Antioxidant Properties of Fermented Green Coffee Beans with Wickerhamomyces anomalus (Strain KNU18Y3)
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Fermentation of Green Coffee Beans
2.3. Coffee Roasting, Grinding, Extraction
2.4. Color Parameters
2.5. Antioxidant Activity
2.5.1. Oxygen Radical Absorbance Capacity
2.5.2. Superoxide Dismutase-Like Activity
2.5.3. 2.,2-diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Assay
2.5.4. Ferric Reducing Antioxidant Power (FRAP)
2.6. Total Phenol Content, Flavonoid Content, and Tannin Content
2.7. Sensory Evaluation
2.8. Statistical Analysis
3. Results and Discussion
3.1. pH and Colony Count (log CFU/mL)
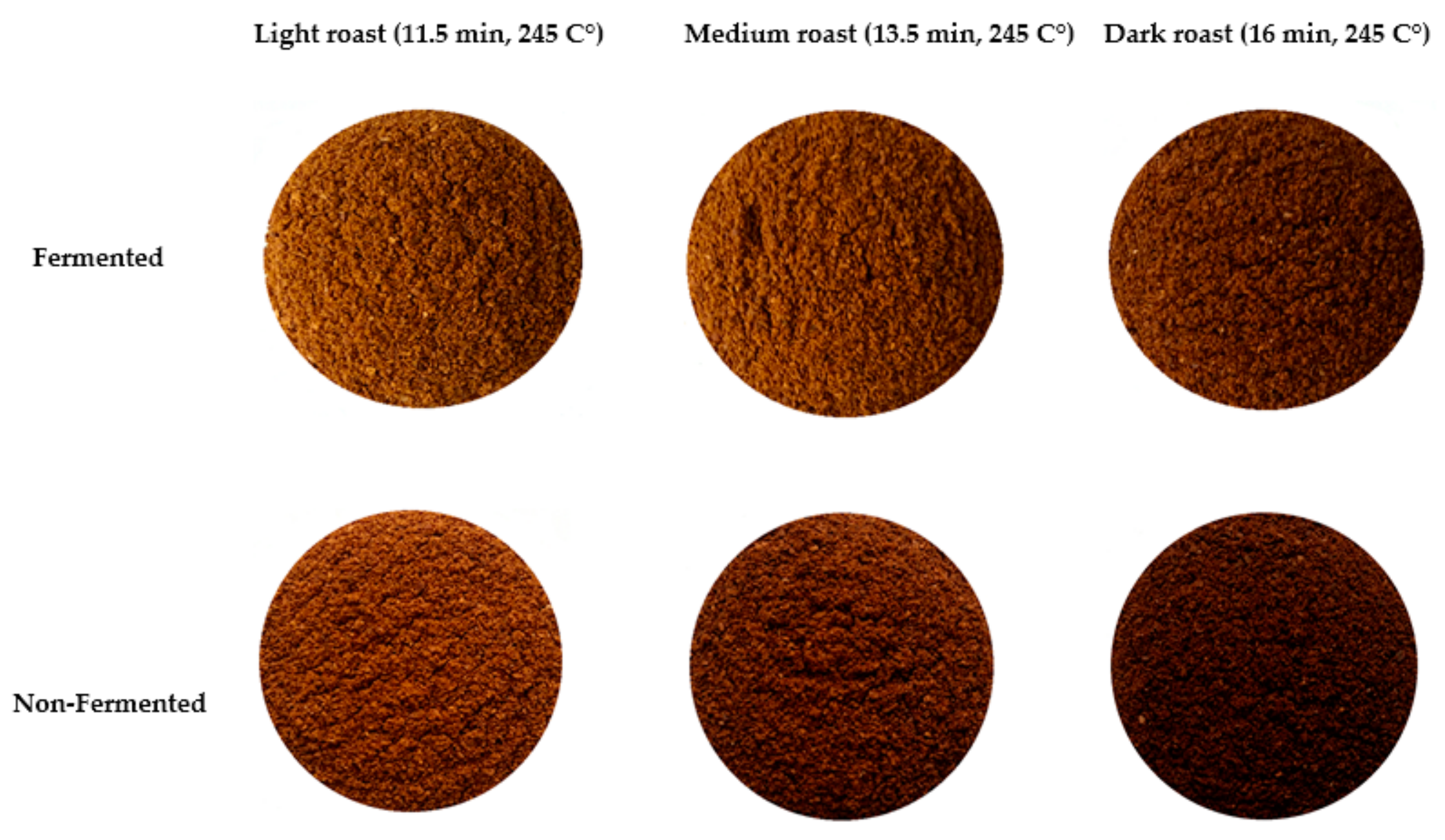
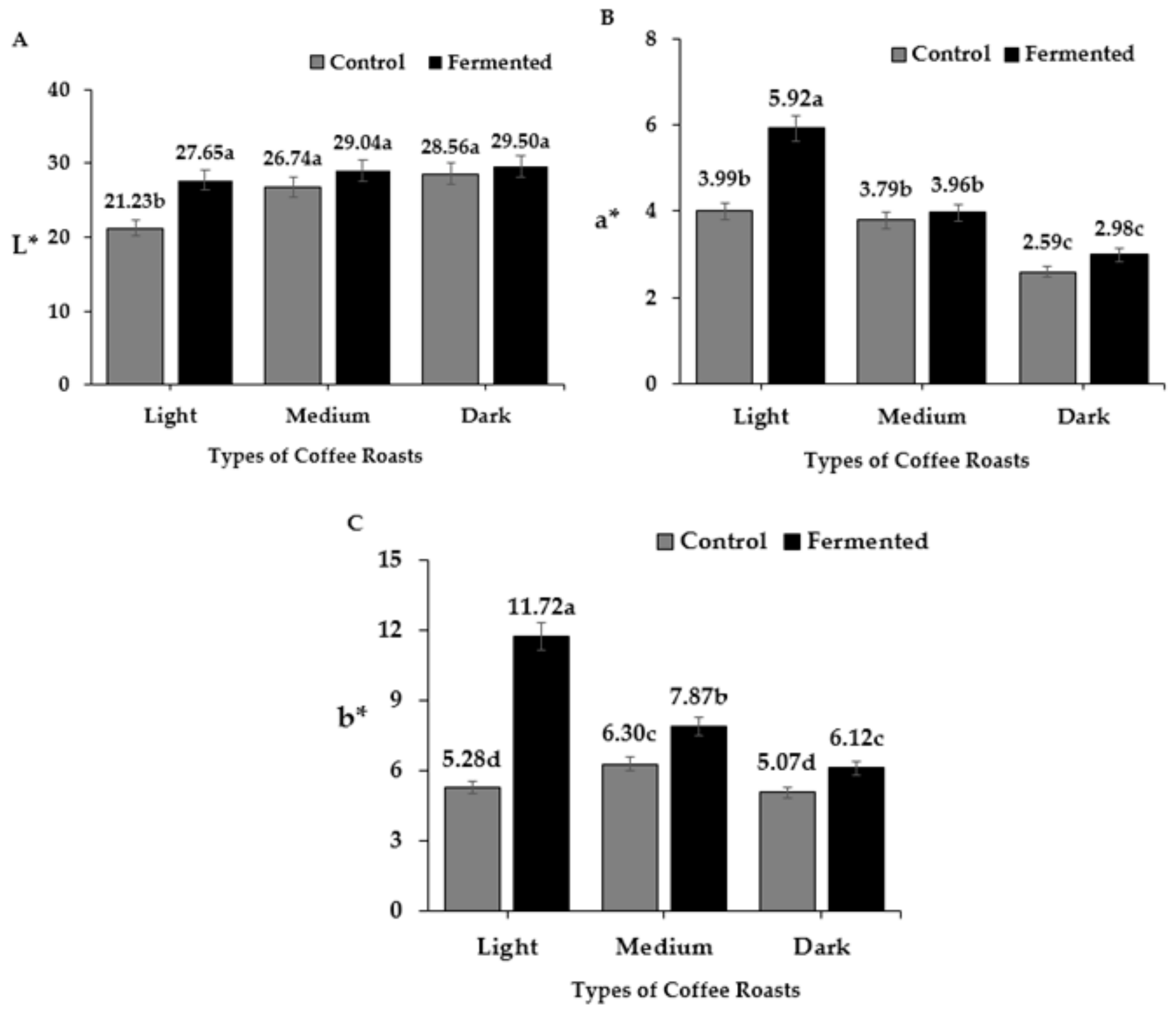
3.2. Colorimeter Data
3.3. Antioxidant Activity
3.3.1. SOD-Like and ORAC
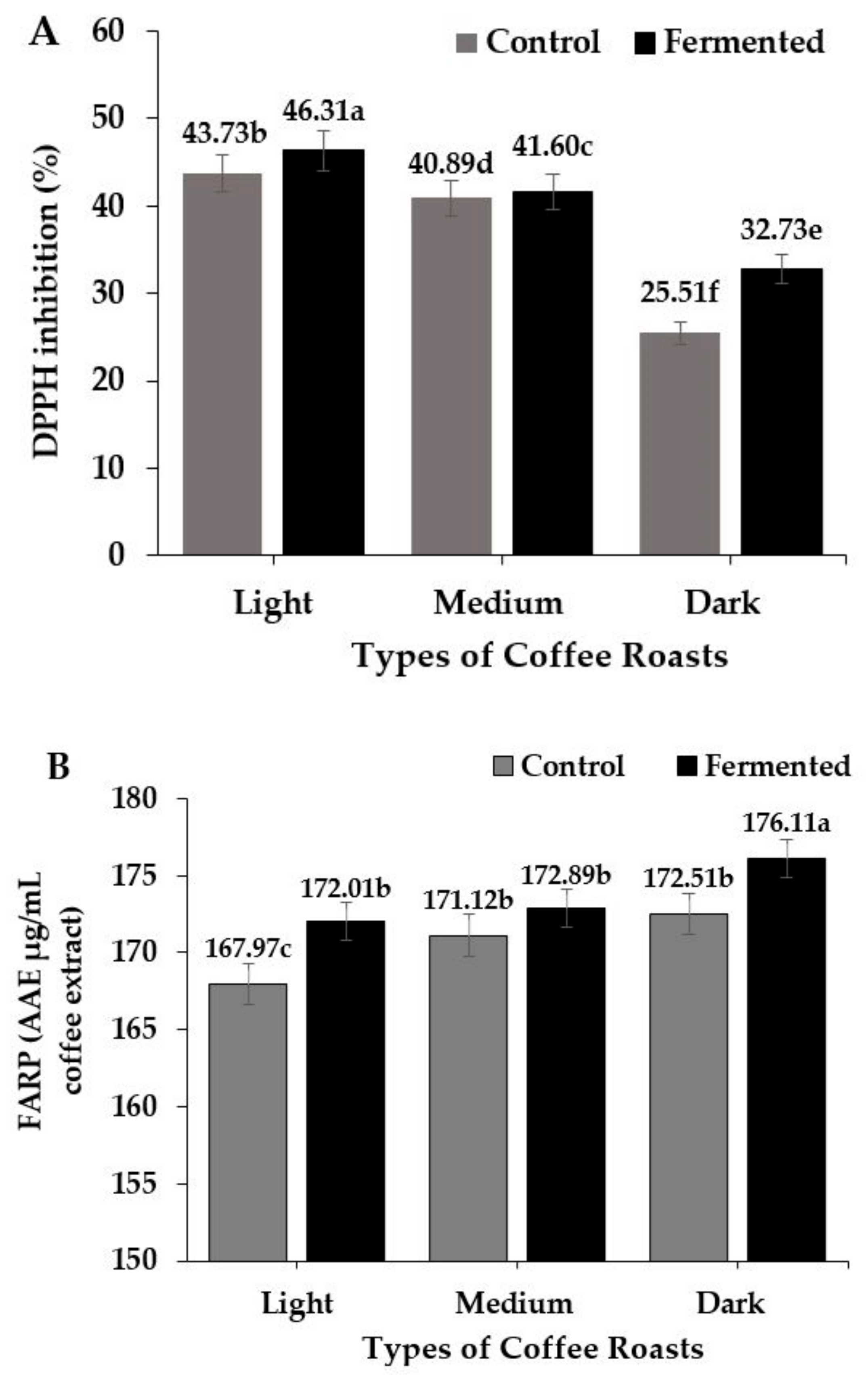
3.3.2. DPPH and FRAP
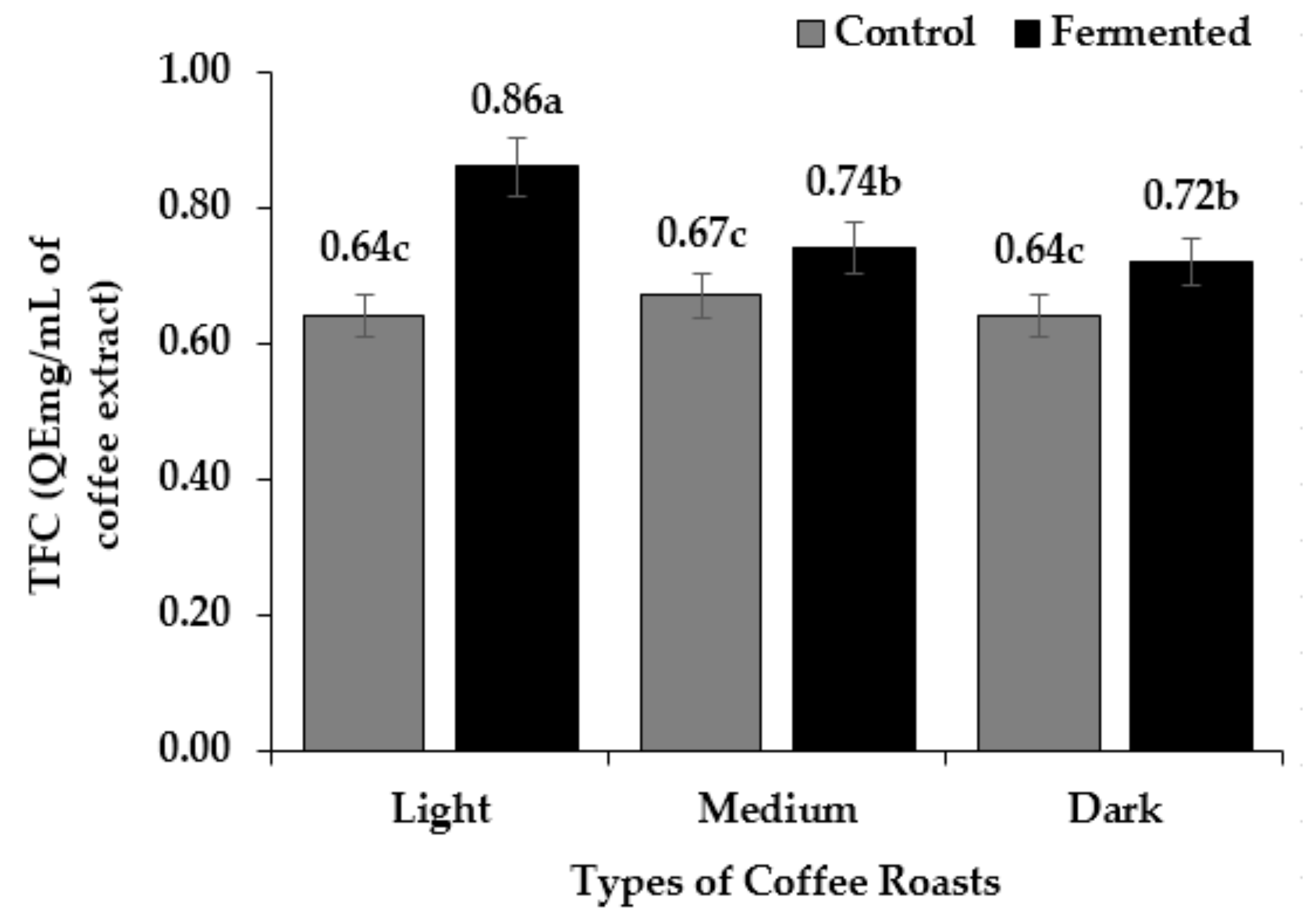
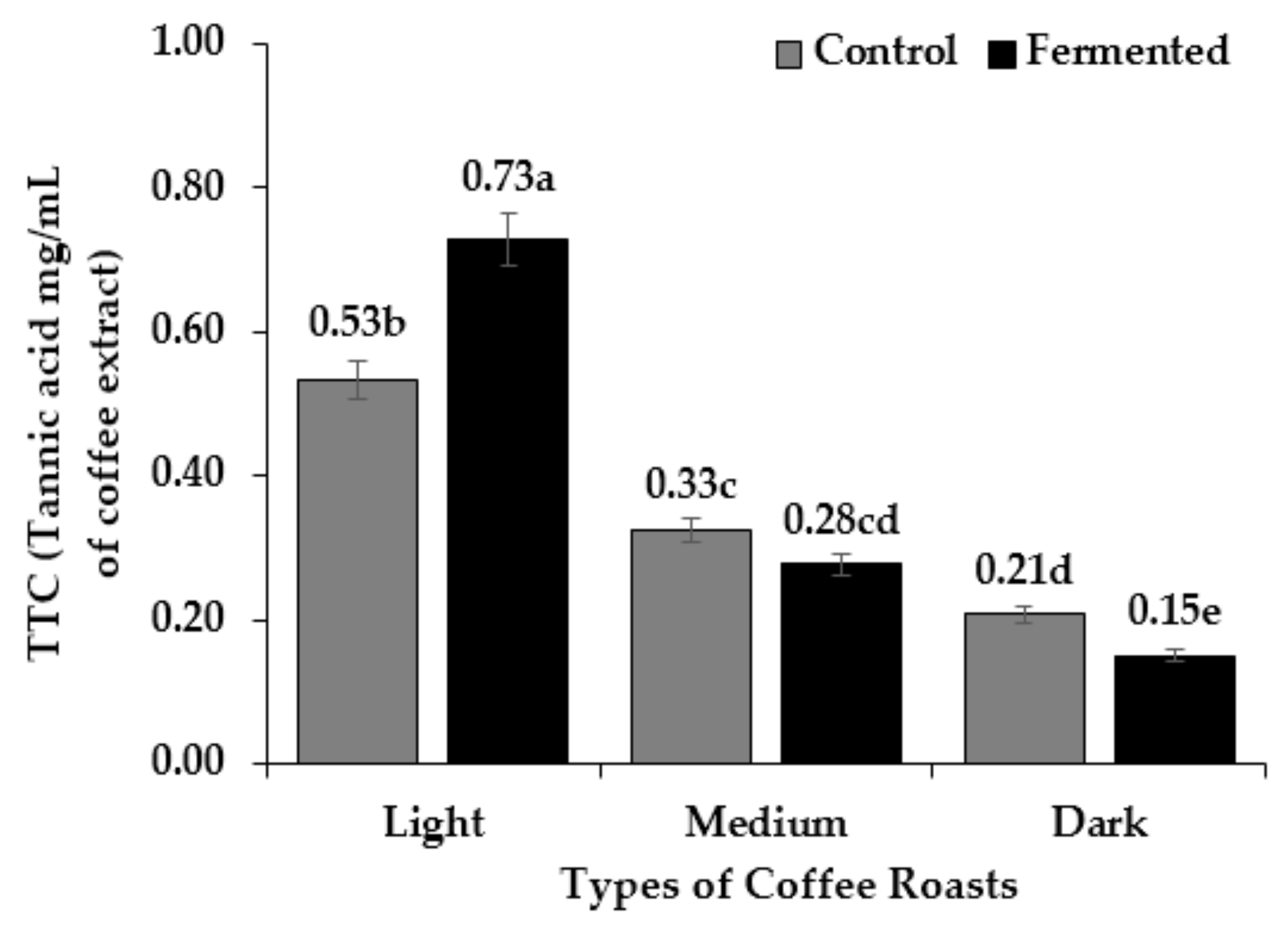
3.4. Total Phenol, Flavonoid and Tannin Content
3.5. Sensory Evaluation
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Mesfin, H.; Kang, W.H. The role of microbes in coffee fermentation and their impact on coffee quality. J. Food Qual. 2019. [Google Scholar] [CrossRef]
- Martins, S.; Mussatto, S.I.; Martínez-Avila, G.; Montañez-Saenz, J.; Aguilar, C.N.; Teixeira, J.A. Bioactive Phenolic Compounds: Production and Extraction by Solid-State Fermentation. A Review. Biotechnol. Adv. 2011, 29, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Torino, M.I.; Limón, R.I.; Martínez-Villaluenga, C.; Mäkinen, S.; Pihlanto, A.; Vidal-Valverde, C.; Frias, J. Antioxidant and Antihypertensive Properties of Liquid and Solid State Fermented Lentils. Food Chem. 2013, 136, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Dulf, F.V.; Vodnar, D.C.; Socaciu, C. Effects of solid-state fermentation with two filamentous fungi on the total phenolic contents, flavonoids, antioxidant activities and lipid fractions of plum fruit (Prunus domestica L.) by-products. Food Chem. 2016, 209, 27–36. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Chen, J.; Li, X.L.; Yi, K.; Ye, Y.; Liu, G.; Wang, Z.G. Dynamic changes in antioxidant activity and biochemical composition of tartary buckwheat leaves during Aspergillus niger fermentation. J. Funct. Foods 2017, 32, 375–381. [Google Scholar] [CrossRef]
- Belitz, H.D.; Grosh, W.; Schieberle, P. Coffee, Tea, Cocoa. In Food Chemistry; Springer-Verlag: Heidelberg/Berlin, Germany; New York, NY, USA, 2009. [Google Scholar]
- Rawel, H.M.; Kulling, S.E. Nutritional contribution of coffee, cacao and tea phenolics to human health. J. Verbraucherschutz Lebensmittelsicherheit 2007, 2, 399–406. [Google Scholar] [CrossRef]
- Richelle, M.; Tavazzi, I.; Offord, E. Comparison of the antioxidant activity of commonly consumed polyphenolic beverages (coffee, cocoa, and tea) prepared per cup serving. J Agric. Food Chem. 2001, 49, 3438–3442. [Google Scholar] [CrossRef]
- Cheynier, V. Polyphenols in foods are more complex than often thought. Am. J. Clin. Nutr. 2005, 81, 223S–229S. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Kumar, S.; Mishra, A.; Pandey, A.K. Antioxidant mediated protective effect of Parthenium hysterophorus against oxidative damage using in vitro models. BMC Complement Altern. Med. 2013, 13, 120. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Phenolic content, reducing power and membrane protective activities of Solanum xanthocarpum root extracts. Vegetos 2013, 26, 301–307. [Google Scholar]
- Yao, L.H.; Jiang, Y.M.; Shi, J.; Tomás-Barberán, F.A.; Datta, N.; Singanusong, R.; Chen, S.S. Flavonoids in food and their health benefits. Plant Foods Hum. Nutr. 2004, 59, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Kurtzman, C.P.; Fell, J.W. The Yeast, a Taxonomical Study, 4th ed.; Elsevier Science: Amsterdam, The Netherlands, 1988; p. 1055. [Google Scholar]
- De Hoog, G.S. Risk assessment of fungi reported from humans and animals. Mycoses 1996, 39, 407–417. [Google Scholar] [CrossRef]
- Comitini, F.; De Ingenis, J.; Pepe, L.; Mannazu, I.; Ciani, M. Pichia anomala and Kluyveromyces wikerhamii killer toxins as new tool against Dekkera/Brettanomyces spoilage yeasts. FEMS Microbiol. Lett. 2004, 238, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Choi, Y.R.; Lee, S.Y.; Park, J.T.; Shim, J.H.; Park, K.H.; Kim, J.W. Screening wild yeast strains for alcohol fermentation from various fruits. Mycobiology 2011, 39, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Yue, T.; Yuan, Y. Effects of sequential mixed cultures of Wickerhamomyces anomalus and Saccharomyces cerevisiae on apple cider fermentation. FEMS Yeast Res. 2014, 14, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Madrigal, T.; Maicas, S.; Tolosa, J.J.M. Glucose and ethanol tolerant enzymes produced by Pichia (Wickerhamomyces) isolates from enological ecosystems. Am. J. Enol. Vitic. 2013, 64, 126–133. [Google Scholar] [CrossRef]
- Zha, M.; Sun, B.; Wu, Y.; Yin, S.; Wang, C. Improving flavor metabolism of Saccharomyces cerevisiae by mixed culture with Wickerhamomyces anomalus for Chinese Baijiu making. J. Biosci. Bioeng. 2018, 126, 189–195. [Google Scholar] [CrossRef]
- Kwak, H.S.; Jeong, Y.; Kim, M. Effect of Yeast Fermentation of Green Coffee Beans on Antioxidant Activity and Consumer Acceptability. J. Food Qual. 2018. [Google Scholar] [CrossRef]
- Mesfin, H.; Kang, W.H. Antioxidant Activity, Total Polyphenol, Flavonoid and Tannin Contents of Fermented Green Coffee Beans with Selected Yeasts. Fermentation 2019, 5, 29. [Google Scholar]
- Lee, L.W.; Cheong, M.W.; Curran, P.; Yu, B.; Liu, S.Q. Modulation of coffee aroma via the fermentation of green coffee beans with Rhizopus oligosporus: I. Green Coffee. Food Chem. 2016, 211, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.W.; Tay, G.Y.; Cheong, M.W.; Curran, P.; Yu, B.; Liu, S.Q. Modulation of the volatile and non-volatile profiles of coffee fermented with Yarrowia lipolytica: I. Green coffee. LWT- Food Sci. Technol. 2017, 77, 225–232. [Google Scholar] [CrossRef]
- Mesfin, H.; Kang, W.H. Isolation, Identification, and Characterization of Pectinolytic Yeasts for Starter Culture in Coffee Fermentation. Microorganisms 2019, 7, 401. [Google Scholar]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Pataro, G.; Sinik, M.; Capitoli, M.M.; Donsì, G.; Ferrari, G. The influence of post-harvest UV-C and pulsed light treatments on quality and antioxidant properties of tomato fruits during storage. Innov. Food Sci. Emerg. Technol. 2015, 30, 103–111. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Dewanto, V.; Wu, X.; Liu, R.H. Processed sweet corn has higher antioxidant activity. J. Agric. Food Chem. 2002, 50, 4959–4964. [Google Scholar] [CrossRef]
- CI, K.C.; Indira, G. Quantitative estimation of total phenolic, flavonoids, tannin and chlorophyll content of leaves of Strobilanthes Kunthiana (Neelakurinji). J. Med. Plants 2016, 4, 282–286. [Google Scholar]
- Coote, N.; Kirsop, B.H. Factors responsible for the decrease in pH during beer fermentations. J. Inst. Brew. 1976, 82, 149–153. [Google Scholar] [CrossRef]
- Rodrigues, C.I.; Marta, L.; Maia, R.; Miranda, M.; Ribeirinho, M.; Máguas, C. Application of solid-phase extraction to brewed coffee caffeine and organic acid determination by UV/HPLC. J. Food Compos. Anal. 2007, 20, 440–448. [Google Scholar] [CrossRef]
- Afoakwa, E.O.; Paterson, A.; Fowler, M.; Ryan, A. Flavor formation and character in cocoa and chocolate: A critical review. Food Sci. Nutr. 2008, 48, 840–857. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.Y.; Choi, K.H.; Kim, S.J.; Choi, D.W.; Kim, Y.S.; Kim, Y.C. Peroxyl radical-scavenging activity of coffee brews. Eur. Food Res. Technol. 2005, 221, 471–477. [Google Scholar]
- Gómez-Ruiz, J.Á.; Leake, D.S.; Ames, J.M. In Vitro Antioxidant Activity of Coffee Compounds and Their Metabolites. J. Agric. Food Chem. 2007, 55, 6962–6969. [Google Scholar] [CrossRef] [PubMed]
- Nicoli, M.C.; Anese, M.; Manzocco, L.; Lerici, C.R. Antioxidant properties of coffee brews in relation to the roasting degree. Lebensm. Wiss. Technol. 1997, 30, 292–297. [Google Scholar] [CrossRef]
- Liu, Y.; Kitts, D.D. Confirmation that the Maillard reaction is the principle contributor to the antioxidant capacity of coffee brews. Food Res. Int. 2011, 44, 2418–2424. [Google Scholar] [CrossRef]
- Vignoli, J.A.; Viegas, M.C.; Bassoli, D.G.; Benassi, M.T. Roasting process affects differently the bioactive compounds and the antioxidant activity of arabica and robusta coffees. Food Res. Int. 2014, 61, 279–285. [Google Scholar] [CrossRef]
- Sánchez-González, I.; Jiménez-Escrig, A.; Saura-Calixto, F. In vitro antioxidant activity of coffees brewed using different procedures (Italian, espresso and filter). Food Chem. 2005, 90, 113–139. [Google Scholar] [CrossRef]
- Chandrasekara, N.; Shahidi, F. Effect of roasting on phenolic content and antioxidant activities of whole cashew nuts, kernels, and testa. J. Agric. Food Chem. 2011, 59, 5006–5014. [Google Scholar] [CrossRef]
- Del Castillo, M.D.; Ames, J.M.; Gordon, M.H. Effect of roasting on the antioxidant activity of coffee brews. J. Agric. Food Chem. 2002, 50, 3698–3703. [Google Scholar] [CrossRef] [PubMed]
- Cämmerer, B.; Kroh, L.W. Antioxidant activity of coffee brews. Eur. Food Res. Technol. 2006, 223, 469–474. [Google Scholar] [CrossRef]
- Perrone, D.; Farah, A.; Donangelo, C.M. Influence of coffee roasting on the incorporation of phenolic compounds into melanoidins and their relationship with antioxidant activity of the brew. J. Agric. Food Chem. 2012, 60, 4265–4275. [Google Scholar] [CrossRef] [PubMed]
- Szymanowska, K.; Wołosiak, R. The effect of coffee roasting on selected parameters of its quality. Res. Teach. Appar. 2014, 19, 77–83. [Google Scholar]
- Duarte, S.M.S.; Abreu, C.M.P.; Menezes, H.C.; Santos, M.H.; Gouvêa, C.M.C.P. Effect of processing and roasting on the antioxidant activity of coffee brews. Ciênc. Tecnol. Aliment. 2005, 25, 387–393. [Google Scholar] [CrossRef]
- Hecimovic, I.; Belscak-Cvitanovic, A.; Horzic, D.; Komes, D. Comparative study of polyphenols and caffeine in different coffee varieties affected by the degree of roasting. Food Chem. 2011, 129, 991–1000. [Google Scholar] [CrossRef]
- Sacchetti, G.; Mattia, C.D.; Pittia, P.; Mastrocola, D. Effect of roasting degree, equivalent thermal effect and coffee type on the radical scavenging activity of coffee brews and their phenolic fraction. J. Food Eng. 2009, 90, 74–80. [Google Scholar] [CrossRef]
- Summa, C.A.; Calle, B.; Brohee, M.; Stadler, R.H.; Anklama, E. Impact of the roasting degree of coffee on the in vitro radical scavenging capacity and content of acrylamide. LWT- Food Sci. Technol. 2007, 40, 1849–1854. [Google Scholar] [CrossRef]
- Adetuyi, F.O.; Ibrahim, T.A. Effect of fermentation time on the phenolic, flavonoid and vitamin C contents and antioxidant activities of okra (Abelmoschus esculentus) seeds. Niger. Food J. 2014, 32, 128–137. [Google Scholar] [CrossRef]
- Abu, F.; Taib, M.; Norma, C.; Moklas, M.; Aris, M.; Mohd Akhir, S. Antioxidant Properties of Crude Extract, Partition Extract, and Fermented Medium of Dendrobium sabin Flower. J. Evid.-Based Complement. Altern. Med. 2017. [Google Scholar] [CrossRef]
- Jung, S.; Kim, M.H.; Park, J.H.; Jeong, Y.; Ko, K.S. Cellular antioxidant and anti-inflammatory effects of coffee extracts with different roasting levels. J. Med. Food. 2017, 20, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Song, J.L.; Asare, T.S.; Kang, M.Y.; Lee, S.C. Changes in Bioactive Compounds and Antioxidant Capacity of Coffee under Different Roasting Conditions. Korean J. Plant Resour. 2018, 31, 704–713. [Google Scholar]
- Moktan, B.; Saha, J.; Sarkar, P.K. Antioxidant activities of soybean as affected by Bacillus-fermentation to kinema. Food Res. Int. 2008, 41, 586–593. [Google Scholar] [CrossRef]
- Ademiluyi, A.O.; Oboh, G. Antioxidant properties of condiment produced from fermented bambara groundnut (Vigna subterranea L. Verdc). J. Food Biochem. 2011, 35, 1145–1160. [Google Scholar] [CrossRef]
- Dajanta, K.; Janpum, P.; Leksing, W. Antioxidant capacities, total phenolics and flavonoids in black and yellow soybeans fermented by Bacillus subtilis: A comparative study of Thai fermented soybeans (thua nao). Int. Food Res. J. 2013, 20, 3125. [Google Scholar]
- Guzmán-Uriarte, M.L.; Sánchez-Magaña, L.M.; Angulo-Meza, G.Y.; Cuevas-Rodríguez, E.O.; Gutiérrez-Dorado, R.; Mora-Rochín, S.; Reyes-Moreno, C. Solid state bioconversion for producing common bean (Phaseolus vulgaris L.) functional flour with high antioxidant activity and antihypertensive potential. Food Nutr. Sci. 2013, 4, 480. [Google Scholar]
- Plaitho, Y.; Kangsadalampai, K.; Sukprasansap, M. The protective effect of Thai fermented pigmented rice on urethane induced somatic mutation and recombination in Drosophila melanogaster. J. Med. Plant Resour. 2013, 7, 91–98. [Google Scholar]
- Amadou, I.; Yong-Hui, S.; Sun, J.; Guo-Wei, L. Fermented soybean products: Some methods, antioxidants compound extraction and their scavenging activity. Asian J. Biochem. 2009, 4, 68–76. [Google Scholar]
- Wang, C.Y.; Sz-Jie, W.; Shyu, Y.T. Antioxidant Properties of Certain Cereals as Affected by Food-Grade Bacteria Fermentation. J. Biosci. Bioeng. 2014, 117, 449–456. [Google Scholar] [CrossRef]
- Shrestha, A.K.; Dahal, N.R.; Ndungutse, V. Bacillus fermentation of soybean: A review. J. Food Sci. Technol. 2010, 6, 1–9. [Google Scholar] [CrossRef]
- Moustakime, Y.; Hazzoumi, Z.; Joutei, K.A. Effect of proteolytic activities in combination with the pectolytic activities on extractability of the fat and phenolic compounds from olives. Springer Plus 2016, 5, 739. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Odžaković, B.; Džinić, N.; Kukrić, Z.; Grujić, S. Effect of roasting degree on the antioxidant activity of different Arabica coffee quality classes. Acta Sci. Pol. Technol. Aliment. 2016, 15, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, B.K.; Brunton, N.P.; Brennan, C. Handbook of plant food phytochemicals: Sources, stability and extraction. John Wiley and Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Kumar, S.; Pandey, A.K. Antioxidant, lipo-protective and antibacterial activities of phytoconstituents present in Solanum xanthocarpum root. Int. Rev. Biophys. Chem. 2012, 3, 42–47. [Google Scholar]
- Hassan, I.A.; El Tinay, A.H. Effect of fermentation on tannin content and in-vitro protein and starch digestibilities of two sorghum cultivars. Food Chem. 1995, 53, 149–151. [Google Scholar] [CrossRef]
- Shang, Y.F.; Cao, H.; Ma, Y.L.; Zhang, C.; Ma, F.; Wang, C.X.; Wei, Z.J. Effect of lactic acid bacteria fermentation on tannins removal in Xuan Mugua fruits. Food Chem. 2019, 274, 118–122. [Google Scholar] [CrossRef]
- Agume, A.; Njintang, N.; Mbofung, C. Effect of soaking and roasting on the physicochemical and pasting properties of soybean flour. Foods 2017, 6, 12. [Google Scholar] [CrossRef]
- Oboh, G.; Ademiluyi, O.A.; Akindahunsi, A.A. The effect of roasting on the nutritional and antioxidant properties of yellow and white maize varieties. Int. J. Food Sci. Technol. 2010, 45, 1236–1242. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haile, M.; Kang, W.H. Antioxidant Properties of Fermented Green Coffee Beans with Wickerhamomyces anomalus (Strain KNU18Y3). Fermentation 2020, 6, 18. https://doi.org/10.3390/fermentation6010018
Haile M, Kang WH. Antioxidant Properties of Fermented Green Coffee Beans with Wickerhamomyces anomalus (Strain KNU18Y3). Fermentation. 2020; 6(1):18. https://doi.org/10.3390/fermentation6010018
Chicago/Turabian StyleHaile, Mesfin, and Won Hee Kang. 2020. "Antioxidant Properties of Fermented Green Coffee Beans with Wickerhamomyces anomalus (Strain KNU18Y3)" Fermentation 6, no. 1: 18. https://doi.org/10.3390/fermentation6010018
APA StyleHaile, M., & Kang, W. H. (2020). Antioxidant Properties of Fermented Green Coffee Beans with Wickerhamomyces anomalus (Strain KNU18Y3). Fermentation, 6(1), 18. https://doi.org/10.3390/fermentation6010018





