Impact of Genotype, Environment, and Malting Conditions on the Antioxidant Activity and Phenolic Content in US Malting Barley
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Barley Samples
2.3. Micro-Malting
2.4. Preparation of Extracts from Barley and Malt
2.5. Radical Scavenging Activities
2.6. Iron (II) Chelating Activity
2.7. Reducing Power
2.8. Total Phenolic Content (TPC)
2.9. Statistical Analysis
3. Results
3.1. Effect of Genotype and Environment on the Total Phenolic Content and Antioxidant Activities
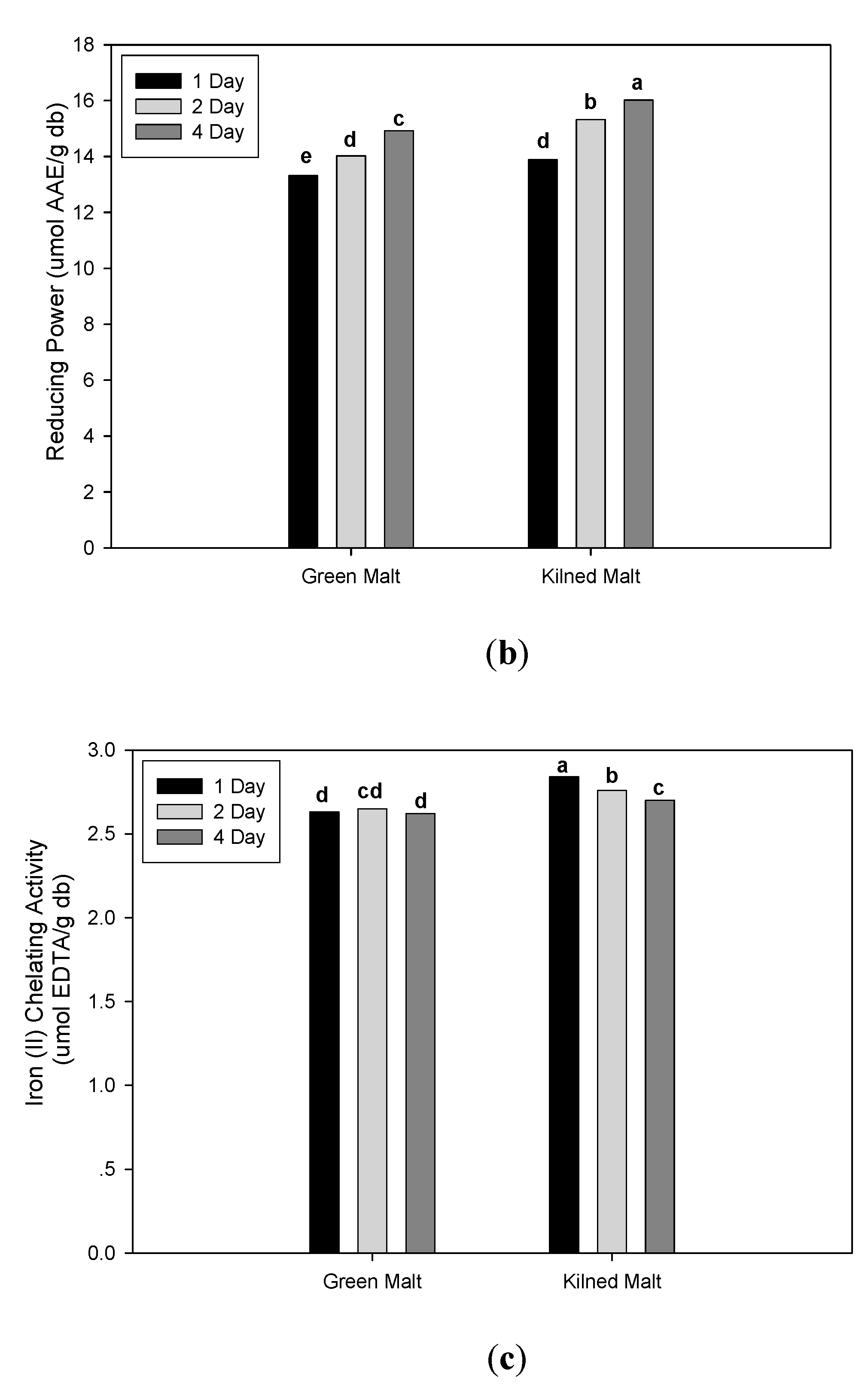
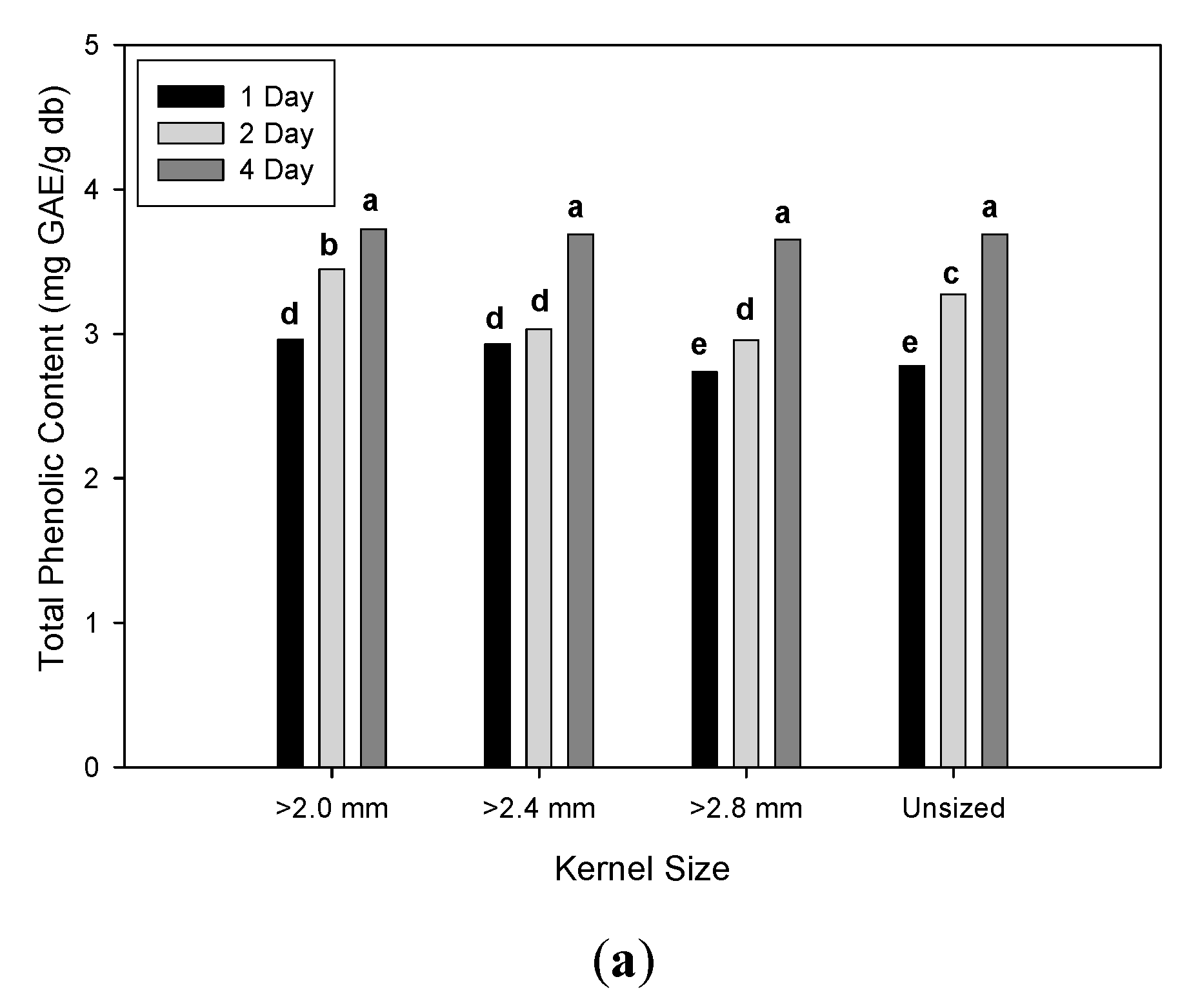
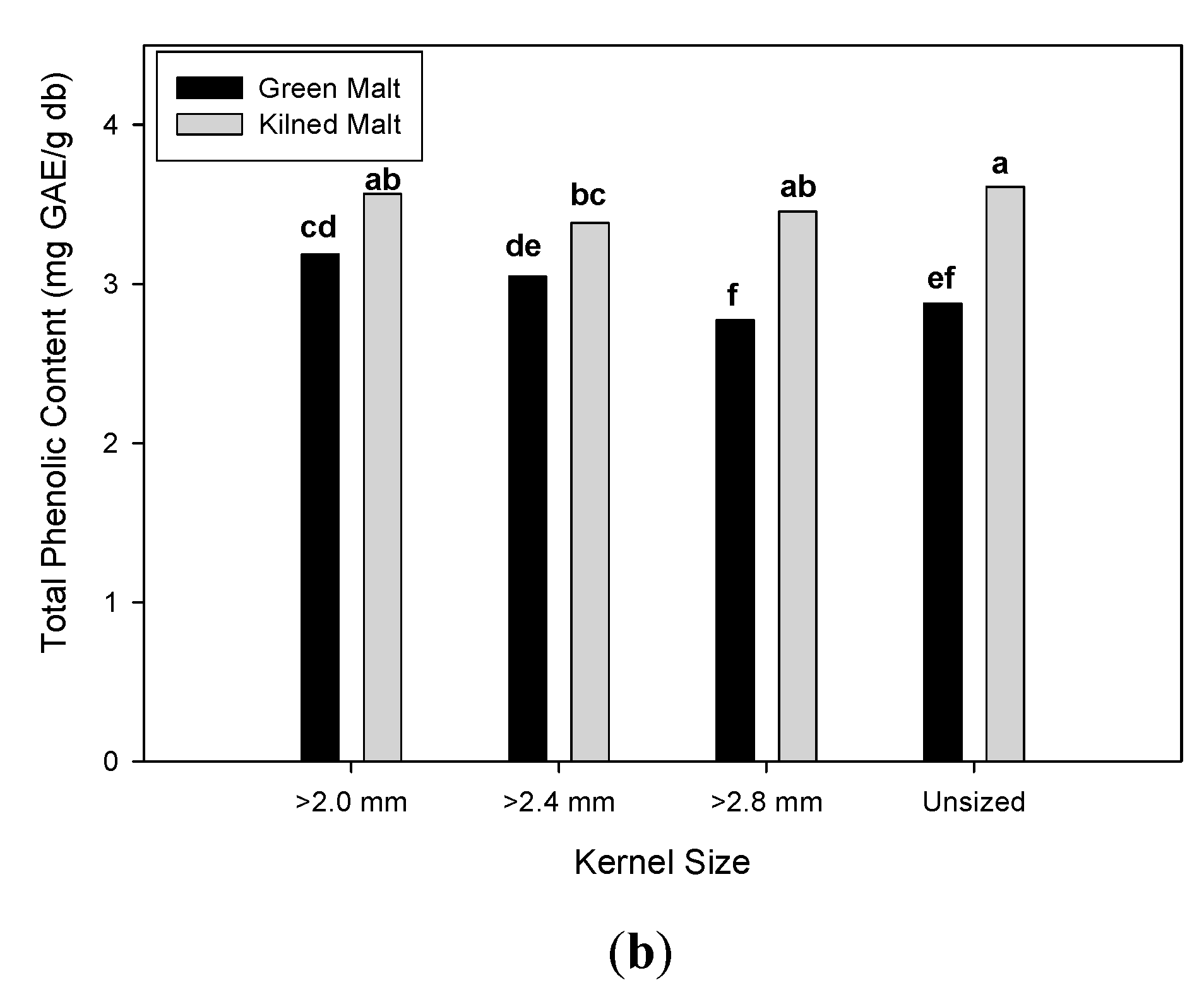
3.2. Effect of Malting Conditions on the Total Phenolic Content and Antioxidant Activities
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Stampfer, M.J.; Hu, F.B.; Giovannucci, E.; Rimm, E.; Manson, J.E.; Hennekens, C.H.; Willett, W.C. Whole-grain consumption and risk of coronary heart disease: Results from the Nurses’ Health Study. Am. J. Clin. Nutr. 1999, 70, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Manson, J.E.; Stampfer, M.J.; Rexrode, K.M.; Hu, F.B.; Rimm, E.B.; Willett, W.C. Whole grain consumption and risk of ischemic stroke in women: A prospective study. JAMA 2000, 284, 1534–1540. [Google Scholar] [CrossRef] [PubMed]
- De Munter, J.S.L.; Hu, F.B.; Spiegelman, D.; Franz, M.; van Dam, R.M. Whole grain, bran, and germ intake and risk of type 2 diabetes: A prospective cohort study and systematic review. PLoS Med. 2007, 4, e40261. [Google Scholar] [CrossRef] [PubMed]
- Priebe, M.; van Binsbergen, J.; de Vos, R.; Vonk, R.J. Whole grain foods for the prevention of type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2008. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Whole grain phytochemicals and health. J. Cereal Sci. 2007, 46, 207–219. [Google Scholar] [CrossRef]
- Jacobs, D.R., Jr.; Marquart, L.; Slavin, J.; Kushi, L.H. Whole-grain intake and cancer: An expanded review and meta-analysis. Nutr. Cancer 1998, 30, 85–96. [Google Scholar] [CrossRef]
- Egeberg, R.; Olsen, A.; Loft, S.; Christensen, J.; Johnsen, N.F.; Overvad, K.; Tjønneland, A. Intake of whole grain products and risk of breast cancer by hormone receptor status and histology among postmenopausal women. Int. J. Cancer 2009, 124, 745–750. [Google Scholar] [CrossRef]
- Tricase, C.; Amicarelli, V.; Lamonaca, E.; Rana, R.L. Economic analysis of the barley market and related uses. In Grasses as Food and Feed; IntechOpen: London, UK, 2018. [Google Scholar]
- Wannenmacher, J.; Gastl, M.; Becker, T. Phenolic substances in beer: Structural diversity, reactive potential and relevance for brewing process and beer quality. Compr. Rev. Food Sci. Food Saf. 2018, 17, 953–988. [Google Scholar] [CrossRef]
- Lentz, M. The impact of simple phenolic compounds on beer aroma and flavor. Fermentation 2018, 4, 20. [Google Scholar] [CrossRef]
- Hernanz, D.; Nuñez, V.; Sancho, A.I.; Faulds, C.B.; Williamson, G.; Bartolomé, B.; Gómez-Cordovés, C. Hydroxycinnamic acids and ferulic acid dehydrodimers in barley and processed barley. J. Agric. Food Chem. 2001, 49, 4884–4888. [Google Scholar] [CrossRef] [PubMed]
- Holtekjølen, A.K.; Kinitz, C.; Knutsen, S.H. Flavanol and bound phenolic acid contents in different barley varieties. J. Agric. Food Chem. 2006, 54, 2253–2260. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Han, Z.; Huang, Y.; Chen, Z.-H.; Zhang, G.; Dai, F. Genetic diversity of individual phenolic acids in barley and their correlation with barley malt quality. J. Agric. Food Chem. 2015, 63, 7051–7057. [Google Scholar] [CrossRef]
- Zhao, H.; Fan, W.; Dong, J.; Lu, J.; Chen, J.; Shan, L.; Lin, Y.; Kong, W. Evaluation of antioxidant activities and total phenolic contents of typical malting barley varieties. Food Chem. 2008, 107, 296–304. [Google Scholar] [CrossRef]
- Li, Q.; Yang, S.; Li, Y.; Huang, Y.; Zhang, J. Antioxidant activity of free and hydrolyzed phenolic compounds in soluble and insoluble dietary fibres derived from hulless barley. LWT 2019, 111, 534–540. [Google Scholar] [CrossRef]
- Šimić, G.; Horvat, D.; Dvojković, K.; Abičić, I.; Vuletić, M.V.; Tucak, M.; Lalić, A. Evaluation of total phenolic content and antioxidant activity of malting and hulless barley grain and malt extracts. Czech J. Food Sci. 2017, 35, 73–78. [Google Scholar]
- Yang, X.-J.; Dang, B.; Fan, M.-T. Free and bound phenolic compound content and antioxidant activity of different cultivated blue highland barley varieties from the Qinghai-Tibet plateau. Molecules 2018, 23, 879. [Google Scholar] [CrossRef]
- Suriano, S.; Iannucci, A.; Codianni, P.; Fares, C.; Russo, M.; Pecchioni, N.; Marciello, U.; Savino, M. Phenolic acids profile, nutritional and phytochemical compounds, antioxidant properties in colored barley grown in southern Italy. Food Res. Int. 2018, 113, 221–233. [Google Scholar] [CrossRef]
- Madhujith, T.; Shahidi, F. Antioxidative and antiproliferative properties of selected barley (Hordeum vulgarae L.) cultivars and their potential for inhibition of low-density lipoprotein (LDL) cholesterol oxidation. J. Agric. Food Chem. 2007, 55, 5018–5024. [Google Scholar] [CrossRef]
- Lugasi, A. Polyphenol content and antioxidant properties of beer. Acta Aliment. 2003, 32, 181–192. [Google Scholar] [CrossRef]
- Fogarasi, A.-L.; Kun, S.; Tankó, G.; Stefanovits-Bányai, É.; Hegyesné-Vecseri, B. A comparative assessment of antioxidant properties, total phenolic content of einkorn, wheat, barley and their malts. Food Chem. 2015, 167, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pejin, J.; Grujić, O.; Čanadanović-Brunet, J.; Vujić, Đ.; Tumbas, V. Investigation of phenolic acids content and antioxidant activity in malt production. J. Am. Soc. Brew. Chem. 2009, 67, 81–88. [Google Scholar] [CrossRef]
- Koren, D.; Kun, S.; Vecseri, B.H.; Kun-Farkas, G. Study of antioxidant activity during the malting and brewing process. J. Food Sci. Technol. 2019, 56, 3801–3809. [Google Scholar] [CrossRef] [PubMed]
- Narwal, S.; Kumar, D.; Verma, R.P.S. Effect of Genotype, Environment and Malting on the Antioxidant Activity and Phenolic Content of Indian Barley. J. Food Biochem. 2016, 40, 91–99. [Google Scholar] [CrossRef]
- O’Brien, L. Genotype and environment effects on feed grain quality. Aust. J. Agric. Res. 1999, 50, 703–720. [Google Scholar] [CrossRef]
- Zhou, K.; Yu, L. Antioxidant properties of bran extracts from Trego wheat grown at different locations. J. Agric. Food Chem. 2004, 52, 1112–1117. [Google Scholar] [CrossRef]
- Yu, L.; Perret, J.; Harris, M.; Wilson, J.; Haley, S. Antioxidant properties of bran extracts from “Akron” wheat grown at different locations. J. Agric. Food Chem. 2003, 51, 1566–1570. [Google Scholar] [CrossRef]
- Yu, L.; Zhou, K. Antioxidant properties of bran extracts from “Platte” wheat grown at different locations. Food Chem. 2005, 90, 311–316. [Google Scholar] [CrossRef]
- Mpofu, A.; Sapirstein, H.D.; Beta, T. Genotype and environmental variation in phenolic content, phenolic acid composition, and antioxidant activity of hard spring wheat. J. Agric. Food Chem. 2006, 54, 1265–1270. [Google Scholar] [CrossRef]
- Yearly Weather Data. Available online: https://ndawn.ndsu.nodak.edu/weather-data-yearly.html (accessed on 14 April 2020).
- Horsley, R.D.; Schwarz, P.B.; Foster, A.E. Effects of gene ant13 on agronomic and malt quality traits of barley. Crop Sci. 1991, 31, 593–598. [Google Scholar] [CrossRef]
- Karababa, E.; Schwarz, P.B.; Horsley, R.D. Effect of kiln schedule on micromalt quality parameters. J. Am. Soc. Brew. Chem. 1993, 51, 163–167. [Google Scholar] [CrossRef]
- Jin, Z.; Zhou, B.; Gillespie, J.; Gross, T.; Barr, J.; Simsek, S.; Brueggeman, R.; Schwarz, P.B. Production of deoxynivalenol (DON) and DON-3-glucoside during the malting of Fusarium infected hard red spring wheat. Food Control 2018, 85, 6–10. [Google Scholar] [CrossRef]
- Yu, L. Wheat Antioxidants; John Wiley & Sons Inc: Hoboken, NJ, USA, 2008. [Google Scholar]
- Xu, M.; Jin, Z.; Peckrul, A.; Chen, B. Pulse seed germination improves antioxidative activity of phenolic compounds in stripped soybean oil-in-water emulsions. Food Chem. 2018, 250, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Granato, D.; Shahidi, F.; Wrolstad, R.; Kilmartin, P.; Melton, L.D.; Hidalgo, F.J.; Miyashita, K.; van Camp, J.; Alasalvar, C.; Ismail, A.B.; et al. Antioxidant activity, total phenolics and flavonoids contents: Should we ban in vitro screening methods? Food Chem. 2018, 264, 471–475. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Donkor, O.N.; Stojanovska, L.; Ginn, P.; Ashton, J.; Vasiljevic, T. Germinated grains--Sources of bioactive compounds. Food Chem. 2012, 135, 950–959. [Google Scholar] [CrossRef]
- Vanbeneden, N.; Gils, F.; Delvaux, F.; Delvaux, F. Variability in the Release of Free and Bound Hydroxycinnamic Acids from Diverse Malted Barley (Hordeum vulgare L.) Cultivars during Wort Production. J. Agric. Food Chem. 2007, 55, 11002–11010. [Google Scholar] [CrossRef]
- Maillard, M.-N.; Berset, C. Evolution of antioxidant activity during kilning: Role of insoluble bound phenolic acids of barley and malt. J. Agric. Food Chem. 1995, 43, 1789–1793. [Google Scholar] [CrossRef]
- Schwarz, P.B.; Horsley, R.D. Malt quality improvement in North American six-rowed barley cultivars since 1910. J. Am. Soc. Brew. Chem. 1995, 53, 14–18. [Google Scholar] [CrossRef]

| DPPH a (µmol TE/g, db) | ABTS (µmol TE/g, db) | SARSA (%) | RP (µmol AAE/g, db) | ICA (µmol EDTAE/g, db) | TPC (mg GAE/g, db) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Mean b | STD c | Mean | STD | Mean | STD | Mean | STD | Mean | STD | Mean | STD |
| Genotype (row- type) | ||||||||||||
| A(6R) | 12.19 c | ± 0.40 | 21.63 ab | ± 2.83 | 13.51 a | ± 4.85 | 14.84 bc | ± 0.79 | 1.90 ab | ± 0.68 | 2.52 ab | ±0.21 |
| B(6R) | 12.43 bc | ± 0.57 | 21.66 ab | ± 2.09 | 15.31 a | ± 4.17 | 15.29 ab | ± 0.52 | 1.65 b | ± 0.54 | 2.61 ab | ±0.21 |
| C(6R) | 12.76 ab | ± 0.36 | 21.75 a | ± 2.30 | 16.72 a | ± 8.19 | 15.22 ab | ± 0.86 | 1.71 ab | ± 0.58 | 2.58 ab | ±0.19 |
| D(6R) | 13.02 a | ± 0.41 | 22.01 a | ± 1.88 | 18.20 a | ± 8.76 | 15.64 a | ± 0.74 | 1.70 ab | ± 0.50 | 2.66 a | ±0.19 |
| E(6R) | 11.29 d | ± 0.38 | 19.75 bc | ± 1.60 | 15.08 a | ± 9.44 | 14.33 c | ± 0.58 | 1.58 b | ± 0.64 | 2.31 dc | ±0.10 |
| F(2R) | 10.27 e | ± 0.51 | 18.09 c | ± 1.28 | 16.81 a | ± 10.38 | 13.31 d | ± 0.84 | 1.72 ab | ± 0.77 | 2.20 d | ±0.19 |
| G(2R) | 12.01 c | ± 0.52 | 21.01 ab | ± 1.86 | 12.56 a | ± 11.10 | 14.73 bc | ± 0.38 | 1.96 ab | ± 0.53 | 2.43 bc | ±0.17 |
| H(2R) | 12.11 c | ± 0.49 | 21.08 ab | ± 2.30 | 13.42 a | ± 8.81 | 14.82 bc | ± 0.72 | 2.18 a | ± 0.45 | 2.43 bc | ±0.19 |
| Location | ||||||||||||
| EI | 11.99 a | ± 1.00 | 20.87 a | ± 2.58 | 16.82 a | ± 8.54 | 14.79 a | ± 0.89 | 1.31 b | ± 0.50 | 2.46 ab | ±0.22 |
| EII | 12.09 a | ± 0.97 | 20.69 a | ± 2.15 | 15.72 a | ± 7.83 | 14.63 a | ± 0.99 | 1.56 b | ± 0.64 | 2.38 b | ±0.23 |
| EIII | 11.95 a | ± 0.99 | 21.06 a | ± 1.83 | 13.06 a | ± 8.26 | 14.91 a | ± 0.89 | 2.54 a | ± 0.34 | 2.56 a | ±0.27 |
| Genotype | Location (E) | ||
|---|---|---|---|
| EI | EII | EIII | |
| DPPH radical scavenging activity (µmol TE/g, db) | |||
| A(6R) | 11.74 cd | 11.81 d | 13.05 a |
| B(6R) | 11.99 bcd | 12.76abc | 12.55 a |
| C(6R) | 12.99 a | 12.89 ab | 12.40 a |
| D(6R) | 12.92 a | 13.08 a | 13.05 a |
| E(6R) | 11.22 d | 11.66 d | 10.97 bc |
| F(2R) | 10.18 e | 10.12 e | 10.51 c |
| G(2R) | 12.20 abc | 12.34 bcd | 11.48 b |
| H(2R) | 12.70 ab | 12.09 cd | 11.55 b |
| Total phenolic content (mg GAE/g, db) | |||
| A(6R) | 2.39 ab | 2.37 ab | 2.79 ab |
| B(6R) | 2.59 a | 2.54 a | 2.69 abc |
| C(6R) | 2.58 a | 2.49 a | 2.81 a |
| D(6R) | 2.61 a | 2.56 a | 2.67 abc |
| E(6R) | 2.30 ab | 2.29 ab | 2.35 c |
| F(2R) | 2.15 b | 2.11 b | 2.35 c |
| G(2R) | 2.50 a | 2.38 ab | 2.39 bc |
| H(2R) | 2.53 a | 2.34 ab | 2.43 abc |
| DPPH (µmol TE/g, db) | ABTS (µmol TE/g, db) | SARSA (%) | RP (µmol AAE/g, db) | ICA (µmol EDTAE/g, db) | TPC (mg GAE/g, db) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Mean b | STD c | Mean | STD | Mean | STD | Mean | STD | Mean | STD | Mean | STD |
| Kernel size fraction | ||||||||||||
| Unsized | 12.81 b | ± 0.86 | 18.78 b | ± 2.28 | 26.51 a | ± 18.51 | 14.43 b | ± 0.90 | 2.69 c | ± 0.11 | 3.25 b | ± 0.56 |
| ≥ 2.8 mm | 12.39 c | ± 0.90 | 17.09 c | ± 1.67 | 17.37 b | ± 15.56 | 14.19 b | ± 1.13 | 2.61 d | ± 0.18 | 3.11 c | ± 0.55 |
| ≥ 2.4 mm | 13.05 b | ± 0.74 | 18.62 b | ± 1.39 | 17.63 b | ± 16.03 | 14.76 a | ± 1.24 | 2.73 b | ± 0.08 | 3.22 b | ± 0.40 |
| ≥ 2.0 mm | 14.07 a | ± 0.69 | 19.90 a | ± 1.37 | 12.55 b | ± 8.73 | 14.94 a | ± 0.91 | 2.79 a | ± 0.07 | 3.37 a | ± 0.39 |
| Germination time | ||||||||||||
| 1 day | 12.51 c | ± 0.94 | 17.84 b | ± 1.54 | 12.18 b | ± 11.34 | 13.60 c | ± 0.51 | 2.74 a | ± 0.12 | 2.85 c | ± 0.31 |
| 2 day | 13.04 b | ± 0.98 | 18.42 b | ± 1.62 | 20.62 a | ± 17.28 | 14.67 b | ± 0.96 | 2.70 b | ± 0.17 | 3.18 b | ± 0.34 |
| 4 day | 13.71 a | ± 0.71 | 19.54 a | ± 2.29 | 22.75 a | ± 16.14 | 15.47 a | ± 0.69 | 2.66 c | ± 0.09 | 3.69 a | ± 0.32 |
| Malt drying type | ||||||||||||
| Green | 12.59 b | ± 0.98 | 17.57 b | ± 1.30 | 25.70 a | ± 12.47 | 14.09 b | ± 0.79 | 2.64 b | ± 0.14 | 2.97 b | ± 0.40 |
| Kilned | 13.57 a | ± 0.75 | 19.63 a | ± 1.96 | 11.32 b | ± 15.12 | 15.08 a | ± 1.08 | 2.77 a | ± 0.08 | 3.50 a | ± 0.39 |
| Parameter | DPPH | ABTS | SARSA | RP | ICA | TPC | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Partial R2 | Cumulative R2 | Partial R2 | Cumulative R2 | Partial R2 | Cumulative R2 | Partial R2 | Cumulative R2 | Partial R2 | Cumulative R2 | Partial R2 | Cumulative R2 | |
| Kernel size fraction | 27% | 27% (1)b | 10% | 60% (3) | 11% | 37% (2) | 5% | 81% (3) | 14% | 41% (2) | 1% | 89% (3) |
| Germination time | 26% | 80% (3) | 16% | 50% (2) | 8% | 45% (3) | 52% | 52% (1) | 6% | 47% (3) | 55% | 55% (1) |
| Malt drying type | 27% | 54% (2) | 34% | 34% (1) | 26% | 26% (1) | 24% | 76% (2) | 27% | 27% (1) | 33% | 88% (2) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, B.; Jin, Z.; Schwarz, P.; Li, Y. Impact of Genotype, Environment, and Malting Conditions on the Antioxidant Activity and Phenolic Content in US Malting Barley. Fermentation 2020, 6, 48. https://doi.org/10.3390/fermentation6020048
Zhou B, Jin Z, Schwarz P, Li Y. Impact of Genotype, Environment, and Malting Conditions on the Antioxidant Activity and Phenolic Content in US Malting Barley. Fermentation. 2020; 6(2):48. https://doi.org/10.3390/fermentation6020048
Chicago/Turabian StyleZhou, Bing, Zhao Jin, Paul Schwarz, and Yin Li. 2020. "Impact of Genotype, Environment, and Malting Conditions on the Antioxidant Activity and Phenolic Content in US Malting Barley" Fermentation 6, no. 2: 48. https://doi.org/10.3390/fermentation6020048
APA StyleZhou, B., Jin, Z., Schwarz, P., & Li, Y. (2020). Impact of Genotype, Environment, and Malting Conditions on the Antioxidant Activity and Phenolic Content in US Malting Barley. Fermentation, 6(2), 48. https://doi.org/10.3390/fermentation6020048





