Reusability of Immobilized Cells for Subsequent Balsamic-Styled Vinegar Fermentations
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Fermentation Medium, Inoculums and Calcium Alginate Solutions
2.2. Cell Immobilization: Gel Entrapment and Cell Adsorption
2.3. Inoculation Procedure for First Cycle Fermentations
2.4. Second Cycle Fermentation Experiments: Recovery and Reuse of Immobilization matrices
2.5. Evaluation of the Integrity of the Immobilization Matrices
2.6. Sensory Evaluation and Statistical Analysis
3. Results and Discussion
3.1. Comparative Analyses of Sugar Consumption Rates for First and Second Cycle BSV Fermentations
3.2. Comparative Analyses of Acetification Rates for First and Second Cycle BSV Fermentations
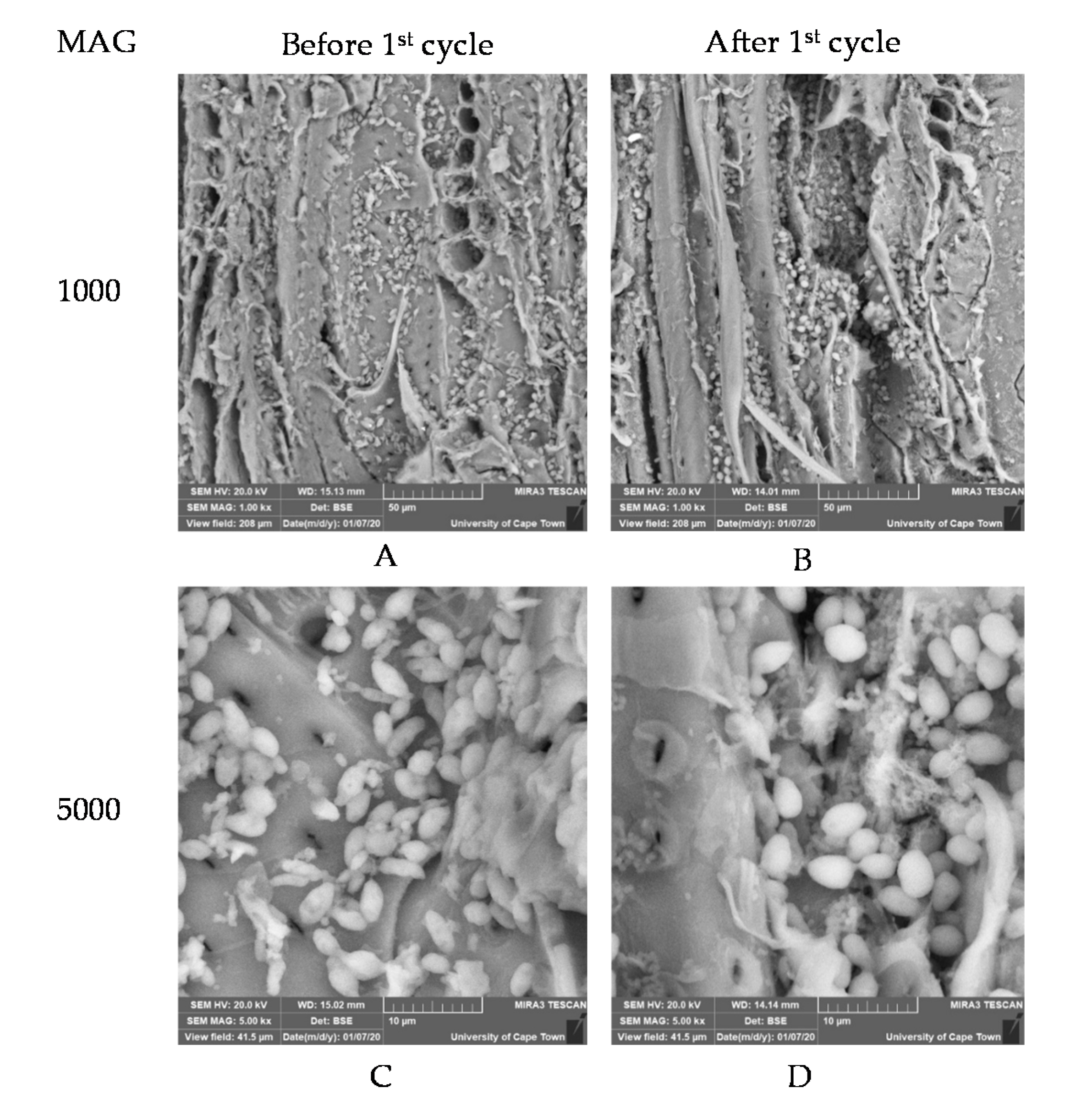
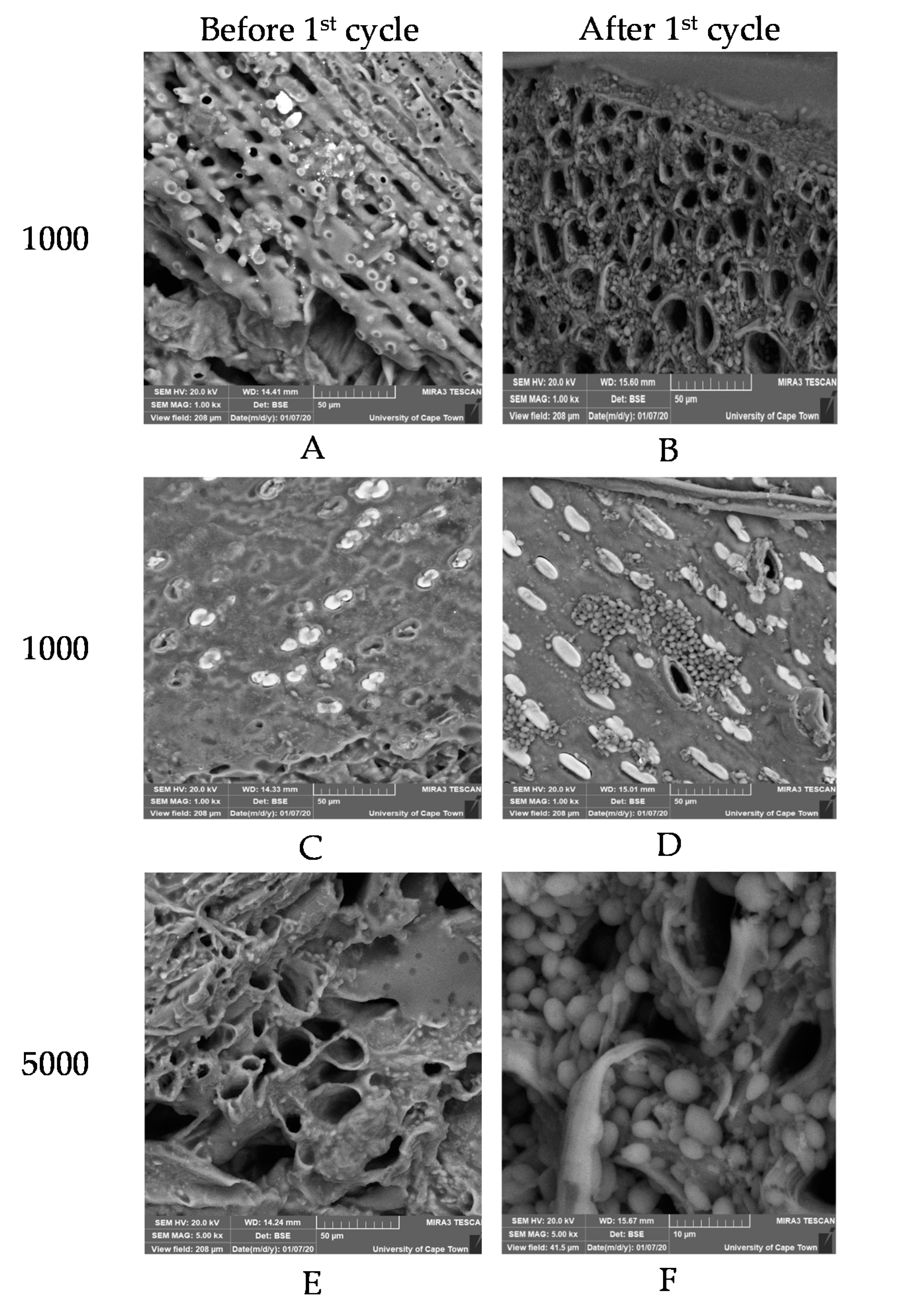
3.3. Structural Morphology of Cell Immobilization Matrices before and after BSV Fermentation
3.3.1. Ca-Alginate Beads
3.3.2. Oakwood Chips
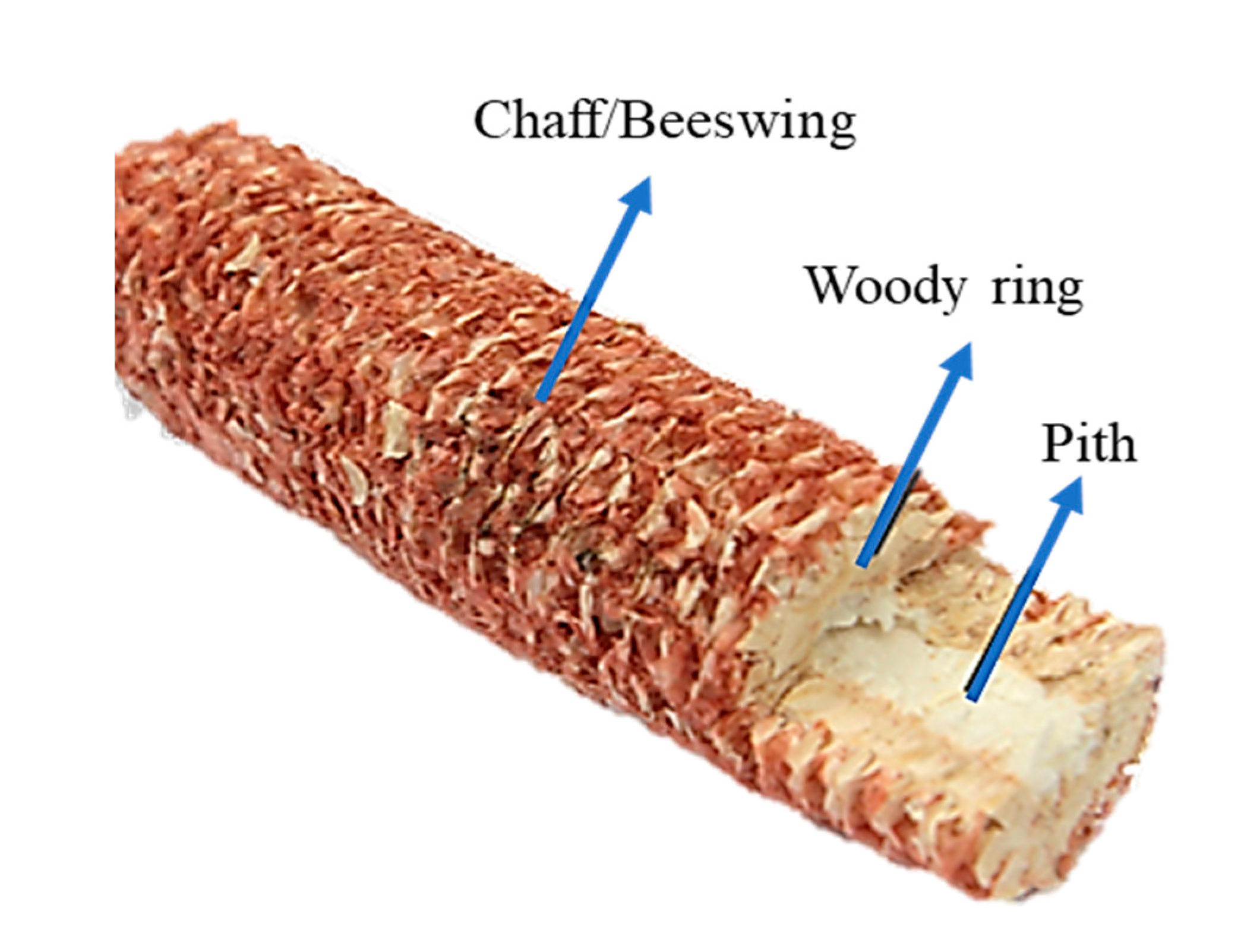
3.3.3. Corncobs
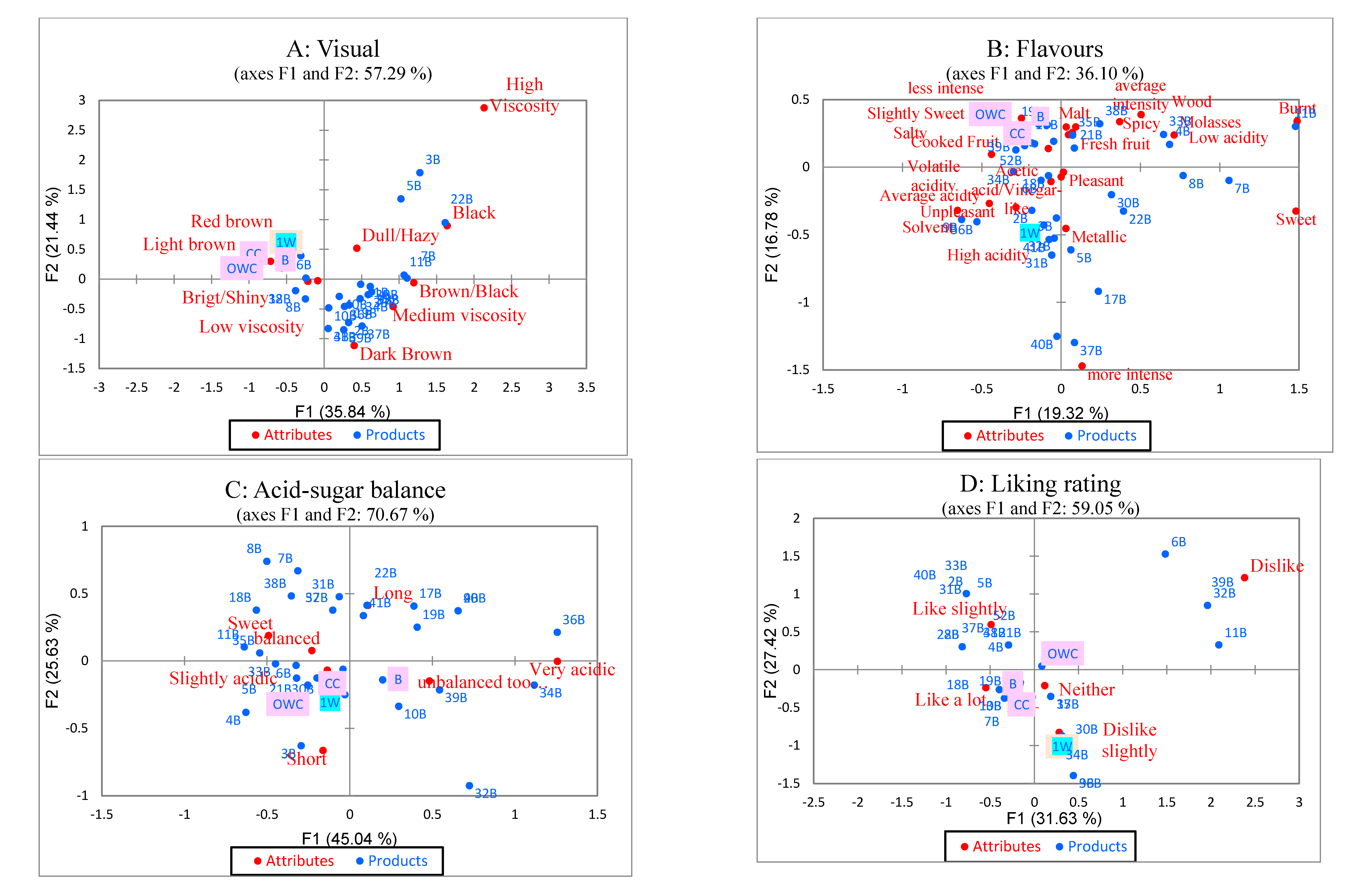
3.4. Sensory Attributes of Balsamic-Styled Vinegar Versus Commercial Balsamic Vinegar
4. Conclusions and Recommendations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tesfaye, W.; Morales, M.L.; Garcıa-Parrilla, M.C.; Troncoso, A.M. Wine vinegar: Technology, authenticity and quality evaluation. Trends Food Sci. Technol. 2002, 13, 12–21. [Google Scholar] [CrossRef]
- Fernández-Pérez, R.; Torres, C.; Sanz, S.; Ruiz-Larrea, F. Strain typing of acetic acid bacteria responsible for vinegar production by the submerged elaboration method. Food Microbiol. 2010, 27, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, U.F.; Ntwampe, S.K.O.; Ngongang, M.M.; Du Plessis, H.W.; Chidi, B.S.; Saulse, C.K.L.N.; Jolly, N.P. Cell immobilization by Gel entrapment in Ca-alginate beads for balsamic-styled vinegar production. In Proceedings of the 10th International Conference on Advances in Science, Engineering, Technology and Healthcare (ASETH-18), Cape Town, South Africa, 19–20 November 2018. [Google Scholar]
- Hutchinson, U.F.; Gqozo, S.; Jolly, N.P.; Chidi, B.S.; Du Plessis, H.W.; Mewa-Ngongang, M.; Ntwampe, S.K. Aeration, agitation and cell immobilization on corncobs and oak wood chips effects on balsamic-styled vinegar production. Foods 2019, 8, 303. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, T.; Seo, C.W.; Marshall, W.E. Removal of selected metal ions from aqueous solution using modified corncobs. Bioresour. Technol. 2001, 78, 133–139. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, Y.; Ma, T.; Bao, X.; Du, F.; Zhuang, G.; Qu, Y. Simultaneous saccharification and fermentation of acid-pretreated corncobs with a recombinant Saccharomyces cerevisiae expressing β-glucosidase. Bioresour. Technol. 2008, 99, 5099–5103. [Google Scholar] [CrossRef] [PubMed]
- Kyraleou, M.; Kallithraka, S.; Chira, K.; Tzanakouli, E.; Ligas, I.; Kotseridis, Y. Differentiation of wines treated with wood chips based on their phenolic content, volatile composition, and sensory parameters. J. Food Sci. 2015, 80, C2701–C2710. [Google Scholar] [CrossRef] [PubMed]
- Laqui-Estaña, J.; López-Solís, R.; Peña-Neira, Á.; Medel-Marabolí, M.; Obreque-Slier, E. Wines in contact with oak wood: The impact of the variety (Carménère and Cabernet Sauvignon), format (barrels, chips and staves), and aging time on the phenolic composition. J. Sci. Food Agric. 2019, 99, 436–448. [Google Scholar] [CrossRef] [PubMed]
- Hecht, H.; Srebnik, S. Structural characterization of sodium alginate and calcium alginate. Biomacromolecules 2016, 17, 2160–2167. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Jin, Y.B.; Chang, H.N. Reusable biosorbents in capsules from Zoogloea ramigera cells for cadmium removal. Biotechnol. Bioeng. 1999, 63, 116–121. [Google Scholar] [CrossRef]
- Kocher, G.S.; Kalra, K.L.; Phutela, R.P. Comparative production of sugarcane vinegar by different immobilization techniques. J. Inst. Brew. 2006, 112, 264–266. [Google Scholar] [CrossRef]
- Rahman, R.N.Z.A.; Ghazali, F.M.; Salleh, A.B.; Basri, M. Biodegradation of hydrocarbon contamination by immobilized bacterial cells. J. Microbiol. 2006, 44, 354–359. [Google Scholar] [PubMed]
- Rao, C.S.; Madhavendra, S.S.; Rao, R.S.; Hobbs, P.J.; Prakasham, R.S. Studies on improving the immobilized bead reusability and alkaline protease production by isolated immobilized Bacillus circulans (MTCC 6811) using overall evaluation criteria. Appl. Biochem. Biotechnol. 2008, 150, 65–83. [Google Scholar]
- Nigam, V.K.; Khandelwal, A.K.; Gothwal, R.K.; Mohan, M.K.; Choudhury, B.; Vidyarthi, A.S.; Ghosh, P. Nitrilase-catalysed conversion of acrylonitrile by free and immobilized cells of Streptomyces sp. J. Biosci. 2009, 34, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Shyamkumar, R.; Moorthy, I.M.G.; Ponmurugan, K.; Baskar, R. Production of L-glutamic acid with Corynebacterium glutamicum (NCIM 2168) and Pseudomonas reptilivora (NCIM 2598): A study on immobilization and reusability. Avicenna J. Med Biotechnol. 2014, 6, 163. [Google Scholar] [PubMed]
- Adams, J.; Williams, A.; Lancaster, B.; Foley, M. Advantages and uses of check-all-that-apply response compared to traditional scaling of attributes for salty snacks. In Proceedings of the 7th Pangborn Sensory Science Symposium, Minneapolis, MN, USA, 12–16 August 2007; Volume 16. [Google Scholar]
- Pramudya, R.C.; Seo, H.S. Using Check-All-That-Apply (CATA) method for determining product temperature-dependent sensory-attribute variations: A case study of cooked rice. Food Res. Int. 2018, 105, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Gullo, M.; Giudici, P. Acetic acid bacteria in traditional balsamic vinegar: Phenotypic traits relevant for starter cultures selection. Int. J. Food Microbiol. 2008, 125, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Bekatorou, A. Current Industrial Vinegar Production. In Advances in Vinegar Production; CRC press Taylor & Francis group: New York, NY, USA, 2019; Volume 1, p. 83. [Google Scholar]
- Hutchinson, U.F.; Jolly, N.P.; Chidi, B.S.; Ngongang, M.M.; Ntwampe, S.K.O. Vinegar Engineering: A Bioprocess Perspective. Food Eng. Rev. 2019, 11, 290–305. [Google Scholar] [CrossRef]
- Anonymous. The Andersons Cob Products. Available online: https://andersonscob.com/about-the-andersons-cob-products/slide_bkgd2/ (accessed on 18 January 2020).
- Giudici, P.; Gullo, M.; Solieri, L.; Falcone, P.M. Technological and microbiological aspects of traditional balsamic vinegar and their influence on quality and sensorial properties. Adv. Food Nutr. Res. 2009, 58, 137–182. [Google Scholar] [PubMed]
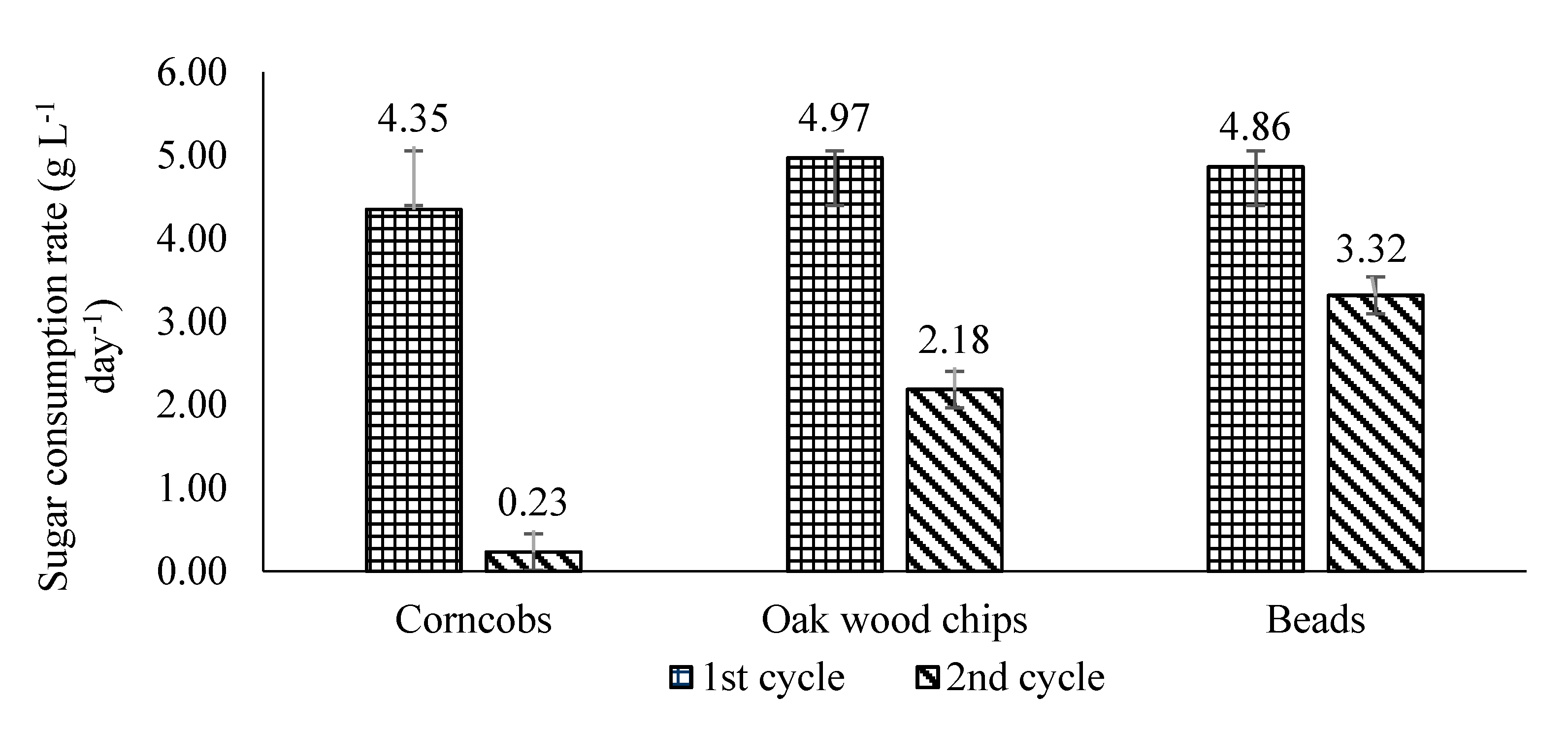


| Adsorption | |||||
|---|---|---|---|---|---|
| Adsorbents | Length (cm) | Width/Diameter (cm) | Circumference (cm) | Surface Area of One Piece (cm2) | Total Adsorbent Surface Area (cm2) |
| Corncobs | 3.0 | 3.9 | 12.25 | 54.29 | 108.58 (2 pieces) |
| Oakwood chips | 2.90 | 1.80 | 9.60 | 19.64 | 117 (6 chips) |
| Entrapment | |||||
| Ca-alginate beads | Volume of Alginate Infused with Cells (mL) | Diameter of Beads (mm) | |||
| 20 | 4.5 | ||||
| Cell Immobilization Matrix | 1 Price (USD) ($) per 250 g | Quantity of Cell Immobilization Matrices Used per 350 mL Fermentation Volume |
|---|---|---|
| Calcium alginate | 63.24 | 0.56 g per bead (20 mL of alginate solution infused with cells) |
| Oakwood chips | 5.96 | 108.58 cm2 |
| Corncobs | 0.043–0.11 (generally sold in tons ~ $170.30 to $454.13 per ton) | 117 cm2 |
| Cell Immobilization Matrix | First Cycle Fermentation Time (Days) | Second Cycle Fermentation Time (Days) |
|---|---|---|
| Corncobs | 21 | Sluggish fermentation |
| Oakwood chips | 40 | Sluggish fermentation |
| Ca-alginate beads | 19 | 50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hutchinson, U.F.; Ntwampe, S.K.O.; Chidi, B.S.; Mewa-Ngongang, M.; du Plessis, H.W.; Booyse, M.; Jolly, N.P. Reusability of Immobilized Cells for Subsequent Balsamic-Styled Vinegar Fermentations. Fermentation 2020, 6, 103. https://doi.org/10.3390/fermentation6040103
Hutchinson UF, Ntwampe SKO, Chidi BS, Mewa-Ngongang M, du Plessis HW, Booyse M, Jolly NP. Reusability of Immobilized Cells for Subsequent Balsamic-Styled Vinegar Fermentations. Fermentation. 2020; 6(4):103. https://doi.org/10.3390/fermentation6040103
Chicago/Turabian StyleHutchinson, Ucrecia F., Seteno K. O. Ntwampe, Boredi S. Chidi, Maxwell Mewa-Ngongang, Heinrich W. du Plessis, Mardé Booyse, and Neil P. Jolly. 2020. "Reusability of Immobilized Cells for Subsequent Balsamic-Styled Vinegar Fermentations" Fermentation 6, no. 4: 103. https://doi.org/10.3390/fermentation6040103
APA StyleHutchinson, U. F., Ntwampe, S. K. O., Chidi, B. S., Mewa-Ngongang, M., du Plessis, H. W., Booyse, M., & Jolly, N. P. (2020). Reusability of Immobilized Cells for Subsequent Balsamic-Styled Vinegar Fermentations. Fermentation, 6(4), 103. https://doi.org/10.3390/fermentation6040103








