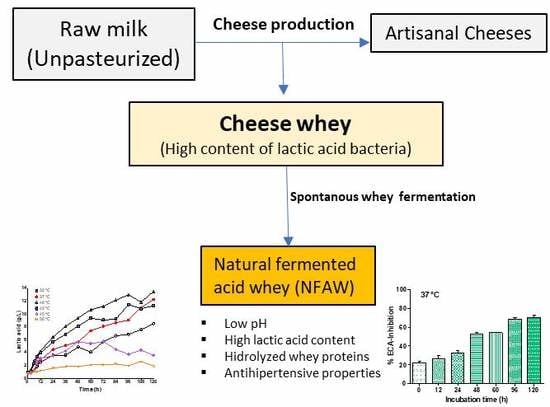
Cheese Whey Fermentation by Its Native Microbiota: Proteolysis and Bioactive Peptides Release with ACE-Inhibitory Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Materials
2.3. Lactic Acid Fermentation of Whey
2.4. Physicochemical Analysis
2.5. Proteolysis Evaluation
2.6. SDS-PAGE Analysis
2.7. ACE-Inhibitory Activity Assay
2.8. Statistical Analysis
3. Results
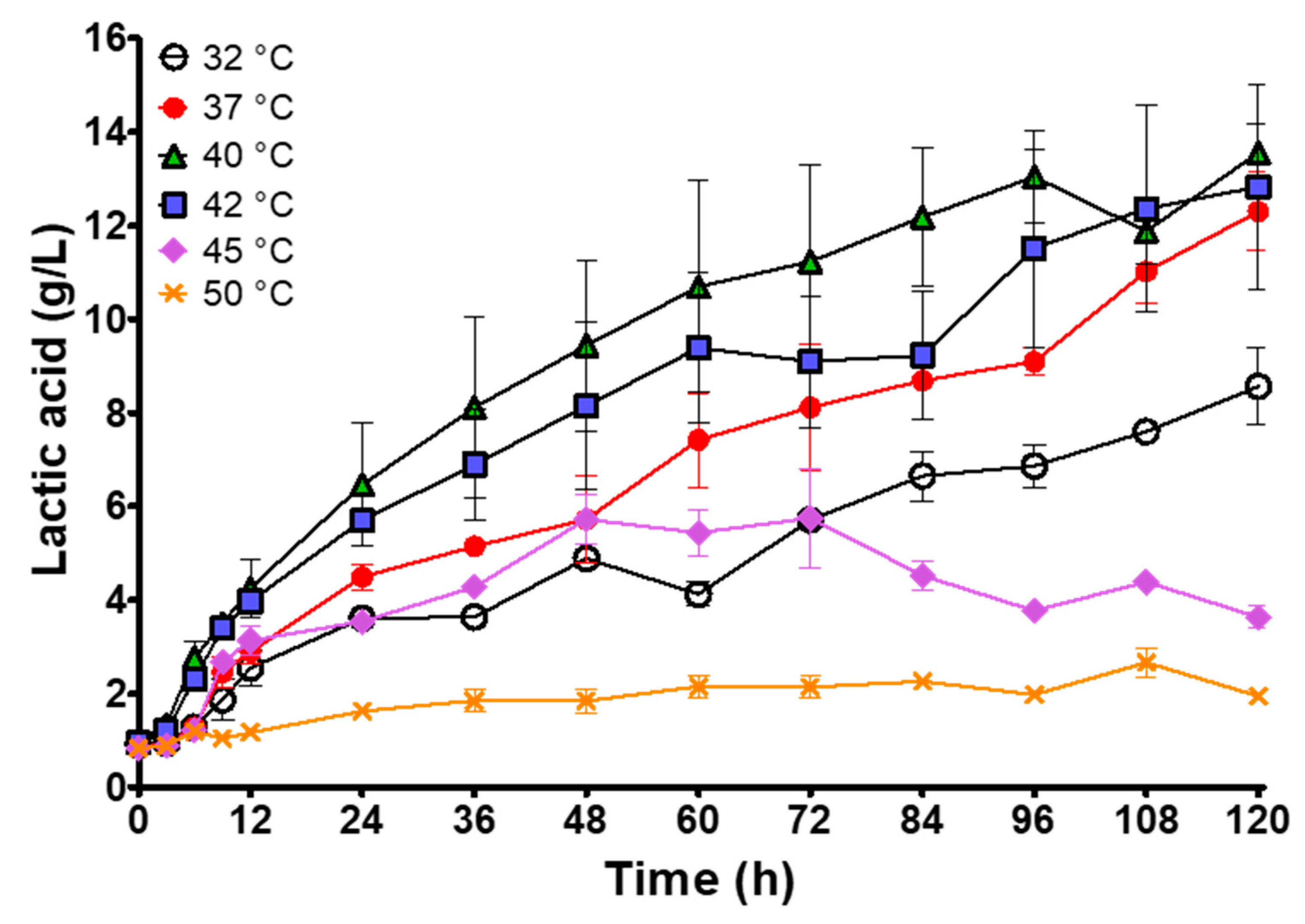
3.1. Temperature Effect on Spontaneous Lactic Acid Whey Fermentation
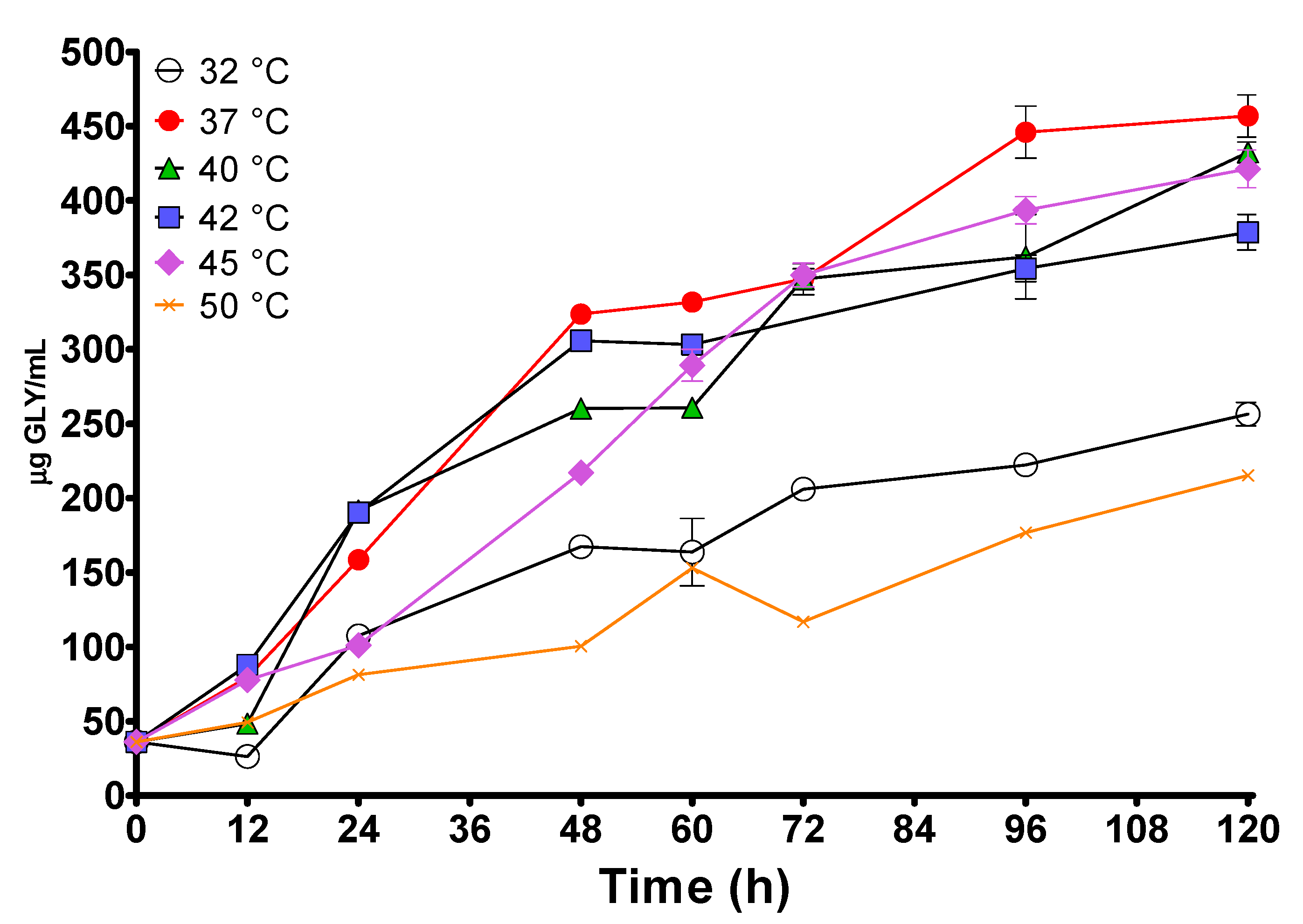
3.2. Proteolysis Occurring during Spontaneous Whey Fermentation
3.3. ACE-Inhibitory Activity in Fermented Whey
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- El-Tanboly, E.S.; El-Hofi, M. Khorshid. Recovery of cheese whey, a by-product from the dairy industry for use as an animal feed. J. Nutr. Health Food Eng. 2017, 6, 148–154. [Google Scholar] [CrossRef][Green Version]
- Yadav, J.S.S.; Yan, S.; Pilli, S.; Kumar, L.; Tyagi, R.D.; Surampalli, R.Y. Cheese whey: A potential resource to transform into bioprotein, functional/nutritional proteins and bioactive peptides. Biotechnol. Adv. 2015, 33, 756–774. [Google Scholar] [CrossRef]
- Mazorra-Manzano, M.A.; Moreno-Hernández, J.M. Properties and options for the valorization of whey from the artisanal cheese industry. CienciaUAT 2019, 14, 133–144. [Google Scholar]
- McSweeney, P.L.H. Biochemistry of cheese ripening. Int. J. Dairy Technol. 2004, 57, 127–144. [Google Scholar] [CrossRef]
- Hernández-Rojas, M.; Vélez-Ruíz, J.F. Suero de leche y su aplicación en la elaboración de alimentos funcionales. Temas Sel. Ing. Aliment. 2014, 8, 13–22. [Google Scholar]
- Brandelli, A.; Daroit, D.J.; Corrêa, A.P.F. Whey as a source of peptides with remarkable biological activities. Food Res. Int. 2015, 73, 149–161. [Google Scholar] [CrossRef]
- Cuevas-González, P.F.; Heredia-Castro, P.Y.; Méndez-Romero, J.I.; Hernández-Mendoza, A.; Reyes-Díaz, R.; Vallejo-Cordoba, B.; González-Córdova, A.F. Artisanal Sonoran cheese (Cocido cheese): An exploration of its production process, chemical composition and microbiological quality. J. Sci. Food Agric. 2017, 97, 4459–4466. [Google Scholar] [CrossRef]
- Mazorra-Manzano, M.A.; Ramírez-Suarez, J.C.; Yada, R.Y. Plant proteases for bioactive peptides release: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 2147–2163. [Google Scholar] [CrossRef]
- Hernández-Ledesma, B.; Contreras, M.M.; Recio, I. Antihypertensive peptides: Production, bioavailability and incorporation into foods. Adv. Colloid Interface Sci. 2011, 165, 23–35. [Google Scholar] [CrossRef]
- Rodríguez-Figueroa, J.C.; González-Córdova, A.F.; Astiazaran-García, H.; Hernández-Mendoza, A.; Vallejo-Cordoba, B. Antihypertensive and hypolipidemic effect of milk fermented by specific Lactococcus lactis strains. J. Dairy Sci. 2013, 96, 4094–4099. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2002. [Google Scholar]
- Church, F.C.; Swisgood, H.E.; Porter, D.H.; Catignani, G.L. Spectrophotometric Assay Using o-Phthaldialdehyde for Determination of Proteolysis in Milk and Isolated Milk Proteins. J. Dairy Sci. 1983, 66, 1219–1227. [Google Scholar] [CrossRef]
- Laemmli, U. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Cushman, D.W.; Cheung, H.S. Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem. Pharmacol. 1971, 20, 1637–1648. [Google Scholar] [CrossRef]
- Wu, J.; Aluko, R.E.; Muir, A.D. Improved method for direct high-performance liquid chromatography assay of angiotensin-converting enzyme-catalyzed reactions. J. Chromatogr. A 2002, 950, 125–130. [Google Scholar] [CrossRef]
- Spalatelu, C. Biotechnological Valorisation of Whey. Innov. Rom. Food Biotechnol. 2012, 10, 1–8. [Google Scholar]
- Blaschek, K.M.; Wendorff, W.L.; Rankin, S.A. Survey of Salty and Sweet Whey Composition from Various Cheese Plants in Wisconsin. J. Dairy Sci. 2007, 90, 2029–2034. [Google Scholar] [CrossRef]
- Tarango-Hernández, S.; Alarcón-Rojo, A.D.; Robles-Sánchez, M.; Gutiérrez-Méndez, N.; Rodríguez-Figueroa, J.C. Short communication: Potential of Fresco-style cheese whey as a source of protein fractions with antioxidant and angiotensin-I-converting enzyme inhibitory activities. J. Dairy Sci. 2015, 98, 7635–7639. [Google Scholar] [CrossRef]
- Panesar, P.S.; Kennedy, J.F.; Gandhi, D.N.; Bunko, K. Bioutilisation of whey for lactic acid production. Food Chem. 2007, 105, 1–14. [Google Scholar] [CrossRef]
- Simanca, M.; Arteaga, M.; Pérez, Y.; Soto, M.; Salcedo, J. Characterization and study of spontaneous fermentation offermented milk product (suero costeño) produced in Monteria. MVZ Córdoba 2010, 15, 1944–1953. [Google Scholar]
- Klupsaite, D.; Juodeikiene, G.; Arbones, E.; Quintáns, A.P.; Zadeike, D.; Bartkiene, E.; Glasner, C.; Dikiy, A.; Shumilina, E. A Comparison Study on the Production and Recovery of Lactic Acid by Fermenting Dairy By-Products with P. acidilactici and Lb. delbrüeckii spp. bulgaricus. Waste Biomass Valoriz. 2019, 10, 1519–1528. [Google Scholar] [CrossRef]
- De Candia, S.; De Angelis, M.; Dunlea, E.; Minervini, F.; McSweeney, P.L.H.; Faccia, M.; Gobbetti, M. Molecular identification and typing of natural whey starter cultures and microbiological and compositional properties of related traditional Mozzarella cheeses. Int. J. Food Microbiol. 2007, 119, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Aldrete-Tapia, A.; Escobar-Ramírez, M.C.; Tamplin, M.L.; Hernández-Iturriaga, M. High-throughput sequencing of microbial communities in Poro cheese, an artisanal Mexican cheese. Food Microbiol. 2014, 44, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Pescuma, M.; Hébert, E.M.; Bru, E.; de Valdez, G.F.; Mozzi, F. Diversity in growth and protein degradation by dairy relevant lactic acid bacteria species in reconstituted whey. J. Dairy Res. 2012, 79, 201–208. [Google Scholar] [CrossRef]
- Pescuma, M.; Hébert, E.M.; Mozzi, F.; Font de Valdez, G. Whey fermentation by thermophilic lactic acid bacteria: Evolution of carbohydrates and protein content. Food Microbiol. 2008, 25, 442–451. [Google Scholar] [CrossRef]
- Pessione, E.; Cirrincione, S. Bioactive Molecules Released in Food by Lactic Acid Bacteria: Encrypted Peptides and Biogenic Amines. Front. Microbiol. 2016, 7, 876. [Google Scholar] [CrossRef] [PubMed]
- Pescuma, M.; Valdez, G.F.D.; Mozi, F. Whey-derived valuable products obtained by microbial fermentation. Appl. Microbiol. Biotechnol. 2015, 99, 6183–6196. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, R.; Tsutsumi, Y.M. Peptides and proteins in whey and their benefits for human health. Austin J. Nutr. Food Sci. 2014, 1, 1002. [Google Scholar]
- Virtanen, T.; Pihlanto, A.; Akkanen, S.; Korhonen, H. Development of antioxidant activity in milk whey during fermentation with lactic acid bacteria. J. Appl. Microbiol. 2007, 102, 106–115. [Google Scholar] [CrossRef]
- Ibrahim, H.R.; Ahmed, A.S.; Miyata, T. Novel angiotensin-converting enzyme inhibitory peptides from caseins and whey proteins of goat milk. J. Adv. Res. 2017, 8, 63–71. [Google Scholar] [CrossRef]
- Mullally, M.M.; Meisel, H.; FitzGerald, R.J. Angiotensin-I-converting enzyme inhibitory activities of gastric and pancreatic proteinase digests of whey proteins. Int. Dairy J. 1997, 7, 299–303. [Google Scholar] [CrossRef]
- Pereira, Á.M.D.S.; de Farias, D.R.B.; de Queiroz, B.B.; Nobre, M.S.D.C.; Cavalcanti, M.T.; Salles, H.O.; Dos Santos, K.M.O.; de Medeiros, A.C.D.; Florentino, E.R.; Alonso Buriti, F.C. Influence of a Co-culture of Streptococcus thermophilus and Lactobacillus casei on the Proteolysis and ACE-Inhibitory Activity of a Beverage Based on Reconstituted Goat Whey Powder. Probiot. Antimicrob. Proteins 2017, 11, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Barrientos, L.M.; Hernández-Mendoza, A.; Torres-Llanez, M.J.; González-Córdova, A.F.; Vallejo-Córdoba, B. Invited review: Fermented milk as antihypertensive functional food. J. Dairy Sci. 2016, 99, 4099–4110. [Google Scholar] [CrossRef] [PubMed]
- Didelot, S.; Bordenave-Juchereau, S.; Rosenfeld, E.; Fruitier-Arnaudin, I.; Piot, J.-M.; Sannier, F. Preparation of angiotensin-I-converting enzyme inhibitory hydrolysates from unsupplemented caprine whey fermentation by various cheese microflora. Int. Dairy J. 2006, 16, 976–983. [Google Scholar] [CrossRef]
- Nabi, X.-H.; Ma, C.-Y.; Manaer, T.; Heizati, M.; Wulazibieke, B.; Aierken, L. Anti-atherosclerotic effect of traditional fermented cheese whey in atherosclerotic rabbits and identification of probiotics. BMC Complement. Altern. Med. 2016, 16, 1–13. [Google Scholar] [CrossRef]
- Hong, S.-M.; Chung, E.-C.; Kim, C.-H. Anti-obesity Effect of Fermented Whey Beverage using Lactic Acid Bacteria in Diet-induced Obese Rats. Korean J. Food Sci. Anim. Resour. 2015, 35, 653–659. [Google Scholar] [CrossRef]
- Zhao, Z.W.; Pan, D.D.; Wu, Z.; Sun, Y.Y.; Guo, Y.X.; Zeng, X.Q. Antialcoholic liver activity of whey fermented by Lactobacillus casei isolated from koumiss. J. Dairy Sci. 2014, 97, 4062–4071. [Google Scholar] [CrossRef]
- AbdulAlim, T.S.; Zayan, A.; Campelo, P.H.; Bakry, A.M. Development of new functional fermented product: Mulberry-whey beverage. J. Nutr. Food Res. Technol. 2018, 1, 64–69. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazorra-Manzano, M.A.; Robles-Porchas, G.R.; González-Velázquez, D.A.; Torres-Llanez, M.J.; Martínez-Porchas, M.; García-Sifuentes, C.O.; González-Córdova, A.F.; Vallejo-Córdoba, B. Cheese Whey Fermentation by Its Native Microbiota: Proteolysis and Bioactive Peptides Release with ACE-Inhibitory Activity. Fermentation 2020, 6, 19. https://doi.org/10.3390/fermentation6010019
Mazorra-Manzano MA, Robles-Porchas GR, González-Velázquez DA, Torres-Llanez MJ, Martínez-Porchas M, García-Sifuentes CO, González-Córdova AF, Vallejo-Córdoba B. Cheese Whey Fermentation by Its Native Microbiota: Proteolysis and Bioactive Peptides Release with ACE-Inhibitory Activity. Fermentation. 2020; 6(1):19. https://doi.org/10.3390/fermentation6010019
Chicago/Turabian StyleMazorra-Manzano, Miguel A., Glen R. Robles-Porchas, Daniel A. González-Velázquez, María J. Torres-Llanez, Marcel Martínez-Porchas, Celia O. García-Sifuentes, Aarón F. González-Córdova, and Belinda Vallejo-Córdoba. 2020. "Cheese Whey Fermentation by Its Native Microbiota: Proteolysis and Bioactive Peptides Release with ACE-Inhibitory Activity" Fermentation 6, no. 1: 19. https://doi.org/10.3390/fermentation6010019
APA StyleMazorra-Manzano, M. A., Robles-Porchas, G. R., González-Velázquez, D. A., Torres-Llanez, M. J., Martínez-Porchas, M., García-Sifuentes, C. O., González-Córdova, A. F., & Vallejo-Córdoba, B. (2020). Cheese Whey Fermentation by Its Native Microbiota: Proteolysis and Bioactive Peptides Release with ACE-Inhibitory Activity. Fermentation, 6(1), 19. https://doi.org/10.3390/fermentation6010019