dhar, K.; Kanwar, S.S.; Arora, P.K. Lipase Catalysis in Organic Solvents: Advantages and Applications. Biol. Proced. Online 2016, 18, 1–11. [Google Scholar] [CrossRef]
- Ravelo, M.; Fuente, E.; Blanco, Á.; Ladero, M.; García-Ochoa, F. Esterification of Glycerol and Ibuprofen in Solventless Media Catalyzed by Free CALB: Kinetic Modelling. Biochem. Eng. J. 2015, 101, 228–236. [Google Scholar] [CrossRef]
- Radzi, S.M.; Basri, M.; Salleh, A.B.; Ariff, A.; Mohammad, R.; Rahman, M.B.A.; Rahman, R.N.Z.R.A. Large Scale Production of Liquid Wax Ester by Immobilized Lipase. J. Oleo Sci. 2005, 54, 203–209. [Google Scholar] [CrossRef]
- Tamayo, J.J.; Ladero, M.; Santos, V.E.; García-Ochoa, F. Esterification of Benzoic Acid and Glycerol to α-Monobenzoate Glycerol in Solventless Media Using an Industrial Free Candida antarctica Lipase B. Process Biochem. 2012, 47, 243–250. [Google Scholar] [CrossRef]
- Conley, A.J.; Kabara, J.J. Antimicrobial Action of Esters of Polyhydric Alcohols. Antimicrob. Agents Chemother. 1973, 4, 501–506. [Google Scholar] [CrossRef]
- Kiran, S.; Kamal, S.; Aslam, N.; Hussain, A.I.; Ghaffar, A.; Bibi, I.; Kamal, A.; Munir, B.; Sultan, N. Synthesis of Ibuprofen Derivatives with Improved Antibacterial Activity. Asian J. Chem. 2015, 27, 3259–3262. [Google Scholar] [CrossRef]
- Ghanem, A. Trends in Lipase-Catalyzed Asymmetric Access to Enantiomerically Pure/Enriched Compounds. Tetrahedron 2007, 63, 1721–1754. [Google Scholar] [CrossRef]
- Chiaradia, V.; Paroul, N.; Cansian, R.L.; Júnior, C.V.; Detofol, M.R.; Lerin, L.A.; Oliveira, J.V.; Oliveira, D. Synthesis of Eugenol Esters by Lipase-Catalyzed Reaction in Solvent-Free System. Appl. Biochem. Biotechnol. 2012, 168, 742–751. [Google Scholar] [CrossRef]
- Giovannini, P.P.; Catani, M.; Massi, A.; Sacchetti, G.; Tacchini, M.; de Oliveira, D.; Lerin, L.A. Continuous Production of Eugenol Esters Using Enzymatic Packed-Bed Microreactors and an Evaluation of the Products as Antifungal Agents. Flavour Fragr. J. 2019, 34, 201–210. [Google Scholar] [CrossRef]
- Ortiz, C.; Ferreira, M.L.; Barbosa, O.; Dos Santos, J.C.S.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Briand, L.E.; Fernandez-Lafuente, R. Novozym 435: The “Perfect” Lipase Immobilized Biocatalyst? Catal. Sci. Technol. 2019, 9, 2380–2420. [Google Scholar] [CrossRef]
- Foresti, M.L.; Galle, M.; Ferreira, M.L.; Briand, L.E. Enantioselective Esterification of Ibuprofen with Ethanol as Reactant and Solvent Catalyzed by Immobilized Lipase: Experimental Andmolecular Modeling Aspects. J. Chem. Technol. Biotechnol. 2009, 84, 1461–1473. [Google Scholar] [CrossRef]
- Trodler, P.; Pleiss, J. Modeling Structure and Flexibility of Candida antarctica Lipase B in Organic Solvents. BMC Struct. Biol. 2008, 8, 1–10. [Google Scholar] [CrossRef]
- Ong, A.L.; Kamaruddin, A.H.; Bhatia, S.; Long, W.S.; Lim, S.T.; Kumari, R. Performance of Free Candida antarctica Lipase B in the Enantioselective Esterification of (R)-Ketoprofen. Enzyme Microb. Technol. 2006, 39, 924–929. [Google Scholar] [CrossRef]
- Nordblad, M.; Adlercreutz, P. Immobilisation Procedure and Reaction Conditions for Optimal Performance of Candida antarctica Lipase B in Transesterification and Hydrolysis. Biocatal. Biotransform. 2013, 31, 237–245. [Google Scholar] [CrossRef]
- Manoel, E.A.; dos Santos, J.C.S.; Freire, D.M.G.; Rueda, N.; Fernandez-Lafuente, R. Immobilization of Lipases on Hydrophobic Supports Involves the Open Form of the Enzyme. Enzyme Microb. Technol. 2015, 71, 53–57. [Google Scholar] [CrossRef]
- Pagliaro, M.; Ciriminna, R.; Kimura, H.; Rossi, M.; Della Pina, C. From Glycerol to Value-Added Products. Angew. Chem. Int. Ed. 2007, 46, 4434–4440. [Google Scholar] [CrossRef]
- Bradbury, S.L.; Jakoby, W.B. Glycerol as an Enzyme-Stabilizing Agent: Effects on Aldehyde Dehydrogenase. Proc. Natl. Acad. Sci. USA 1972, 69, 2373–2376. [Google Scholar] [CrossRef] [PubMed]
- Ohtake, S.; Kita, Y.; Arakawa, T. Interactions of Formulation Excipients with Proteins in Solution and in the Dried State. Adv. Drug Deliv. Rev. 2011, 63, 1053–1073. [Google Scholar] [CrossRef] [PubMed]
- Vagenende, V.; Yap, M.G.S.; Trout, B.L. Mechanisms of Protein Stabilization and Prevention of Protein Aggregation by Glycerol. Biochemistry 2009, 48, 11084–11096. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Chari, R.; Sharma, V.K.; Kalonia, D.S. Modulation of the Thermodynamic Stability of Proteins by Polyols: Significance of Polyol Hydrophobicity and Impact on the Chemical Potential of Water. Int. J. Pharm. 2011, 413, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Wolfson, A.; Atyya, A.; Dlugy, C.; Tavor, D. Glycerol Triacetate as Solvent and Acyl Donor in the Production of Isoamyl Acetate with Candida antarctica Lipase B. Bioprocess Biosyst. Eng. 2010, 33, 363–366. [Google Scholar] [CrossRef]
- Wolfson, A.; Dlugy, C.; Shotland, Y. Glycerol as a Green Solvent for High Product Yields and Selectivities. Environ. Chem. Lett. 2007, 5, 62–71. [Google Scholar] [CrossRef]
- Ravelo, M.; Wojtusik, M.; Ladero, M.; García-Ochoa, F. Synthesis of Ibuprofen Monoglyceride in Solventless Medium with Novozym®435: Kinetic Analysis. Catalysts 2020, 10, 76. [Google Scholar] [CrossRef]
- Katzung, B.G. Basic & Clinical Pharmacology; McGraw-Hill Educ.: New York, NY, USA, 2018. [Google Scholar]
- Zhao, H.; Song, Z. Migration of Reactive Trace Compounds from Novozym® 435 into Organic Solvents and Ionic Liquids. Biochem. Eng. J. 2010, 49, 113–118. [Google Scholar] [CrossRef]
- Kobayashi, T.; Matsuo, T.; Kimura, Y.; Adachi, S. Thermal Stability of Immobilized Lipase from Candida antarctica in Glycerols with Various Water Contents at Elevated Temperatures. JAOCS, J. Am. Oil Chem. Soc. 2008, 85, 1041–1044. [Google Scholar] [CrossRef]
- Foresti, M.L.; Pedernera, M.; Bucalá, V.; Ferreira, M.L. Multiple Effects of Water on Solvent-Free Enzymatic Esterifications. Enzyme Microb. Technol. 2007, 41, 62–70. [Google Scholar] [CrossRef]
- Bouaziz, A.; Horchani, H.; Salem, N.B.; Chaari, A.; Chaabouni, M.; Gargouri, Y.; Sayari, A. Enzymatic Propyl Gallate Synthesis in Solvent-Free System: Optimization by Response Surface Methodology. J. Mol. Catal. B Enzym. 2010, 67, 242–250. [Google Scholar] [CrossRef]
- van Vuuren, S.F. Antimicrobial Activity of South African Medicinal Plants. J. Ethnopharmacol. 2008, 119, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Piper, J.D.; Piper, P.W. Benzoate and Sorbate Salts: A Systematic Review of the Potential Hazards of These Invaluable Preservatives and the Expanding Spectrum of Clinical Uses for Sodium Benzoate. Compr. Rev. Food Sci. Food Saf. 2017, 16, 868–880. [Google Scholar] [CrossRef]
- Doležálková, I.; Máčalík, Z.; Butkovičová, A.; Janiš, R.; Buňková, L. Monoacylglycerols as Fruit Juices Preservatives. Czech J. Food Sci. 2012, 30, 567–572. [Google Scholar] [CrossRef]
- Chaibi, A.; Ababouch, L.H.; Busta, F.F. Inhibition by Monoglycerides of L-Alanine-Triggered Bacillus cereus and Clostridium botulinum Spore Germination and Outgrowth. J. Food Prot. 1996, 59, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.K.; Jackman, J.A.; Valle-González, E.R.; Cho, N.J. Antibacterial Free Fatty Acids and Monoglycerides: Biological Activities, Experimental Testing, and Therapeutic Applications. Int. J. Mol. Sci. 2018, 19, 1114. [Google Scholar] [CrossRef] [PubMed]

| Tested Drugs Concentrations (mg/mL−1) | ||||||
|---|---|---|---|---|---|---|
| S. cerevisiae | 0.05 | 0.075 | 0.100 | 0.125 | 0.150 | 0.175 |
| S. griseus | 0.15 | 0.25 | 0.50 | 0.75 | 1.00 | 1.25 |
| Streptomyces griseus | DD |
| Chloramphenicol | 18 mm ± 0.3 |
| Sorbic acid | / |
| Glycerol sorbate | / |
| Potassium sorbate | / |
| Saccharomyces cerevisiae | |
| Clotrimazole | 18 mm ± 0.2 |
| Sorbic acid | 10 mm ± 0.4 |
| Glycerol sorbate | 11.5 mm ± 0.1 |
| Potassium sorbate | 12.6 ± 0.2 |
| S. Ccerevisiae | ||
|---|---|---|
| MIC50 1 | MIC100 | |
| Sorbic acid | 0.097 ± 0.0016 | 0.150 ± 0.001 |
| Glycerol sorbate | 0.090 ± 0.001 | 0.140 ± 0.0008 |
| Potassium Sorbate | 0.080 ± 0.001 | 0.125 ± 0.003 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zappaterra, F.; Summa, D.; Semeraro, B.; Buzzi, R.; Trapella, C.; Ladero, M.; Costa, S.; Tamburini, E. Enzymatic Esterification as Potential Strategy to Enhance the Sorbic Acid Behavior as Food and Beverage Preservative. Fermentation 2020, 6, 96. https://doi.org/10.3390/fermentation6040096
Zappaterra F, Summa D, Semeraro B, Buzzi R, Trapella C, Ladero M, Costa S, Tamburini E. Enzymatic Esterification as Potential Strategy to Enhance the Sorbic Acid Behavior as Food and Beverage Preservative. Fermentation. 2020; 6(4):96. https://doi.org/10.3390/fermentation6040096
Chicago/Turabian StyleZappaterra, Federico, Daniela Summa, Bruno Semeraro, Raissa Buzzi, Claudio Trapella, Miguel Ladero, Stefania Costa, and Elena Tamburini. 2020. "Enzymatic Esterification as Potential Strategy to Enhance the Sorbic Acid Behavior as Food and Beverage Preservative" Fermentation 6, no. 4: 96. https://doi.org/10.3390/fermentation6040096
APA StyleZappaterra, F., Summa, D., Semeraro, B., Buzzi, R., Trapella, C., Ladero, M., Costa, S., & Tamburini, E. (2020). Enzymatic Esterification as Potential Strategy to Enhance the Sorbic Acid Behavior as Food and Beverage Preservative. Fermentation, 6(4), 96. https://doi.org/10.3390/fermentation6040096









